Abstract
Invadopodia are actin-rich protrusions that degrade the extracellular matrix and are required for penetration through the basement membrane, stromal invasion and intravasation. Invadopodia are enriched in actin regulators, such as cortactin, cofilin, N-WASp, Arp2/3 and fascin. Much of the work to date has centered around identifying the proteins involved in regulating actin polymerization and matrix degradation. Recently, there have been significant advances in characterization of the very early stages of invadopodium precursor assembly and the role of adhesion proteins, such as β1 integrin, talin, FAK and Hic-5, in promoting invadopodium maturation. This review summarizes these findings in the context of our current model of invadopodial function and highlights some of the important unanswered questions in the field.
Keywords: invadopodia, invasion, metastasis, β1 integrin, Arg, talin, moesin, NHE-1, cofilin, cdc42
Introduction
Tumor cell metastasis is a multistep process that involves escape from the primary tumor, migration through the stroma, entry into the vasculature and dissemination to distant sites. Invadopodia are finger-like, actin-rich protrusions that are formed by metastatic tumor cells to degrade the extracellular matrix (ECM). Analogous to podosomes formed by hematopoietic cells and rosettes found in Src-transformed fibroblasts, smooth muscle and endothelial cells, invadopodia are formed by tumor cells to facilitate breach of the basement membrane surrounding carcinoma in situ, invasive cancer cell migration through the dense stromal ECM and degradation of the endothelial basement membrane for entry into the blood (Bravo-Cordero et al., 2012; Destaing et al., 2010; Eckert et al., 2011; Linder et al., 1999; Moreau et al., 2003; Yamaguchi et al., 2005). Pioneering work by Chen and colleagues demonstrated that invadopodia are capable of degrading many different types of ECM, including collagen types I and IV, laminin and fibronectin (Kelly et al., 1994). Subsequent work demonstrated that invadopodia also degrade native, tissue-derived basement membranes (Parekh et al., 2011; Schoumacher et al., 2010). As a number of reviews have discussed the similarities and differences between invadopodia and podosomes (Block et al., 2008; Destaing et al., 2011; Gimona et al., 2008; Linder et al., 2011; Murphy and Courtneidge, 2011), this review will focus on recent advances in characterizing the early stages of invadopodium precursor assembly as well as the invadopodium maturation phase (actin polymerization and matrix degradation).
Invadopodium precursor formation
Stimuli of invadopodium assembly
Invadopodium precursors are defined as complexes of invadopodial proteins that do not degrade the ECM. Many stimuli have been reported to induce invadopodium precursor formation (Figure 1 - Stage 1). These stimuli can be grouped into the following categories: growth factors, oncogenic transformation, induction of the epithelial-mesenchymal transition (EMT), hypoxia and matrix metalloprotease (MMP) activity (presumably through the generation of degraded ECM fragments; Clark et al., 2007; Eckert et al., 2011; Pignatelli et al., 2012b; Yamaguchi et al., 2005). Although the epidermal growth factor (EGF) is the best characterized growth factor stimulus, transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF) and heparin binding EGF (HB-EGF) have been shown to induce invadopodium precursor formation in a number of different tumor cell types (Diaz et al., 2013; Eckert et al., 2011; Pignatelli et al., 2012b; Rajadurai et al., 2012; Yamaguchi et al., 2005). EGF receptor (EGFR) ligands, EGF and HB-EGF, induce the formation of invadopodium precursors in starved breast cancer cells and are sufficient to activate the pathways leading to actin polymerization and ultimately matrix degradation (Busco et al., 2010; Zhou et al., 2013). TGF-β and PDGFR ligands, on the other hand, are able to induce de novo invadopodium formation in normal breast and mammary epithelial cells, respectively, which do not normally form these structures (Eckert et al., 2011; Pignatelli et al., 2012b). Finally, it should be noted that tumor cells can also form invadopodia in the absence of external stimuli via autocrine signaling, likely involving the abovementioned growth factors.
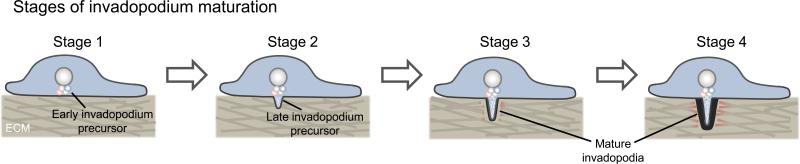
Figure 1. Stages of invadopodium maturation.
Stage 1 (early precursor stage): invadopodia initially form as non-degradative precursors that consist of a core structure containing actin, cortactin, cofilin, N-WASp, Tks5 and other proteins. Stage 2 (late precursor stage): kinases are activated, β1 integrin and talin are recruited and Tks5 anchors the precursor to PI(3,4)P2. Stages 3-4 (mature invadopodium stage): in stage 3, actin polymerization is activated by stimulation of the NHE-1-cofilin pathway, and continued actin polymerization drives invadopodial elongation and stabilization. In stage 4, microtubule and intermediate filament recruitment facilitates further elongation of the protrusion, and matrix proteases are recruited to degrade the ECM (modified from Artym et al., 2006; Oser et al., 2009; Schoumacher et al., 2010; Sharma et al., 2013).
Invadopodium-like invasive protrusions were initially identified in chicken embryonic fibroblasts that were transformed with Rous sarcoma virus (Chen, 1989). In breast and pancreatic epithelial cells, Src and Ras transformation are sufficient to induce de novo invadopodium precursor formation (Neel et al., 2012; Pignatelli et al., 2012b). TGF-β-induced transformation or induction of EMT by Twist1 can also stimulate invadopodium formation in epithelial cells (Eckert et al., 2011; Pignatelli et al., 2012b). Finally, other microenvironmental factors, such as hypoxia and perhaps degraded ECM products, can promote invadopodium formation (Arsenault et al., 2013; Clark et al., 2007; Diaz et al., 2013). Hypoxia, for example, stimulates invadopodium function via ADAM12-dependent release of HB-EGF in multiple cancer cell lines (Diaz et al., 2013).
Although invadopodia form in response to multiple extracellular cues, these pathways appear to converge at the level of the Rho family GTPase, Cdc42. In contrast to the other major Rho GTPases (RhoA, RhoC and Rac; Box 1), Cdc42 depletion in mammary adenocarcinoma cells completely abrogates EGF-induced invadopodium precursor formation as well as invadopodium formation at steady state (Desmarais et al., 2009; Yamaguchi et al., 2005). Similarly, Cdc42 is required for actin punctum formation in pancreatic tumor cells (Razidlo et al., 2014).
Rho GTPases are activated by guanine exchange factors (GEFs), which stabilize the GTP-bound (active) form of the GTPase, leading to activation of downstream GTPase targets (Rossman et al., 2005). A number of Cdc42 GEFs have been implicated in invadopodium formation, including Vav1, β-PIX and Fgd1 (Ayala et al., 2009; Md Hashim et al., 2013; Razidlo et al., 2014). Recently, Src has been shown to activate Vav1, which, in turn, activates Cdc42 to induce invadopodium formation (Razidlo et al., 2014). β-PIX is essential for hypoxia-induced invadopodium formation, while the Cdc42-specific GEF Fgd1 also promotes invadopodium formation (Ayala et al., 2009; Md Hashim et al., 2013). Interestingly, as all three GEFs are activated by EGF-induced Src phosphorylation (Feng et al., 2010; Miyamoto et al., 2003; Razidlo et al., 2014), it is tempting to speculate that the EGFR-Src-GEF-Cdc42 axis may represent a major pathway for initiation of invadopodium precursor assembly (Figure 2). However, since Src is not required for precursor formation in all cases (Mader et al., 2011), an important future direction will be to better characterize the multiple input pathways that initiate assembly of the precursor core structure.
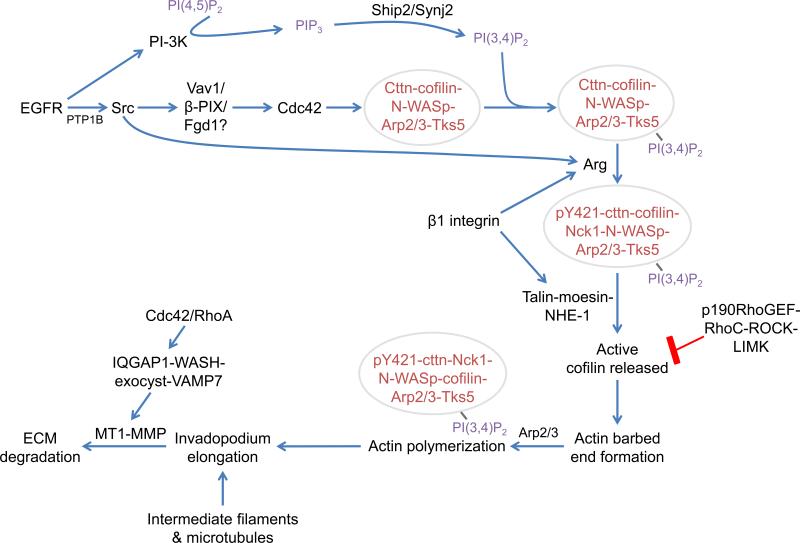
Figure 2. Integrative signaling diagram of invadopodial assembly and maturation.
Invadopodia initially form as precursors in response to EGF or other stimuli (e.g. TGF-β or PDGF). Src is activated either directly by EGFR or by PTP1B (Cortesio et al., 2008). These stimuli induce Cdc42 activation, leading to assembly of the precursor core structure (red text within circle; Razidlo et al., 2014; Yamaguchi et al., 2005). The invadopodium precursor is then anchored by binding to PI(3,4)P2 and further stabilized by β1 integrin-mediated adhesion to the ECM (Beaty et al., 2013; Sharma et al., 2013). Invadopodium maturation begins as β1 integrin activates Arg, which phosphorylates cortactin on Y421 to recruit Nck1 (Beaty et al., 2013; Oser et al., 2010). Talin localizes to the structure and recruits a complex of moesin and NHE-1 through a direct binding interaction with moesin (Beaty et al., 2014). The intracellular pH increases in response to NHE-1 activity, which disrupts the inhibitory interaction between cortactin and cofilin (Busco et al., 2010; Magalhaes et al., 2011). Cofilin severs F-actin to form barbed ends that are used to elongate filaments, on which Nck1 induces N-WASp-Arp2/3-dependent dendritic nucleation (DesMarais et al., 2004). Actin polymerization is required for MMP-dependent matrix degradation at invadopodia, possibly through MMP recruitment (Oser et al., 2009; Sakurai-Yageta et al., 2008; Yamaguchi et al., 2005). MT1-MMP is delivered by the IQGAP1-WASH-exocyst complex and fuses to the membrane via the v-SNARE VAMP7, resulting in matrix degradation (Monteiro et al., 2013; Sakurai-Yageta et al., 2008; Steffen et al., 2008). Cortactin, cttn; synaptojanin2, synj2.
Invadopodium precursor assembly and anchoring
Recently, high temporal resolution microscopy has demonstrated that invadopodium precursors are assembled in a highly orchestrated manner (Sharma et al., 2013). Invadopodial core proteins, cortactin, cofilin and N-WASp form an initial nucleus, or core structure, that is associated with an actin filament (Figure 1 - Stage 1; Artym et al., 2006; Sharma et al., 2013). The adaptor protein Tks5 joins the complex approximately 20 seconds later in order to anchor it to the phosphoinositide PI(3,4)P2 via its PX domain (Sharma et al., 2013). Formation of PI(3,4)P2 at the plasma membrane is thought to occur in a step-wise manner, in which EGFR activates phosphoinositide-3 kinase (PI-3K) to convert PI(4,5)P2 into PI(3,4,5)P3. PIP3 is then dephosphorylated by the 5’-phosphatases Ship2 or synaptojanin2 to form PI(3,4)P2 (Figure 2; Chuang et al., 2004; Sharma et al., 2013; Yamaguchi et al., 2011). Around 2-3 minutes after invadopodium precursor assembly begins, Tks5 binds to PI(3,4)P2 to anchor the structure to the membrane (Sharma et al., 2013). The adhesion receptor β1 integrin is then recruited to the structure, activated and binds to ECM ligands to further stabilize the structure and trigger the maturation process (i.e. actin polymerization and matrix degradation; Figure 1 - Stage 2; Figure 2; Beaty et al., 2013; Sharma et al., 2013).
Invadopodium maturation
An emerging role for adhesion proteins at invadopodia
A long-standing question in the invadopodium field has been: do invadopodia adhere to the ECM (Gimona et al., 2008; Linder et al., 2011)? Many adhesion proteins have been reported to localize to invadopodia, including vinculin, paxillin, filamin, NEDD9, as well as integrins (α3, α5 and β1 integrins, but not β3 integrin; Beaty et al., 2013; Branch et al., 2012; Coopman et al., 1996; Mueller et al., 1999; Sharma et al., 2013; Takkunen et al., 2010). Integrins are adhesion receptors that have three conformational states: inactive (bent/closed), activated (extended) and adherent (extended and bound to the ECM; Askari et al., 2010; Frelinger et al., 1988; Mould and Humphries, 2004; Nishida et al., 2006; Xiong et al., 2001). We have recently shown that β1 integrin is activated in the invadopodium core and that stimulation of β1 integrin-mediated adhesion accelerates precursor maturation into matrix-degrading invadopodia (Figure 1 - Stages 2-4; Beaty et al., 2013). Although β1 integrin does not affect invadopodium precursor assembly, it is required for invadopodium stability through adhesion to the ECM and activating actin polymerization (see below).
β1 integrin promotes invadopodium maturation, specifically by interacting with the tyrosine kinase Arg (Beaty et al., 2013). Interestingly, β1 integrin-EGFR crosstalk is required for Arg activation, as neither β1 integrin activation nor EGF stimulation alone is sufficient to induce Arg activation for invadopodium maturation (Beaty et al., 2013). Arg binding to the β1 integrin cytoplasmic tail is thought to disrupt its autoinhibitory conformation, unmasking Y272 for Arg autophosphorylation (Tanis et al., 2003). EGFR-Src-mediated phosphorylation of Arg Y439 on its activation loop then results in full Arg activation (Bradley and Koleske, 2009; Tanis et al., 2003). Arg phosphorylates cortactin on Y421 and Y466, which recruit Nck1, an adapter protein that binds N-WASp to facilitate Arp2/3 activation (Mader et al., 2011; Oser et al., 2010). This ultimately leads to synergistic cofilin-Arp2/3-dependent actin polymerization (Figure 2).
Like β1 integrin, the focal adhesion protein talin does not regulate precursor assembly (Beaty et al., 2014); rather, talin is essential for invadopodium maturation and tumor cell metastasis, as depletion of talin blocks invadopodial matrix degradation, invasion through 3D ECM and intravasation and spontaneous lung metastasis in vivo (Beaty et al., 2014). Talin is recruited to precursors by binding to actin via the I/LWEQ domain in its C-terminus (talin rod; Beaty et al., 2014). Talin binds directly to the ezrin-radixin-moesin (ERM) family protein, moesin, in vitro and is required for moesin recruitment to invadopodia in MDA-MB-231 cells (Beaty et al., 2014). Moesin, in turn, recruits the sodium-hydrogen exchanger-1 (NHE-1) to invadopodia, where it extrudes H+ from the cell in order to acidify the ECM as well as increase the intracellular pH to disrupt the inhibitory interaction between cortactin and the F-actin severing protein, cofilin (Beaty et al., 2014; Busco et al., 2010; Denker et al., 2000; Magalhaes et al., 2011). This allows active cofilin to sever F-actin to generate free actin barbed ends, which elongate new filaments that support nucleation by the Arp2/3 complex (and potentially direct nucleation by cofilin) for dendritic actin polymerization (Andrianantoandro and Pollard, 2006; Bravo-Cordero et al., 2013; DesMarais et al., 2004). Thus, by regulating moesin and NHE-1 recruitment to invadopodia, talin promotes cofilin-dependent actin polymerization and matrix degradation (Figure 1 - Stages 3-4; Figure 2).
Cofilin activity is tightly regulated in a spatiotemporal manner. In addition to being regulated by binding to cortactin, cofilin activity is controlled by phosphorylation on serine 3 (Yang et al., 1998). This residue is phosphorylated by LIM domain kinases (LIMK) and TES kinases (TESK) and is dephosphorylated by slingshot and chronophin (Gohla et al., 2005; Niwa et al., 2002; Toshima et al., 2001; Yang et al., 1998). At invadopodia, cofilin activity is stimulated by the talin-moesin-NHE-1 complex and suppressed by the RhoC-ROCK-LIMK pathway (Beaty et al., 2014; Bravo-Cordero et al., 2011; Magalhaes et al., 2011). p190RhoGEF, a RhoC GEF, localizes in a ring around the invadopodium core to locally activate RhoC, whereas p190RhoGAP, an inactivator of RhoC, is enriched at the invadopodium core (Bravo-Cordero et al., 2011). The result is RhoC-ROCK-mediated LIMK activation and cofilin phosphorylation (inactivation) outside of invadopodia and active cofilin concentrated at the invadopodium core, where RhoC is inactive (Bravo-Cordero et al., 2011). Interestingly, β1 integrin induces Arg-dependent p190RhoGAP phosphorylation and activation in fibroblasts and neurons (Bradley et al., 2006; Kerrisk et al., 2013; Warren et al., 2012). Thus, it will be interesting to explore the possibility that β1 integrin-Arg signaling may act as a master upstream regulator of invadopodial cofilin activity through Arg-mediated phosphorylation of both cortactin on Y421 and p190RhoGAP at the invadopodia core to relieve cortactin- and RhoC-dependent suppression of cofilin activity, respectively.
The focal adhesion proteins, focal adhesion kinase (FAK), Hic-5, integrin-linked kinase (ILK) and NEDD9, also regulate invadopodia. FAK regulates invadopodium formation indirectly by sequestering active Src at focal adhesions (Chan et al., 2009). FAK knockdown leads to redistribution of Src from focal adhesions to invadopodia, resulting in increased invadopodium formation, but impaired invasion through fibronectin due to reduced focal adhesion turnover and degradation capacity per invadopodium (Chan et al., 2009; Oser et al., 2009). Ectopic Hic-5 expression or knockdown of the endocytic adaptor protein β2-adaptin induce invadopodium matrix degradation via Src activation in normal MCF10A epithelial cells (Pignatelli et al., 2012a; Pignatelli et al., 2012b). Thus, FAK, Hic-5 and β2-adaptin regulate Src-dependent invadopodium function.
ILK and the docking protein NEDD9 regulate MMP surface expression at invadopodia. ILK recruits the scaffold protein IQGAP to invadopodia to induce membrane type-I MMP (MT1-MMP) exocytosis, while NEDD9 limits the accumulation of tissue inhibitor of metalloproteinase-2 (TIMP2), an endogenous MT1-MMP inhibitor, at invadopodia to promote MT1-MMP-mediated matrix degradation (Branch et al., 2012; McLaughlin et al., 2014). Taken together, adhesion proteins regulate invadopodium maturation by enhancing actin polymerization and MMP-mediated matrix degradation, while having limited effects on invadopodium precursor assembly.
Unbranched actin polymerization
While much of the work done on invadopodia has focused on the dendritic actin network generated by Arp2/3, there is strong evidence that invadopodia also contain linear, bundled actin filaments (Li et al., 2010; Schoumacher et al., 2010). Diaphanous-related formins (DRF) are a family of actin nucleators that induce the formation of linear actin networks, such as those found in stress fibers and filopodia (Lizarraga et al., 2009). mDia2 (mouse orthologue of DRF3) localizes to invadopodia, and DRF1-3 have been shown to promote invadopodium maturation in MDA-MB-231 cells (Lizarraga et al., 2009). More specifically, mDia2 has been shown to promote invadopodial elongation and stability in 3D ECM (Figure 1 – Stage 4; Lizarraga et al., 2009; Schoumacher et al., 2010). Similarly, fascin, an actin bundling protein, localizes to invadopodia to promote stability, elongation and matrix degradation (Li et al., 2010; Schoumacher et al., 2010). Thus, regulators of linear actin filaments play an important role in invadopodium maturation.
The role of microtubules and intermediate filaments in invadopodium maturation
Mature invadopodia also contain microtubules and intermediate filaments, namely vimentin (Schoumacher et al., 2010). Although these cytoskeletal elements have not been investigated as extensively as actin-associated proteins, a number of recent studies point to their importance in invadopodium function (Kikuchi and Takahashi, 2008; Schoumacher et al., 2010). Interestingly, disruption of microtubules by treating cells with the microtubule-stabilizing agent paclitaxel, microtubule-destabilizing agent nocodazole, or knockdown of vimentin does not affect initial invadopodium formation (Kikuchi and Takahashi, 2008; Schoumacher et al., 2010); however, microtubules and intermediate filaments are required for invadopodial elongation (Figure 1 - Stages 4; Schoumacher et al., 2010). Taken together, data indicate that actin is critical for all stages of invadopodium formation and maturation, whereas microtubules and intermediate filaments regulate the later stages of invadopodium maturation.
Protease recruitment: the culmination of invadopodium maturation
Invadopodium maturation is a complex process that requires coordination of many different proteins and culminates in the accumulation of a number of proteases, including seprase, cathepsins and MMPs (Brisson et al., 2013; Mueller et al., 1999; Sakurai-Yageta et al., 2008). RhoA and Cdc42 stimulate the association of the polarity protein IQGAP1 with the exocyst complex, which cooperates with endosomal Wiskott-Aldrich syndrome protein and Scar homolog (WASH) to promote MT1-MMP delivery to invadopodia (Figure 2; Monteiro et al., 2013; Sakurai-Yageta et al., 2008). Cortactin also regulates invadopodial MT1-MMP trafficking and MMP-2 and MMP-9 secretion (Clark et al., 2007). The v-SNARE VAMP7 then localizes to invadopodia and facilitates MT1-MMP vesicle anchoring at invadopodia for MT1-MMP insertion into the plasma membrane (Steffen et al., 2008). Last, MMP-2 and MMP-9 are delivered to invadopodia, through a Rab40b- and VAMP4-dependent mechanism, and synergize with MT1-MMP to degrade the surrounding ECM at invadopodia (Jacob et al., 2013; Marrero-Diaz et al., 2009; Sakurai-Yageta et al., 2008).
MT1-MMP trafficking is also regulated by Cdc42-interacting protein-4 (CIP4). CIP4 is an F-BAR protein that promotes MT1-MMP endocytosis to limit ECM degradation; however, when Src is activated at invadopodia, it phosphorylates and inactivates CIP4, resulting in MT1-MMP accumulation at the plasma membrane (Hu et al., 2011). Integrins have also been shown to regulate MT1-MMP trafficking. β1 integrin abrogates MT1-MMP endocytosis in human endothelial cells, and integrin-mediated adhesion induces MT1-MMP exocytosis at 3D invadopodia in a Rab8-dependent manner (Bravo-Cordero et al., 2007; Galvez et al., 2002). As β1 integrin localizes to invadopodia (Coopman et al., 1996), this raises the intriguing possibility that β1 integrin may cooperate with Src-CIP4 to stabilize MT1-MMP at the cell surface of invadopodia and facilitate matrix degradation.
Conclusion
Significant advances have been made in understanding the initiation and function of invadopodia in tumor cells. Recent advances in characterizing the early stages of invadopodium precursor formation and the molecular mechanisms of invadopodium maturation have increased our understanding of the regulation of these structures and their in vivo functions in cancer progression. An important role for adhesion proteins has recently emerged: these proteins localize to late invadopodium precursors at the onset of the maturation process (Figure 1 – Stage 2) to promote actin polymerization and matrix degradation (Figure 1 - Stages 3-4). However, there are a number of open questions in the field that remain unresolved.
First, how is the early invadopodium precursor assembled? While a role for EGFR-Src-Vav1-Cdc42 has been recently identified (Desmarais et al., 2009; Razidlo et al., 2014), the molecular mechanisms by which Cdc42 induces precursor assembly are poorly understood. The upstream GEFs and GAPs that regulate Cdc42 activity and its downstream effectors during the very early stages of invadopodium precursor assembly are not known. For example, it is assumed that N-WASp is the primary Cdc42 effector activated during invadopodium precursor formation, yet Cdc42 knockdown completely abrogates precursor formation, whereas N-WASp depletion only partially blocks their formation in mammary adenocarcinoma cells (Desmarais et al., 2009). This suggests that Cdc42 activates multiple downstream targets at invadopodia. In addition, it is not clear what other Cdc42-independent pathways stimulate invadopodium formation in other tumor types. Thus, an important area of future study is to identify the proteins that are necessary for invadopodium precursor formation using synchronized starvation-growth factor stimulation assays and to determine the relative contributions of the different Cdc42 effectors in precursor assembly.
Another important issue is the development of consensus markers that uniquely identify invadopodia. In the past, matrix degradation was thought to be a distinguishing feature of invadopodia; however, recent work has demonstrated that structures traditionally considered to be “non-degradative” (e.g. focal adhesions and filopodia) actually degrade matrix in some contexts (Starnes et al., 2014; Wang and McNiven, 2012). Thus, a minimum of two invadopodial markers is needed to differentiate these structures. Using actin or cortactin as solitary markers is not sufficient since these proteins are present in other structures that degrade the matrix. We and others have used actin-associated proteins (e.g. cortactin) together with an actin-independent marker (e.g. Tks5 or MT1-MMP) to definitively identify bona fide invadopodium precursors (not associated with ECM degradation) or mature invadopodia (associated with ECM degradation; Artym et al., 2006; Beaty et al., 2013; Sakurai-Yageta et al., 2008).
Second, what determines the subcellular location of invadopodium formation in 2D and 3D ECM contexts? Although there is evidence that invadopodia and podosomes form at the proximal tips of focal adhesions in smooth muscle cells and MTLn3 cells (Burgstaller and Gimona, 2004; Sharma et al., 2013), this is not the case for many other cell types, including MDA-MB-231 cells (unpublished observation). Moreover, it is not clear that focal adhesions per se are required for invadopodium precursor formation. When MTLn3 and MDA-MB-231 cells are plated on poly-L-lysine to prevent integrin-mediated adhesion and focal adhesion formation (Lo et al., 1998), invadopodium precursors still form in response to growth factor stimulation (unpublished observation). Consistent with this finding, disruption of focal adhesion signaling by knockdown of key adhesion proteins either has no effect on invadopodium precursors (i.e. talin and β1 integrin) or increases precursor formation (i.e. FAK; Beaty et al., 2013; Beaty et al., 2014; Chan et al., 2009). This suggests that the formation of invadopodium precursors at the proximal tips of focal adhesions is due to the specific characteristics of this site (e.g. decreased contractility), rather than their specific association with adhesion proteins (Burgstaller and Gimona, 2004). In support of this notion, myosin II is dispersed from and p190RhoGAP is enriched at sites of prospective podosome formation to reduce local cytoskeletal contractility and allow for podosome assembly to occur (Burgstaller and Gimona, 2004). The generality of this finding has yet to be confirmed, but testing this hypothesis in tumor cells will provide important insights into potential mechanisms of invadopodium assembly.
Third, what proteins recruit RhoGTPase GEFs and GAPs to invadopodia? In other systems, p190RhoGEF and p190RhoGAP are recruited to focal adhesions through binding to FAK, but since FAK does not localize to invadopodia, it is not clear how these proteins are recruited (Chan et al., 2009; Lim et al., 2008; Tomar et al., 2009; Yu et al., 2011). Fourth, how are matrix degradation, adhesion, protrusion and translocation through degraded ECM coordinated in a 3D matrix setting? Finally, do invadopodia play a role in extravasation and colonization of secondary organs? While a role for invadopodia has been demonstrated in the case of stromal invasion and intravasation (Eckert et al., 2011; Roh-Johnson et al., 2013), it is yet to be determined if invadopodia are required for later stages of the metastatic cascade. Answers to these questions will add to our growing understanding of invadopodium function and allow us to better evaluate how specific invadopodial proteins may be targeted for clinical applications.
Box 1. Rho GTPases in invadopodia.
Rho GTPases are important regulators of actin dynamics at invadopodia. Cdc42 is one of the few proteins that is essential for initial invadopodium precursor assembly (see main text for further details; Desmarais et al., 2009; Sakurai-Yageta et al., 2008). RhoA, on the other hand, is dispensable for invadopodium precursor formation, but drives invadopodium maturation. Using a RhoA biosensor, we demonstrated that RhoA activity is low in the core of invadopodium precursors (Bravo-Cordero et al., 2011). Rather, RhoA appears to be specifically important for invadopodium maturation, as RhoA knockdown impairs ECM degradation, but only has a modest effect on the total number of invadopodia/cell (e.g. precursors and mature invadopodia; Bravo-Cordero et al., 2011). Consistent with this finding, RhoA cooperates with Cdc42 to stimulate exocyst-mediated MT1-MMP delivery to invadopodia (Sakurai-Yageta et al., 2008), and heterotypic cell contact between tumor cells and macrophages induces RhoA activation, leading to increased numbers of mature invadopodia (Roh-Johnson et al., 2013).
RhoC is another Rho GTPase isoform that regulates invadopodium function. A role for RhoC in invasion was first described by the Hynes group (Clark et al., 2000). Their work demonstrated that RhoC is necessary for extravasation in melanoma cells. In mammary adenocarcinoma cells, RhoC confines cofilin-dependent actin polymerization to the invadopodium core for efficient protrusion formation and focused matrix degradation (see main text for further details; Bravo-Cordero et al., 2011). RhoC depletion leads to the formation of shorter invadopodium protrusions associated with larger areas of shallow matrix degradation, leading to inefficient tumor cell invasion (Bravo-Cordero et al., 2011). Thus, similar to FAK knockdown, RhoC depletion results in increased matrix degradation, but impaired invasion, highlighting the importance of coordination between the actin machinery and matrix degradation during tumor cell invasion.
Although Rac1 has been shown to promote matrix degradation in melanoma and glioma cells (Chuang et al., 2004; Nakahara et al., 2003), Rac1 knockdown dramatically increases invadopodium matrix degradation in breast cancer cells, without affecting invadopodium precursor formation (Moshfegh et al., 2014). Using a Rac1 biosensor, we were able to show that Rac1 is inactive in invadopodium precursors, but is transiently activated immediately prior to invadopodium disassembly (Moshfegh et al., 2014). Localized stimulation of Rac1 by photoactivatable Rac induces rapid invadopodium disassembly, suggesting that the primary role of Rac1 in breast cancer cells is to regulate invadopodium disassembly, not formation or maturation (Moshfegh et al., 2014).
ACKNOWLEDGEMENTS
We thank Jose-Javier Bravo-Cordero, Jeanine Pignatelli and Ved Sharma for their thoughtful discussion. Research in the Condeelis lab has been funded by NIH CA150344 (J.C.) and the MSTP training grant T32-GM007288 (B.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Arsenault D, Brochu-Gaudreau K, Charbonneau M, Dubois CM. HDAC6 deacetylase activity is required for hypoxia-induced invadopodia formation and cell invasion. Plos One. 2013;8:e55529. doi: 10.1371/journal.pone.0055529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic Interactions of Cortactin and Membrane Type 1 Matrix Metalloproteinase at Invadopodia: Defining the Stages of Invadopodia Formation and Function. Cancer Res. 2006;66:3034–3043. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- Askari JA, Tynan CJ, Webb SED, Martin-Fernandez ML, Ballestrem C, Humphries MJ. Focal adhesions are sites of integrin extension. J Cell Biol. 2010;188:891–903. doi: 10.1083/jcb.200907174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala I, Giacchetti G, Caldieri G, Attanasio F, Mariggio S, Tete S, Polishchuk R, Castronovo V, Buccione R. Faciogenital Dysplasia Protein Fgd1 Regulates Invadopodia Biogenesis and Extracellular Matrix Degradation and Is Up-regulated in Prostate and Breast Cancer. Cancer Res. 2009;69:747–752. doi: 10.1158/0008-5472.CAN-08-1980. [DOI] [PubMed] [Google Scholar]
- Beaty BT, Sharma VP, Bravo-Cordero JJ, Simpson MA, Eddy RJ, Koleske AJ, Condeelis J. β1 integrin regulates Arg to promote invadopodial maturation and matrix degradation. Mol Biol Cell. 2013;24:1661–1675. doi: 10.1091/mbc.E12-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaty BT, Wang Y, Bravo-Cordero JJ, Sharma VP, Miskolci V, Hodgson L, Condeelis J. Talin regulates moesin-NHE-1 recruitment to invadopodia and promotes mammary tumor metastasis. J Cell Biol. 2014;205:737–751. doi: 10.1083/jcb.201312046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block M, Badowski C, Millonfremillon A, Bouvard D, Bouin A, Faurobert E, Gerberscokaert D, Planus E, Albigesrizo C. Podosome-type adhesions and focal adhesions, so alike yet so different. European journal of cell biology. 2008;87:491–506. doi: 10.1016/j.ejcb.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin Signaling through Arg Activates p190RhoGAP by Promoting Its Binding to p120RasGAP and Recruitment to the Membrane. Mol Biol Cell. 2006;17:4827–4836. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci. 2009;122:3441–3454. doi: 10.1242/jcs.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch KM, Hoshino D, Weaver AM. Adhesion rings surround invadopodia and promote maturation. Biology Open. 2012;1:1–12. doi: 10.1242/bio.20121867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Hodgson L, Condeelis J. Directed cell invasion and migration during metastasis. Current opinion in cell biology. 2012;24:277–283. doi: 10.1016/j.ceb.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Magalhaes MAO, Eddy RJ, Hodgson L, Condeelis J. Functions of cofilin in cell locomotion and invasion. Nat Rev Mol Cell Bio. 2013;14:405–415. doi: 10.1038/nrm3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Marrero-Diaz R, Megias D, Genis L, Garcia-Grande A, Garcia MA, Arroyo AG, Montoya MC. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. Embo J. 2007;26:1499–1510. doi: 10.1038/sj.emboj.7601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Cordero JJ, Oser M, Chen X, Eddy R, Hodgson L, Condeelis J. A Novel Spatiotemporal RhoC Activation Pathway Locally Regulates Cofilin Activity at Invadopodia. Curr Biol. 2011;21:635–644. doi: 10.1016/j.cub.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson L, Driffort V, Benoist L, Poet M, Counillon L, Antelmi E, Rubino R, Besson P, Labbal F, Chevalier S, Reshkin SJ, Gore J, Roger S. Na(V)1.5 Na+ channels allosterically regulate the NHE-1 exchanger and promote the activity of breast cancer cell invadopodia. J Cell Sci. 2013;126:4835–4842. doi: 10.1242/jcs.123901. [DOI] [PubMed] [Google Scholar]
- Burgstaller G, Gimona M. Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci. 2004;117:223–231. doi: 10.1242/jcs.00839. [DOI] [PubMed] [Google Scholar]
- Busco G, Cardone RA, Greco MR, Bellizzi A, Colella M, Antelmi E, Mancini MT, Dell'Aquila ME, Casavola V, Paradiso A, Reshkin SJ. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. Faseb J. 2010;24:3903–3915. doi: 10.1096/fj.09-149518. [DOI] [PubMed] [Google Scholar]
- Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–370. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed-cells. Journal of Experimental Zoology. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- Chuang YY, Tran NL, Rusk N, Nakada M, Berens ME, Symons M. Role of synaptojanin 2 in glioma cell migration and invasion. Cancer Res. 2004;64:8271–8275. doi: 10.1158/0008-5472.CAN-04-2097. [DOI] [PubMed] [Google Scholar]
- Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000;406:532–535. doi: 10.1038/35020106. [DOI] [PubMed] [Google Scholar]
- Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–4235. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- Coopman PJ, Thomas DM, Gehlsen KR, Mueller SC. Integrin alpha 3 beta 1 participates in the phagocytosis of extracellular matrix molecules by human breast cancer cells. Mol Biol Cell. 1996;7:1789–1804. doi: 10.1091/mbc.7.11.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortesio CL, Chan KT, Perrin BJ, Burton NO, Zhang S, Zhang ZY, Huttenlocher A. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–971. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H+ translocation. Mol Cell. 2000;6:1425–1436. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- DesMarais V, Macaluso F, Condeelis J, Bailly M. Synergistic interaction between the Arp2/3 complex and cofilin drives stimulated lamellipod extension. J Cell Sci. 2004;117:3499–3510. doi: 10.1242/jcs.01211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarais V, Yamaguchi H, Oser M, Soon L, Mouneimne G, Sarmiento C, Eddy R, Condeelis J. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009;66:303–316. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Destaing O, Block MR, Planus E, Albiges-Rizo C. Invadosome regulation by adhesion signaling. Current opinion in cell biology. 2011;23:597–606. doi: 10.1016/j.ceb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Destaing O, Planus E, Bouvard D, Oddou C, Badowski C, Bossy V, Raducanu A, Fourcade B, Albiges-Rizo C, Block MR. Beta1A Integrin Is a Master Regulator of Invadosome Organization and Function. Mol Biol Cell. 2010;21:4108–4119. doi: 10.1091/mbc.E10-07-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz B, Yuen A, Iizuka S, Higashiyama S, Courtneidge SA. Notch increases the shedding of HB-EGF by ADAM12 to potentiate invadopodia formation in hypoxia. J Cell Biol. 2013;201:279–292. doi: 10.1083/jcb.201209151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J. Twist1-Induced Invadopodia Formation Promotes Tumor Metastasis. Cancer Cell. 2011;19:372–386. doi: 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Baird D, Yoo S, Antonyak M, Cerione RA. Phosphorylation of the cool-1/beta-Pix protein serves as a regulatory signal for the migration and invasive activity of Src-transformed cells. J Biol Chem. 2010;285:18806–18816. doi: 10.1074/jbc.M109.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger AL, Lam SCT, Plow EF, Smith MA, Loftus JC, Ginsberg MH. Occupancy of an Adhesive Glycoprotein Receptor Modulates Expression of an Antigenic Site Involved in Cell-Adhesion. J Biol Chem. 1988;263:12397–12402. [PubMed] [Google Scholar]
- Galvez BG, Matias-Roman S, Yanez-Mo M, Sanchez-Madrid F, Arroyo AG. ECM regulates MT1-MMP localization with beta 1 or alpha v beta 3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Current opinion in cell biology. 2008;20:235–241. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- Hu JH, Mukhopadhyay A, Truesdell P, Chander H, Mukhopadhyay UK, Mak AS, Craig AWB. Cdc42-interacting protein 4 is a Src substrate that regulates invadopodia and invasiveness of breast tumors by promoting MT1-MMP endocytosis. J Cell Sci. 2011;124:1739–1751. doi: 10.1242/jcs.078014. [DOI] [PubMed] [Google Scholar]
- Jacob A, Jing J, Lee J, Schedin P, Gilbert SM, Peden AA, Junutula JR, Prekeris R. Rab40b regulates trafficking of MMP2 and MMP9 during invadopodia formation and invasion of breast cancer cells. J Cell Sci. 2013;126:4647–4658. doi: 10.1242/jcs.126573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T, Mueller SC, Yeh Y, Chen WT. Invadopodia promote proteolysis of a wide variety of extracellular matrix proteins. Journal of cellular physiology. 1994;158:299–308. doi: 10.1002/jcp.1041580212. [DOI] [PubMed] [Google Scholar]
- Kerrisk ME, Greer CA, Koleske AJ. Integrin alpha3 is required for late postnatal stability of dendrite arbors, dendritic spines and synapses, and mouse behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:6742–6752. doi: 10.1523/JNEUROSCI.0528-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Takahashi K. WAVE2-and microtubule-dependent formation of long protrusions and invasion of cancer cells cultured on three-dimensional extracellular matrices. Cancer Science. 2008;99:2252–2259. doi: 10.1111/j.1349-7006.2008.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu XZ, Konig I, Anderson K, Machesky LM. The Actin-Bundling Protein Fascin Stabilizes Actin in Invadopodia and Potentiates Protrusive Invasion. Curr Biol. 2010;20:339–345. doi: 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Nelson D, Weiss M, Aepfelbacher M. Wiskott-Aldrich syndrome protein regulates podosomes in primary human macrophages. Proc Natl Acad Sci U S A. 1999;96:9648–9653. doi: 10.1073/pnas.96.17.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder S, Wiesner C, Himmel M. Degrading Devices: Invadosomes in Proteolytic Cell Invasion. Annual review of cell and developmental biology. 2011;27:185–211. doi: 10.1146/annurev-cellbio-092910-154216. [DOI] [PubMed] [Google Scholar]
- Lizarraga F, Poincloux R, Romao M, Montagnac G, Le Dez G, Bonne I, Rigaill G, Raposo G, Chavrier P. Diaphanous-Related Formins Are Required for Invadopodia Formation and Invasion of Breast Tumor Cells. Cancer Res. 2009;69:2792–2800. doi: 10.1158/0008-5472.CAN-08-3709. [DOI] [PubMed] [Google Scholar]
- Lo YYC, Luo L, McCulloch CAG, Cruz TF. Requirements of Focal Adhesions and Calcium Fluxes for Interleukin-1-induced ERK Kinase Activation and c-fos Expression in Fibroblasts. J Biol Chem. 1998;273:7059–7065. doi: 10.1074/jbc.273.12.7059. [DOI] [PubMed] [Google Scholar]
- Mader CC, Oser M, Magalhaes MAO, Bravo-Cordero JJ, Condeelis J, Koleske AJ, Gil-Henn H. An EGFR-Src-Arg-Cortactin Pathway Mediates Functional Maturation of Invadopodia and Breast Cancer Cell Invasion. Cancer Res. 2011;71:1730–1741. doi: 10.1158/0008-5472.CAN-10-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes MAO, Larson DR, Mader CC, Bravo-Cordero JJ, Gil-Henn H, Oser M, Chen X, Koleske AJ, Condeelis J. Cortactin phosphorylation regulates cell invasion through a pH-dependent pathway. The Journal of Cell Biology. 2011;195:903–920. doi: 10.1083/jcb.201103045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero-Diaz R, Bravo-Cordero JJ, Megias D, Garcia MA, Bartolome RA, Teixido J, Montoya MC. Polarized MT1-MMP-CD44 Interaction and CD44 Cleavage During Cell Retraction Reveal an Essential Role for MT1-MMP in CD44-Mediated Invasion. Cell Motil Cytoskel. 2009;66:48–61. doi: 10.1002/cm.20325. [DOI] [PubMed] [Google Scholar]
- McLaughlin SL, Ice RJ, Rajulapati A, Kozyulina PY, Livengood RH, Kozyreva VK, Loskutov YV, Culp MV, Weed SA, Ivanov AV, Pugacheva EN. NEDD9 Depletion Leads to MMP14 Inactivation by TIMP2 and Prevents Invasion and Metastasis. Molecular cancer research : MCR. 2014;12:69–81. doi: 10.1158/1541-7786.MCR-13-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md Hashim NF, Nicholas NS, Dart AE, Kiriakidis S, Paleolog E, Wells CM. Hypoxia-induced invadopodia formation: a role for beta-PIX. Open biology. 2013;3:120159. doi: 10.1098/rsob.120159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Itoh H. Src Kinase Regulates the Activation of a Novel FGD-1-related Cdc42 Guanine Nucleotide Exchange Factor in the Signaling Pathway from the Endothelin A Receptor to JNK. J Biol Chem. 2003;278:29890–29900. doi: 10.1074/jbc.M301559200. [DOI] [PubMed] [Google Scholar]
- Monteiro P, Rosse C, Castro-Castro A, Irondelle M, Lagoutte E, Paul-Gilloteaux P, Desnos C, Formstecher E, Darchen F, Perrais D, Gautreau A, Hertzog M, Chavrier P. Endosomal WASH and exocyst complexes control exocytosis of MT1-MMP at invadopodia. J Cell Biol. 2013;203:1063–1079. doi: 10.1083/jcb.201306162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau V, Tatin F, Varon C, Genot E. Actin can reorganize into podosomes in aortic endothelial cells, a process controlled by Cdc42 and RhoA. Molecular and cellular biology. 2003;23:6809–6822. doi: 10.1128/MCB.23.19.6809-6822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshfegh Y, Bravo-Cordero JJ, Miskolci V, Condeelis J, Hodgson L. A Trio-Rac1-Pak1 signalling axis drives invadopodia disassembly. Nat Cell Biol. 2014;16:574–586. doi: 10.1038/ncb2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, Humphries MJ. Regulation of integrin function through conformational complexity: not simply a knee-jerk reaction? Current opinion in cell biology. 2004;16:544–551. doi: 10.1016/j.ceb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Ghersi G, Akiyama SK, Sang QXA, Howard L, Pineiro-Sanchez M, Nakahara H, Yeh Y, Chen WT. A novel protease-docking function of integrin at invadopodia. J Biol Chem. 1999;274:24947–24952. doi: 10.1074/jbc.274.35.24947. [DOI] [PubMed] [Google Scholar]
- Murphy DA, Courtneidge SA. The ‘ins’ and ‘outs’ of podosomes and invadopodia: characteristics, formation and function. Nat Rev Mol Cell Bio. 2011;12:413–426. doi: 10.1038/nrm3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara H, Otani T, Sasaki T, Miura Y, Takai Y, Kogo M. Involvement of Cdc42 and Rac small G proteins in invadopodia formation of RPMI7951 cells. Genes to cells : devoted to molecular & cellular mechanisms. 2003;8:1019–1027. doi: 10.1111/j.1365-2443.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Neel NF, Rossman KL, Martin TD, Hayes TK, Yeh JJ, Der CJ. The RalB small GTPase mediates formation of invadopodia through a GTPase-activating protein-independent function of the RalBP1/RLIP76 effector. Molecular and cellular biology. 2012;32:1374–1386. doi: 10.1128/MCB.06291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida N, Xie C, Shimaoka M, Cheng YF, Walz T, Springer TA. Activation of leukocyte beta(2) integrins by conversion from bent to extended conformations. Immunity. 2006;25:583–594. doi: 10.1016/j.immuni.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Oser M, Mader CC, Gil-Henn H, Magalhaes M, Bravo-Cordero JJ, Koleske AJ, Condeelis J. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. J Cell Sci. 2010;123:3662–3673. doi: 10.1242/jcs.068163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser M, Yamaguchi H, Mader CC, Bravo-Cordero JJ, Arias M, Chen X, DesMarais V, van Rheenen J, Koleske AJ, Condeelis J. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. The Journal of Cell Biology. 2009;186:571–587. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A, Ruppender NS, Branch KM, Sewell-Loftin MK, Lin J, Boyer PD, Candiello JE, Merryman WD, Guelcher SA, Weaver AM. Sensing and modulation of invadopodia across a wide range of rigidities. Biophys J. 2011;100:573–582. doi: 10.1016/j.bpj.2010.12.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli J, Jones MC, LaLonde DP, Turner CE. Beta2-Adaptin Binds Actopaxin and Regulates Cell Spreading, Migration and Matrix Degradation. Plos One. 2012a:7. doi: 10.1371/journal.pone.0046228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli J, Tumbarello DA, Schmidt RP, Turner CE. Hic-5 promotes invadopodia formation and invasion during TGF-beta-induced epithelial-mesenchymal transition. The Journal of Cell Biology. 2012b;197:421–437. doi: 10.1083/jcb.201108143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadurai CV, Havrylov S, Zaoui K, Vaillancourt R, Stuible M, Naujokas M, Zuo D, Tremblay ML, Park M. Met receptor tyrosine kinase signals through a cortactin–Gab1 scaffold complex, to mediate invadopodia. J Cell Sci. 2012;125:2940–2953. doi: 10.1242/jcs.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razidlo GL, Schroeder B, Chen J, Billadeau DD, McNiven MA. Vav1 as a central regulator of invadopodia assembly. Curr Biol. 2014;24:86–93. doi: 10.1016/j.cub.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh-Johnson M, Bravo-Cordero JJ, Patsialou A, Sharma VP, Guo P, Liu H, Hodgson L, Condeelis J. Macrophage contact induces RhoA GTPase signaling to trigger tumor cell intravasation. Oncogene. 2013 doi: 10.1038/onc.2013.377. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature reviews. Molecular cell biology. 2005;6:167–180. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- Sakurai-Yageta M, Recchi C, Le Dez G, Sibarita JB, Daviet L, Camonis J, D'Souza-Schorey C, Chavrier P. The interaction of IQGAP1 with the exocyst complex is required for tumor cell invasion downstream of Cdc42 and RhoA. The Journal of Cell Biology. 2008;181:985–998. doi: 10.1083/jcb.200709076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoumacher M, Goldman RD, Louvard D, Vignjevic DM. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J Cell Biol. 2010;189:541–556. doi: 10.1083/jcb.200909113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Ved P., Eddy R, Entenberg D, Kai M, Gertler Frank B., Condeelis J. Tks5 and SHIP2 Regulate Invadopodium Maturation, but Not Initiation, in Breast Carcinoma Cells. Curr Biol. 2013;23:2079–2089. doi: 10.1016/j.cub.2013.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnes TW, Bennin DA, Bing X, Eickhoff JC, Grahf DC, Bellak JM, Seroogy CM, Ferguson PJ, Huttenlocher A. The F-BAR protein PSTPIP1 controls extracellular matrix degradation and filopodia formation in macrophages. Blood. 2014;123:2703–2714. doi: 10.1182/blood-2013-07-516948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen A, Le Dez G, Poincloux R, Recchi C, Nassoy P, Rottner K, Galli T, Chavrier P. MT1-MMP-dependent invasion is regulated by TI-VAMP/VAMP7. Curr Biol. 2008;18:926–931. doi: 10.1016/j.cub.2008.05.044. [DOI] [PubMed] [Google Scholar]
- Takkunen M, Hukkanen M, Liljestrom M, Grenman R, Virtanen I. Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia. Journal of cellular and molecular medicine. 2010;14:1569–1593. doi: 10.1111/j.1582-4934.2009.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis KQ, Veach D, Duewel HS, Bornmann WG, Koleske AJ. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Molecular and cellular biology. 2003;23:3884–3896. doi: 10.1128/MCB.23.11.3884-3896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima J, Toshima JY, Amano T, Yang N, Narumiya S, Mizuno K. Cofilin phosphorylation by protein kinase testicular protein kinase 1 and its role in integrin-mediated actin reorganization and focal adhesion formation. Mol Biol Cell. 2001;12:1131–1145. doi: 10.1091/mbc.12.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, McNiven MA. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA, Reichardt LF, Greer CA, Taylor JR, Koleske AJ. Integrin Beta1 Signals through Arg to Regulate Postnatal Dendritic Arborization, Synapse Density, and Behavior. Journal of Neuroscience. 2012;32:2824–2834. doi: 10.1523/JNEUROSCI.3942-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong JP, Stehle T, Diefenbach B, Zhang RG, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Crystal structure of the extracellular segment of integrin alpha V beta 3. Science. 2001;294:339–345. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Lorenz M, Kempiak S, Sarmiento C, Coniglio S, Symons M, Segall J, Eddy R, Miki H, Takenawa T, Condeelis J. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. The Journal of Cell Biology. 2005;168:441–452. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Yoshida S, Muroi E, Yoshida N, Kawamura M, Kouchi Z, Nakamura Y, Sakai R, Fukami K. Phosphoinositide 3-kinase signaling pathway mediated by p110 alpha regulates invadopodia formation. J Cell Biol. 2011;193:1275–1288. doi: 10.1083/jcb.201009126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yu HG, Nam JO, Miller NL, Tanjoni I, Walsh C, Shi L, Kim L, Chen XL, Tomar A, Lim ST, Schlaepfer DD. p190RhoGEF (Rgnef) promotes colon carcinoma tumor progression via interaction with focal adhesion kinase. Cancer Res. 2011;71:360–370. doi: 10.1158/0008-5472.CAN-10-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZN, Sharma VP, Beaty BT, Roh-Johnson M, Peterson EA, Van Rooijen N, Kenny PA, Wiley HS, Condeelis JS, Segall JE. Autocrine HBEGF expression promotes breast cancer intravasation, metastasis and macrophage-independent invasion in vivo. Oncogene. 2013 doi: 10.1038/onc.2013.363. [DOI] [PMC free article] [PubMed] [Google Scholar]