Abstract
Purpose
To determine whether stereopsis of infants treated for monocular cataracts varies with the type of optical correction used.
Design
Randomized prospective clinical trial
Methods
The Infant Aphakia Treatment Study randomized 114 patients with unilateral cataracts at age 1 to 7 months to either primary intraocular lens (IOL) or contact lens correction. At 4.5 years of age a masked examiner assessed stereopsis on these patients using three different tests: 1) Frisby; 2) Randot Preschool; and 3) Titmus fly.
Results
Twenty-eight patients (25%) had a positive response to at least one of the stereopsis tests. There was no statistically significant difference in stereopsis between the two treatment groups. Frisby (contact lens, 6 (11%); IOL, 7 (13%); p=0.99), Randot (contact lens, 3 (6%); IOL, 1 (2%); p=0.62) or Titmus: (contact lens, 8 (15%); IOL, 13 (23%); p=0.34). The median age at surgery for patients with stereopsis was younger than for those without stereopsis (1.2 versus 2.4 months; p=0.002). The median visual acuity for patients with stereopsis was better than for those without stereopsis (20/40 vs. 20/252; p=0.0003).
Conclusion
The type of optical correction did not influence stereopsis outcomes. However, two other factors did: age at surgery and visual acuity in the treated eye at age 4.5 years. Early surgery for unilateral congenital cataract and the presence of visual acuity better than or equal to 20/40 appear to be more important than the type of initial optical correction used for the development of stereopsis.
Introduction
The ability to perceive depth based on image disparity between the two eyes (stereopsis) is the hallmark of human binocular vision. Patients with a unilateral congenital cataract disabling enough to require surgery do not generally demonstrate stereopsis despite best efforts at visual rehabilitation.1,2 Infants who have undergone surgery for a unilateral cataract do not experience the typical level of equal and simultaneous visual input to both eyes, which is required for the development of critical cortical connections underlying stereoscopic vision. Anecdotal accounts of good stereopsis following unilateral congenital cataract surgery have been reported but treatment regimens and stereopsis testing were not standardized.3–10 The Infant Aphakia Treatment Study randomized infants who had surgery for a unilateral cataract between 1 and 7 months of age to either primary intraocular lens or contact lens correction.11,12 This paper examines differences in stereopsis at 4.5 years of age between the two treatment groups.
Methods
The Infant Aphakia Treatment Study is a randomized clinical trial involving 12 sites supported by a cooperative agreement with the National Eye Institute of the National Institutes of Health. The study design, surgical techniques, patching and optical correction regimens, evaluation methods, patient characteristics at baseline, monocular visual acuity at age 4.5 years and clinical findings at age 5 years have been reported previously.11,12 Only the elements pertinent to the current paper will be briefly described here. The study and data collection were carried out with approval from the appropriate Institutional Review Board at each site. Informed Consent for the research was obtained from the parents of the participants, and the study is in accordance with the Health Insurance Portability and Accountability regulations. The off-label research use of the Acrysof SN60AT and MA60AC Intraocular Lenses (Alcon Laboratories, Fort Worth, Texas) is covered by US Food and Drug Administration investigational device exemption G020021.
Optical Correction
Patients randomized to the contact lens group were fit with a Silsoft (Bausch + Lomb, Rochester, New York) or a rigid gas permeable contact lens within a week after surgery. The power of the lens included a 2.0 diopter (D) overcorrection to provide a near point focus. The intraocular lens power for infants randomized to that group was calculated based on the Holladay 1 formula targeting an 8 D undercorrection for infants 4–6 weeks of age and a 6 D undercorrection for infants older than 6 weeks. For this group, a 2.0 D overcorrection was achieved by prescribing spectacles, beginning as early as the 1-month postoperative visit, when any of the following conditions existed in the treated eye: hyperopia >1.0 D, myopia >3.0 D or astigmatism >1.5 D. At 2 years of age, all patients received bifocal glasses with near correction and were asked to wear them at all times while awake. These spectacles included distance correction for patients in the intraocular lens group. Aphakic patients continued to wear a contact lens for their distance refractive correction.
Patching Regimen
Starting the second postoperative week, parents were instructed to have their child wear an adhesive occlusive patch over the unoperated eye for 1 hour/day for each month of age until age 8 months. Thereafter, patching was prescribed for one-half of waking hours every day or all waking hours every other day, achieving half-time patching. This regimen was continued until the patients exited from the study at 5 years of age.
Visual Acuity Assessment
Monocular optotype acuity was assessed at age 4.5 years (window +1 month) by a masked traveling examiner using the Amblyopia Treatment Study (ATS)-HOTV test13. Patients were tested wearing their best correction (updated at their last study visit three months earlier). Visual acuity was tested first in the aphakic/pseudophakic eye. The eye not being tested was occluded using a translucent occluder mounted in child sunglass frames (Good-Lite, Elgin, Illinois) to minimize the amplitude of latent nystagmus under monocular conditions. The initial testing distance was 3 meters. If the child was unable to see the HOTV letters, this distance was decreased to 1 meter. If the child still could not identify the letters, the Low Vision Card (Teller Acuity Card 0.32 cy/cm) was used to test for pattern vision. If the child did not respond to the Low Vision Card, the eye was assessed for light perception using a penlight in a room with no overhead lighting. If the child did not respond to the penlight, the vision was categorized as no light perception.
Stereopsis Testing
Stereopsis was evaluated at 4.5 years of age (window +1 month) by a masked traveling examiner using three different tests; 1) Frisby Stereotest (Richmond Products, New Mexico), 2) Randot Preschool Stereoacuity Test (Stereo Optical, Chicago, Illinois) and 3) Titmus Fly Test (Stereo Optical, Chicago, Illinois).
The Frisby Stereotest consists of a series of square plates with four square patterned areas, composed of randomly placed triangular shapes. One of the four squares has a set of the triangular shapes placed in such a way that they create a circle, which can only be detected if the patient has some degree of stereopsis. The Frisby Stereotest began with familiarizing the child with 2-dimensional images of the patterns. They were then tested with a “practice” plate (6 mm). The viewing distance for this plate varied from 20 to 40 cm. The plate was presented up to four times, rotating the location of the “circle” each time. If the child was able to detect the circular pattern on this plate 3 out of 4 times, the child was tested using the 6 mm plate at a testing distance of 40 cm. If the child correctly identified the circular pattern on 3 out of 4 presentations with the 6 mm plate, the same procedure was repeated with a 3 mm plate and then a 1.5 mm plate. If the child was unable to detect the circular pattern on the 6 mm plate at 40 cm, the child was tested at 30 cm. If the child was unable to detect the circular pattern on the 6 mm plate at 30 cm, the test was stopped.
The Randot Preschool test began with a pretest using non-stereo figures in Book 3 to familiarize the child with the shapes. After the pretest, polarizing glasses were placed on the child and the child was asked to identify the Randot shapes beginning with Book 3 (800 and 400 seconds of arc) followed by Book 1 (200 and 100 seconds of arc) and finally Book 2 (60 and 40 seconds of arc). If two shapes at a given level were identified correctly, testing proceeded to the next level. If the child could not correctly identify two shapes at a level, testing was stopped. Stereopsis was determined as the lowest level at which two shapes were correctly identified.
The Titmus Fly test was used to assess gross stereopsis. The child wore polarizing glasses for this test and the viewing distance was approximately 40 cm. The child was given time to adjust to the image and then asked to touch the wings of the fly. If the child obviously pinched the wings above the test plate, credit was given for that level of stereopsis.
For each test, the examiner provided a confidence rating based on her assessment of the child’s behavior and cooperation with the testing. The three-tiered confidence level was intended to specify the extent to which the examiner considered the results reliable: “very confident,” “somewhat confident” or “not confident”
Statistical Methods
The percent of patients with stereopsis was compared between treatment groups using Fisher’s exact test. The median age at surgery and median visual acuity at age 4.5 years was compared between patients with and without stereopsis at age 4.5 years using the Wilcoxon rank-sum test. Multiple logistic regression was used to assess the combined effect of age at surgery and visual acuity at age 4.5 on the presence of stereopsis.
Results
Study Population
There were 114 children enrolled in the study with 57 randomized to each treatment group. One patient in the intraocular lens group was lost to follow-up at age 18 months. The remaining 113 patients had visual acuity and stereopsis assessed at age 4.5 years (mean, 4.5 years; range, 4.5–4.9 years) and a clinical exam at age 5 years (mean, 5.0 years; range, 4.7–5.4 years) with an average length of follow-up of 4.8 years (range, 4.4–5.3 years) after cataract surgery. Significantly more secondary surgeries were required in the patients who received intraocular lenses compared with the patients who were left aphakic (41 versus 12 eyes). 12
Stereopsis
Of the 113 patients examined at age 4.5, 107 completed all three stereopsis tests and 6 patients were missing data for one or more of the tests: 3 patients were missing all the tests (phthisical eye, uncooperative child with poor vision, tester error); 2 patients were missing the Frisby and Randot Preschool tests, (developmental delay, tester error); 1 patient was missing the Titmus test (afraid of the fly). Thus there were 110 patients with data for at least one of the three stereopsis tests.
Stereopsis for Each Test: Intraocular Lens versus Contact Lens Treatment Groups
For the Frisby test, 13 of 108 patients (12.0%) had some stereopsis (2 with 170 seconds of arc and 11 with 340 seconds of arc). The results did not differ significantly between the treatment groups (Contact lens: 6 of 54 (11.1%), Intraocular lens: 7 of 54 (13.0%), p = 0.99). For the Randot Preschool test, 4 of 108 patients (3.7%) had some stereopsis (2 with 400 seconds of arc and 2 with 800 seconds of arc). The percentages of patients with stereopsis were not significantly different between the treatment groups (Contact lens: 3 of 54 (5.6%), Intraocular lens: 1 of 54 (1.9%), p=0.62). For the Titmus test, 21 of 109 patients (19.3%) had some stereopsis (3000 seconds of arc). The treatment groups were not significantly different (Contact lens: 8 of 53 (15.1%), Intraocular lens: 13 of 56 (23.2%), p = 0.34).
Among the 110 patients completing at least one of the tests, 28 patients (25.5%) had some stereopsis on one or more of the tests completed. Of these 28 patients, the number of patients showing positive stereopsis was as follows: all three tests: 3; Frisby and Titmus: 4; Frisby only: 6; Randot only: 1; Titmus only: 14. The percentages of patients with stereopsis on at least one of the tests were not significantly different between the treatment groups (Contact lens: 11 of 54 (20.4%), Intraocular lens: 17 of 56 (30.4%), p=0.28).
Examiner Confidence Ratings
The percentage of patients for which the tester was very confident, somewhat confident or not confident on the 3 stereopsis tests was as follows: Frisby – n=107, very: 96 (90%), somewhat: 10 (9%), not: 1 (1%); Randot – n=103, very: 99 (96%), somewhat: 3 (3%), not: 1 (1%); Titmus – n=106, very: 99 (93%), somewhat: 5 (5%), not: 2 (2%).
Randot Stereopsis Responders
All 4 patients with Randot stereopsis results had acuity of 20/32 or better in the treated eye. Three of these patients (1 intraocular lens, 2 contact lens) had positive results on the other two stereopsis tests as well. All of these patients had surgery prior to 1.2 months of age. The examiner was very confident in the Frisby, Randot (400 seconds of arc) and Titmus responses in 2 patients, and somewhat confident in the Randot response (800 seconds of arc) in the third. The examiner was not confident in the fourth patient who only had Randot stereopsis of 800 seconds of arc and no response to the other two tests.
Frisby Plus Titmus Responders
Four additional patients (2 intraocular lens and 2 contact lens) had positive responses to the Frisby and Titmus tests, but not to the Randot Preschool Stereoacuity test. Acuity in this group was also good (20/40 or better). Three of these patients had surgery prior to 1.1 months of age; the fourth patient had surgery at 6.0 months. The examiner was very confident in all of the results, both positive and negative, except for one “somewhat confident” on the Titmus fly.
Frisby or Titmus Only Responders
Twenty patients demonstrated stereopsis on only one test (14 Titmus and 6 Frisby). Although many had acuities better than 20/40, 4 had acuity of 20/1333 or worse in the operated eye. Despite a vision that would preclude stereopsis on the test, the examiner was very confident of the results for 2 of these patients.
Best Stereopsis Responders
Two patients are of particular interest, because they demonstrated equal and excellent acuity and the highest level of stereopsis among those with positive stereopsis findings. Furthermore, the examiner’s rating was very confident for all of their stereopsis test results. These were the only patients to get 20/170 on the Frisby and 20/400 on the Randot Preschool Stereoacuity Test. Both were contact lens patients (Table 1).
Table 1.
Age at Surgery and Visual Acuity of Patients with Best Stereopsis Outcome in the Infant Aphakia Treatment Study
| Age at Surgery (months) | Visual Acuity at 4.5 Years | Stereopsis Tests (arc seconds) | |||
|---|---|---|---|---|---|
| Treated Eye | Fellow Eye | Frisby | Randot | Titmus | |
| 1.0 | 20/25 | 20/25 | 170 | 400 | 3000 |
| 1.1 | 20/12 | 20/12 | 170 | 400 | 3000 |
Stereopsis versus Age at Surgery and Visual Acuity at Age 4.5 Years
Age at Surgery
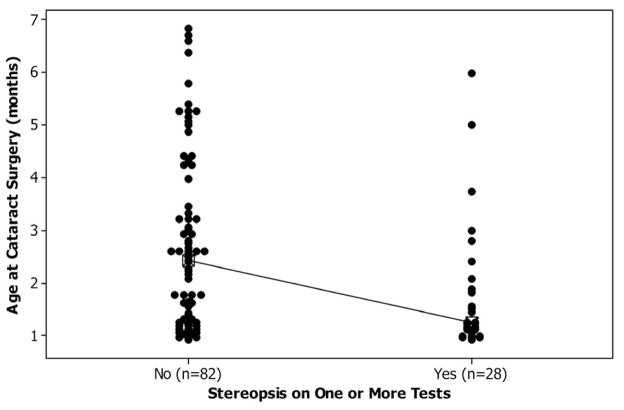
The median age at surgery for patients with stereopsis on at least one of the tests was significantly younger than for patients without stereopsis (1.2 months versus 2.4 months, p = 0.002, Table 2, Figure 1 top panel). A significantly higher percentage of patients who had surgery before 49 days of age had some stereopsis compared to the patients who had surgery after 49 days of age (19 of 48 patients (40%) versus 9 t 62 patients (15%), p = 0.004).
Table 2.
Stereopsis at 4.5 Years versus Age at Surgery and Visual Acuity at 4.5 Years in Patients in the Infant Aphakia Treatment Study
| Characteristic | Stereopsis at Age 4.5 | p-value | |||
|---|---|---|---|---|---|
| Yes (n = 28) | No (n = 82†) | ||||
| Median | IQR* | Median | IQR* | ||
| Age at Surgery (months) | 1.2 | 1.0–2.0 | 2.4 | 1.2–3.4 | 0.002 |
| Visual Acuity at 4.5 Years (logMAR) (Snellen) | 0.30 20/40 |
0.20–0.75 20/32–20/112 |
1.10 20/252 |
0.50–1.70 20/63–20/1002 |
0.0003 |
VA = Visual Acuity, IQR = Interquartile Range
For VA, the sample size for patients without stereopsis was 81 because VA could not be measured for one patient due to developmental delay.
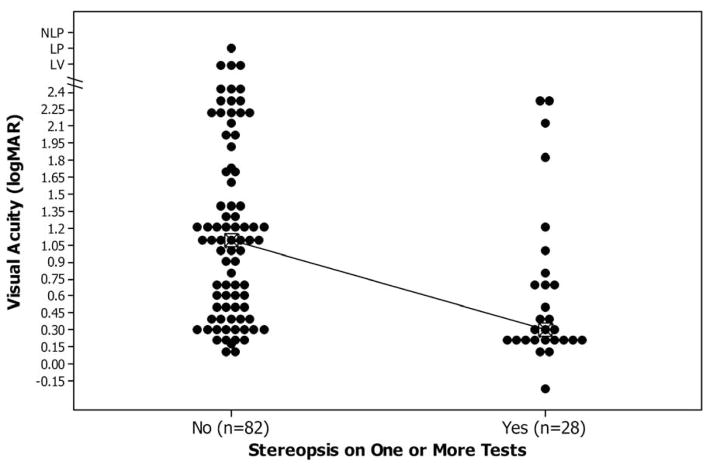
Figure 1.
Data plots showing presence or absence of stereopsis vs. age at cataract surgery (months) or visual acuity at age 4.5 years in the Infant Aphakia Treatment Study.
Top Panel: Individual value plot comparing age at cataract surgery with presence or absence of stereopsis at age 4.5 years.
Bottom Panel: Individual value plot comparing recognition visual acuity for the treated eye with the presence or absence of stereopsis at age 4.5 years.
Visual Acuity at Age 4.5 Years
The median visual acuity at age 4.5 years was significantly better for patients with stereopsis compared to patients without stereopsis (20/40 versus 20/252, p = 0.0003) (Table 2, Figure 1 bottom panel).
Multivariate Analysis
In a multiple logistic regression model, both age at surgery (p = 0.03) and visual acuity at age 4.5 (p = 0.01) demonstrated statistically significant relationships to the presence of stereopsis at age 4.5. Isolating each factor by controlling for the second factor yielded the following odds ratios: 1) Given a one month decrease in age at surgery, the odds ratio for having stereopsis (adjusted for visual acuity) was 1.5 (95% CI = 1.04–2.2) and 2) Given a 2 line improvement in visual acuity (logMAR = 0.2) (adjusted for age at surgery) the odds ratio for having stereopsis was 1.2 (95% CI = 1.05–1.4). The Hosmer-Lemmeshow test indicated that the regression model was well calibrated to the data (p = 0.26), or that the expected and observed events were similar. Table 3 illustrates these relationships by detailing the numbers of patients who demonstrated stereopsis grouped by age at surgery and two categories of visual acuity at 4.5 years.
Table 3.
Number of Patients Demonstrating Stereopsis by Age at Surgery and Visual Acuity at 4.5 Years in the Infant Aphakia Treatment Study
| Age at Surgery | Visual Acuity at 4.5 Years | Total Number of Patients | Stereopsis Present at 4.5 Years n (%) |
|---|---|---|---|
| 28–48 days | < 20/200 | 31 | 15 (48%) |
| ≥ 20/200 | 16 | 4 (25%) | |
| 49–210 days | < 20/200 | 26 | 7 (27%) |
| ≥ 20/200 | 36 | 2 (6%) |
Strabismus
An equal number of patients (11 in each treatment group) were orthophoric at near at the 4.5 year outcome exam.12 The association of stereopsis results, strabismus and strabismus surgery will be addressed in detail in a subsequent publication.
Discussion
Gross stereopsis was found in 28 (25.5%) of the patients in our study and was not associated with treatment group. Seven patients had positive responses to two of the tests (Table 4). Randot stereopsis was found in only 4 patients. All of these patients had good visual acuity in both eyes (20/32 or better) and had undergone surgery earlier in life. The two patients with the best stereopsis outcomes also had the best acuities and earlier surgery.
Table 4.
Age at Surgery and Visual Acuity by Treatment Group of Patients in the Infant Aphakia Treatment Study Demonstrating Stereopsis on at Least Two Tests
| Treatment Group | Age at Surgery (months) | Visual Acuity at 4.5 Years | Stereopsis Tests (arc seconds) | |||
|---|---|---|---|---|---|---|
| Treated Eye | Fellow Eye | Frisby | Randot | Titmus | ||
| Contact Lens | 1.0 | 20/25 | 20/25 | 170 | 400 | 3000 |
| 1.1 | 20/12 | 20/12 | 170 | 400 | 3000 | |
| 1.1 | 20/32 | 20/32 | 340 | 0 | 3000 | |
| 1.1 | 20/25 | 20/20 | 340 | 0 | 3000 | |
| Intraocular Lens | 0.9 | 20/32 | 20/20 | 340 | 800* | 3000 |
| 0.9 | 20/40 | 20/25 | 340 | 0 | 3000 | |
| 5.9 | 20/32 | 20/32 | 340 | 0 | 3000* | |
Examiner confidence level was “somewhat” for these tests.
For all other tests, the examiner indicated “very confident.”
Stereopsis testing can be difficult in young children due to lack of understanding of the test. The Frisby Stereotest has the distinct advantage of presenting real-depth images, which do not require glasses to separate the target images, however the larger disparities can be detected monocularly if head motion is allowed.14,15 Our testing protocol utilized a specific holder to stabilize the test plates and the examiner was aware of the need for the child to hold their head in a stable position during the testing. The Randot Preschool Stereoacuity test requires polarizing glasses, has no monocular cues, and is reliably performed by 89–93% of typically developing 3- to 5-year-old children.16 The Titmus Fly test requires polarizing glasses, has monocular cues, but represents a very gross level of stereopsis.
It is useful to confirm stereopsis test results with repeated testing or using several measures, as well as the examiner’s rating of the reliability of the responses. In our study, we found a marked difference in the positive responses with these three stereopsis tests. We found the lowest positive response with the Randot Preschool Stereoacuity test. The precision of the test images and the requirement to fuse the images through polarizing lenses may have been more challenging for our patients, although this test is reliably performed by the majority of typically developing children at the same age as our patients. The Frisby and Titmus tests are considered easier for the child to interpret, however, it is possible that a limited number of the positive results with the Frisby and Titmus tests were false positives due to non stereo cues.17,18 The Titmus test was administered only in one position, with the fly’s wings protruding from the page. We did not rotate the image 180 degrees to confirm a positive response. Under these conditions, the wings of the fly would have appeared to be behind the page and the child’s response should have been to touch the page. Nonetheless, we were very careful in ascribing a positive response to the Titmus Fly only when the child clearly attempted to touch the wings above the surface of the page.
Earlier surgical removal of a cataractous lens followed by optical correction and appropriate occlusion therapy may increase the likelihood of developing stereopsis. There are a few reports of patients achieving measurable stereopsis, with some as isolated case reports. Gregg and Parks3 describe an 8 year old with 50 seconds of arc of stereopsis on Titmus and Randot testing who underwent cataract surgery at one day of life and whose final acuity in the operated eye was 20/25. Yagasaki et al7 reported stereopsis of 1200–1500 seconds of arc in a patient who had surgery at 19 weeks of age. Birch and coworkers4 found demonstrable Randot stereopsis in 3 of 8 children who had surgery before 6 weeks of age compared with only 1 of 6 patients who had surgery between 2 and 8 months of age. Our results add to the published data on stereopsis vision in infantile monocular cataracts (Table 5). Earlier surgery decreases the period of monocular deprivation and would be expected to enhance the development of stereopsis.
Table 5.
References of Documented Stereopsis Cases in Infantile Cataract Surgery
| Study | # Pts | Age at Surgery | Vision (Snellen) | Stereopsis Test |
|---|---|---|---|---|
| Birch et al4 | 3 | < 6 weeks | 20/20-20/100* | Randot 200″–310″ |
| Tytla et al8 | 2 | 3.2–4.9 months | 20/40 | Titmus Randot 200–400″ |
| Gregg and Parks3 | 1 | 1 day | 20/25 | Randot 50″ |
| Wright et al9 | 3 | 2–5 weeks | 20/30-20/70 | Titmus 200–3000″ |
| Yagasaki et al8 | 1 | 19 weeks | 0.7 logMAR (20/100) | Randot 1200–1500″ |
| Brown et al5 | 8 | 10 days–3.5 months | 20/25-20/80 | Titmus 100–200″ |
| Hwang et al10 | 3 | 2–7 weeks | 20/25-20/40 | Randot 50–500″ |
| Infant Aphakia Treatment Study12 | 28 | 0.95–5.9 months | 20/32-20/112 | Randot 200–400 Frisby 170–340 Titmus 3000 |
Vision includes all study patients since 3 patients with stereo were not specified.
Intensive post-operative occlusion regimens, while important to manage amblyopia, may interfere with optimal binocular stimulation. Pratt – Johnson and Tilson1 reported no stereopsis in 4 patients operated in the first few months of life and occluded > 90% of the time, despite vision of 20/40 or better in both eyes. In Birch’s study4, occlusion regimens were higher in the first year of life, and afterwards similar to the Infant Aphakia Treatment study protocol. Jeffrey et al6 compared intensive (> 80% of waking hours in the first 2 years of life) to reduced (50% of waking hours) occlusion regimens to assess the effects on binocular sensory outcomes. In this study the final acuity was similar in the two groups but the reduced occlusion group had a higher incidence of stereopsis using the Randot Preschool Stereoacuity and Titmus Fly tests. Although the number of patients was smaller in the reduced occlusion group, 2 of 8 patients demonstrated Randot stereopsis of 800 seconds of arc, versus 2 of 29 patients in the intensive occlusion group who demonstrated 3000 seconds of arc with the Titmus Fly. The mean age at surgery was younger in the reduced occlusion group (20.3 days vs 32.5 days) but one patient in the intense occlusion group with Titmus stereo had surgery at 65 days. Of note is that none of the intensive occlusion patients demonstrated stereopsis using the Randot Preschool Stereoacuity test. Brown et al5 found a high incidence (62%) of stereopsis responses in 13 patients with early surgery (< 4 months) and a reduced occlusion regimen. Limitations of this study are that the data were retrospective and stereopsis was assessed only with the Titmus Fly.
It is apparent that stereopsis can be achieved in patients with monocular infantile cataracts. Our findings provide evidence that earlier surgery and better acuity in the treated eye play a critical role. Patients with better acuity demonstrated the best stereopsis. However, not all patients with good acuity demonstrated stereopsis.
Acknowledgments
Funding/Support: Supported through a cooperative agreement from the National Institutes of Health Grants U10 EY13272 and U10 EY013287 and in part by National Institutes of Health Departmental Core Grant EY006360 and Research to Prevent Blindness, Inc, New York, New York
Other Acknowledgments: none
Biographies

E. Eugenie Hartmann, PhD is a Professor in the Department of Vision Sciences at the University of Alabama at Birmingham. Her training encompasses Developmental Psychology as well as Vision Sciences. She has expertise in both behavioral and electrophysiological assessments of visual function in infants and young children. She has participated in multi-center trials since 1987, including studies with Ross Laboratories demonstrating the advantages of supplemented formulas for preterm infants based on visual evoked potential findings.

Ann U Stout MD has joined Houston Eye Associates after fourteen years at Casey Eye Institute in Portland, Oregon. She attended Baylor College of Medicine and was chief resident at Baylor’s Cullen Eye Institute. She completed a pediatric ophthalmology fellowship at Childrens Hospital Los Angeles, and stayed as faculty for ten years. In 2000 she joined Oregon Health Sciences. Her research includes the Pediatric Eye Disease Investigator Group studies and early detection of cerebrotendinous xanthomatosis.
Appendix 1: The Infant Aphakia Treatment Study Group
Administrative Units and Participating Clinical Centers
Clinical Coordinating Center (Emory University): Scott R. Lambert, MD (Study Chair); Lindreth DuBois, MEd, MMSc, CO, COMT (National Coordinator)
Data Coordinating Center (Emory University): Michael Lynn MS (Director), Betsy Bridgman, BS; Marianne Celano PhD; Julia Cleveland, MSPH; George Cotsonis, MS; Carey Drews-Botsch, PhD; Nana Freret, MSN; Lu Lu, MS; Seegar Swanson; Thandeka Tutu-Gxashe, MPH
Visual Acuity Testing Center (University of Alabama, Birmingham): E. Eugenie Hartmann, PhD (Director); Anna K Carrigan, MPH; Clara Edwards
Eye Movement Reading Center (University of Alabama, Birmingham and Retina Foundation of the Southwest, Dallas, TX): Claudio Busettini, PhD; Samuel Hayley; Eleanor Lewis, Alicia Kindred, Joost Felius, PhD
Steering Committee: Scott R. Lambert, MD; Edward G. Buckley, MD; David A. Plager, MD; M. Edward Wilson, MD; Michael Lynn, MS; Lindreth DuBois, MEd, MMSc; Carolyn Drews-Botsch, PhD; E. Eugenie Hartmann, PhD; Donald F. Everett, MA. Rotating: Joost Felius, PhD; Margaret Bozic, CCRC, COA; Ann Holleschau, BA
Contact Lens Committee: Buddy Russell, COMT; Michael Ward, MMSc
Participating Clinical Centers (In order by the number of patients enrolled)
Medical University of South Carolina; Charleston, South Carolina (14): M. Edward Wilson, MD; Margaret Bozic, CCRC, COA; Carol Bradham, COA, CCRC
Harvard University; Boston, Massachusetts (14): Deborah K. Vanderveen, MD; Theresa A. Mansfield, RN; Kathryn Bisceglia Miller, OD
University of Minnesota; Minneapolis, Minnesota (13): Stephen P. Christiansen, MD; Erick D. Bothun, MD; Ann Holleschau, B.A.; Jason Jedlicka, OD; Patricia Winters, OD; Jacob Lang, OD
Cleveland Clinic; Cleveland, Ohio (10): Elias I. Traboulsi, MD; Susan Crowe, BS, COT; Heather Hasley Cimino, OD. Case Western Reserve: Faruk Orge, MD; Megin Kwiatkowski; Beth Colon
Baylor College of Medicine; Houston, Texas (10): Kimberly G. Yen, MD; Maria Castanes, MPH; Alma Sanchez, COA; Shirley York, OD; Stacy Malone, COA; Margaret Olfson
Oregon Health and Science University; Portland, Oregon (9): David T Wheeler, MD; Ann U. Stout, MD; Paula Rauch, OT, CRC; Kimberly Beaudet, CO, COMT; Pam Berg, CO, COMT
Emory University; Atlanta, Georgia (9): Scott R. Lambert, MD; Amy K. Hutchinson, MD; Lindreth Dubois, MEd, MMSc, CO, COMT; Rachel Robb, MMSc, CO, COMT; Marla J. Shainberg, CO
Duke University; Durham, North Carolina (8): Edward G. Buckley, MD; Sharon F. Freedman, MD; Lois Duncan, BS, CO, COMT; B.W. Phillips, FCLSA; John T. Petrowski, OD
Vanderbilt University: Nashville, Tennessee (8): David Morrison, MD; Sandy Owings COA, CCRP; Ron Biernacki, CO, COMT; Christine Franklin, COT
Indiana University, Indianapolis, Indiana (7): David A. Plager, MD; Daniel E. Neely, MD; Michele Whitaker, COT; Donna Bates, COA; Dana Donaldson, OD
Miami Children’s Hospital, Miami, Florida (6): Stacey Kruger, MD; Charlotte Tibi, CO; Susan Vega
University of Texas Southwestern; Dallas, Texas (6): David R. Weakley, MD; David R. Stager Jr M.D.; Joost Felius, PhD; Clare Dias, CO; Debra L. Sager; Todd Brantley, OD
Data and Safety Monitoring Committee: Robert Hardy, PHD (Chair); Eileen Birch, PhD; Ken Cheng, MD; Richard Hertle, MD; Craig Kollman, PhD; Marshalyn Yeargin-Allsopp, MD (resigned); Cyd McDowell; Donald F. Everett, MA
Medical Safety Monitor: Allen Beck, MD
Footnotes
Financial Disclosures: No financial disclosures
Trial Registration clinicaltrials.gov Identifier NCT00212134
Author Contributions: Dr. Lambert has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Design and conduct of the study: Lambert, Lynn, Hartmann. Acquisition of data: all authors. Analysis and interpretation of data: Stout, Lambert, Lynn, Hartmann. Drafting of the manuscript: Stout, Hartmann, Lambert, Lynn. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Lynn. Administrative, technical, or material support: Lambert, Lynn. Study supervision: Lambert, Lynn
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pratt-Johnson JA, Tillson G. Unilateral congenital cataract: binocular status after treatment. J Pediatr Ophthalmol Strabismus. 1989;26(2):72–5. doi: 10.3928/0191-3913-19890301-07. [DOI] [PubMed] [Google Scholar]
- 2.Allen RJ, Speedwell L, Russell-Eggitt I. Long-term visual outcome after extraction of unilateral congenital cataracts. Eye (Lond) 2010;24(7):1263–7. doi: 10.1038/eye.2009.295. [DOI] [PubMed] [Google Scholar]
- 3.Gregg FM, Parks MM. Stereopsis after congenital monocular cataract extraction. Am J Ophthalmol. 1992;114(3):314–7. doi: 10.1016/s0002-9394(14)71797-0. [DOI] [PubMed] [Google Scholar]; Anketell PM, Saunders KJ, Little JA. Stereoacuity norms for school-age children using the Frisby stereotest. J AAPOS. 2013;17(6):582–7. doi: 10.1016/j.jaapos.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Birch EE, Swanson WH, Stager DR, et al. Outcome after very early treatment of dense congenital unilateral cataract. Invest Ophthalmol Vis Sci. 1993;34(13):3687–99. [PubMed] [Google Scholar]
- 5.Brown SM, Archer S, Del Monte MA. Stereopsis and binocular vision after surgery for unilateral infantile cataract. J AAPOS. 1999;3(2):109–13. doi: 10.1016/s1091-8531(99)70080-7. [DOI] [PubMed] [Google Scholar]
- 6.Jeffrey BG, Birch EE, Stager DR, Weakley DR. Early binocular visual experience may improve binocular sensory outcomes in children after surgery for congenital unilateral cataract. J AAPOS. 2001;5(4):209–16. doi: 10.1067/mpa.2001.115591. [DOI] [PubMed] [Google Scholar]
- 7.Yagasaki T, Sato M, Nomura H, et al. A case with stereopsis following early surgery for unilateral congenital cataract. Nihon Ganka Gakkai Zasshi. 1994;98(1):111–6. [PubMed] [Google Scholar]
- 8.Tytla ME, Lewis TL, Maurer D, Brent HP. Stereopsis after congenital cataract. Invest Ophthalmol Vis Sci. 1993;34(5):1767–73. [PubMed] [Google Scholar]
- 9.Wright KW, Matsumoto E, Edelman PM. Binocular fusion and stereopsis associated with early surgery for monocular congenital cataracts. Arch Ophthalmol. 1992;110(11):1607–9. doi: 10.1001/archopht.1992.01080230107032. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JM, Matsumoto ER, Borchert MS. The relationship between stereopsis and monocular optokinetic nystagmus after infantile cataracts. J AAPOS. 1999;3(4):221–6. doi: 10.1016/s1091-8531(99)70006-6. [DOI] [PubMed] [Google Scholar]
- 11.Lambert SR, Buckley EG, Drews-Botsch C, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128(1):21–7. doi: 10.1001/archophthalmol.2009.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambert SR, Lynn MJ, Hartmann EE, et al. Comparison of contact lens and intraocular lens correction of monocular aphakia during infancy: a randomized clinical trial of HOTV optotype acuity at age 4. 5 years and clinical findings at age 5 years. JAMA Ophthalmol. 2014;132(6):676–82. doi: 10.1001/jamaophthalmol.2014.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthalmol. 2001;132(6):903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 14.Anketell PM, Saunders KJ, Little JA. Stereoacuity norms for school-age children using the Frisby stereotest. J AAPOS. 2013;17(6):582–7. doi: 10.1016/j.jaapos.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Archer SM. Stereotest artifacts and the strabismus patient. Graefes Arch Clin Exp Ophthalmol. 1988;226(4):313–6. doi: 10.1007/BF02172957. [DOI] [PubMed] [Google Scholar]
- 16.Birch E, Williams C, Hunter J, Lapa MC. Random dot stereoacuity of preschool children. ALSPAC “Children in Focus” Study Team. J Pediatr Ophthalmol Strabismus. 1997;34(4):217–22. doi: 10.3928/0191-3913-19970701-08. quiz 47–8. [DOI] [PubMed] [Google Scholar]
- 17.Cooper J, Warshowsky J. Lateral displacement as a response cue in the Titmus Stereo test. Am J Optom Physiol Opt. 1977;54(8):537–41. doi: 10.1097/00006324-197708000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hall C. The relationship between clinical stereotests. Ophthalmic Physiol Opt. 1982;2(2):135–43. [PubMed] [Google Scholar]