Abstract
Background
Climate change is likely to increase threat of wildfires, and little is known about how wildfires affect health in exposed communities. A better understanding of the impacts of the resulting air pollution has important public health implications for the present day and the future.
Method
We performed a systematic search to identify peer-reviewed scientific studies published since 1986 regarding impacts of wildfire smoke on health in exposed communities. We reviewed and synthesized the state of science of this issue including methods to estimate exposure, and identified limitations in current research.
Results
We identified 61 epidemiological studies linking wildfire and human health in communities. The U.S. and Australia were the most frequently studied countries (18 studies on the U.S., 15 on Australia). Geographic scales ranged from a single small city (population about 55,000) to the entire globe. Most studies focused on areas close to fire events. Exposure was most commonly assessed with stationary air pollutant monitors (35 of 61 studies). Other methods included using satellite remote sensing and measurements from air samples collected during fires. Most studies compared risk of health outcomes between 1) periods with no fire events and periods during or after fire events, or 2) regions affected by wildfire smoke and unaffected regions. Daily pollution levels during or after wildfire in most studies exceeded U.S. EPA regulations. Levels of PM10, the most frequently studied pollutant, were 1.2 to 10 times higher due to wildfire smoke compared to non-fire periods and/or locations. Respiratory disease was the most frequently studied health condition, and had the most consistent results. Over 90% of these 45 studies reported that wildfire smoke was significantly associated with risk of respiratory morbidity.
Conclusion
Exposure measurement is a key challenge in current literature on wildfire and human health. A limitation is the difficulty of estimating pollution specific to wildfires. New methods are needed to separate air pollution levels of wildfires from those from ambient sources, such as transportation. The majority of studies found that wildfire smoke was associated with increased risk of respiratory and cardiovascular diseases. Children, the elderly and those with underlying chronic diseases appear to be susceptible. More studies on mortality and cardiovascular morbidity are needed. Further exploration with new methods could help ascertain the public health impacts of wildfires under climate change and guide mitigation policies.
Keywords: Wildfire, Air pollution, Health, Smoke, Forest Fire
1. Introduction
Much remains unknown regarding the public health impacts of forest fire smoke, but interest in the topic is growing as forest fire incidence rises in many parts of the world (Dimopoulou and Giannikos 2004). There is broad consensus that climate change is increasing the threat of forest fires (Albertson et al., 2010; Balling et al., 1992; Flannigan and Vanwagner 1991; Keeton et al., 2007; Malevsky-Malevich et al., 2008; Spracklen et al., 2009), with fires that burn more intensely, occur more frequently, and can spread faster (Fried et al., 2008; Fried et al., 2004; Parry et al., 2007; Westerling and Bryant 2008). The U.S. Forest Service noted that forest fires have already become more intense and that the forest fire season has expanded (U.S. Forest Service 2009). While an increasing frequency of forest fires has often been attributed to many factors including changes in land use, higher spring and summer temperatures may be more relevant (Westerling et al., 2006). The Intergovernmental Panel on Climate Change (IPCC) anticipates that climate change will lengthen the window of high summertime forest fire risk in North America by 10–30%, and result in increased frequency of forest fires in many other parts of the world (Parry et al., 2007). As a result, exposure to air pollution from forest fires is anticipated to increase in coming decades (Interagency Working Group on Climate Change and Health 2010).
The U.S. Forest Service recognizes forest fire smoke as a hazard to human health and identifies airborne particulate matter (PM) as the component of greatest concern for the public (U.S. Forest Service 2010). Numerous studies have demonstrated links between airborne particles and health outcomes including mortality and hospital admissions (Lepeule et al., 2012; Medina-Ramon et al., 2006; Peng et al., 2008; Pope and Dockery 2006). However, not all particles appear to be equally toxic as research indicates that the size and chemical composition of airborne particles affect its impact on health (Ebisu and Bell 2012; Franck et al., 2011; Zanobetti et al., 2009). In general, effects are stronger for smaller particles, which can deposit deeper in the respiratory tract (Valavanidis et al., 2008). The specific mechanistic pathways to adverse health outcomes remain unclear, but chemical composition, particle size, number, and shape have been identified as of putative importance. As the chemical composition of forest fire smoke is likely to differ from those of other sources (e.g., vehicles) (Mao et al., 2011; Pio et al., 2008; Robinson et al., 2011), the observed health associations for more commonly studied air pollutants and sources, such as particulate matter in urban settings, may not be generalizable to pollution from forest fires. Thus, scientific evidence is needed on the health burden from forest fire smoke specifically.
Understanding how forest fire smoke affects public health has the potential to inform intervention-focused policies to protect public health in the present day, climate change mitigation policies, research on health impacts from a changing climate, and economic estimates of the health costs of forest fires. We reviewed and summarized the published literature regarding the public health impacts of forest fire smoke with the goals of synthesizing existing information and identifying gaps in scientific knowledge.
2. Methods
Eligibility criteria
We reviewed peer-reviewed journal articles on the topic of forest fire/wildfire smoke and health, published between 1 Jan 1986 and 30 May 2014. We included studies written in English or Portuguese (with English abstract), and excluded papers written in other languages. We considered all papers relevant to non-occupational exposure to wildfire smoke and physical health impact. We excluded experimental/chamber studies because it is not clear how relevant the exposure level/composition is to those experienced by the community. We excluded conference abstracts, unpublished studies, and non-research publications, such as commentaries. Natural fires were included and controlled prescribed burns were excluded. We did not exclude studies based on type or diversity of vegetation, such as trees peat bog or savannah. All fires are referred to as ‘wildfire’ hereon. We excluded studies of indoor and outdoor wood burning for heating or cooking purposes. Studies that investigated occupational exposures were excluded, as the focus of this review was impacts on communities or broader populations. Therefore, we excluded studies of fire fighters. Since mental health issues are not direct physical health consequences from exposure to wildfire smoke, we excluded studies that investigated only mental health outcomes. As this review focussed on wildfire smoke we also excluded studies that investigated non-smoke related morbidities, such as burns and accidents. Thus, we focused on wildfire smoke and its physical health impacts on the general population.
Information sources
We considered papers indexed in PubMed, a database of biomedical literature and life science journals, managed by the U.S. National Library of Medicine (NIH 2011) and Scopus, a comprehensive database of research literature (Elsevier 2013). References of the resulting papers were examined to better ensure a complete assessment of the literature.
Search terms
Detailed information on the search terms is provided in the supplemental material. Briefly, key words included “wildfire”, “forest fire”, or “bushfire” with any of the following: “health”, “hospital*”, “respir*”, “pulmon*”, “asthma*”, “cardiac”, “cardiovascular”, or “mortality”, where “*” stands for any combination of letters (e.g., hospital* can represent hospitalizations or hospital) (Appendix A).
Summary measures
We summarized the papers with respect to study setting, study design, exposure and outcome assessment, participant vulnerability, key findings, and estimates of association (e.g., odds ratios) when provided.
Study assessment
As exposure assessment is a critical challenge in the study of health impacts from wildfire smoke, we described the approaches used by identified studies to estimate exposures. We assessed the overall state of scientific evidence on associations between wildfire smoke and health outcomes for respiratory morbidity, cardiovascular morbidity, mortality, and other outcomes. The approaches to assess health outcomes are diverse, and we summarized the sources of health data for each study. We grouped the studies by health outcomes and summarized the results on health effects. We described factors that might have influenced the summary of evidence based on the studies reviewed. Finally, we highlighted the limitations of these studies and identified needs for future research.
3. Results
The database searches identified 926 papers. We then excluded 277 duplicates (i.e., papers identified by more than one search). We eliminated papers that did not meet the inclusion criteria, by first screening the titles and abstracts (526 papers excluded) and then by a review of the full articles (62 papers excluded). We also excluded studies for which wildfire smoke exposure was not a dominant component relative to other ambient sources (e.g. Sarnat et al., 2008). The final review included 61 studies of human health impacts of wildfires in community populations (Table 1).
Table 1.
Summary of studies on wildfire smoke and population health
| Study | Location | Background population or cohort size |
Time of fire |
Major health outcome |
Exposure metric |
|---|---|---|---|---|---|
| Aditama (2000) | Multiple provinces in Indonesia | 12,360,000 residents exposed to smoke | major fire: July–Oct. 1997 | Respiratory symptoms | CO, SO2, PM10, TSP, NOx, O3, organic compounds |
| Analitis et al., (2012) | Athens, Greece | More than 3 million residents | 1994–2004 | Mortality | Sizes of area burned |
| Azevedo et al., (2011) | Northern coast of Portugal | Elderly among Porto (total population 1.4 million) | June to Aug. 2005 | Cardiovascular (CVD), respiratory admissions | O3 |
| Caamano-Isorna et al., (2011) | Galicia, Spain | About 2 million inhabitants | Summer 2006 | Respiratory medicine usage | Exposure classified into three categories based on number of fires |
| Cameron et al., (2009) | Victoria, Australia | 5.2 million residents | Feb. 2009 | Injuries | Not specified |
| do Carmo et al., (2010) | Alta Floresta municipality, Mato Grasso, Brazil | 51,136 residents in Alta Floresta, Mato Grosso (9% children <5y, 5% elderly >64y) | Jan. 2004 – Dec. 2005 | Respiratory admissions | PM2.5 |
| Castro et al., (2009) | State of Rondônia, western Brazil | 1.6 million residents | 1998–2005 | Mortality | Number of fire “hotspots” |
| Centers for Disease Control and Prevention (CDC) (1999) | Central Florida | Not specified | Jun.– Jul. 1998 | Respiratory and cardiovascular Emergency Room (ER) visits | Wildfire v. non-wildfire periods |
| Centers for Disease Control and Prevention (CDC) (2007) | Panhandle region and 9 other counties, Texas, U.S. | Not specified | March 12–20, 2006 | Mortality | Presence of wildfire smoke |
| Centers for Disease Control and Prevention (CDC) (2008) | San Diego Co., California, U.S. | Not specified | Oct. 22–26, 2007 | Respiratory ER visits | Wildfire v. non-wildfire periods |
| Chen et al., (2006) | Brisbane, Australia | Not specified | Fire seasons 1997–2000 | Respiratory admissions | PM10 |
| Cleland et al., (2011) | Melbourne, Australia | Not specified | Feb. 2007 | Injuries | Not specified |
| Crabbe (2012) | Darwin, Australia | 110,000 residents | 1993–1998 | Respiratory, CVD ER visits | PM10, black carbon |
| Delfino et al., (2009) | Southern California, U.S. | 20.5 million residents | Oct. 21–30, 2003 | CVD, respiratory admissions | PM2.5 |
| Dohrenwend et al., (2013) | San Diego Co., California, U.S. | Not specified | Oct 21–Nov 6, 2007 | Respiratory ER visits | Wildfire v. non-wildfire periods |
| Duclos et al., (1990) | 6 counties in California, U.S. | Residents in 6 counties (population size not specified) | Aug. 30–Sep. 3, 1987 | Respiratory ER visits | PM10, TSP |
| Elliott et al., (2013) | British Columbia (BC), Canada | Residents from 29 local health areas (LHA) in BC; population ranges 7,024–352,783 people | Fire seasons 2003–2010 | Respiratory medicine usage | PM2.5, PM10 |
| Emmanuel (2000) | Singapore | > 3 million residents | End of Aug. to early Nov. 1997 | Respiratory admissions; all-cause mortality | PM10, SO2, NO2, O3, CO, total hydrocarbon |
| Frankenberg et al., (2005) | Kalimantan and Sumatra, Indonesia | 10,869 subjects > 30y | July–Oct., 1997 | Respiratory illness/symptoms; physical strength, overall health | Aerosol |
| Hanigan et al., (2008) | Darwin, Australia | 110,000 residents | Dry seasons (Apr. – Nov.) of 1996–2005 | Respiratory, CVD admissions | PM10 |
| Hänninen et al., (2009) | 11 provinces in southern Finland | 3.4 million residents | Aug. 26–Sep. 8, 2002 | Mortality | PM2.5, PM10 |
| Henderson et al., (2011) | Southeastern corner of BC, Canada | 281,711 subjects | Summer 2003 | CVD, Respiratory admissions | PM10 |
| Holstius et al., (2012) | South Coast Air Basin, California, U.S. | 886,034 infants in exposed group; 747,590 infants in control group | Oct. 2003 | Birth weight | Exposed or unexposed to fire during pregnancy |
| Huttunen et al., (2012) | Kotka, Finland | 52 elderly people (>50 y) with ischemic heart disease | Apr. 25–May 6, 2006 | Blood concentration of inflammatory markers | PM2.5 |
| Ignotti et al., (2010) | Microregions in northern states of Brazilian Amazon, with Mato Grosso and Maranhão | 24 million inhabitants affected; subpopulations: Children (<5 y), elderly (>64), and an intermediate age group (5–64 y) | 2004–2005 | Respiratory admissions | PM2.5 |
| Jalaludin et al., (2000) | Sydney, Australia | 32 children | Jan. 1994 | Peak expiratory flow rates (PEFR) | PM10, NO2, O3 |
| Jayachandran (2009) | Indonesia | ~1.3 million children (<3 y), infants or fetuses | Aug.–Oct. 1997 | Mortality | Aerosols |
| Johnston et al., (2002) | Darwin, Australia | 115,000 residents | Apr. 1–Oct. 31, 2000 | Asthma ER visits | PM10 |
| Johnston et al., (2006) | Darwin, Australia | 251 asthmatic adults and children, about half < 18y | 7 months in 2004 | Asthmatic symptoms | PM2.5, PM10 |
| Johnston et al., (2007) | Darwin, Australia | 110,000 residents | Fire seasons of 2000, 2004, 2005 | Respiratory, CVD admissions | PM10 |
| Johnston et al., (2011) | Sydney, Australia | ~ 4 million residents | 1994–2007 | Mortality | PM10, O3 |
| Johnston et al., (2012) | Global | Not specified | 1997–2006 | Mortality | PM2.5 |
| Kolbe and Gilchrist (2009) | Albury, New South Wales, Australia | 389 interviewees | Jan–Feb, 2002 | Respiratory symptoms | PM10 |
| Kunii et al., (2002) | Jambi, Sumatra (affected) and Jakarta, Java (control), Indonesia | 543 subjects in Jambi | July–Oct. 1997 | Respiratory symptoms | CO, CO2, SO2, NO2, O3, PM10, inorganic ions, PAHs |
| Kunzli et al., (2006) | 16 communities in Southern California, U.S. | 873 high school students, 5551 elementary school students | Oct. 2003 | Respiratory symptoms | PM10 |
| Lee et al., (2009) | Hoopa Indian Reservation, California, U.S. | 2,633 residents | Late summer and fall 1999 | Respiratory, CVD, diabetes admissions | PM10 |
| Martin et al., (2013) | Sydney, Newcastle and Wollongong, Australia | About 4.5 million residents | Fire seasons 1994–2007 | All non-trauma admissions | PM10, PM2.5 |
| Mascarenhas et al., (2008) | Rio Branco, Brazil | 19,581 ER visits | Sep. 1–30, 2005 | Respiratory ER visits | PM2.5 |
| de Mendonca et al., (2006) | 261 districts in Brazilian Amazon | Residents in Amazon regions (population size not specified) | Fire seasons 1996–2000 | Respiratory admissions | hot pixels from satellite data |
| Mirabelli et al., (2009) | 12 counties in California, U.S. | 465 non-asthmatic students (16–19 y) in the Children’s Health Study | Oct. – Nov. 2003 | Respiratory symptoms | Number of days subjects smelled smoke |
| Moore et al., (2006) | Kelowna and Kamloops regions in British Columbia, Canada | 146,199 residents in Kaelowna; 100,548 residents in Kamloops | Aug. 2003 | Respiratory, CVD | PM10, PM2.5 |
| Morgan et al., (2010) | Sydney, Australia | ~ 3.48 million residents | Jan. 1994–June 2002 | Respiratory admissions; Mortality | PM10 |
| Mott et al., (2002) | Hoopa Reservation, California | 289 residents in Humboldt Co. interviewed (26% of population) | Aug. 23–Nov. 3, 1999 | Respiratory admissions | PM10 |
| Mott et al., (2005) | Kuching, Malaysia | ~400,000 residents affected | Aug. 1–Dec. 31, 1997 | Respiratory symptoms | PM10 |
| Nunes et al., (2013) | 107 micro areas in Brazilian Amazon | Not specified | Dry season 2005 | Mortality due to circulatory diseases | Annual % hours with PM2.5 greater than 25µg/m3 |
| Prass et al., (2012) | Porto Velho, Amazon region | 22,012 live births | 2001–2006 | Birth weight | Number of fires |
| Rappold et al., (2011) | 42 contiguous counties in eastern North Carolina, U.S. | Not specified | June 2008 | Respiratory, CVD ER visits | Aerosol optical depth (AOD) |
| Rappold et al., (2012) | 40 mostly rural counties, North Carolina, U.S. | Not specified | June to July, 2008 | Asthma, CVD ER visits | PM2.5 |
| Sastry (2002) | Kuala Lumpur and Kuching, Malaysia | Not specified | July–Dec. 1997 | Mortality | PM10 |
| Schranz et al., (2010) | San Diego Co., California, U.S. | Not specified | Oct. 21–24. 2007 | Respiratory ER visits | PM2.5 |
| Shaposhnikov et al., (2014) | Moscow, Russia | 11.5 million residents | Jul–Aug 2010 | Mortality | PM10, O3 |
| Shusterman et al., (1993) | Alameda Co., California, U.S. | Not specified | Oct. 20–21, 1991 | Respiratory, injury ER visits | Not specified |
| Smith et al., (1996) | Western Sydney, Australia | 907,450 residents | Jan. 5–12,1994 | Respiratory, asthma ER visits | PM10, NO2 |
| Sutherland et al., (2005) | Denver, Colorado, U.S. | 21 residents who are >40 y, smoke, and with pre-existing COPD | June 8 to July 18, 2002 | Respiratory symptoms | PM2.5, PM10, CO |
| Tan et al., (2000) | Singapore | 30 male volunteers | Sep.–Oct. 1997 | Bone marrow content | SO2, PM10, NO2, O3; CO |
| Tham et al., (2009) | Northeastern and Alpine district, Victoria, Australia | Not specified | Jan.–March, 2003 | Respiratory ER visits | PM10 |
| Thelen et al., (2013) | San Diego Co., California, U.S. | Not specified | Oct. 2007 | Respiratory ER visits | PM2.5, PM10 |
| Vedal and Dutton (2006) | Denver, Colorado, U.S. | ~ 2 million residents | June 9–18, 2002 | Mortality | PM2.5, PM10 |
| Viswanathan et al., (2006) | San Diego Co., California, U.S. | 2.8 million residents | Oct. 2003 | Respiratory, CVD, diarrhea admissions | PM2.5, PM10, O3, NO2, SO2, CO |
| Vora et al., (2011) | San Diego Co., California, U.S. | 8 subjects in downtown San Diego with asthma | Oct. 2007 | Respiratory function, rescue medication use | PM2.5 |
| (Wiwatanadate and Liwsrisakun (2011)) | Chiang Mai, Northern Thailand | 1.7 million residents | Aug. 2005 – June 2006 | PEFR, asthma symptoms | CO, O3, NO2, SO2, PM2.5, PM10 |
Study setting
More studies were identified for more recent years, with 4 studies published before 2000 and 35 studies published in the last 5 years. Most studies focused on the Brazilian Amazon, Southeast Asia and the Pacific, the North American West, and the Mediterranean, where wildfires are common. The U.S. and Australia were the most frequently studied countries (18 U.S. studies, 15 Australian studies). Southeast Asia was also frequently studied (9 studies). No studies were set in Africa. Geographic scales ranged from a single small city (population about 55,000) (Huttunen et al., 2012) to the entire globe (Johnston et al., 2012). Most studies focused on cities or regions close to fire events.
Study design
The majority of studies were based on either spatially or temporally aggregated populations, such as ecological studies (37 of 61 studies). There were relatively fewer cohort or panel studies (14 of 61 studies). Most of the studies compared the risk of health outcomes between 1) periods with no fire events and periods during or after the fire events, or 2) regions not affected by wildfire smoke and regions affected by wildfire smoke. The selection of model adjustment variables was not universal, but can be classified as 1) meteorological; 2) air pollutants other than the pollutants of interest; 3) community-level socio-demographics; and 4) temporal effects (seasonal or secular trend). Of these, meteorological factors were the most prevalent adjustment variables. Some studies controlled for individual variables, such as age group and sex, by stratification (Analitis et al., 2012; Castro et al., 2009; Delfino et al., 2009; Frankenberg et al., 2005; Henderson et al., 2011; Mott et al., 2005; Nunes et al., 2013; Prass et al., 2012; Rappold et al., 2011; Sarnat et al., 2008)
Health outcomes investigated and outcome assessment
Respiratory disease was the most frequently studied outcome (45 studies (74% of 61 studies)) (Supplementary Table A.4). The outcomes included contacts with emergency departments (ED), hospitals or other primary care providers (33 studies (54%)), respiratory symptoms or lung function measurements (9 studies (15%)), and dispensation or consumption of medication (three studies (5%)). Relatively few studies examined cardiovascular morbidity (14 studies) or mortality (13 studies) (Table 2).
Table 2.
Summary of studies based on health outcome and observed associations
Other outcomes investigated were diarrhea due to power outage after wildfire events (identified from surveillance records), birth weight (obtained from hospital birth records), blood biomarkers for systemic inflammation and bone marrow content. The studies of lung-function, blood biomarker concentration and bone marrow content were all cohort studies measuring subjects’ lung function or blood samples both before and after fire events.
The most common source of information for health outcomes was the use of datasets maintained by governmental agencies or statistical bureaus (32 studies), followed by hospital admission records or billing records (19 studies), interviews or surveys (10 studies), and subject tests such as lung function or blood samples (seven studies). Some studies used multiple methods to assess health outcomes. All mortality data came from governmental agencies or bureaus. Use of individual surveys (e.g., “smell of wildfire smoke indoors” (Kunzli et al., 2006)) was the most employed method in assessing personal exposure and self-reported symptoms for short-term studies.
Exposure assessment
The most commonly used method for either designating a fire period or area, or assessing exposure for previously designated fire and non-fire periods or areas, was use of measurements from land-based air pollutant monitors (35 studies), followed by satellite-based imagery or models (11 studies), air quality modelling (six studies) and personal exposure from individual surveys, personal reports, or personal photometers (three studies) (Supplementary Table A.3). Of the 61 studies, seven studies used other methods to assess exposure, such as air sample analysers. Satellite-based methods became popular in studies from recent years.
Pollutant data from air monitors were usually obtained by governmental agencies or research institutions and were used as the exposure variable in statistical models. The monitoring data usually covered pre-, during- and post-fire periods. Most of the studies determined “exposed period” based on the start/end dates of fire events but did not specify how the start/end days were identified. Some studies used thresholds of air monitoring data to categorize days, for example, high PM days with aerodynamic diameter <2.5µm (PM2.5) >40µg/m3, low PM days with PM2.5<10µg/m3 (e.g., Johnston et al., 2002). Personal surveys and reports generally asked questions such as “did you smell any smoke?” or “did you have any health symptoms?” plus the respondents’ personal characteristics, such as age and education. Personal photometers were used to measure personal exposure to PM2.5 (Huttunen et al., 2012).
Satellite-based imagery or models are increasingly common in the recent studies to aid exposure assessment. Some satellite-based studies used satellite images to detect “hotspots”, which were used as indicators of fire events (e.g., Castro et al., 2009; de Mendonca et al., 2006)). Some studies determined “exposed region” based on either satellite images or proximity to fire events (e.g., Kunii et al., 2002). The majority of the studies using satellite-based methods measured exposure for at least 5 years. In contrast, studies using individual photometers or reports usually investigated individual-specific exposure among subjects of a prospective cohort for a shorter period of a few days to a few months (Frankenberg et al., 2005; Kunii et al., 2002; Kunzli et al., 2006).
The length of exposure measurement varies from a few days to over a dozen years. Huttunen et al. assessed daily average exposure of PM2.5 and PM with aerodynamic diameter < 10µm (PM10) during a 12-day fire that occurred in Kotka, Finland from Apr. 25 to May 6, 2006 (2012). Many studies compared longer-term exposure across months or seasons (Hanigan et al., 2008; Johnston et al., 2007; Smith et al., 1996). Elliott et al. (2013) measured exposure during fire seasons (Apr. 1 to Sep. 30) in each year (2003–2010) and compared the health risk during fire seasons with non-fire seasons. Evaluation of long-term exposure was more common in regions with distinct fire seasons, such as Australia (e.g., Hanigan et al., 2008; Johnston et al., 2011; Morgan et al., 2010; Smith et al., 1996) and Canada (Elliott et al., 2013). Johnston et al. (2011) investigated long-term mortality effect by measuring PM10 exposure attributed to wildfires over 13.5 years, from 1994 to 2007 in Sydney, Australia.
Other studies compared exposure and health during the period when forests were burning to the periods before and/or after the fire (Supplementary Table A.3). Of these studies, Duclos et al. (1990), Frankenberg et al. (2005), and Moore et al. (2006) compared exposure and health during the fire events or seasons with control periods in preceding and/or subsequent years. Many studies estimated short-term (e.g., a few days to one or two weeks) exposure under a certain fire event and compared the health risk during the fire event with that during short pre- or post-fire periods (e.g., Schranz et al., 2010; Sutherland et al., 2005; Vora et al., 2011). This exposure timeframe was common in studies based on local populations and a single fire event. Many studies compared longer-term exposure across months or seasons (e.g., Hanigan et al., 2008; Johnston et al., 2007; Smith et al., 1996).
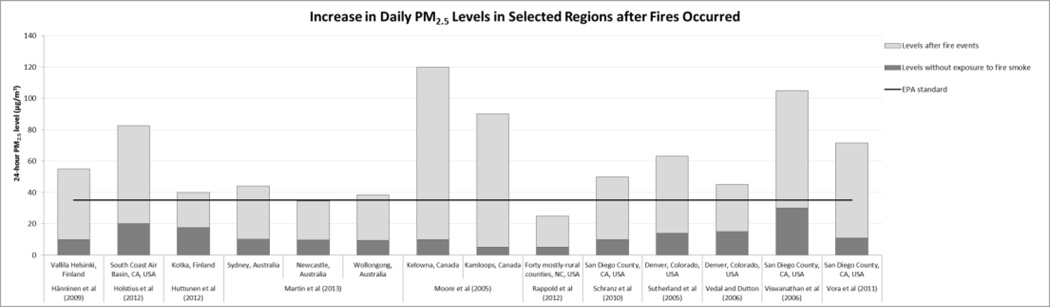
Almost all studies mentioned that air pollutant levels, especially particulate matter levels, increase dramatically during wildfire events. Figure 1 shows estimated air pollutant levels during fire periods compared with levels in control periods. PM2.5 levels in most studies exceeded the U.S. EPA National Ambient Air Quality Standard for 24-hour PM2.5 (35µg/m3). Some studies indicated particulate levels during fire periods over 100 µg/m3 for PM2.5 and over 500 µg/m3 for PM10 (e.g. Hänninen et al., 2009; Holstius et al., 2012; Kolbe and Gilchrist 2009; Kunii et al., 2002)
Figure 1.
PM2.5 (top) and PM10 levels (bottom) during wildfire events and non-fire periods
3.1 Association between wildfire smoke and health outcomes
3.1.1 Respiratory morbidity
Of the health outcomes examined, respiratory morbidity had the strongest evidence of an association with wildfire smoke, with a statistically significant adverse association reported for 43 of the 45 respiratory studies (Supplementary Table A.4). Analysis of respiratory-related contacts with primary care providers constituted 31 studies that reported associations and 2 studies that did not detect an adverse association. ED contacts for asthma in Darwin, Australia were 2.4 (95% confidence interval 1.5–3.9) times greater on a fire day (PM10>40µg/m3) than on a non-fire day (PM10<10 µg/m3) (Johnston et al., 2002). Two other Australian studies reported greater risk of hospital admission for elevated exposure two days before the hospital admission day (Morgan et al., 2010) and five days before the admission day (Chen et al., 2006). Associations for longer lags (greater than five days) between exposure and hospitalization were not directly investigated in any study. From cross-sectional studies there were increases in primary care contacts for a 12-week period of exposure to wildfire smoke in California (Lee et al., 2009) and a five-week exposure period in Canada (Moore et al., 2006) compared to the same period in previous years when there were no fires. However, it remains unclear as to whether admissions increased due to high acute exposures over short periods (days) and/or lower levels accumulated over a longer period (months). Associations were consistently reported between wildfire related exposure and respiratory symptoms or dispensation/use of medication (all 12 studies). Adverse associations were observed for cough, wheeze and eye irritation (Supplementary Table A.4).
A statistically significant association between exposure to wildfire smoke and hospital or emergency room admissions for respiratory diseases was not reported in two of the 45 studies (Azevedo et al., 2011; Smith et al., 1996). A study of Sydney compared ED records in seven hospitals during a two-week fire period with that during the same period in the previous year. The researchers found no difference in asthma ED visits during the two periods (Smith et al., 1996). The Northern Portugal study reported that high ozone level (greater than 100µg/m3) during the three-month fire period was not associated with respiratory disease admissions.
3.1.2 Cardiovascular morbidity
Of the 14 studies that assessed the relationship between wildfires and cardiovascular morbidity, six reported a statistically significant increase in risk of cardiovascular outcomes with exposure to wildfire smoke. Some authors reported change in risk per unit (such as per 100 µg/m3) increase in daily measurement of certain wildfire-promoted pollutants, such as ozone, PM10 or PM2.5 (Azevedo et al., 2011; Lee et al., 2009; Rappold et al., 2012). Others reported changes in risks comparing regions or time periods of wildfires with non-wildfire regions or times (Delfino et al., 2009; Rappold et al., 2011). PM10 was the most commonly studied pollutant for cardiovascular diseases and most of the PM10-CVD studies (eight out of nine) did not find any significant association. Other air pollutants from wildfires were less studied and their impact on cardiovascular illness remains unclear. Study findings varied geographically, with no report of a statistically significant cardiovascular impact of wildfire smoke in any study from Australia and Canada (seven out of 14) (Crabbe 2012; Hanigan et al., 2008; Henderson et al., 2011; Johnston et al., 2007; Martin et al., 2013; Moore et al., 2006; Morgan et al., 2010). Contrastingly, five out of six U.S. studies reported that exposure to wildfire smoke was associated with hospital admissions for cardiovascular diseases, such as cardiac arrests, or symptoms such as chest pain (Delfino et al., 2009; Lee et al., 2009; Rappold et al., 2012; Rappold et al., 2011). All studies assessed cardiovascular disease by hospital admissions or emergency room visits. A U.S. study found that a 100µg/m3 increase in wildfire smoke-related PM2.5 was associated with a significant 42% (95%CI: 5%–93%) increase in emergency room visits for congestive heart failure (CHF) (Rappold et al., 2012). However, there were too few studies on specific cardiovascular endpoints, such as ischemic heart disease (e.g., Azevedo et al., 2011; Crabbe 2012; Moore et al., 2006) to establish consistency of associations.
3.1.3 Mortality
Mortality was associated with wildfire smoke for nine of 13 studies. Only three of these studies assessed non-accidental mortality (Analitis et al., 2012; Johnston et al., 2011; Vedal and Dutton 2006). Two investigated cause-specific mortality for respiratory and COPD (Castro et al., 2009; Nunes et al., 2013). Other studies examined total all-cause mortality. The increase in mortality under exposure to wildfire smoke, compared with periods of no fires, ranged from 1.2% for children during the fire event (Jayachandran 2009) to 92.0% for respiratory mortality during days with large fires (Analitis et al., 2012). Large fires (>3000 hectares burned) had larger estimated associations with mortality than smaller fires (Analitis et al., 2012). As wildfire events occur more often in summer, Shaposhnikov et al., (2014) examined the interaction between heat and wildfire smoke. They found that temperature and PM10 (largely due to wildfires) collectively contributed to over 2000 deaths. One of the three studies that investigated shorter-term exposure and did not report a statistically significant association did not provide numeric results (Vedal and Dutton 2006) while the effect estimates reported in the other two studies were in the positive direction, i.e., adverse mortality effects (Hänninen et al. (2009) and Morgan et al. (2010)).
3.1.4 Other health outcomes
Eleven studies investigated other health outcomes in relation to wildfire smoke. These included studies on birth weight (Holstius et al., 2012; Prass et al., 2012), bone marrow content (Tan et al., 2000), systematic inflammation (Huttunen et al., 2012), physical strength and overall health (Frankenberg et al., 2005), diarrhea (Viswanathan et al., 2006), diabetes (Lee et al., 2009), and injuries (Cameron et al., 2009; Cleland et al., 2011). For the two studies that investigated birth weight, results were inconsistent (Holstius et al., 2012; Prass et al., 2012). All three cohort studies reported significant adverse associations between wildfires and health: systemic inflammation (Huttunen et al., 2012), bone marrow content (Tan et al., 2000), and physical strength and overall health (Frankenberg et al., 2005). Diarrhea and diabetes were mentioned as health outcomes of interest in multiple studies (Aditama 2000; Jalaludin et al., 2000; Lee et al., 2009; Viswanathan et al., 2006), but only two reported the results (Lee et al., 2009; Viswanathan et al., 2006). Exposure to wildfire smoke did not show discernible effects on either diarrhea or diabetes.
Vulnerable sub-populations
A limited number of studies assessed whether some populations face higher health risk from exposure to wildfire smoke than others, examining population characteristics such as age categories. The age cut-offs for age categories varied by study. Larger positive associations between wildfire smoke and cardiorespiratory morbidities were observed for middle-aged adults (Henderson et al., 2011) and older adults compared to other age groups (Analitis et al., 2012; Castro et al., 2009; Delfino et al., 2009; Frankenberg et al., 2005; Morgan et al., 2010; Nunes et al., 2013; Shaposhnikov et al., 2014). Elevated levels of wildfire smoke had larger risk estimates for asthma hospitalizations among adults aged 40–64 years (Mott et al., 2005), 15–64 years (Morgan et al., 2010), and 19–64 years (Rappold et al., 2011) compared to other age groups. Risk of respiratory-related hospital contacts associated with wildfire smoke was higher for children (<5 years) compared with other age groups (Ignotti et al., 2010).
Men and women may have different health risks when exposed to wildfire smoke. Risks for asthma-related symptoms or visits in relation to wildfire smoke were greater for women than men (Lee et al., 2009; Rappold et al., 2011). However, Henderson et al. (2011) and Prass et al. (2012) did not find differences in wildfire effect estimates between men and women in respiratory and cardiovascular physician visits, and birth weight, respectively.
Three studies reported effect modification by socio-economic status (SES), race, or co-morbidities. Larger risk estimates between wildfire smoke and risk of asthma and congestive heart failure were observed among counties of lower SES compared to higher SES counties (Rappold et al., 2012). Aboriginal Australians had higher risk of respiratory admissions and emergency admissions than other races when exposed to PM10 (Hanigan et al., 2008; Johnston et al., 2007). Johnston et al., (2007) did not detect an association between PM10 and cardiovascular admissions for the general population, but restriction of analyses to the Aboriginal population with ischemic heart disease resulted in findings of the greatest risk of respiratory-related hospital admissions three days after exposure (Johnston et al., 2007). It is plausible that associations at longer lags might have only been observable for such high-risk sub-populations, most susceptible to wildfire. Lee et al. (2009) and Mirabelli et al., (2009) reported that adults with pre-existing respiratory conditions or weakness (i.e. small airway size) were more likely to seek care or have additional symptoms after wildfire exposure than persons without those conditions. However, Künzli et al. (2002) reported opposite results, as children without pre-existing asthmatic conditions had greater increase in respiratory symptoms under exposure than did other children. The authors suggested that children with pre-existing asthmatic conditions tended to be on medication and have better access to care, hence their smaller increase in symptoms when exposed to wildfire smoke. In an Australian study, no adverse association was observed between wildfire related PM10 and lung function (peak expiratory flow) except when analysis was restricted to children with no bronchial hyper-reactivity (Jalaludin et al., 2000).
4. Discussion
Overall, wildfire smoke exposures, as measured by proxies such as criteria air pollutants, were consistently associated with mortality and respiratory morbidities. Respiratory-related effects of wildfire smoke included increases in risk of hospitalization, use of respiratory medication, cough, wheeze and eye irritation. In one study, risk of emergency department contact for asthma could be more than two times greater after exposure to wildfire smoke (Johnston et al., 2002). As most mortality studies investigated all-cause mortality, further research is needed to better identify the specific causes of mortality most strongly associated with wildfire smoke exposures. The magnitude of the effects on mortality varied by study. Respiratory mortality almost doubled from exposure to a wildfire in Greece (Analitis et al., 2012), but some wildfires were not associated with changes in the mortality rate (Morgan et al., 2010). The only global study posited that 339,000 deaths per year were attributable to wildfires, with Sub-Saharan Africa and Southeast Asia the most affected regions (Johnston et al., 2012). However, this review highlighted disproportionately fewer studies in Southeast Asia and no other studies conducted in Sub-Saharan Africa. Some parts of the world such as Sub-Saharan Africa are affected by wildfire events but have not been studied. Those places, usually the less-developed regions, may contribute the most to the global burden of many diseases. It is also unlikely that these parts of the world can respond to such risk as well as more developed nations. Therefore, more studies are needed in these less studied countries.
Although our review of studies on forest fires and health is the most extensive to date, past reviews on related topics have also contributed substantially towards knowledge on the health effects of wildfire smoke. An early review by Naeher et al. (2007) focused on the toxicity of wood smoke, thereby establishing biological plausibility of the association, and called for further studies on the topic. Two later reviews investigated effects on respiratory outcomes of bushfire smoke (Dennekamp and Abrahmson 2011) and on respiratory outcomes for forest fires (Henderson and Johnston 2012). Dennekamp and Abramson (2011) identified that elevated PM concentrations from bushfire smoke explained associations with increased respiratory morbidity. Henderson and Johnston (2012) confirmed consistency of associations with acute respiratory outcomes and identified the need for studies in equatorial regions with rainforest depletion. Finlay et al. (2012) included non-respiratory outcomes and focused on demonstrating the current stage of investigation on this issue in the U.K. and identified literature gaps for the U.K. Finlay et al. identified the potential burden on cardiovascular and ophthalmic outcomes. Our review confirms that there still remain too few studies on these endpoints to establish consistency. The findings of our comprehensive review add to those of the previous reviews that focused on specific types of wildfire, health outcomes, or countries. Our review also quantified the substantial increase in exposure levels from wildfires and how these increases differed across studies. This was the first review to identify the dearth of studies from sub-Saharan Africa and paucity of studies in Southeast Asia, which are regions that experience a large health burden and are less able to respond to the increasing frequency and intensity of wildfires that accompany climate change. Our review also identified the shift in exposure assessment from the dominant use of measurements from ground-based air monitors to use of satellite imagery and chemical transport models.
In our review we found that results were most consistent among cohort studies, as almost all cohort studies found significant impact of wildfire smoke on health in at least one of the health outcomes and part of the population studied. Studies involving direct physiological measurements on recruited patients, such as bone marrow (Tan et al., 2000) and Peak Expiratory Flow Rates PFFR (e.g. Jalaludin et al., 2000), also tend to discern significant impacts. Ecological studies generally had inconsistent results. However, it is difficult to draw conclusions as to how study design and methods affected the reported associations because of heterogeneity in these and other design factors across studies, significant difference between pollutant levels during wildfire and non-wildfire periods, and how this difference varied across studies.
Studies consistently reported substantially higher levels of air pollution during fire periods and locations compared to non-fire periods and areas. Daily average PM10 levels in an exposed city (Jambi, Indonesia) exceeded 1800µg/m3 during fire events (Kunii et al., 2002), which was 12 times the WHO interim target-1 standard (150µg/m3 24-hour) and 36 times the WHO air quality guideline (50µg/m3 24-hour). Daily average PM2.5 levels during wildfires exceeded 150µg/m3, more than 6 times greater than the WHO air quality guideline (25µg/m3 24-hour) (Moore et al., 2006). Levels of carbon monoxide can increase 30–40% during wildfire periods compared with periods with no fires (Sutherland et al., 2005; Tan et al., 2000). These results indicate that wildfire events can result in severe levels of exposures. In addition to high levels, the chemical composition of wildfire smoke is distinctive. Wildfire smoke is accompanied by elevated levels of black carbon (Crabbe 2012), and polycyclic aromatic hydrocarbons can be 15 times higher than background levels (Aditama 2000).
4.1 Methods used to assess exposure to wildfire smoke
This review identified assessment of exposure as a key challenge in health studies of wildfires, with a range of methods applied. It is difficult to identify a direct marker that can represent air pollutants only from wildfires. Studies used indicators such as criteria air pollutants, aerosol optical depth or area burnt as indirect proxies. Although use of indirect proxies can be a useful approach, it is difficult to ascertain the fraction of health morbidity due to wildfire smoke excluding health morbidities due to those proxies in non-wildfire periods and from other sources during wildfire periods. The most commonly used marker for wildfire smoke used in the reviewed studies was particulate matter (PM) (Phuleria et al., 2005). Although the fine fraction of particulate matter (PM2.5) has been more consistently associated with adverse health effects than larger particles in studies of particulate matter more generally (Pope and Dockery 2006), fewer studies investigated the health effects of wildfire smoke-related PM2.5. Notably, in all countries, the measurement of PM2.5 began more recently than PM10. A further exposure-related limitation of many of the reviewed studies was the coarse spatial resolution of exposure, due primarily to the use of ground-based ambient air monitors and the available monitoring network. An exception to this was studies that used remotely sensed satellite-derived imagery of area burnt (de Mendonca et al., 2006). However, it is unclear as to whether area burnt is a suitable proxy for wildfire smoke exposure because it must be interpreted relative to population’s distance to the wildfire, wind speed and direction, and atmospheric mixing depth (Naeher et al., 2007; Ward 1990). Wildfire smoke also varies with vegetation type as, for example, wood from eucalypt forest has more oil content and releases higher concentrations of PM10 than pine, acacia or cork oak (Goncalves et al., 2010).
Exposure assessment is an ongoing challenge in epidemiological studies of wildfire smoke. Ground-based monitors do not measure the complicated mixture of pollution from the source of wildfires specifically. Monitors measure the level of a specific pollutant, such as PM2.5, and cannot measure the pollution solely from fires as opposed to other sources. Therefore, it is difficult to separate the health effect of wildfire-emitted pollutants from that of pollutants from other sources. Moreover, ground-based air pollution monitors are not located in all places or time periods with affected populations. Exposure estimates based on satellite data provide more comprehensive spatial coverage (Kloog et al., 2011; Lee et al., 2011), but do not address the issue of specificity of the exposure estimates for wildfire smoke. It is critical to better understand the levels of wildfire smoke-specific pollutants (e.g., particulate matter from wildfires), as the range of health responses to the chemical signature specific to wildfire smoke is currently unclear (Wegesser et al., 2009). Recent developments in chemical transport models may help address this limitation in future work. Chemical transport models, such as GEOS-Chem models, can estimate air pollutants specifically from wildfires (e.g. Singh et al., 2010). Johnston et al (2012) employed this method to estimate the global exposure to wildfire-emitted PM2.5. They found that 339,000 deaths could be attributed to wildfires annually. One limitation of using chemical transport models is that the wildfire-specific pollutant estimates may be difficult to validate. Modeled data could also be computationally expensive and requires collaboration efforts of atmospheric scientists (Kleeman et al., 2009).
4.2 Health outcomes affected by wildfire smoke
The health endpoints investigated by the reviewed studies mainly focused on mortality and respiratory morbidity. Over 90% of the studies on respiratory morbidity and about 70% of the studies on mortality found significant association with wildfire smoke. There was insufficient evidence to conclude a consistent association between wildfire smoke and cardiovascular morbidities due to the relatively fewer number of studies. Despite the inconsistent association for cardiovascular morbidities globally, the association was mostly consistent in North America (five out of six studies found significant impact), where prevalence of cardiovascular diseases are higher than many other study areas. Causal links have been established between PM10 more generally and a range of cardiovascular endpoints (Brook et al., 2010). Other potential health endpoints that have been studied in the context of air pollution are hypertensive disorders (e.g. van den Hooven et al., 2011), ophthalmic outcomes (e.g. Versura et al., 1999), adverse pregnancy outcomes (e.g. Ritz et al., 2002), and non-respiratory atopic disease (Morgenstern et al., 2008). Future studies on the health impacts from wildfires may investigate these outcomes.
4.3 Susceptibility/Vulnerability
Among other factors, variation in the magnitude and statistical significance of observed effect estimates across the reviewed studies was likely attributable, in part, to differences in the underlying characteristics of the study population, including biological susceptibility, sociodemographic vulnerability, or other factors. Air pollution research more broadly has acknowledged population characteristics that can lead to greater biological susceptibility or sociodemographic vulnerability (Gouveia and Fletcher 2000). However, for wildfire smoke exposure, our review identified a paucity of studies on potentially vulnerable/susceptible subpopulations. There was some indication of elevated vulnerability to adverse health-effects of wildfire smoke among certain sub-populations: young children, older adults, and individuals of lower socioeconomic status. It is plausible that individuals with pre-existing respiratory morbidities are more susceptible to the respiratory effects of wildfire smoke possibly due to elevated sensitivity to environmental hazards by weaker immune systems. Pre-existing morbidities, such as asthma, that may not be fully controlled by medication might lead to greater susceptibility to adverse health effects of wildfire smoke. Although not specific to wildfire smoke, PM10 has been associated with poorly controlled asthma among adults (Jacquemin et al., 2012) and the effect of air pollutants on respiratory exacerbation among asthmatic children appears to be greater for those not on anti-inflammatory medication (Delfino et al., 2002).
In the identified studies, five of six U.S. studies reported associations between wildfire smoke and cardiovascular hospital admissions, whereas associations were not observed in studies for other locations, including Australia and Canada. Cardiovascular diseases are more prevalent in U.S. adults (more than 1 in 3 adult Americans have cardiovascular diseases) (Lloyd-Jones et al., 2010) than in Australia (about 1 in 6) (The Heart Foundation 2011). The mortality rates due to cardiovascular diseases are also higher in the U.S. than in Canada or Australia (Lloyd-Jones et al., 2010). The different findings by region may result from higher risk for cardiovascular responses from wildfire smoke for population with high CVD prevalence.
4.4 Recommendations for future research
More studies in wildfire-affected but less-developed regions, such as Africa and Southeast Asia are needed. These regions face the highest health risk to wildfire smoke because they lack well-developed health care infrastructure and resources (Watson et al., 2007). They are also less able to adapt to climate change compared to the developed world (Matthes 2008), leading to even higher risk to wildfires in the future. The populations are particularly vulnerable because behavioral interventions are complex (e.g., remaining indoors might increase exposure due to use of solid fuels, and chronic exposure to indoor solid fuels can lead to higher susceptibility to respiratory diseases (Po et al., 2011)) (Smith et al., 2004).
More large-scale studies are needed to obtain more reliable results on health impact of wildfires. Most of the identified studies were based on single-episode fire events, with fewer long-term studies. Studies based on multiple-episode fire events might be useful to identify consistency of an association over time or change in vulnerability or behavioral adaptation (e.g., remaining indoors) to wildfire smoke exposure. Similarly, most studies focused on local regions, with few studies at national or other large geographic scales. Investigating larger geographies will introduce greater sociodemographic variation that might reveal communities at the greatest risk of wildfire smoke-related health responses. Large-scale studies can also help policy-makers by identifying the most vulnerable communities and populations for policy reference.
In addition, future studies could also adapt more new technologies to advance exposure assessment. Chemical transport models, dispersion models and satellite-based models could help address the limitations of assessing wildfire smoke exposure using air monitors. Moreover, as wildfire potential has been projected to increase in the future (Liu et al., 2010), studies that estimate future wildfire-related health impact are needed. In our review, no identified studies projected the future health risk from wildfires under climate change, or identified high-risk regions or populations under future conditions. Studies projecting future health impact of wildfires can raise awareness of the health impact of wildfires in communities, promote preventive public health programs in high-risk communities, and aid in our understanding of the health consequences of a changing climate.
5. Conclusion
Our review indicates that wildfire events have potential to induce a substantial health burden. As wildfires are likely to occur more frequently and intensely under the impact of climate change, this health burden may increase in the future. Air pollution from wildfires was consistently associated with respiratory outcomes, and more studies are needed to investigate cardiovascular morbidity and mortality in community populations. Most of the current studies were based on single episodes and local populations. Conducting multiple episode and larger scale studies may reveal effects of wildfire smoke and help elucidate changes in wildfire frequency and possible adaptation. It was not possible to separate completely the health effect of wildfires from that of other ambient sources for the reviewed studies. Key challenges in current research include the assessment of exposure of wildfire-specific pollutants and the health risk modelling for source-specific air pollutant estimates. More research is needed to investigate the health effects of fine particulate matter from wildfires in Africa and Southeast Asia, the susceptible/vulnerable populations under exposure to wildfire smoke, and future health burden from wildfires under climate change.
Supplementary Material
Highlights.
Wildfire smoke dramatically increased ambient air pollutant levels
Wildfire smoke consistently associated with increased risk of respiratory disease
Suggestive evidence wildfire smoke linked with cardiovascular diseases & mortality
Key challenge of exposure assessment: estimating fire-specific pollutants
Acknowledgements
This work was funded by NIH (R21ES021427), the U.S. EPA through the Harvard Clean Air Center (83479801), and the Yale Institute for Biospheric Studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jia C. Liu, Email: coco.liu@yale.edu.
Gavin Pereira, Email: gavin.pereira@yale.edu.
Sarah A. Uhl, Email: sarah.uhl@gmail.com.
Mercedes A. Bravo, Email: Mercedes.bravo@yahoo.com.
Michelle L. Bell, Email: michelle.bell@yale.edu.
References
- Aditama TY. Impact of haze from forest fire to respiratory health: Indonesian experience. Respirology (Carlton, Vic) 2000;5:169–174. doi: 10.1046/j.1440-1843.2000.00246.x. [DOI] [PubMed] [Google Scholar]
- Albertson K, et al. Climate change and the future occurrence of moorland wildfires in the Peak District of the UK. Clim Res. 2010;45:105–118. [Google Scholar]
- Analitis A, et al. Forest fires are associated with elevated mortality in a dense urban setting. Occupational and environmental medicine. 2012;69:158–162. doi: 10.1136/oem.2010.064238. [DOI] [PubMed] [Google Scholar]
- Azevedo JM, et al. Long-range ozone transport and its impact on respiratory and cardiovascular health in the north of Portugal. International journal of biometeorology. 2011;55:187–202. doi: 10.1007/s00484-010-0324-2. [DOI] [PubMed] [Google Scholar]
- Balling RC, et al. Climate Change in Yellowstone-National-Park - Is the Drought-Related Risk of Wildfires Increasing. Climatic Change. 1992;22:35–45. [Google Scholar]
- Brook RD, et al. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Cameron PA, et al. Black Saturday: the immediate impact of the February 2009 bushfires in Victoria, Australia. The Medical journal of Australia. 2009;191:11–16. doi: 10.5694/j.1326-5377.2009.tb02666.x. [DOI] [PubMed] [Google Scholar]
- Castro HA, et al. Trend of mortality from respiratory disease in elderly and the forest fires in the state of Rondonia/Brazil - period between 1998 and 2005. Ciencia & saude coletiva. 2009;14:2083–2090. doi: 10.1590/s1413-81232009000600015. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Surveillance of Morbidity During Wildfires -- Central Florida, 1998. MMWR Morbidity and mortality weekly report. 1999;48:78–79. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Wildfire-related deaths--Texas. [March 12–20, 2006];MMWR Morbidity and mortality weekly report. 2007 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Monitoring health effects of wildfires using the biosense system--San Diego County, California, October 2007. MMWR Morbidity and mortality weekly report. 2008 [PubMed] [Google Scholar]
- Chen LP, et al. Air particulate pollution due to bushfires and respiratory hospital admissions in Brisbane, Australia. International journal of environmental health research. 2006;16:181–191. doi: 10.1080/09603120600641334. [DOI] [PubMed] [Google Scholar]
- Cleland HJ, et al. Multidisciplinary team response to a mass burn casualty event: outcomes and implications. Med J Australia. 2011;194:589–593. doi: 10.5694/j.1326-5377.2011.tb03110.x. [DOI] [PubMed] [Google Scholar]
- Crabbe H. Risk of respiratory and cardiovascular hospitalisation with exposure to bushfire particulates: new evidence from Darwin, Australia. Environmental geochemistry and health. 2012;34:697–709. doi: 10.1007/s10653-012-9489-4. [DOI] [PubMed] [Google Scholar]
- de Mendonca MJ, et al. Estimation of damage to human health due to forest burning in the Amazon. J Popul Econ. 2006;19:593–610. [Google Scholar]
- Delfino RJ, et al. The relationship of respiratory and cardiovascular hospital admissions to the southern California wildfires of 2003. Occupational and environmental medicine. 2009;66:189–197. doi: 10.1136/oem.2008.041376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, et al. Association of asthma symptoms with peak particulate air pollution and effect modification by anti-inflammatory medication use. Environmental health perspectives. 2002;110:A607–A617. doi: 10.1289/ehp.021100607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennekamp M, Abramson MJ. The effects of bushfire smoke on respiratory health. Respirology (Carlton, Vic) 2011;16:198–209. doi: 10.1111/j.1440-1843.2010.01868.x. [DOI] [PubMed] [Google Scholar]
- Dimopoulou M, Giannikos I. Towards an integrated framework for forest fire control. Eur J Oper Res. 2004;152:476–486. [Google Scholar]
- do Carmo CN, et al. Association between particulate matter from biomass burning and respiratory diseases in the southern region of the Brazilian Amazon. Revista panamericana de salud publica = Pan American journal of public health. 2010;27:10–16. doi: 10.1590/s1020-49892010000100002. [DOI] [PubMed] [Google Scholar]
- Dohrenwend P, et al. The Impact on Emergency Department Visits for Respiratory Illness During the Southern California Wildfires West. J Emerg Med. 2013;14:79–84. doi: 10.5811/westjem.2012.10.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duclos P, et al. The 1987 forest fire disaster in California: assessment of emergency room visits. Archives of environmental health. 1990;45:53–58. doi: 10.1080/00039896.1990.9935925. [DOI] [PubMed] [Google Scholar]
- Ebisu K, Bell ML. Airborne PM2.5 Chemical Components and Low Birth Weight in the Northeastern and Mid-Atlantic Regions of the United States. Environmental health perspectives. 2012;120:1746–1752. doi: 10.1289/ehp.1104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CT, et al. Time series analysis of fine particulate matter and asthma reliever dispensations in populations affected by forest fires. Environmental health : a global access science source. 2013;12:11. doi: 10.1186/1476-069X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel SC. Impact to lung health of haze from forest fires: the Singapore experience. Respirology (Carlton, Vic) 2000;5:175–182. doi: 10.1046/j.1440-1843.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- Finlay SE, et al. Health Impacts of Wildfires. PLOS Currents Disasters. 2012 doi: 10.1371/4f959951cce2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannigan MD, Vanwagner CE. Climate Change and Wildfire in Canada. Can J Forest Res. 1991;21:66–72. [Google Scholar]
- Franck U, et al. The effect of particle size on cardiovascular disorders - The smaller the worse. Sci Total Environ. 2011;409:4217–4221. doi: 10.1016/j.scitotenv.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Frankenberg E, et al. Health consequences of forest fires in Indonesia. Demography. 2005;42:109–129. doi: 10.1353/dem.2005.0004. [DOI] [PubMed] [Google Scholar]
- Fried JS, et al. Predicting the effect of climate change on wildfire behavior and initial attack success. Climatic Change. 2008;87:S251–S264. [Google Scholar]
- Fried JS, et al. The impact of climate change on wildfire severity: A regional forecast for northern California. Climatic Change. 2004;64:169–191. [Google Scholar]
- Goncalves C, et al. Characterisation of PM10 emissions from woodstove combustion of common woods grown in Portugal. Atmos Environ. 2010;44:4474–4480. [Google Scholar]
- Gouveia N, Fletcher T. Time series analysis of air pollution and mortality: effects by cause, age and socioeconomic status. J Epidemiol Commun H. 2000;54:750–755. doi: 10.1136/jech.54.10.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan IC, et al. Vegetation fire smoke, indigenous status and cardio-respiratory hospital admissions in Darwin, Australia, 1996–2005: a time-series study. Environmental health : a global access science source. 2008;7:42. doi: 10.1186/1476-069X-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänninen OO, et al. Population exposure to fine particles and estimated excess mortality in Finland from an East European wildfire episode. Journal of exposure science & environmental epidemiology. 2009;19:414–422. doi: 10.1038/jes.2008.31. [DOI] [PubMed] [Google Scholar]
- Henderson SB, et al. Three measures of forest fire smoke exposure and their associations with respiratory and cardiovascular health outcomes in a population-based cohort. Environmental health perspectives. 2011;119:1266–1271. doi: 10.1289/ehp.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson SB, Johnston FH. Measures of forest fire smoke exposure and their associations with respiratory health outcomes. Current opinion in allergy and clinical immunology. 2012;12:221–227. doi: 10.1097/ACI.0b013e328353351f. [DOI] [PubMed] [Google Scholar]
- Holstius DM, et al. Birth weight following pregnancy during the 2003 Southern California wildfires. Environmental health perspectives. 2012;120:1340–1345. doi: 10.1289/ehp.1104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen K, et al. Low-level exposure to ambient particulate matter is associated with systemic inflammation in ischemic heart disease patients. Environmental research. 2012;116:44–51. doi: 10.1016/j.envres.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Ignotti E, et al. Impact on human health of particulate matter emitted from burnings in the Brazilian Amazon region. Revista de saude publica. 2010;44:121–130. doi: 10.1590/s0034-89102010000100013. [DOI] [PubMed] [Google Scholar]
- Interagency Working Group on Climate Change and Health. A Human Health Perspective on ClimateChange: A Report Outlining the Research Needs on the Human Health Effects of Climate Change. In: NIEHS E.H.P.a, editor. 2010. [Google Scholar]
- Jacquemin B, et al. Air pollution and asthma control in the Epidemiological study on the Genetics and Environment of Asthma. J Epidemiol Commun H. 2012;66:796–802. doi: 10.1136/jech.2010.130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalaludin B, et al. Acute effects of bushfires on peak expiratory flow rates in children with wheeze: a time series analysis. Australian and New Zealand journal of public health. 2000;24:174–177. doi: 10.1111/j.1467-842x.2000.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Jayachandran S. Air Quality and Early-Life Mortality Evidence from Indonesia's Wildfires. J Hum Resour. 2009;44:916–954. [Google Scholar]
- Johnston FH, et al. Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. Bmc Public Health. 2007;7 doi: 10.1186/1471-2458-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston FH, et al. Extreme air pollution events from bushfires and dust storms and their association with mortality in Sydney, Australia 1994–2007. Environmental research. 2011;111:811–816. doi: 10.1016/j.envres.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Johnston FH, et al. Estimated global mortality attributable to smoke from landscape fires. Environmental health perspectives. 2012;120:695–701. doi: 10.1289/ehp.1104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston FH, et al. Exposure to bushfire smoke and asthma: an ecological study. The Medical journal of Australia. 2002;176:535–538. doi: 10.5694/j.1326-5377.2002.tb04551.x. [DOI] [PubMed] [Google Scholar]
- Johnston FH, et al. Vegetation fires, particulate air pollution and asthma: A panel study in the Australian monsoon tropics. International journal of environmental health research. 2006;16:391–404. doi: 10.1080/09603120601093642. [DOI] [PubMed] [Google Scholar]
- Keeton WS, et al. Climate Variability, Climate Change, and WesternWildfire with Implications for the Urban-Wildland Interface. In: Howarth R, editor. Advances in the Economics of Environmental Resources. Emerald Group Publishing; 2007. [Google Scholar]
- Kleeman MJ, et al. Enhanced Air Pollution Epidemiology using a Epidemiology using a Source-Oriented Chemical Transport Model. EPA. 2009 [Google Scholar]
- Kloog I, et al. Assessing temporally and spatially resolved PM2.5 exposures for epidemiological studies using satellite aerosol optical depth measurements. Atmos Environ. 2011;45:6267–6275. [Google Scholar]
- Kolbe A, Gilchrist KL. An extreme bushfire smoke pollution event: health impacts and public health challenges. New South Wales public health bulletin. 2009;20:19–23. doi: 10.1071/nb08061. [DOI] [PubMed] [Google Scholar]
- Kunii O, et al. The 1997 haze disaster in Indonesia: Its air quality and health effects. Archives of environmental health. 2002;57:16–22. doi: 10.1080/00039890209602912. [DOI] [PubMed] [Google Scholar]
- Kunzli N, et al. Health effects of the 2003 Southern California wildfires on children. American journal of respiratory and critical care medicine. 2006;174:1221–1228. doi: 10.1164/rccm.200604-519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, et al. A novel calibration approach of MODIS AOD data to predict PM2.5 concentrations. Atmos Chem Phys. 2011;11:7991–8002. [Google Scholar]
- Lee TS, et al. Risk factors associated with clinic visits during the 1999 forest fires near the Hoopa Valley Indian Reservation, California, USA. International journal of environmental health research. 2009;19:315–327. doi: 10.1080/09603120802712750. [DOI] [PubMed] [Google Scholar]
- Lepeule J, et al. Chronic Exposure to Fine Particles and Mortality: An Extended Follow-up of the Harvard Six Cities Study from 1974 to 2009. Environmental health perspectives. 2012;120:965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, et al. Trends in global wildfire potential in a changing climate. Forest Ecology and Management. 2010;259:685–697. [Google Scholar]
- Lloyd-Jones D, et al. Heart Disease and Stroke Statistics-2010 Update A Report From the American Heart Association. Circulation. 2010;121:E46–E215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Malevsky-Malevich SP, et al. An assessment of potential change in wildfire activity in the Russian boreal forest zone induced by climate warming during the twenty-first century. Climatic Change. 2008;86:463–474. [Google Scholar]
- Mao YH, et al. Biomass burning contribution to black carbon in the Western United States Mountain Ranges. Atmos Chem Phys. 2011;11:11253–11266. [Google Scholar]
- Martin KL, et al. Air pollution from bushfires and their association with hospital admissions in Sydney, Newcastle and Wollongong, Australia 1994–2007. Australian and New Zealand journal of public health. 2013;37:238–243. doi: 10.1111/1753-6405.12065. [DOI] [PubMed] [Google Scholar]
- Mascarenhas MD, et al. Anthropogenic air pollution and respiratory disease-related emergency room visits in Rio Branco, Brazil--September, 2005. Jornal brasileiro de pneumologia : publicacao oficial da Sociedade Brasileira de Pneumologia e Tisilogia. 2008;34:42–46. doi: 10.1590/s1806-37132008000100008. [DOI] [PubMed] [Google Scholar]
- Matthes FC. Climate change 2007. The physical science basis, impacts, adaptation and vulnerability mitigation of climate change. Int Politik. 2008;63:130–132. [Google Scholar]
- Medina-Ramon M, et al. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: A national multicity study. Am J Epidemiol. 2006;163:579–588. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- Mirabelli MC, et al. Respiratory symptoms following wildfire smoke exposure: airway size as a susceptibility factor. Epidemiology (Cambridge, Mass) 2009;20:451–459. doi: 10.1097/EDE.0b013e31819d128d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D, et al. Population health effects of air quality changes due to forest fires in British Columbia in 2003: estimates from physician-visit billing data. Canadian journal of public health = Revue canadienne de sante publique. 2006;97:105–108. doi: 10.1007/BF03405325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G, et al. Effects of bushfire smoke on daily mortality and hospital admissions in Sydney, Australia. Epidemiology (Cambridge, Mass) 2010;21:47–55. doi: 10.1097/EDE.0b013e3181c15d5a. [DOI] [PubMed] [Google Scholar]
- Morgenstern V, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. American journal of respiratory and critical care medicine. 2008;177:1331–1337. doi: 10.1164/rccm.200701-036OC. [DOI] [PubMed] [Google Scholar]
- Mott JA, et al. Cardiorespiratory hospitalizations associated with smoke exposure during the 1997, Southeast Asian forest fires. International journal of hygiene and environmental health. 2005;208:75–85. doi: 10.1016/j.ijheh.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Mott JA, et al. Wildland forest fire smoke: health effects and intervention evaluation, Hoopa, California, 1999. The Western journal of medicine. 2002;176:157–162. doi: 10.1136/ewjm.176.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeher LP, et al. Woodsmoke health effects: a review. Inhalation toxicology. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Nunes KVR, et al. Circulatory disease mortality rates in the elderly and exposure to PM2.5 generated by biomass burning in the Brazilian Amazon in 2005. Cadernos de saude publica. 2013;29:589–598. doi: 10.1590/s0102-311x2013000300016. [DOI] [PubMed] [Google Scholar]
- Parry ML, et al. Technical Summary. Climate Change 2007: Impacts, Adaptation, and Vulnerability. In: Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CE, editors. Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Peng RD, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. JAMA : the journal of the American Medical Association. 2008;299:2172–2179. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phuleria HC, et al. Air quality impacts of the October 2003 Southern California wildfires. J Geophys Res-Atmos. 2005;110 [Google Scholar]
- Pio CA, et al. Chemical composition of atmospheric aerosols during the 2003 summer intense forest fire period. Atmos Environ. 2008;42:7530–7543. [Google Scholar]
- Po JYT, et al. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax. 2011;66:232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- Pope CA, Dockery DW. Health effects of fine particulate air pollution: Lines that connect. J Air Waste Manage. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- Prass TS, et al. Amazon Forest Fires Between 2001 and 2006 and Birth Weight in Porto Velho. B Environ Contam Tox. 2012;89:1–7. doi: 10.1007/s00128-012-0621-z. [DOI] [PubMed] [Google Scholar]
- Rappold AG, et al. Cardio-respiratory outcomes associated with exposure to wildfire smoke are modified by measures of community health. Environmental health : a global access science source. 2012;11:71. doi: 10.1186/1476-069X-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappold AG, et al. Peat bog wildfire smoke exposure in rural North Carolina is associated with cardiopulmonary emergency department visits assessed through syndromic surveillance. Environmental health perspectives. 2011;119:1415–1420. doi: 10.1289/ehp.1003206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, et al. Ambient air pollution and risk of birth defects in southern California. Am J Epidemiol. 2002;155:17–25. doi: 10.1093/aje/155.1.17. [DOI] [PubMed] [Google Scholar]
- Robinson MS, et al. Characterization of PM2.5 collected during broadcast and slash-pile prescribed burns of predominately ponderosa pine forests in northern Arizona. Atmos Environ. 2011;45:2087–2094. doi: 10.1016/j.atmosenv.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnat JA, et al. Fine particle sources and cardiorespiratory morbidity: an application of chemical mass balance and factor analytical source-apportionment methods. Environmental health perspectives. 2008;116:459–466. doi: 10.1289/ehp.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry N. Forest fires, air pollution, and mortality in southeast Asia. Demography. 2002;39:1–23. doi: 10.1353/dem.2002.0009. [DOI] [PubMed] [Google Scholar]
- Schranz CI, et al. The 2007 San Diego Wildfire impact on the Emergency Department of the University of California, San Diego Hospital System. Prehospital and disaster medicine. 2010;25:472–476. doi: 10.1017/s1049023x0000858x. [DOI] [PubMed] [Google Scholar]
- Shaposhnikov D, et al. Mortality related to air pollution with the moscow heat wave and wildfire of 2010. Epidemiology (Cambridge, Mass) 2014;25:359–364. doi: 10.1097/EDE.0000000000000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman D, et al. Immediate health effects of an urban wildfire. The Western journal of medicine. 1993;158:133–138. [PMC free article] [PubMed] [Google Scholar]
- Singh HB, et al. Pollution influences on atmospheric composition and chemistry at high northern latitudes: Boreal and California forest fire emissions. Atmos Environ. 2010;44:4553–4564. [Google Scholar]
- Smith KR, et al. Indoor air pollution from household use of solid fuels. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. 2004;2:1435–1493. [Google Scholar]
- Smith MA, et al. Asthma presentations to emergency departments in western Sydney during the January 1994 Bushfires. International journal of epidemiology. 1996;25:1227–1236. doi: 10.1093/ije/25.6.1227. [DOI] [PubMed] [Google Scholar]
- Spracklen DV, et al. Impacts of climate change from 2000 to 2050 on wildfire activity and carbonaceous aerosol concentrations in the western United States. J Geophys Res-Atmos. 2009;114 [Google Scholar]
- Sutherland ER, et al. Wildfire smoke and respiratory symptoms in patients with chronic obstructive pulmonary disease. The Journal of allergy and clinical immunology. 2005;115:420–422. doi: 10.1016/j.jaci.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Tan WC, et al. The human bone marrow response to acute air pollution caused by forest fires. American journal of respiratory and critical care medicine. 2000;161:1213–1217. doi: 10.1164/ajrccm.161.4.9904084. [DOI] [PubMed] [Google Scholar]
- Tham R, et al. The impact of smoke on respiratory hospital outcomes during the 2002–2003 bushfire season, Victoria, Australia. Respirology (Carlton, Vic) 2009;14:69–75. doi: 10.1111/j.1440-1843.2008.01416.x. [DOI] [PubMed] [Google Scholar]
- The Heart Foundation. Data and Statistics. Information for professionals. 2011 [Google Scholar]
- Thelen B, et al. Modeling acute respiratory illness during the 2007 San Diego wildland fires using a coupled emissions-transport system and generalized additive modeling. Environ Health-Glob. 2013;12 doi: 10.1186/1476-069X-12-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Forest Service. Today’s Challenges and Opportunities: climate change Briefing paper. 2009 [Google Scholar]
- U.S. Forest Service. Wildland Fire Smoke. 2010 [Google Scholar]
- Valavanidis A, et al. Airborne Particulate Matter and Human Health: Toxicological Assessment and Importance of Size and Composition of Particles for Oxidative Damage and Carcinogenic Mechanisms. J Environ Sci Heal C. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, et al. Air Pollution, Blood Pressure, and the Risk of Hypertensive Complications During Pregnancy The Generation R Study. Hypertension. 2011;57:406-U138. doi: 10.1161/HYPERTENSIONAHA.110.164087. [DOI] [PubMed] [Google Scholar]
- Vedal S, Dutton SJ. Wildfire air pollution and daily mortality in a large urban area. Environmental research. 2006;102:29–35. doi: 10.1016/j.envres.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Versura P, et al. Eye discomfort and air pollution. Ophthalmologica. 1999;213:103–109. doi: 10.1159/000027401. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, et al. An analysis of effects of San Diego wildfire on ambient air quality. Journal of the Air & Waste Management Association (1995) 2006;56:56–67. doi: 10.1080/10473289.2006.10464439. [DOI] [PubMed] [Google Scholar]
- Vora C, et al. 2007 San Diego Wildfires and Asthmatics. J Asthma. 2011;48:75–78. doi: 10.3109/02770903.2010.535885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DE. Factors influencing the emissions of gases and particulate matter from biomass burning. Fire in the Tropical Biota: Springer Berlin Heidelberg. 1990 [Google Scholar]
- Watson JT, et al. Epidemics after natural disasters. Emerging infectious diseases. 2007;13:1–5. doi: 10.3201/eid1301.060779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegesser TC, et al. California wildfires of 2008: coarse and fine particulate matter toxicity. Environmental health perspectives. 2009;117:893–897. doi: 10.1289/ehp.0800166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerling AL, Bryant BP. Climate change and wildfire in California. Climatic Change. 2008;87:S231–S249. [Google Scholar]
- Westerling AL, et al. Warming and earlier spring increase western US forest wildfire activity. Science. 2006;313:940–943. doi: 10.1126/science.1128834. [DOI] [PubMed] [Google Scholar]
- Wiwatanadate P, Liwsrisakun C. Acute effects of air pollution on peak expiratory flow rates and symptoms among asthmatic patients in Chiang Mai, Thailand. International journal of hygiene and environmental health. 2011;214:251–257. doi: 10.1016/j.ijheh.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, et al. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health-Glob. 2009;8 doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.