Summary
Minimal residual disease (MRD) is a strong prognostic factor in children and adolescents with acute myeloid leukaemia (AML) but nearly one-quarter of patients who achieve MRD-negative status still relapse. The adverse prognostic factors among MRD-negative patients remain unknown. We analysed the AML02 study cohort to identify demographic and genetic prognostic factors. Among the presenting features, certain 11q23 abnormalities, such as t(6;11) and t(10;11), acute megakaryoblastic leukaemia without the t(1;22), and age ≥10 years were associated with inferior outcome in patients who had MRD-negative status after either remission induction I or II. By contrast, those with rearrangement of CBF genes had superior outcome. Our study identifies patient populations for whom close post-remission MRD monitoring to detect and treat emerging relapse and adjustment in treatment intensity might be indicated.
Keywords: acute myeloid leukaemia, minimal residual disease, paediatric, prognostic factor
Introduction
Despite significant progress in treating paediatric acute myeloid leukaemia (AML), relapse rates still range between 20% and 41% in contemporary trials, with overall survival rates commonly below 75% (Tsukimoto, et al 2009; Rubnitz, et al 2010a; Abrahamsson, et al 2011; Gibson, et al 2011; Cooper, et al 2012; Hasle, et al 2012; Creutzig, et al 2013; Pession, et al 2013). Refinement in predictions of which patients will relapse may allow further improvements in outcomes.
Tracking residual AML cells beyond the resolution of standard morphological methods (i.e. minimal residual disease, MRD) by flow cytometry allows a more accurate definition of early treatment response (Coustan-Smith, et al 2003; Langebrake, et al 2006; Rubnitz, et al 2010a; van der Velden, et al 2010; Buccisano, et al 2012; Loken, et al 2012; Coustan-Smith and Campana 2013). However, attaining low levels of MRD by flow cytometry does not exclude subsequent relapse: for example, AML may recur in about 20% of patients with negative MRD after remission induction therapy (Rubnitz, et al 2010a; van der Velden, et al 2010; Loken, et al 2012). Polymerase chain reaction (PCR) monitoring of leukaemic fusion transcripts also does not provide further insights in prognosis among patients who are MRD-negative by flow cytometry (Inaba, et al 2012). Until a more sensitive technique to detect MRD is developed, we reasoned that presenting features may help identify those with a higher risk of relapse among these patients. We therefore analysed the prognostic impact of clinical and biological parameters determined at diagnosis among a cohort of children and adolescents with AML enrolled in the St. Jude AML02 study who achieved MRD-negative status at the end of remission induction therapy.
Methods
Patients and therapy
Between 2002 and 2008, 232 non-Down syndrome children with AML were enrolled on the multicentre AML02 study (NCT00136084); 24 were excluded from the present analysis (2 were not entered in the scheduled randomization of remission induction therapy, 14 had mixed-lineage leukaemia and 8 did not complete induction II; Sup. Fig 1). Patients with PML-RARa (acute promyelocytic leukaemia) were excluded from therapy on AML02. Full details of the treatment plan and risk assignment have previously been described (Rubnitz, et al 2010a).
MRD was measured by flow cytometry, as previously described (Rubnitz, et al 2010a; Inaba, et al 2012). Briefly, a patient-specific, leukaemia-associated immunophenotype was identified at diagnosis, with combinations of markers chosen to allow detection of MRD of at least 0.1% (i.e., 1 cell in 1,000), and then applied in follow-up bone marrow evaluations. Samples were evaluated for structural abnormalities using conventional cytogenetics. Reverse transcription PCR (RT-PCR) for KMT2A (MLL)-MLLT3 (AF9), Rearrangement of CBF genes (RUNX1-RUNX1T1 and CBFB-MYH11), RBM15-MKL1 and FLT3-internal tandem duplication (ITD) was performed on all samples as previously described (Raimondi, et al 1999; Coustan-Smith, et al 2003). FLT3-ITD/wild type allelic ratio data was not obtained in this study. Samples were also evaluated for KMT2A (MLL) rearrangement by fluorescent in-situ hybridization in all cases without a common chromosomal rearrangement.
Patients with -7, FLT3-ITD, t(6;9), megakaryoblastic leukaemia, treatment-related AML, or AML arising from myelodysplastic syndrome were designated as high risk and were eligible for haematopoietic stem cell transplantation following induction chemotherapy, as were standard risk patients (no features of high or low risk) with a matched sibling donor. Patients with poor responses to therapy (>25% blasts after induction I or persistent MRD after three courses of therapy) were also considered high risk. All other patients received consolidation chemotherapy after remission induction. Patients with t(8;21)/RUNX1-RUNX1T1, inv(16) or t(16;16)/CBFB-MHY11, or t(9;11)/KMT2A (MLL)-MLLT3 were considered low risk.
The study was approved by the institutional review boards at the participating institutions. Informed consent was obtained from the patients, parents or guardians and assent from the patients, as appropriate.
Statistical analysis
Event-free survival was defined as the time from diagnosis while disease-free survival (DFS) and overall survival (OS) were defined as the time from the end of induction II to an adverse event (withdrawal, relapse, second malignancy, or death) or death, respectively, estimated by the Kaplan-Meier method, and compared by the exact log-rank test. The exact χ2 test was used to compare categorical features and MRD status. The association of presenting features with outcome among MRD-defined cohorts was assessed by Cox proportional hazards regression models; a bi-directional, stepwise procedure was used to determine the final model with Akaike information criteria (Manola et al 2000) and results from the univariate analysis. The multivariate models were fitted by using patients for whom data about all the variables were available. The possible variables were: age, race, sex, white blood cell (WBC) count, provisional assessment of standard and high risk, t(9;11), t(6;11) and t(10;11), other 11q23 abnormalities, acute megakaryoblastic leukaemia (AMKL) without t(1;22), Rearrangement of CBF genes, FLT3-ITD, and duration of induction therapy. Monte-Carlo approximations based on 104 permutations were used to compute the P values for the χ2 and log-rank tests. P values were two-sided with values less than 0.05 considered to indicate statistical significance. The competing-risks regression method was used to model the risk of relapse while adjusting for competing risks (i.e., induction failure, study withdrawal, second malignancy and death).
Results
Patient characteristics
Demographics of the 208 patients analysed are shown in Table I. Five-year DFS was 63.5% ± 4.6% (standard deviation) and 5-year OS was 72.1% ± 4.2%. After remission induction I, 126 (64.0%) of the 197 patients who were analysed by flow cytometry achieved MRD-negative status; 5-year DFS and OS rates for these groups were 75.3% ± 5.5% and 81.4% ± 4.9%, respectively, a significantly better outcome than that of the 71 patients with detectable MRD (DFS 38.9% ± 7.4% and OS 52.9% ± 7.4%; P<0.001 for both comparisons). After remission induction II, 155 (80.3%) of 193 evaluable patients were MRD-negative. Again, 5-year DFS (71.4% ± 5.1%) and OS (77.3% ± 4.7%) rates for this group were superior to those of the 38 patients with detectable MRD (28.1% ± 7.9% and 48.2% ± 9.3%, respectively; P< 0.001 for both comparisons).
Table I.
Association of patient demographics with minimal residual disease after induction therapy
| Presenting feature | No. of patients | Induction I MRD status | Induction II MRD status | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Positive | Negative | P | Positive | Negative | P | ||
| Age (years) | |||||||
| < 10 | 108 | 33 | 68 | <0.001 | 20 | 80 | < 0.001 |
| ≥ 10 | 100 | 38 | 58 | 18 | 75 | ||
|
| |||||||
| Leucocyte counts (x 109/l) | |||||||
| < 50 | 153 | 51 | 97 | 0.49 | 28 | 117 | 0.84 |
| ≥ 50 | 55 | 20 | 29 | 10 | 38 | ||
|
| |||||||
| Sex | |||||||
| Male | 117 | 42 | 70 | 0.66 | 22 | 88 | 1.0 |
| Female | 91 | 29 | 56 | 16 | 67 | ||
|
| |||||||
| Race | |||||||
| White | 146 | 49 | 90 | 0.75 | 28 | 107 | 0.16 |
| Black | 38 | 12 | 24 | 9 | 27 | ||
| Other | 23 | 9 | 12 | 1 | 20 | ||
|
| |||||||
| FAB Subtype | 0.01 | 0.32 | |||||
| M0 | 2 | 1 | 1 | 1.0 | 0 | 2 | 1.0 |
| M1 | 23 | 15 | 7 | 0.002 | 6 | 14 | 0.24 |
| M2 | 35 | 10 | 25 | 0.34 | 5 | 29 | 0.47 |
| M4 | 55 | 18 | 37 | 0.62 | 10 | 44 | 1.0 |
| M5 | 52 | 10 | 35 | 0.034 | 6 | 39 | 0.29 |
| M6 | 2 | 1 | 1 | 1.0 | 1 | 1 | 0.36 |
| M7 (AMKL) | 25 | 9 | 14 | 0.82 | 5 | 18 | 0.78 |
| NA | 14 | 7 | 6 | 0.23 | 5 | 8 | 0.14 |
|
| |||||||
| Karyotype | <0.001 | <0.001 | |||||
| t (9;11) | 15 | 4 | 11 | 0.58 | 2 | 13 | 0.74 |
| 11q23 non-t(9;11) | 25 | 5 | 17 | 0.24 | 2 | 20 | 0.26 |
| t(1;11) | 2 | 2 | 0 | 0.13 | 0 | 2 | 1.0 |
| t(4;11) | 1 | 0 | 0 | NA | 0 | 0 | NA |
| t(6;11) | 2 | 0 | 2 | 0.54 | 0 | 2 | 1.0 |
| t(10;11) | 9 | 0 | 9 | 0.028 | 1 | 8 | 1.0 |
| t(11;19) | 9 | 2 | 5 | 1.0 | 1 | 6 | 1.0 |
| Other 11q23 | 2 | 1 | 1 | 1.0 | 0 | 2 | 1.0 |
| t (8;21) | 30 | 6 | 24 | 0.062 | 1 | 28 | 0.02 |
| inv (16)/t(16;16) | 25 | 0 | 25 | <0.001 | 0 | 25 | 0.005 |
| t(1;22) | 5 | 0 | 4 | 0.30 | 0 | 4 | 1.0 |
| Normal | 49 | 28 | 19 | <0.001 | 14 | 31 | 0.034 |
| Other | 54 | 25 | 25 | 0.026 | 17 | 32 | 0.004 |
| NA | 5 | 3 | 1 | 0.13 | 2 | 2 | 0.17 |
|
| |||||||
| FLT3 status | |||||||
| ITD | 27 | 20 | 6 | <0.001 | 13 | 12 | < 0.001 |
| Mutation | 8 | 1 | 6 | 1 | 6 | ||
| Wild-type | 158 | 46 | 105 | 23 | 125 | ||
| Unknown | 15 | 4 | 9 | 1 | 12 | ||
|
| |||||||
| Study Arm | |||||||
| High-dose Ara-C | 99 | 29 | 64 | 0.19 | 15 | 76 | 0.37 |
| Low-dose Ara-C | 109 | 42 | 62 | 23 | 79 | ||
MRD, minimal residual disease; FAB, French-American-British classification; ITD, internal tandem duplication; NA, not available; AMKL, acute megakaryoblastic leukaemia; Ara-C, cytarabine.
Total numbers may be unequal due to incomplete data. The exact χ2 test was used to compare categorical features and MRD status.
Patients younger than 10 years were more likely than older patients to be MRD-negative after induction I (P<0.001) and induction II (P<0.001). There was an association between French-American-British subtype and MRD status after induction I but not induction II. Karyotypes were associated with MRD status after both induction I (P<0.001) and induction II (P<0.001), with inv(16)/t(16;16), t(10;11) (for induction I only), and t(8;21) (for induction II only) associated with lower rates of MRD, while normal karyotype was associated higher rates of MRD. FLT3-ITD was associated with increased occurrence of MRD at both time points (P<0.001 for inductions I and II).
Identification of risk populations among MRD-negative patients
Supplemental Table I summarizes results of univariate analyses in the cohorts of patients who were MRD-negative after induction I and II. For these analyses, 11q23 abnormalities except the t(9;11) were initially grouped together, termed as 11q23 non-t(9;11). Patients with the t(9;11) were excluded from 11q23 analysis because the protocol classified these patients as low risk. Evaluation of patients with AMKL excluded patients with the t(1;22), a group with favourable outcome in AML02 (O’Brien, et al 2013).
Univariate analysis showed that 11q23 non-t(9;11) and AMKL without t(1;22) were significant adverse predictors for both DFS and OS among patients who were MRD-negative after induction I and II. Subgroup analysis of the non-t(9;11) 11q23 cohort based on KMT2A (MLL) translocation partners indicated that this effect was due primarily to patients with the t(6;11) and the t(10;11), henceforth termed high-risk KMT2A (MLL) [HR-KMT2A (MLL)]. Patients with core-binding factor (CBF) leukaemia had a favourable outcome compared to other MRD-negative patients after induction I and II. Patients with FLT3-ITD who achieved MRD-negative status had a trend toward adverse risk after induction II but not induction I.
In multivariate analyses, HR-KMT2A (MLL), AMKL without the t(1;22) and age ≥10 years were all associated with lower DFS and OS rates in patients who were MRD-negative after induction I (Table II). The same factors (as well as black race) were also significant adverse prognostic factors in patients who were MRD-negative after induction II. In contrast, patients with CBF-rearranged AML had a significantly better outcome than other patients who were MRD-negative after induction I or II (Table II).
Table II.
Independent factors associated with outcome among minimal residual disease–negative patients in multivariate model
| Cohort | Prognostic subgroup | N | N of relapses | DFS | OS | ||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR | 95% CI | P | HR | 95% CI | P | ||||
| Induction I (N =126) | t(6;11)/t(10;11) | 10 | 6 | 5.57 | 1.97–15.73 | 0.001 | 9.660 | 3.09–30.15 | <0.001 |
| AMKL without t(1;22) | 10 | 4 | 5.43 | 1.58–18.59 | 0.007 | 7.980 | 1.85–34.43 | 0.005 | |
| CBF-rearrangement | 49 | 2 | 0.18 | 0.06–0.57 | 0.004 | 0.150 | 0.03–0.75 | 0.02 | |
| Age ≥ 10 years | 58 | 9 | 6.08 | 2.29–16.16 | <0.001 | 6.650 | 2.03–21.79 | 0.002 | |
|
| |||||||||
| Induction II (N =155) | t(6;11)/t(10;11) | 9 | 4 | 4.25 | 1.53–11.77 | 0.005 | 7.470 | 2.45–22.73 | <0.001 |
| AMKL without t(1;22) | 13 | 6 | 4.01 | 1.25–12.91 | 0.02 | 7.690 | 2.06–28.71 | 0.002 | |
| CBF-rearrangement | 52 | 2 | 0.14 | 0.04–0.45 | <0.001 | 0.210 | 0.06–0.79 | 0.02 | |
| Age ≥ 10 years | 68 | 11 | 3.220 | 1.37–7.55 | 0.007 | 3.330 | 1.17–9.43 | 0.02 | |
| FLT3-ITD | 11 | 0 | 1.260 | 0.45–3.55 | 0.66 | 2.590 | 0.85–7.84 | 0.09 | |
| Race (Black) | 25 | 6 | 2.620 | 1.1–6.24 | 0.03 | 2.090 | 0.83–5.23 | 0.12 | |
| Race (Other) | 19 | 3 | 0.410 | 0.1–1.77 | 0.23 | 0.260 | 0.03–2.01 | 0.2 | |
DFS, disease-free survival; OS, overall survival; HR, hazard ratio; MRD, minimal residual disease; CI, confidence interval; AMKL, acute megakaryoblastic leukaemia; CBF, core-binding factor group of genes; ITD, internal tandem duplication. Hazard ratios for black and other race are relative to the risk in white patients.
The associations observed for DFS and OS were also reflected in the analyses of incidence of leukaemia relapse. Thus, MRD-negative patients with HR-KMT2A (MLL), AMKL without the t(1;22), or ≥10 years at diagnosis had a higher risk of relapse, while the risk was lower for patients with CBF-rearranged leukaemia (Table III). Among patients with HR-KMT2A (MLL), relapse occurred in 6 of 10 patients negative for MRD after induction I and 4 of 9 negative for MRD after induction II. On the other hand, among patients without t(9;11) or HR-KMT2A (MLL) rearrangements, relapse occurred in only 1 of 6 with negative MRD after induction I and 2 of 10 with negative MRD after induction II. Both patients with t(1;11) were long term survivors despite positive MRD after induction I (but negative MRD after induction II). Among 13 patients who had AMKL without t(1;22) and negative MRD after induction II, relapse occurred in 3 of 8 after haematopoietic stem cell transplantation and in 3 of 5 patients treated with chemotherapy. Of 62 patients considered low risk based on cytogenetics and negative MRD after 2 courses of induction therapy, 3 of 13 patients with t(9;11) and 2 of 25 patients with inv(16) [or t(16;16)] relapsed. Such low risk patients rarely had positive MRD after 2 courses of induction therapy: 2 patients with the t(9;11) and 1 patient with the t(8;21). One of the 2 patients with t(9;11) relapsed and the patient with the t(8;21) remains in remission.
Table III.
Factors associated with risk of relapse among minimal residual disease–negative patients in multivariate analysis
| Cohort | Prognostic subgroup | HR | 95% CI | P |
|---|---|---|---|---|
| Induction I | t(6;11)/t(10;11) | 5.55 | 2.18–14.17 | <0.001 |
| AMKL without t(1;22) | 5.56 | 1.83–16.89 | 0.002 | |
| CBF-rearrangement | 0.18 | 0.06–0.59 | 0.004 | |
| Age ≥ 10 years | 6.34 | 2.64–15.23 | <0.001 | |
| High-dose Ara-C arm | 0.49 | 0.21–1.13 | 0.095 | |
|
| ||||
| Induction II | t(6;11)/t(10;11) | 3.82 | 1.72–8.48 | 0.001 |
| AMKL without t(1;22) | 4 | 1.46–10.99 | 0.007 | |
| CBF-rearrangement | 0.13 | 0.04–0.39 | <0.001 | |
| Age ≥ 10 years | 3.26 | 1.46–7.29 | 0.004 | |
| Race (Black) | 2.25 | 1–5.03 | 0.049 | |
| Race (Other) | 0.49 | 0.17–1.4 | 0.18 | |
| High-dose Ara-C arm | 0.64 | 0.33–1.27 | 0.21 | |
HR, hazard ratio; CI, confidence interval; AMKL, acute megakaryoblastic leukaemia; CBF, core-binding factor group of genes; Ara-C, cytarabine.
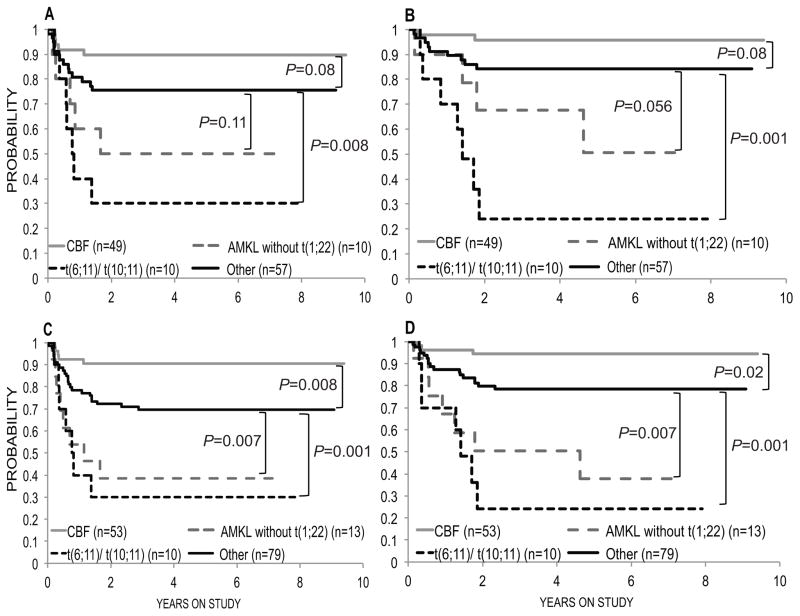
To evaluate for interactions between age and the morphologically and genetically defined groups, patients were categorized as either having AMKL without t(1;22), HR-KMT2A (MLL), a rearrangement of CBF genes, or as a group comprising all other patients. Among these groups, HR-KMT2A (MLL) was a highly significant adverse predictor for both DFS and OS among patients who were MRD-negative after induction I. Among those MRD-negative after induction II, HR-KMT2A (MLL) and AMKL without the t(1;22) were adverse predictors whereas CBF was a favourable prognostic factor (Fig 1).
Fig 1. Kaplan-Meyer curves for minimal residual disease–negative patients.
(A) Disease-free survival and (B) overall survival after induction I. (C) Disease-free survival and (D) overall survival after induction II. AMKL, acute megakaryoblastic leukaemia; CBF, core-binding factor. Other, patients not in the other 3 groups.
Although positive MRD levels were defined as 0.1% or above in this study, 8 of 126 patients after induction I and 10 of 155 after induction II had a detectable leukaemia-associated phenotype below 0.1%. Such patients had significantly worse DFS and OS when compared with those who had undetectable MRD after induction II therapy but not after induction I (Table IV). These patients were heterogeneous, including 1 patient with the t(8;21), 1 with the t(1;19)(q23;p13.3), 3 with normal cytogenetics and 5 with miscellaneous cytogenetic abnormalities.
Table IV.
Comparison of outcomes for patients with detectable minimal residual disease below 0.01% following induction therapy
| MRD | Negative | >0 to 0.001 | P | |
|---|---|---|---|---|
| Induction I | N | 118 | 8 | |
| 3-year DFS | 75.3%±4.2% | 75.0%±14.2% | 0.72 | |
| 3-year OS | 83.4%±3.7% | 75.0%±14.2% | 0.41 | |
|
| ||||
| Induction II | N | 145 | 10 | |
| 3-year DFS | 73.6%±3.8% | 40.0%±13.9% | 0.006 | |
| 3-year OS | 81.0%±3.4% | 40.0%±13.9% | 0.002 | |
MRD, minimal residual disease; DFS, disease-free survival; OS, overall survival
Discussion
The current study identifies certain biological subtypes of childhood AML, i.e. AML with 11q23 abnormalities [t(6;11) and t(10;11)] or AMKL without the t(1;22), which confer a significant risk of relapse despite excellent response to initial therapy. The t(6;11) and t(10;11) were previously identified as high-risk genetic abnormalities in an international study of paediatric 11q23/KMT2A (MLL)-rearranged AML (Balgobind, et al 2009). Half of the patients with these high-risk genetic rearrangements relapsed and these patients may benefit from novel therapeutic agents. In this regard, inhibitors of bromodomain-containing protein 4 (BRD4) or the histone methyltransferase DOT1L target the KMT2A (MLL) complex and associated epigenetic regulators in 11q23-rearranged leukaemia (Zuber, et al 2011; Daigle, et al 2013).
Although many AMKL patients receive allogeneic haematopoietic stem cell transplants in first complete remission despite MRD negative status, those without t(1;22) have a generally dismal prognosis (O’Brien, et al 2013). Thus, these patients may also benefits from novel therapeutic agents. Aurora kinase A inhibitors can promote many features of terminal megakaryocyte differentiation in AMKL cell lines, including the induction of polyploidization, which inhibits cellular proliferation and induces apoptosis (Thiollier, et al 2012; Wen, et al 2012). Rearrangements of CBF genes were associated with a lower likelihood of having MRD after both inductions I and II. While the favourable prognosis associated with CBF rearranged leukaemia has long been known, our data also demonstrate superior outcomes even when compared to other rapidly responding patients.
Age ≥10 years was associated with a worse outcome in MRD-negative patients. A similar trend was shown in the whole AML02 patient population when multiple clinical and biological factors were considered in analysis (Rubnitz, et al 2010a). For younger age groups, there was no difference in DFS or OS between patients <2 years of age and those 2–10 years of age (data not shown). The poor prognosis of age ≥10 years may be partially due to the excess therapy-related toxicity previously shown in this group (Rubnitz, et al 2012). The higher risk of relapse of patients aged ≥10 years also suggests a contribution from disease-related factors, such as FLT3-ITD (Zwaan, et al 2003). Further identification of these factors may further inform treatment decisions for this group. Additionally, black race has previously been shown to be an adverse risk factor in paediatric AML studies (Gurney, et al 1995; Aplenc, et al 2006; Loken, et al 2012). Our data show that this is true even among patients who experience MRD-defined remission.
The efficacy of allogeneic haematopoietic stem cell transplant in first remission for high-risk patients is controversial (Niewerth, et al 2010). However, our recent transplantation results have improved regardless of donor type, possibly because of better DNA-based human leucocyte antigen typing of donors and recipients, better supportive care for infection and comprehensive killer-cell immunoglobulin-like receptor (KIR) typing of donor natural killer (NK) cells (Leung, et al 2011; Bari, et al 2013). Cellular therapy with infusions of NK cells or chimeric antigen receptor T cells can be considered as alternatives to transplantation (Rubnitz, et al 2010b; Mardiros, et al 2013). In our current AML08 study (NCT00703820), we administer KIR-ligand mismatched parental NK cells to MRD negative standard-risk patients. Selection of candidates for transplantation in first remission requires optimal risk stratification.
Although positive MRD levels were defined as 0.1% or above in this study, patients who had detectable MRD below 0.1% level had a worse prognosis after induction II therapy. Thus, patients may benefit from more sensitive methods to detect residual disease. In this regard, future MRD evaluation by flow cytometry will be aided by the integration of new leukaemia-specific markers (Coustan-Smith and Campana 2013).
In conclusion, our study identifies patients with certain 11q23 abnormalities, such as the t(6;11) and the t(10;11), patients with AMKL without t(1;22) and patients aged ≥10 years as being at high risk for therapy failure despite apparent response to remission induction therapy. Changes in therapeutic regimens for these patients may be needed in future trials to improve their outcomes.
Supplementary Material
Acknowledgments
This study was supported, in part, by Cancer Center Core Grant CA21765 from the National Institutes of Health and by the American Lebanese Syrian Associated Charities (ALSAC). Dr. Pui is an American Cancer Society Professor. The authors thank Soheil Meshinchi, MD, PhD (Fred Hutchinson Cancer Research Center, Seattle, WA) and Gladstone Airewele, MD (Texas Children’s Cancer Center, Houston, TX) for entering patients in AML02 and Cherise Guess (St. Jude) for editorial assistance.
Footnotes
Conflict-of-interest disclosure: The authors have no potential conflicts of interest, including specific financial interests, relationships, or affiliations relevant to the subject of this manuscript.
Contribution: S.E.K. and H.I. designed the research. E.C-S. and D.C. performed minimal residual disease analyses. X.C. performed the statistical analyses. E.C-S., S.A.S., S.C.R., J.K.C., R.C.R., G.V.D., W.P.B., J.W.T., B.D., W.L., J.R.D., C.H.P., J.E.R., D.C. and H.I. contributed patient samples and/or contributed to the clinical trial. S.E.K. and H.I. wrote the paper with contributions from all authors.
References
- Abrahamsson J, Forestier E, Heldrup J, Jahnukainen K, Jonsson OG, Lausen B, Palle J, Zeller B, Hasle H. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. doi: 10.1200/JCO.2010.30.6829. [DOI] [PubMed] [Google Scholar]
- Aplenc R, Alonzo TA, Gerbing RB, Smith FO, Meshinchi S, Ross JA, Perentesis J, Woods WG, Lange BJ, Davies SM. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Blood. 2006;108:74–80. doi: 10.1182/blood-2005-10-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, Beverloo HB, Chang M, Creutzig U, Dworzak MN, Forestier E, Gibson B, Hasle H, Harrison CJ, Heerema NA, Kaspers GJ, Leszl A, Litvinko N, Nigro LL, Morimoto A, Perot C, Pieters R, Reinhardt D, Rubnitz JE, Smith FO, Stary J, Stasevich I, Strehl S, Taga T, Tomizawa D, Webb D, Zemanova Z, Zwaan CM, van den Heuvel-Eibrink MM. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114:2489–2496. doi: 10.1182/blood-2009-04-215152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari R, Rujkijyanont P, Sullivan E, Kang G, Turner V, Gan K, Leung W. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J Clin Oncol. 2013;31:3782–3790. doi: 10.1200/JCO.2012.47.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccisano F, Maurillo L, Del Principe MI, Del Poeta G, Sconocchia G, Lo-Coco F, Arcese W, Amadori S, Venditti A. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012;119:332–341. doi: 10.1182/blood-2011-08-363291. [DOI] [PubMed] [Google Scholar]
- Cooper TM, Franklin J, Gerbing RB, Alonzo TA, Hurwitz C, Raimondi SC, Hirsch B, Smith FO, Mathew P, Arceci RJ, Feusner J, Iannone R, Lavey RS, Meshinchi S, Gamis A. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118:761–769. doi: 10.1002/cncr.26190. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Campana D. Should evaluation for minimal residual disease be routine in acute myeloid leukemia? Curr Opin Hematol. 2013;20:86–92. doi: 10.1097/MOH.0b013e32835dd90a. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Ribeiro RC, Rubnitz JE, Razzouk BI, Pui CH, Pounds S, Andreansky M, Behm FG, Raimondi SC, Shurtleff SA, Downing JR, Campana D. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, Klingebiel T, Kremens B, Lehrnbecher T, von Neuhoff C, Ritter J, Sander A, Schrauder A, von Stackelberg A, Stary J, Reinhardt D. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. [DOI] [PubMed] [Google Scholar]
- Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, Allain CJ, Klaus CR, Raimondi A, Scott MP, Waters NJ, Chesworth R, Moyer MP, Copeland RA, Richon VM, Pollock RM. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122:1017–1025. doi: 10.1182/blood-2013-04-497644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson BE, Webb DK, Howman AJ, De Graaf SS, Harrison CJ, Wheatley K. Results of a randomized trial in children with Acute Myeloid Leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155:366–376. doi: 10.1111/j.1365-2141.2011.08851.x. [DOI] [PubMed] [Google Scholar]
- Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States. Sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–2195. doi: 10.1002/1097-0142(19950415)75:8<2186::aid-cncr2820750825>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Hasle H, Abrahamsson J, Forestier E, Ha SY, Heldrup J, Jahnukainen K, Jonsson OG, Lausen B, Palle J, Zeller B. Gemtuzumab ozogamicin as postconsolidation therapy does not prevent relapse in children with AML: results from NOPHO-AML 2004. Blood. 2012;120:978–984. doi: 10.1182/blood-2012-03-416701. [DOI] [PubMed] [Google Scholar]
- Inaba H, Coustan-Smith E, Cao X, Pounds SB, Shurtleff SA, Wang KY, Raimondi SC, Onciu M, Jacobsen J, Ribeiro RC, Dahl GV, Bowman WP, Taub JW, Degar B, Leung W, Downing JR, Pui CH, Rubnitz JE, Campana D. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J Clin Oncol. 2012;30:3625–3632. doi: 10.1200/JCO.2011.41.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langebrake C, Creutzig U, Dworzak M, Hrusak O, Mejstrikova E, Griesinger F, Zimmermann M, Reinhardt D. Residual disease monitoring in childhood acute myeloid leukemia by multiparameter flow cytometry: the MRD-AML-BFM Study Group. J Clin Oncol. 2006;24:3686–3692. doi: 10.1200/JCO.2005.05.4312. [DOI] [PubMed] [Google Scholar]
- Leung W, Campana D, Yang J, Pei D, Coustan-Smith E, Gan K, Rubnitz JE, Sandlund JT, Ribeiro RC, Srinivasan A, Hartford C, Triplett BM, Dallas M, Pillai A, Handgretinger R, Laver JH, Pui CH. High success rate of hematopoietic cell transplantation regardless of donor source in children with very high-risk leukemia. Blood. 2011;118:223–230. doi: 10.1182/blood-2011-01-333070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA, Ho PA, Franklin J, Cooper TM, Gamis AS, Meshinchi S. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood. 2012;120:1581–1588. doi: 10.1182/blood-2012-02-408336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manola J, Atkins M, Ibrahim J, Kirkwood J. Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol. 2000;18:3782–93. doi: 10.1200/JCO.2000.18.22.3782. [DOI] [PubMed] [Google Scholar]
- Mardiros A, Dos Santos C, McDonald T, Brown CE, Wang X, Budde LE, Hoffman L, Aguilar B, Chang WC, Bretzlaff W, Chang B, Jonnalagadda M, Starr R, Ostberg JR, Jensen MC, Bhatia R, Forman SJ. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood. 2013;122:3138–3148. doi: 10.1182/blood-2012-12-474056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewerth D, Creutzig U, Bierings MB, Kaspers GJ. A review on allogeneic stem cell transplantation for newly diagnosed pediatric acute myeloid leukemia. Blood. 2010;116:2205–2214. doi: 10.1182/blood-2010-01-261800. [DOI] [PubMed] [Google Scholar]
- O’Brien MM, Cao X, Pounds S, Dahl GV, Raimondi SC, Lacayo NJ, Taub J, Chang M, Weinstein HJ, Ravindranath Y, Inaba H, Campana D, Pui CH, Rubnitz JE. Prognostic features in acute megakaryoblastic leukemia in children without Down syndrome: a report from the AML02 multicenter trial and the Children’s Oncology Group Study POG 9421. Leukemia. 2013;27:731–734. doi: 10.1038/leu.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pession A, Masetti R, Rizzari C, Putti MC, Casale F, Fagioli F, Luciani M, Lo Nigro L, Menna G, Micalizzi C, Santoro N, Testi AM, Zecca M, Biondi A, Pigazzi M, Rutella S, Rondelli R, Basso G, Locatelli F. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood. 2013;122:170–178. doi: 10.1182/blood-2013-03-491621. [DOI] [PubMed] [Google Scholar]
- Raimondi SC, Chang MN, Ravindranath Y, Behm FG, Gresik MV, Steuber CP, Weinstein HJ, Carroll AJ. Chromosomal abnormalities in 478 children with acute myeloid leukemia: clinical characteristics and treatment outcome in a cooperative pediatric oncology group study-POG 8821. Blood. 1999;94:3707–3716. [PubMed] [Google Scholar]
- Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J, Pounds S, Razzouk BI, Lacayo NJ, Cao X, Meshinchi S, Degar B, Airewele G, Raimondi SC, Onciu M, Coustan-Smith E, Downing JR, Leung W, Pui CH, Campana D. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010a;11:543–552. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010b;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubnitz JE, Pounds S, Cao X, Jenkins L, Dahl G, Bowman WP, Taub JW, Pui CH, Ribeiro RC, Campana D, Inaba H. Treatment outcome in older patients with childhood acute myeloid leukemia. Cancer. 2012;118:6253–6259. doi: 10.1002/cncr.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiollier C, Lopez CK, Gerby B, Ignacimouttou C, Poglio S, Duffourd Y, Guegan J, Rivera-Munoz P, Bluteau O, Mabialah V, Diop M, Wen Q, Petit A, Bauchet AL, Reinhardt D, Bornhauser B, Gautheret D, Lecluse Y, Landman-Parker J, Radford I, Vainchenker W, Dastugue N, de Botton S, Dessen P, Bourquin JP, Crispino JD, Ballerini P, Bernard OA, Pflumio F, Mercher T. Characterization of novel genomic alterations and therapeutic approaches using acute megakaryoblastic leukemia xenograft models. J Exp Med. 2012;209:2017–2031. doi: 10.1084/jem.20121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukimoto I, Tawa A, Horibe K, Tabuchi K, Kigasawa H, Tsuchida M, Yabe H, Nakayama H, Kudo K, Kobayashi R, Hamamoto K, Imaizumi M, Morimoto A, Tsuchiya S, Hanada R. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. doi: 10.1200/JCO.2008.18.7948. [DOI] [PubMed] [Google Scholar]
- van der Velden VH, van der Sluijs-Geling A, Gibson BE, te Marvelde JG, Hoogeveen PG, Hop WC, Wheatley K, Bierings MB, Schuurhuis GJ, de Graaf SS, van Wering ER, van Dongen JJ. Clinical significance of flowcytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia. 2010;24:1599–1606. doi: 10.1038/leu.2010.153. [DOI] [PubMed] [Google Scholar]
- Wen Q, Goldenson B, Silver SJ, Schenone M, Dancik V, Huang Z, Wang LZ, Lewis TA, An WF, Li X, Bray MA, Thiollier C, Diebold L, Gilles L, Vokes MS, Moore CB, Bliss-Moreau M, Verplank L, Tolliday NJ, Mishra R, Vemula S, Shi J, Wei L, Kapur R, Lopez CK, Gerby B, Ballerini P, Pflumio F, Gilliland DG, Goldberg L, Birger Y, Izraeli S, Gamis AS, Smith FO, Woods WG, Taub J, Scherer CA, Bradner JE, Goh BC, Mercher T, Carpenter AE, Gould RJ, Clemons PA, Carr SA, Root DE, Schreiber SL, Stern AM, Crispino JD. Identification of regulators of polyploidization presents therapeutic targets for treatment of AMKL. Cell. 2012;150:575–589. doi: 10.1016/j.cell.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, Taylor MJ, Johns C, Chicas A, Mulloy JC, Kogan SC, Brown P, Valent P, Bradner JE, Lowe SW, Vakoc CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, Podleschny M, Hahlen K, Pieters R, Zimmermann M, Reinhardt D, Harbott J, Creutzig U, Kaspers GJ, Griesinger F. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.