Abstract
ATP-binding cassette (ABC) drug transporters consuming ATPs for drug efflux is a common mechanism by which clinical cancers develop multidrug resistance (MDR). We hypothesized that MDR phenotypes could be suppressed by administration of “ersatzdroges”, non-chemotherapy drugs that are, nevertheless, ABC substrates. We reasoned that, through prolonged activation of the ABC pumps, ersatzdroges will force MDR cells to divert limited resources from proliferation and invasion thus delaying disease progression. We evaluated ABC substrates as ersatzdroge by comparing their effects on proliferation and survival of MDR cell lines (MCF-7/Dox and 8226/Dox40) with the effects on the drug-sensitive parental lines (MCF-7 and 8226/s, respectively) in glucose-limited condition. The changes in glucose and energy demands were also examined in vitro and in vivo. MCF-7/Dox showed higher ATP demand and susceptibility to glucose resource limitation. Ersatzdroges significantly decreased proliferation of MCF-7/Dox when the culture media contained physiological glucose concentrations (1.0 g/L) or less, but had no effect on MCF-7. Similar evidence was obtained from 8226/Dox40 and 8226/s comparison. In vivo 18F-FDG-PET imaging demonstrated that glucose uptake was increased by systemic administration of an ersatzdroge in tumors composed of MDR. These results suggest that administration of ersatzdroges, by increasing the metabolic cost of resistance, can suppress proliferation of drug-resistance phenotypes. This provides a novel and relatively simple application model of evolution-based strategy which can exploit the cost of resistance to delay proliferation of drug-resistant cancer phenotypes. Furthermore, suggested is the potential of ersatzdroges to identify tumors or regions of tumors that express the MDR phenotype.
Introduction
Chemotherapy that targets continuously proliferating tumor cells remains a common therapeutic strategy in many cancers. Unfortunately, as with other therapies, tumor cell populations exposed to the strong selection forces generated by cytotoxic chemotherapy eventually become resistant often by upregulation of xenobiotic metabolism. ATP-binding cassette (ABC) drug transporters are well-known drug efflux proteins that confer multidrug resistance (MDR) 1 by reducing intracellular concentrations of cytotoxic agents. The most extensively characterized ABCs include ABCB1 (also known as P-glycoprotein and MDR1), ABCC1 (also known as MRP1) and ABCG2 (also known BCRP). These pumps export the substrates including chemo-reagents by consuming ATP. ABCB1, for example, requires 2 ATPs to export one substrate molecule 2.
The ABCB1 transporter is commonly found in normal tissues such as kidneys and intestine where it likely serves as a protective mechanism for blood-borne or ingested toxins 3. In contrast, the expression of ABCB1 proteins in untreated primary breast cancers is typically observed in less than 10% of cells 4 but increases upon administration of various chemotherapeutic agents 5, 6. The potential benefit of inhibiting the ABCB1 transporter and, thus, reversing therapy resistance is well recognized. Substrates that either bind to and reduce pump activity or act as competitive inhibitors to reduce drug efflux have been extensively investigated. While some success in reversing chemotherapy resistance has been observed, this approach has not generally shown significant clinical benefit 1.
The emergence of therapy resistance is generally viewed as an evolutionary process in which cancer cells adapt to selection pressures mediated by cytotoxic drugs 7. However, evolutionary dynamics are only rarely explicitly incorporated into therapy design 8. We have previously proposed that while evolution of resistant phenotypes is virtually inevitable, proliferation of resistant populations is not and is potentially vulnerable to Darwinian perturbations. In particular, the fitness cost of therapy resistance can be exploited to inhibit population expansion. That is, as noted above, resistance to therapy requires energy and other resources which are, thus, diverted from proliferation and invasion. Prior work has demonstrated that proliferation of chemo-resistant populations could be delayed and even prevented by exploiting the fitness cost of their resistance mechanisms. 9, 10. Here we propose an additional evolutionary approach in which the resistant phenotype is actively targeted with non-toxic therapy that, nevertheless, increases the metabolic cost of resistance and reduces proliferation.
In this study, we focused specifically on the metabolic cost of the MDR phenotype. A cell using the ABC pump for drug resistance must expend resources for synthesizing and transporting the membrane proteins as well as the ATP cost of their activity (roughly 2 ATP per molecule of drug extruded). In the presence of cytotoxic drug, this energy expenditure increases survival and, therefore, confers increased fitness. However, in the absence of chemotherapy, the cost of ABC pumps serves no survival benefit and, therefore, reduces fitness because of the added energetic cost. This cost is evident experimentally as, for example, doxorubicin must be continuously added to culture media to maintain resistance in the MCF-7/Dox cell line. That is, in the absence of a fitness benefit, the substrate require for synthesizing and expressing ABC proteins is sufficiently high that there are selection pressures against their expression. These additional resources must be either acquired from the microenvironment or diverted from other cellular functions such as proliferation, invasion, and survival. Our goal in this work is to test the hypothesis that increasing the evolutionary cost of resistance, at physiological resource levels, will further diminish the fitness of the resistant population and reduce proliferation.
Here we propose the novel concept of an “ersatzdroge”. An ersatzdroge is a non-toxic or less toxic drug substitute for chemotherapeutic agent, which is, nevertheless, a substrate for the ABC pump. In the presence of an ersatzdroge, MDR cells will continue to activate their membrane pumps to extrude the ersatzdroge as though it were a cytotoxic agent. This requires significant energy consumption for no survival benefit (sweat but no gain) thus causing a decrease in fitness by limiting available resources for proliferation and invasion. In contrast, cells without ABC transporter will be unchanged in the presence of an ersatzdroge – no sweat no gain. While, of course, the ersatzdroge does not affect chemotherapy sensitive populations – these remain controllable by the current therapy. Thus, an ersatzdroge is designed to increase the fitness difference between drug-sensitive and drug-resistant population in the absence of chemotherapy to prevent proliferation of uncontrollable, therapy-resistant cells while maintaining a stable population of therapy-sensitive phenotype. The net clinical benefit is prolongation of progression-free survival.
Methods
In vitro proliferation and survivability
MCF-7 and its Doxorubicin resistant variant MCF-7/Dox were obtained and cultured as described previously10. The similar culture condition was applied for the multiple myeloma cell line 8226/s and 8226/Dox40 which were generously provided by Dr. William Dalton at H. Lee Moffitt Cancer Center 11. Doxorubicin, verapamil, cyclosporin A, clarithromycin, and chloroquine were obtained from Sigma. Calcein-AM and Hoechst 33342 were purchased from Life Technology.
We examined the drug susceptibility and/or proliferation of MCF-7 and MCF-7/Dox by comparing the number of survived cells in the absence of various drug concentrations. Cells were plated on 96-well plate at 2×104 cells/well and cultured overnight. The glucose level in the media was adjusted by adding D-glucose (Sigma) to glucose-free RPMI-1640 (Life Technology). The amounts of survived cells were measured by crystal violet staining after fixation using 3.7% formaldehyde for MCF-7 and MCF-7/Dox. For multiple myeloma cells, the cell amount was measured by using CellTiter-Glo Luminescent Cell Viability Assay (Promega). The cells were seeded at 1×104 cells/well and culture in the presence of difference concentration of glucose and/or verapamil for 48 hrs.
ATP measurements
Cells were grown on 96-well plates with opaque walls and bottoms at 2×105 cells/ml overnight. After cells were incubated at 37°C in glucose- and serum-free RPMI-1640 for the time indicated, CellTiter-Glo Assay reagent (Promega) was added to each well. After 10 min incubation at room temperature, the bioluminescence was measured by using VICTOR X Multilabel Plate Reader (Perkin Elmer) to compare the relative amount of ATP.
Dual MFP Xenograft Model
MCF-7 and MCF-7/Dox cell tumors were orthotopically generated in two opposite mammary fat pads (MFPs) of a mouse to compare the tumor quality in the identical host. The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of South Florida. Animals were maintained and evaluated under pathogen free conditions in accordance with IACUC standards of care at the H. Lee Moffitt Cancer Center & Research Institute (Tampa, FL). Briefly, the cell suspension at 1×108 cells/ml in 50% Matrigel™ (BD Bioscience) was inoculated (~1×107 cells/mouse) into MFPs of 6-8 week-old female nu/nu mice (Harlan) in which an estrogen pellet (0.72 mg, Innovative Research of America) was engrafted 2-3 days prior to the cell injection. When the tumor volume reached 300 mm3 according to caliper measurements, mice were subjected to PET scans as well as MRI. Two-dimensional tumor measurements were performed twice a week, and tumor volume was calculated according to the formula; volume = π (short diameter2) × (long diameter)/6.
MRI Scan
The tumor size was monitored by MRI. All MR data was acquired using a 7-T horizontal magnet (ASR 310, Agilent Technologies) equipped with nested 205/120/HDS gradient insert and a bore size of 310 mm. Prior to imaging, animals anesthetized using 2% isoflurane in medical grade O2. Following induction, animals were restrained in a specific holder and inserted into the magnet while constantly receiving isoflurane. Body temperature was maintained at 37±1°C using a specific hot-air pump and respiratory functions were monitored with the SAII animal system (Small Animal Instruments Inc.) Temperature control of the imaging gradients was achieved by means of a water chiller (Nestle Waters) and maintained at 12°C for all acquisitions. Using a 35-mm quadrature coil (Doty Scientific), axial T2-weighted fast spin-echo multislice (FSEM) sequences were acquired with TE/TR = 72/1000 ms, field of view = 35 × 35 mm, Matrix = 128 ×128, slice thickness of 1.5 mm and 6 averages over 1.5 minutes. Spatial resolution for these scans were 273 μm. Applying the same axial slice plane, a diffusion weighted sequence using three b-values (50, 500, 1000) and TE/TR = 36/1325 ms also was acquired over 6.5 minutes. Image reconstruction and volumetric analysis were performed in VnmrJ (Agilent Technologies). Tumor volumes were obtained from the high-resolution T2-weighted FSE datasets and measured by manually drawn regions of interest (ROI) encompassing the entire tumors (VnmrJ).
18F-FDG-PET
In order to measure the 18F-FDG uptake by MCF-7 and MCF-7/Dox tumors, two mice bearing both tumors at the opposite mammary fat pads were scanned by PET (Inveon PET/SPECT/CT, Siemens). The tumor sizes were measured by MRI a day prior to PET scan. Following the MRI session, the mouse pellets were removed from the cages overnight and fasting was induced in order to reduce background signal for the 18F-FDG uptake. On the scanning day, the body weights were measured to standardize the uptake value before the scanning. Prior to imaging, each mouse was anesthetized using 2% isoflurane and a catheter was installed in the tail vein. The mouse was i.v. injected with 18F-FDG (~200 μCi) and kept awake for 30 min after the catheter was removed. The animal was again anesthetized using isoflurane at 0.5-1 hr after the injection and moved to the scanning bed. A dynamic scanning was acquired up to 1 hr, A 2-dimensional ordered subset expectation maximum algorithm was used for image reconstruction. Decay, attenuation, and scatter corrections were all performed during the image histogram and reconstruction processes.
The obtained signal was standardized using the body weight. Regions of interest (ROIs) were manually defined on the PET images using the SIEMENS IRW software, the standardized uptake value (SUV; defined as tissue concentration at a particular time normalized to injected dose per body-surface area) was calculated. In order to see the effect of ersatzdroge, 5 mg/kg verapamil was i.p. injected into a mouse 1 hour prior to the 18F-FDG injection. During scan, as well as MRI, the body temperature was maintained at 37±1°C and respiratory functions were monitored with the SAII animal system.
Statistical Analysis
In this study, all data are expressed as means ± SD except for the in vivo tumor growth in Figure 5 where they are presented as a means ± SEM. A Student t test was used to evaluate the statistical significance by using GraphPad Prism software.
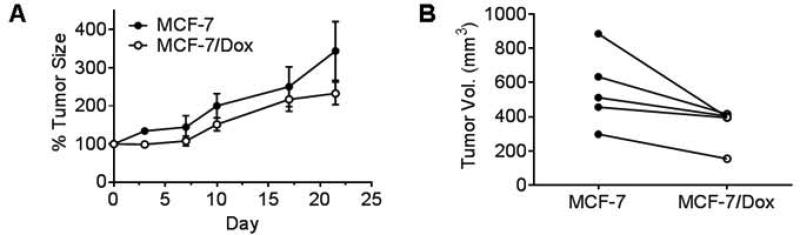
Figure 5.
Tumor growth comparison in vivo. MCF-7 and MCF-7/Dox tumors were developed in two MFPs of a mouse, and the tumor volumes were periodically measured by MRI (n=5). MCF-7 tumor tends to grow faster than MCF-7/Dox. A. Tumor growth rate was monitored by comparing the relative tumor volume increase form the time point when the volume was 100 mm3 or larger. B. Tumor volumes on day 21 in each mouse were paired.
Results
Evolving resistant cells lines that over-express ABCB1
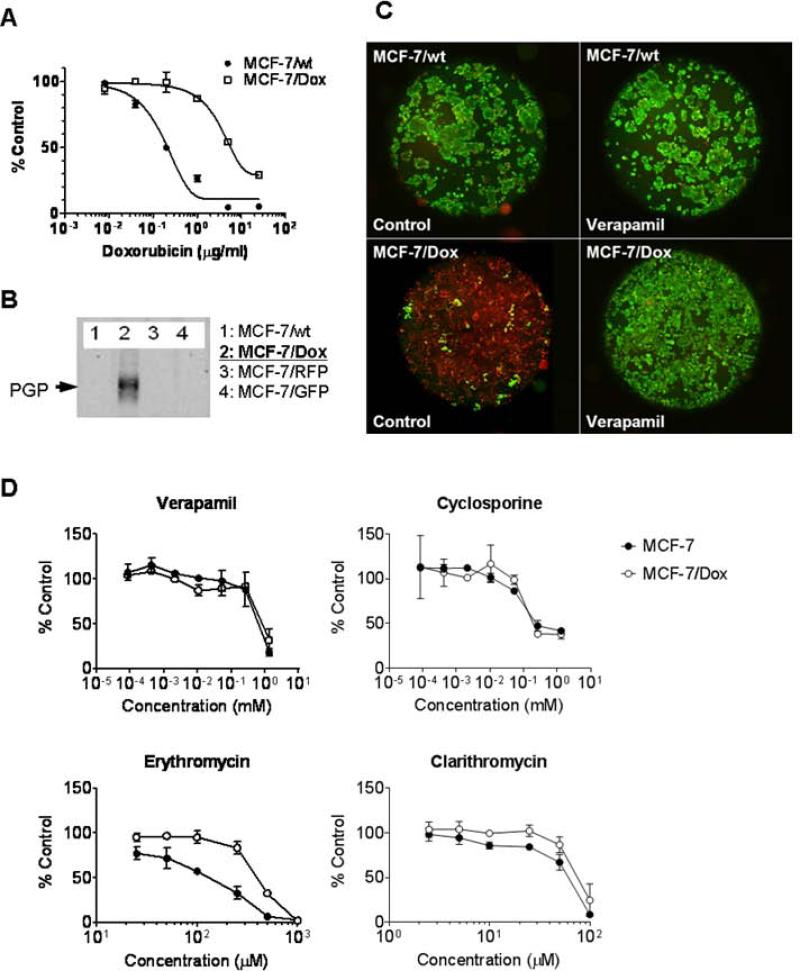
MCF-7/Dox is a cell line originated from MCF-7 and, through chronic exposure to progressively higher concentrations of doxorubicin, evolved resistance mediated by upregulation of ABCB1 10 (Figure 1A). The ABCB1 expression was confirmed by immunoblot (Figure 1B) and the pump activity by measuring the exclusion of calcein-AM, a well-known ABCB1 substrate emitting green fluorescence upon the internalization (Figure 1C). The calcein-AM exclusion was inhibited by applying excess amount of ABCB1-substrates verapamil. To test the potential use of ABCB1 substrate as ersatzdroge, we compared cytotoxicities of several compounds previously reported as potent ABCB1 substrates: verapamil, cyclosporine, erythromycin and clarithromycin. As shown in Figure 1D, all four substrates showed low cytotoxicity compared to anticancer drug like doxorubicin. Whereas IC50 of doxorubicin even for MCF-7/Dox is ~0.63 μg/ml (~1.1 μM), the IC50s of the other substrates were higher than 10 μM. There was no significant difference in the survival rate between MCF-7 and ABCB1 expressing MCF-7/Dox in the presence of substrates at micromolar to sub-millimolar level except for erythromycin. MCF-7/Dox slightly more resistant to erythromycin (IC50 =0.41 mM) than MCF-7 (IC5-= 0.14mM).
Figure 1.
MDR phenotype upon ABCB1-expression in MCF-7/Dox cell line. A. The survived cell amounts of MCF-7 and MCF-7/Dox after 72 hour culture in the presence of doxorubicin were compared. B. The ABCB1-expression in MCF-7/Dox was confirmed by immunoblot. C. Calcein-AM (green)-exclusion from MCF-7 or MCF-7/Dox in the presence or absence of verapamil (10 μM) was monitored by fluorescence microscopy. Cells were counter-labeled by nuclear staining (red). D. Drug sensitivity to ABCB1-substrates. MCF-7 and MCF-7/Dox were cultured 48 hr in the presence of non-chemotherapeutic agents known as ABCB1 substrates; verapamil, cyclosporine, erythromycin, and clarithromycin.
In some experiments we additionally investigated the doxorubicin-resistant multiple myeloma cell line 8226/Dox40 compared to the wild type 8226/s. The 8226/Dox40 has been extensively investigated and its upregulation of ABCB1 is well documented 11.
Quantifying the cost of ABCB1 synthesis and actions
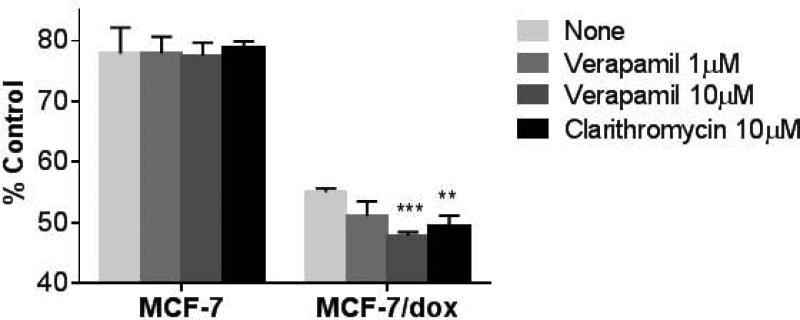
After 30 minutes of glucose deprivation, ATP concentrations in MCF-7/Dox cells were 55 ± 0.63 % of baseline compared to 78 ± 4.3 % in wild type MCF-7 (Figure 2). This is consistent with prior investigations 10 and provides an estimate of the metabolic cost required to maintain the ABCB1 proteins. Following addition of 10μg/ml verapamil for 30 minutes, ATP concentrations fell by 50% in MCF-7/dox cells but were not changed in wt MCF-7. A decline of about 40% was observed following addition of 10 μg/ml clarithromycin for 30 minutes with no change in wild type MCF-7.
Figure 2.
The relative ATP level change upon glucose deprivation in the absence or presence of ersatzdroge. The relative level of ATP was assessed by measuring luminescence by CellTiter-Glo ATP assay system (Promega). The % value indicates the relative ATP level remaining in cells that have been glucose-deprived for 1 hr by replacing the media to glucose-free and serum-free RPMI-1640 compared to controls which were not deprived (<1 min). The ATP reduction in MCF-7/Dox was enhanced in the presence of ersatzdroge. (**, p<0.005; ***, p<0.0005)
Changes in proliferation and survival due to resistance cost
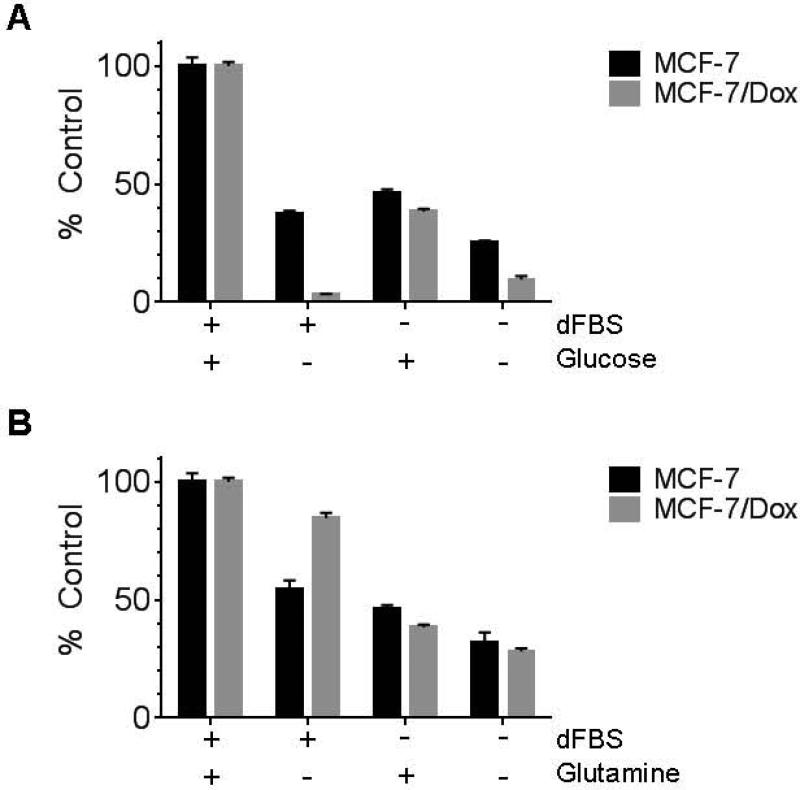
In results that paralleled the ATP experiments, we found removal of glucose from the culture media resulted in decreased survival (3%) of MCF-7/Dox cells after 48 hours compared to 37 % of the wild type MCF-7 cells still survived (Figure 3A). In contrast, following serum starvation in the presence of 2.0 g/L glucose and 0.3 g/L glutamine, 46 ± 1.6 % (error bars) of MCF-7 survived compared to 38 ± 1.1 % survival in of MCF-7/Dox,. Interestingly, MCF-7/Dox populations grew faster than MCF-7 in a conventional culture condition with 2.0 g/L glucose but proliferated less quickly than MCF-7 when the glucose level was physiological (0.9g/L) (Supplemental Figure 1). In contrast to glucose limitation, MCF-7/Dox showed less susceptibility (85% survival) to the deprivation of glutamine, an alternative metabolic resource, compared to MCF-7 (55% survival) (Figure 3B).
Figure 3.
Effect of limitation of culture media resources, glucose (A) and glutamine (B). MCF-7 and MCF-7/Dox cells were plated and cultured overnight. Cells were cultured further 48 hours after the media was changed to resource-deprived media as indicated. In order to exclude any residual resource in serum, dialyzed FBS (dFBS) was used. Cells grown in a fully supplemented medium containing regular FBS was used as a control group. The amount of survived cells in each group was measured by crystal violet staining (see Materials and Methods) and compared with the control group.
ABCB1 substrates effect as ersatzdroge at physiological glucose level
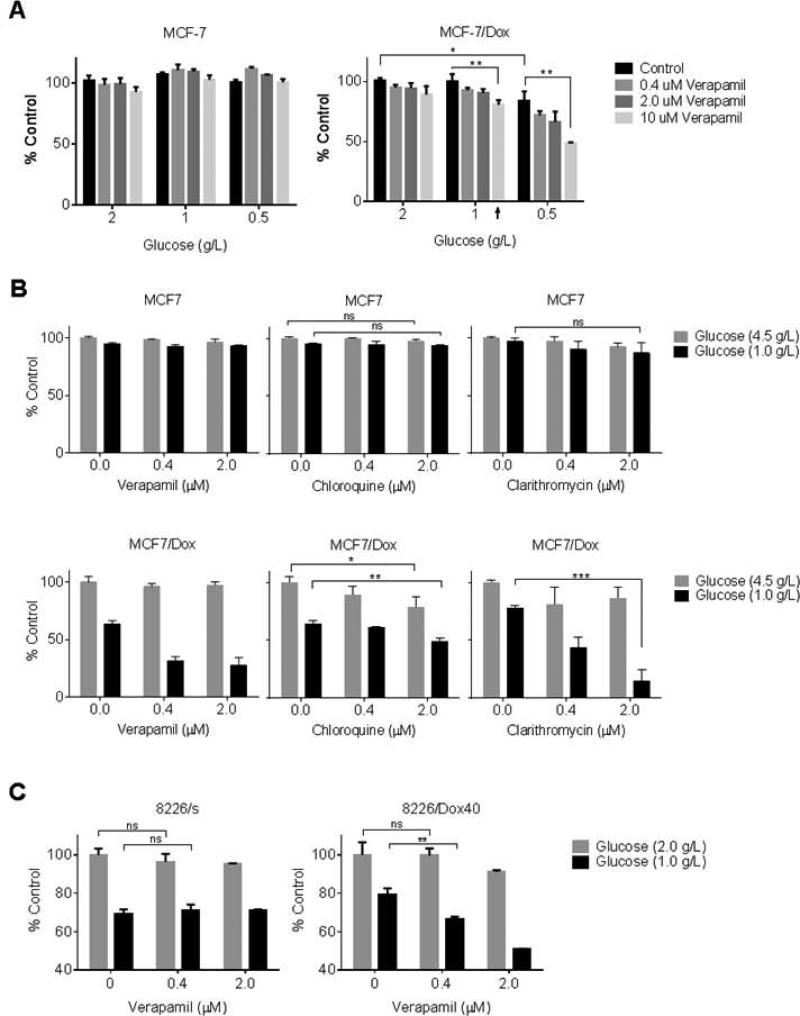
We examined the effect of glucose concentration on the susceptibility of ABCB1-expressing cells to potential ersatzdroges. In agreement with data in Figure 1, verapamil up to 10 μM did not alter survival and proliferation of MCF-7 and MCF-7/Dox within 48 hr in normal culture condition containing 2.0 g/L glucose (Figure 4A). Glucose concentration closer to physiological level in the culture media (1 g/L) did not result in any significant difference for MCF-7 or MCF-7/Dox survival. When glucose was further limited to 0.5 g/L, MCF-7/Dox cell proliferation/survival was reduced to 84 ± 7.6 % (p < 0.05) compared to the cells cultured in the conventional culture media (2.0 g/L of glucose). In contrast, there was no difference in MCF-7 survival and proliferation in the tested glucose concentrations (p > 0.5). The data also showed that 10 μM verapamil can decrease the survived cell amount further than glucose limitation alone (p < 0.005). Interestingly, verapamil could suppress MCF-7/Dox even in the presence of 1 g/L glucose which showed little effect by itself (arrowed).
Figure 4.
Glucose-dependent ersatzdroge effect. A. The proliferated and survived cell amounts of MCF-7 and MCF-7/Dox after 48 hr culture in the presence of various glucose and/or verapamil were measured by crystal violet staining. Data were presented as % value of control group maintained in the presence of 2.0 g/L glucose and in the absence of verapamil. B. The proliferation/survival of MCF-7 and MCF-7/Dox were measured after 72 hr culture in the presence of glucose at 4.5 g/L or 1.0 g/L by varying ersatzdroge type and concentration. The control is the cell amount of each cell type raised in the presence of 4.5 g/L glucose without any additional compound. C. The survived cell amount of 8226/s and 8226/Dox40 was measured and compared after 48 h culture in the presence of verapamil. (ns, not significant; *, p<0.05; **, p<0.005, ***, p<0.0005)
Based on the verapamil effect in the physiological glucose-condition shown in Figure 4A, the effect of other ersatzdroge candidates were evaluated by the similar method. Erythromycin was excluded from the candidates because MCF-7/Dox may have higher fitness by the protective effect of ABCB1 as shown in Figure 1D. The live cell amount after 72 hr culture in the absence and presence of several ABCB1 substrates were compared by varying glucose concentration in media – high glucose (4.5 g/L) vs. physiological glucose (1 g/L). The high glucose culture condition (4.5 g/L) is widely used for many cell lines including MCF-7 itself to provide unlimited glucose resource. As shown in lower graphs of Figure 4B, all three ersatzdroges could suppress MCF-7/Dox survival even in concentrations of 2 μM or less when glucose was limited to physiological level. The upper graphs in Figure 4B showed again that MCF-7 proliferation/survival was not perturbed at all by any of ABCB1 substrates tested regardless of glucose level. The survived cell amount was decreased by more than 50 % within 72 hr by verapamil treatment even by 0.4 μM concentration compared to the cells maintained by physiological level glucose in the absence of any ersatzdroge; from 64 % to 31 % survival. Clarithromycin also showed MCF-7/Dox-specific suppressive effect in glucose level-dependent manner and the survival rate was reduced down by 80% (77 % to 14 %) upon 2 μM treatment. Chloroquine suppressed MCF-7/Dox proliferation and survival glucose level-dependently but appeared to be less effective (reduction by 24 %, p < 0.005) compared to verapamil or clarithromycin.
To extend these results, we examined the effects of ersatzdroges on cell lines 8226/Dox40 (Figure 4C). Similar to the other experiments we found proliferation/survival was suppressed by verapamil when glucose level in the culture medium was reduced to 1.0 g/L or less. At 1.0 g/L, the survival of 8226/s and 8226/Dox40 was reduced to 69 ± 2.1% and 79 ± 3.4 % in the absence of any drugs. The additions of 0.4 μM verapamil further decreased live 8226/Dox40 cells to 67 ± 1.0 % (reduction by 15% than glucose limitation only, p < 0.005, ). Verapamil at 2 μM reduced the survival rate to 51 ± 0.15 % (further reduction by 35%, p < 0.0005) in the presence of physiological level glucose. In contrast, in the presence of 2.0 g/L glucose, 2 μM verapamil decreased the survival slightly (~ 9%) but it was statistically insignificant (p=0.078). Similar to the MCF-7 cells, the drug-sensitive parental cell line 8226/s was not inhibited by verapamil treatment regardless of glucose availability.
MDR tumor growth disadvantage and increased glucose uptake in vivo
To examine the effects of ABCB1 growth in vivo, we implanted MCF-7 and MCF-7/Dox into left and right mammary fat pads (MFPs) of a given mouse respectively. The tumor growth was monitored by MRI volume measurements performed twice per week. As shown in Figure 5A, MCF-7/Dox tumor volume increased at a slower rate than the wild type MCF-7; 353 ± 50 % and 557 ± 98 % increase within 21 day window (p = 0.054). When the tumor size paired for each mouse, MCF-7/Dox tumors are generally smaller than MCF-7 tumor (Figure 5B, p < 0.05 at ratio paired t-test).
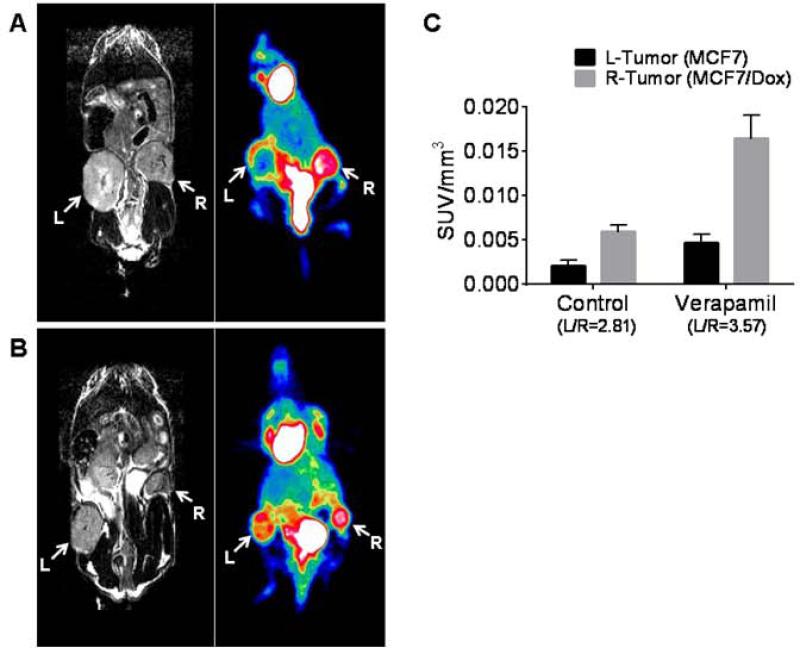
To investigate the possibility that the metabolic cost of ABCB1 expression could be observed with clinical imaging, we examine glucose consumption inMCF-7/Dox and. MCF-7 tumors using 18F-FDG-Positron Emission Tomography (PET). In agreement with prior in vitro data (10), 18F-FDG accumulation in MCF-7/Dox tumor was higher than the MCF-7 (Figure 6A). The standardized uptake value (SUV) of PET signal normalized using tumor volume measured by MRI was 2.8 fold higher than MCF-7 (Figure 6C, left bars).
Figure 6.
Comparison of 18F-FDG-PET signal with T2-weighted MRI. (A) A control mouse bearing MCF-7 and MCF-7/Dox in the dual MFP model was imaged by MRI and PET . (B) A mouse bearing dual tumors was injected with 5 mg/kg verapamil (i.p.) 1 hour prior to the 18F-FDG injection. MRI and PET were acquired using the similar scan plane. (C) PET signal was normalized by the tumor volume measured by MRI (L, left tumor of MCF-7; R, right tumor of MCF-7/Dox). From the dynamic scanning data, SUV at 24 min was used as the representative data.
To examine the metabolic cost of the ABC pumps following administration of an ersatzdroge we measure the glucose uptake in tumors before and after injection of verapamil (5mg/kg IV). As shown in Figures 6B and 6C, the normalized SUV in the MCF-7/Dox tumor changed from 0.0059 to 0.016 while the SUV in the MCF-7 tumor changed from 0.0021 to 0.0046.
Discussion
Although many chemotherapeutic drugs are initially effective, treatment almost inevitably fails due to emergence and proliferation of resistant populations. While cancers can develop many mechanisms of resistance, a common and well-studied strategy is extrusion of cytotoxic drugs through upregulation of membrane proteins such as ABCB1. Because xenobiotic metabolism can act on a wide range of substrate, this commonly results in multi-drug resistance (MDR). Although the Darwinian advantage of resistance during chemotherapy is apparent, there is a significant disadvantage in the absence of therapy because every resistance mechanism entails a metabolic cost. In the case of MDR, extra resources are required for synthesis of the membrane proteins and the ATP cost for the pump activity. In the intratumoral environment of limited resources, the energy cost of resistance must be compensated by reduction in other activities including proliferation and invasion. As a result, drug-resistant phenotypes will generally be less fit than the sensitive phenotypes in the absence of therapy. This is evident, for example, in the rare expression of ABCB1 proteins in breast cancer prior to therapy typically remain a minor population. That is, the expenditure of energy for resistance proteins is not evolutionarily favored unless it confers a significant survival benefit as occurs when chemotherapy is administered.
We have previously demonstrated that this difference in fitness could be exploited to prolong response to chemotherapy 9, 10. Specifically we have found that if small populations of chemosensitive cells are allowed to persist they will suppress the proliferation of resistant cells when treatment is withdrawn.
Here we address an additional mechanism to gain evolutionary advantage through administration of a non-toxic drug that is a substrate for ABCB1 protein and forces MDR cells to activate their membrane pumps. We hypothesized that this will generate a significant energetic cost requiring diversion of resources away from proliferation and invasion. Administration of this “ersatzdroge” will, thus, decrease the proliferation of drug-resistant tumor populations.
Our experimental work builds of prior studies that demonstrated perturbations in energy metabolism upon MDR acquisition including elevated glycolysis 12-14 or energy resource alteration 15. Similarly, we demonstrate that resistant cells expend increased energy to maintain then MDR phenotype even in the absence of substrate. This manifested as increased glycolysis and glycolytic capacity as well as the increased uptake of 2-NBDG, a fluorescent analog of 2-deoxyglucose 10. Here we demonstrate that this energy cost results in decreased proliferation and survival in physiological and sub-physiological glucose concentrations but not in supra-physiological glucose concentrations. The latter indicates that in an environment of abundant resources the metabolic effects of resistance are minimized.
Our results demonstrate that the increased energy demand for acquisition of the MDR phenotype has two components. First, there is the necessary cost to produce and maintain ABCB1 pumps on the cell membrane. This is a “fixed” cost and will be observed even in the absence of drug. Second, the MDR phenotype has a variable cost that represents the ATP necessary to extruded substrates that are administered as therapy. Interestingly, our results demonstrate that the MDR phenotype utilizes glycolysis as a major source of ATP for pumping activity so that part of the fixed cost will include upregulation of glycolytic enzymes to provide ATP for pump activity. In fact, prior reports have demonstrated a similar fixed cost with increased glycolytic capacity and high glycolysis activity of MCF-7/Dox even in the absence of verapamil.10 The results suggest a genetic or epigenetic link between glycolysis and the acquisition of ABCB1 expression. Consistent with this drug efflux pump expression in liver cancer cells can be modulated depending on glucose availability and extracellular ATP. 16, 17 However, the precise molecular mechanisms for this remain unknown.
Critically, we find that the administration of an ersatzdroge increases the cost of resistance and further diminishes proliferation. Prior studies have investigated MDR substrate as potential competitive inhibitors but this represents, to the best of our knowledge, the first attempt to use non-toxic substrate to consume the resources of resistant cells and thus reduce their capacity for proliferation and invasion. Further detailed studies will be required for screening of effective ersatzdroges and appropriate administration design. Importantly, we find ersatzdroge candidates should be evaluated in the presence of physiological glucose concentration not in high glucose culture condition according to our data. Also, the screening and administration design may need to consider additional pharmacological factors such as turn over rate at plasma membrane and half life in blood.
Finally, we note that the cost of resistance could be exploited to detect resistant tumors or regions of tumors using clinical imaging. We demonstrated that resistant tumors exhibit increased glucose uptake on 18F-FDG PET scans compared to sensitive tumors and that the administration of an ersatzdroge further increased glucose utilization. Thus, 18F-FDG-PET scans before and after the administration of a cytotoxic drug or and ersatzdroge could be used to detect emergence of resistant tumors or regions within a tumor.
In conclusion, we demonstrate the potential value of ersatzdroges – non toxic (or minimally toxic) drugs that are substrate for xenobiotic membrane pumps – in systemic therapy for disseminated or locally unresectable cancers. We demonstrate that we can use these compounds to increase the energy expenditure in resistant cancer cells thus reducing their fitness and proliferation. This property may be useful clinically to detect the presence of resistant tumors during therapy using 18F-FDG PET imaging and prolong progression-free survival by delaying or preventing growth of resistant populations. Finally, while we used clinically available compounds as ersatzdroges in this study, clinical translation will require further drug development to limit toxicity and optimize interactions with membrane pumps. However, our findings suggest this treatment strategy may be valuable in delaying or preventing emergence of drug-resistance during common cancer therapies.
Supplementary Material
Supplementary Figure 1. The growth patterns of MCF-7 and MCF-7/Dox in the absence or presence of verapamil varying the glucose level in culture media. The glucose concentration of each culture condition was indicated in parenthesis.
Novelty and Impact Statements.
A common mechanism for acquired resistance to cancer chemotherapy is upregulation of the ABCB1 pump. We present a novel evolutionary strategy to suppress proliferation of cells expressing ABCB1 by administering nontoxic pump substrates – “Ersatzdroges”. We demonstrate that Eresatzdroges increase the metabolic energy cost of the therapy-resistant phenotype requiring diversion of resources with reduction of the cell's ability to proliferate and invade. Since many Ersatzdroges are currently available (e.g. Verapamil), this strategy could be applied clinically.
Acknowledgement
This work was supported by National Cancer Institute at the National Institutes of Health 1U54CA143970-01 and RO1CA170595; and the James S. McDonnell Foundation 21st Century Science Initiative Grant “Cancer Therapy: Perturbing a Complex Adaptive System”.
Footnotes
Conflict of Interest: None
References
- 1.Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature reviews Cancer. 2010;10:147–56. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 2.Wind NS, Holen I. Multidrug resistance in breast cancer: from in vitro models to clinical studies. International journal of breast cancer. 2011;2011:967419. doi: 10.4061/2011/967419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchetti S, Mazzanti R, Beijnen JH, Schellens JH. Concise review: Clinical relevance of drug drug and herb drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein). The oncologist. 2007;12:927–41. doi: 10.1634/theoncologist.12-8-927. [DOI] [PubMed] [Google Scholar]
- 4.Wishart GC, Plumb JA, Going JJ, McNicol AM, McArdle CS, Tsuruo T, Kaye SB. P-glycoprotein expression in primary breast cancer detected by immunocytochemistry with two monoclonal antibodies. British journal of cancer. 1990;62:758–61. doi: 10.1038/bjc.1990.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider J, Bak M, Efferth T, Kaufmann M, Mattern J, Volm M. P-glycoprotein expression in treated and untreated human breast cancer. British journal of cancer. 1989;60:815–8. doi: 10.1038/bjc.1989.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanfilippo O, Ronchi E, De Marco C, Di Fronzo G, Silvestrini R. Expression of P-glycoprotein in breast cancer tissue and in vitro resistance to doxorubicin and vincristine. Eur J Cancer. 1991;27:155–8. doi: 10.1016/0277-5379(91)90476-t. [DOI] [PubMed] [Google Scholar]
- 7.Gerlinger M, Swanton C. How Darwinian models inform therapeutic failure initiated by clonal heterogeneity in cancer medicine. British journal of cancer. 2010;103:1139–43. doi: 10.1038/sj.bjc.6605912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aktipis CA, Kwan VS, Johnson KA, Neuberg SL, Maley CC. Overlooking evolution: a systematic analysis of cancer relapse and therapeutic resistance research. PloS one. 2011;6:e26100. doi: 10.1371/journal.pone.0026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res. 2009;69:4894–903. doi: 10.1158/0008-5472.CAN-08-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva AS, Kam Y, Khin ZP, Minton SE, Gillies RJ, Gatenby RA. Evolutionary approaches to prolong progression-free survival in breast cancer. Cancer Res. 2012;72:6362–70. doi: 10.1158/0008-5472.CAN-12-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalton WS, Durie BG, Alberts DS, Gerlach JH, Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Res. 1986;46:5125–30. [PubMed] [Google Scholar]
- 12.Broxterman HJ, Pinedo HM, Kuiper CM, Kaptein LC, Schuurhuis GJ, Lankelma J. Induction by verapamil of a rapid increase in ATP consumption in multidrug-resistant tumor cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1988;2:2278–82. doi: 10.1096/fasebj.2.7.3350243. [DOI] [PubMed] [Google Scholar]
- 13.Broxterman HJ, Pinedo HM, Kuiper CM, Schuurhuis GJ, Lankelma J. Glycolysis in P-glycoprotein-overexpressing human tumor cell lines. Effects of resistance-modifying agents. FEBS letters. 1989;247:405–10. doi: 10.1016/0014-5793(89)81380-8. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan O, Navon G, Lyon RC, Faustino PJ, Straka EJ, Cohen JS. Effects of 2-deoxyglucose on drug-sensitive and drug-resistant human breast cancer cells: toxicity and magnetic resonance spectroscopy studies of metabolism. Cancer Res. 1990;50:544–51. [PubMed] [Google Scholar]
- 15.Harper ME, Antoniou A, Villalobos-Menuey E, Russo A, Trauger R, Vendemelio M, George A, Bartholomew R, Carlo D, Shaikh A, Kupperman J, Newell EW, et al. Characterization of a novel metabolic strategy used by drug-resistant tumor cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2002;16:1550–7. doi: 10.1096/fj.02-0541com. [DOI] [PubMed] [Google Scholar]
- 16.Ledoux S, Yang R, Friedlander G, Laouari D. Glucose depletion enhances P-glycoprotein expression in hepatoma cells: role of endoplasmic reticulum stress response. Cancer Res. 2003;63:7284–90. [PubMed] [Google Scholar]
- 17.Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, Bergen AA, Gorgels TG, Borst P, van de Wetering K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20206–11. doi: 10.1073/pnas.1319582110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. The growth patterns of MCF-7 and MCF-7/Dox in the absence or presence of verapamil varying the glucose level in culture media. The glucose concentration of each culture condition was indicated in parenthesis.