Abstract
The insulin-like growth factor 2 (IGF2) is an important target for cancer therapy. We have previously proposed an approach for fast and irreversible removal of IGF2 from the circulation by using monoclonal antibodies (mAbs) that bind to two or more non-overlapping epitopes on the same molecule. We provided initial evidence for the formation of oligomeric antibody-ligand complexes that can bind to cells expressing Fc gamma receptors (FcγRs) with high avidity using an antibody domain with relatively low affinity as one of the anti-IGF2 mAbs. Recently, we identified a mAb, m708.5, in a scFv format which binds to both IGF2 and IGF1 with very high (pM) affinity. Interestingly, and rather surprisingly, this mAb did not compete with our other high affinity mAb, m610.27, for binding to IGF2. Therefore, we generated a new bispecific mAb, m67, by combining m708.5 and m610.27. As expected m67 potently inhibited binding of IGF2 to cells expressing the IGF1R and its phosphorylation, and resulted in formation of multimolecular complexes when incubated with IGF2 and bound with high avidity to cells expressing FcγRII; the complexes were internalized in a macrophage-like cell line. However, although m67 exhibited a reasonably long half-life (6.4±0.6 days) in cynomolgus macaques and high stability in serum, its administration to three animals did not result in any measurable decrease in the IGF2 concentration likely due to the complexity of the IGF2 interactions in the blood and the relatively low (2 mg/kg) dose of the mAb leading to a relatively low maximal blood concentration of 120 nM. In spite of the lack of effect on the IGF2 concentration in this particular experimental setup, m67 exhibited good drugability properties and could be highly effective in other animal models and in humans. Studies with animal models of cancer are ongoing to evaluate the potential of m67 as a new candidate mAb-based therapeutic.
Keywords: IGF ligand, bispecific antibodies, half-life, cynomolgus macaques
Background
The IGF signaling pathway has been implicated in the growth and metastasis of many tumor types (Lasota et al., 2013; Miettinen et al., 2013). The pathway has multiple ligands (insulin, insulin-like growth factor 1 and 2 or IGF1 & 2), and several known receptors, IGF-1R, insulin receptor and hybrid receptor. The low molecular weight ligands, IGF1 and IGF2, are mainly in tri-molecular complexes with IGF binding proteins (BPs) and the acid labile subunit (ALS) (Corvaia et al., 2013). Only small percentage of IGFs exists in free form, and only the free form can bind to the receptors. In the past decade more than 10 therapeutic monoclonal antibodies (mAbs) targeting IGF-1R have been tested in early clinical trials in varieties of cancers (Corvaia et al., 2013; Feng and Dimitrov, 2008b; Pollak, 2008; Rajan et al., 2014). A common issue found in these studies is that inhibiting IGF-1R alone is not sufficient to abolish the signaling from IGFs, the redundant receptors could still transduce signals and bypass the antibody inhibition. Severe adverse effects caused by IGF-1R antibodies also have curbed the enthusiasm for the target (Langer et al., 2014; Robertson et al., 2013). Because many tumors, especially many childhood tumors such as neuroblastoma, Ewing sarcoma, rhabdomyosarcoma and osteosarcoma, often express elevated levels of IGF2 and the tumor growth is driven by the over-expressed IGF2 (Bid et al., 2012; Visser et al., 1997), we have been developed antibodies against these ligands. Unlike IGF1 homeostasis, which is regulated by an elaborate feedback control to the growth hormone (GH) and pituitary gland, IGF2 does not have such feedback control in mice or humans.
We have identified and characterized several fully human mAbs against IGF2, m610.27 (Feng et al., 2006; Kimura et al., 2010) and mAb cross-reactive to IGF1 & 2, m708.5 (Zhao et al., 2011). Due to the stable nature of antibody molecules, IGF2/antibody complexes will also have an extended half-life. Over a long term treatment with such anti-ligand antibody, the complexed ligand amount will increase, even though the ligand is isolated from its receptor. Indeed, a study have shown that the anti-IGF1 & 2 antibody BI836845 treatment in mice leads to increase in total IGF1 (IGF2 was not reported in that study) (Mireuta et al., 2014). In the case of VEGF, treatment of patients with Bevacizumab (Avastin), causes an increase in the plasma VEGF, although there is clinical benefits with the treatment (Yang et al., 2003).
We have hypothesized that bi-specific antibodies composed of antibodies binding to different epitopes on IGF2 allows the formation of complexes between IGF2 and antibodies. When multiple antibody molecules bind to a protein, they promote the engagement of low affinity Fc gamma receptors, which normally do not bind to soluble IgGs. Engagement of Fc gamma receptors on macrophages activate the phagocytosis process and lead to the degradation of IGF2 (Chen et al., 2012). While a mono-specific IGF2 antibody can prevent binding of IGF2 to its receptors, the bi-specific antibody provides the possibility to metabolize IGF2 and remove it from the circulation. Due to the long half life of antibody molecules, a mono-specific Ab/IGF2 complex will also stay in plasma for a long time and may serve as a reservoir, which still has the possibility of binding to its receptor when it dissociates from the complex during the dynamic equilibrium. Although the theory is new in the growth factor field, studies in botulinum toxin have indicated that administering multiple antibodies against different epitopes on the toxin has far better protections than any of the single antibodies (Nowakowski et al., 2002).
Here we describe a new bispecific antibody designated m67. The two arms of m67 are m610.27 (anti-mature and long IGF2) (Feng et al., 2006) and m708.5 (cross-reactive to IGF1/2) (Zhao et al., 2011). m67 was capable of forming multi-molecular complexes with IGF2, and these complexes triggered endocytosis by macrophages, which is an important step to clear IGF2 from the circulation. We also found that the antibody reached ~120 nM maximal concentration in plasma and had a half-life of approximate 6.4 days. However, it did not change IGF2 levels during the study.
Materials and methods
Cells
MCF-7 from ATCC were cultured in DMEM+10% FBS and 100 ug/ml penicillin, 100 ug/ml streptomycin. BJAB and U937 cells were cultured in RPMI+10% FBS and 100 ug/ml penicillin, 100 ug/ml streptomycin. U937 cells were treated with 10 ng/ml phorbol myristic acid (PMA, dissolved in DMSO) for 24 hours for differentiation into macrophage-like cells. 293 FreeStyle cells were purchased from Invitrogen and cultured as in the Invitrogen manual.
Construction of bispecific antibody
Primers used in the study were listed in Table 1. To generate the bispecific antibody m67, the heavy chain leader peptide gene fragment (Hleader) and scFv m610.27 were PCR-amplified with primer pairs H1/H2 and H3/H4, respectively. The product was digested with XbaI and SacI, and cloned into vector pDR12. To fuse scFv m708.5 to the N terminus of the human IgG1 kappa light chain constant region, the light chain leader peptide (Lleader), scFv m708.5, and the human IgG1 kappa light chain constant region (CK) were amplified by PCR with primer pairs L1/L3, L2/L4, and L5/L6, respectively. The Lleader-scFv m708.5-CK fragment was then digested with EcoRI and HindIII, and cloned into the pDR12 (courtesy of Dr. Burton, The Scripps Research Institute) already containing scFv m610.27.
Table 1.
Primers used for construction of m67.
| Names | Sequences |
|---|---|
| H1 | 5′-GTGTTCTAGAGCCGCCACCATGGAATGGAGCTGGGTCTTTCTCTTC-3′ |
| H2 | 5′-ACTACAGGTGTCCACTCCCAAGTGCAGCTGGTGCAG-3′ |
| H3 | 5′-GGAGTGGACACCTGTAGTTACTGACAGGAAGAAGAGAAAGAC-3′ |
| H4 | 5′CCTTGGAGCTCGATCCGCCACCGCCAGAGCCACCTCCGCCTGAACCGCCTCCACCTCGTTTGATCTCCACC-3′ |
| L1 | 5′-GTGTAAGCTTACCATGGGTGTGCC-3′ |
| L2 | 5′-GGGTGGCTGAGTCAGCACGGACTGACATCTGGCATCTGTAAGCC-3′ |
| L3 | 5′-GGCTTACAGATGCCAGATGTCAGGTACAGCTGCAGCAAC-3′ |
| L4 | 5′-GACCCACCGCCCCCACTGCCGCCTCCACCTTGTTTAATCTCCAGTC-3′ |
| L5 | 5′-AGTGGGGGCGGTGGGTCCGGGGGTGGAGGCTCGACTGTGGCTGCACCATCTGTC-3′ |
| L6 | 5′-GTG TGAATTCTCTAGAATTAACACTCTCCCCTGTTGAAGCT-3′ |
Expression and purification of m67
The antibody m67was expressed in 293 FreeStyle cells. CellFectin (Invitrogen) was used to transfect 293 FreeStyle cells according to the manufacturer’s instruction. Four days post-transfection, the culture supernatant was harvested, and m67 was purified on a protein G column. The final preparation of m67 was buffer exchanged to PBS
Size exclusion chromatography
A Superdex 200 10/300 GL column (GE Healthcare, Piscataway, NJ) was calibrated with protein molecular mass standards of 14 kDa (ribonuclease A), 25 kDa (chymotrypsin), 44 kDa (ovalbumin), 67 kDa (albumin), 158 kDa (aldolase), 232 kDa (catalase), 440 kDa (ferritin), and 669 kDa (thyroglobulin). Proteins in PBS (pH7.4) were loaded onto the pre-equilibrated column and eluted with PBS at a flow rate of 0.5 ml/min.
ELISA
For competition ELISA, IgG1 m610.27 (200 ng/well) was coated on 96-well ELISA plates overnight at 4°C. IGF2 (200 ng) was captured by coated IgG1 m610.27 for 1 h. VHm630.3 or scFv m708.5 with different dilutions was incubated with captured antigens for 1 h. The bound antibodies were detected with anti-Flag-HRP secondary antibody. For stability analysis, antibodies were first incubated with equal volume of human sera for 1–9 days. Antibodies were then diluted into different concentrations and incubated with coated antigens for 1 h. Bound antibodies were detected with anti-human Fc-HRP mAb (1:2,000; Invitrogen). The ABTS substrate (Roche) was added and the reaction was read at 405 nm.
Flow cytometric analysis
To block the interactions of IGF1 and 2 with MCF-7 cells, 5 nM biotinylated IGF1 or 1 nM biotinylated IGF2 were mixed with or without antibodies in 200 μl PBS containing 0.1% BSA (PBSA). After 30 min incubation at room temperature, the mixtures were added to approximately 5×105 MCF-7 cells and incubated for 30 min on ice. After washing, cells were incubated with a 1:50 dilution of R-phycoerythrin conjugated streptavidin for 30 min on ice, then washed again and then subjected to FACS analysis. For detection of the expression of FcγRII, 5×105 BJAB in 200 μl PBSA were mixed at different ratios (v/v) with FITC-conjugated mouse anti-human CD32 (FcγRII) antibody, and incubated for 30 min on ice. The cells were washed twice with 200 μl PBSA and then used for FACS analysis. Antibodies binding to BJAB and U937 cells in the absence or presence of IGF2 were detected by FITC-conjugated goat F(ab′)2 anti-human IgG (Fc-specific) antibody at a 1:250 dilution (v/v).
Phosphorylation assays
MCF-7 cells were seeded in a six-well plate at 1×106 cells per well in the complete growth medium (DMEM with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin). After overnight culture, cells were rinsed with the serum-free DMEM and then starved in the serum-free DMEM for 5 h. The treatment medium was made by adding 1 nM IGF1 or 5 nM IGF2 and various concentrations of antibodies in serum-free DMEM. After pre-incubation for 30 min at room temperature, the treatment was added to cells. Thirty minutes after addition of ligands and antibodies, cells were chilled on ice, rinsed in cold PBS, and lysed in 1 mL of lysis buffer [50 mM HEPES (pH 7.4), 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 2 mM sodium vanadate, and protease inhibitors]. Lysates were kept on ice for 30 min, followed by centrifugation at 17,000×g for 30 min. The supernatant was used for immunoprecipitation: 20 μL of protein G Sepharose 4B and 3 μg of rabbit anti-IGF1R β IgG (C-20, Santa Cruz) at 4°C overnight. After extensive washings, the immunoprecipitates were run on a 4–12% NUPAGE, transferred to polyvinylidene difluoride membrane, and blotted with anti-phosphotyrosine mAb PY20 (Sigma) to detect the phosphorylated IGFIR. The membrane was stripped and reprobed with C-20 polyclonal antibody to detect the total IGFIR in the immunoprecipitates. A similar procedure was used to detect the phosphorylation of IR but immunoprecipitation and Western blots were done with rabbit anti-IR β pAb (C-19, Santa Cruz).
Treatment of cynomolgus macaques
Female cynomolgus monkeys (Macaca fascicularis) at age 7.5–12 years and body weight 2.7–5.0 kg (±0.5 kg) were selected for the study. Animals passing serum chemistry, hematology and coagulation screening and meeting facility health criteria were used for the study. Animals were housed in social groups of two or three in cages that comply with the Animal Welfare Act and Care and Use of Laboratory Animals (NRC 2011). Animals were fed twice daily and fresh drinking water was provided ad libitum to all animals. Enrichment toys and produce treats were routinely supplied. Animals were acclimated to the study room for 7 days prior to the initiation of dosing. m67 was injected at 2 mg/kg body weight via intravenous (IV) bolus injection followed by a 1-ml saline flush. Clinical observations were performed twice daily for each animal. Body weights of animals were monitored on day −2 and weekly after injection of m67. Plasma were collected at following times: day 0, 2 hr, 4 hr, 8 hr, 16 hr, daily from day 2 to 8 and on days 11, 15, 18, 22, 25 and 30. The study was performed in SNBL USA, Ltd. (Everett, WA) in compliance with Good Laboratory Practice (GLP) regulations. The study protocol was approved by SNBL USA Institutional Animal Care and Use Committee (IACUC).
Measurement of the m67 concentrations in plasma
Recombinant human IGF2 (from R and D Systems) was coated on ELISA plates at 50 ng/well in 50 ul PBS overnight. m67 standard curve was established by adding known amount of m67 to cynomolgus plasma collected from an unrelated animal, from 5 nM to 0.039 nM. The test plasma were diluted into 1:100 (for early samples) or 1:3 (late samples) to make sure the final reading falls in the linear range of the m67 standard curve. After the wells were blocked with 2% non-fat dry milk in PBS (MPBS), m67 serially diluted solutions and test plasma were added to wells in duplicates. The ELISA plates were incubated at 37°C for 2 hours, and washed with PBS+0.05% Tween 20 (PBST) for 4 times. To detect bound m67 on plate, a goat anti-human Fc antibody coupled with horse radish peroxidase (Jackson ImmunoResearch Lab) was used at 1:1000. After wash, the substrate ABTS was added and the color reaction was monitored at 405 nm wavelength. The concentration of tested plasma was calculated from the standard curve of m67. The averages from 3 monkeys were plotted on graph.
Measurement of total IGF2 concentrations in plasma
To extract total IGF2 from its binding protein and antibodies, the plasma was first diluted 1:3 in PBS to a total volume of 50 ul, and mixed with 200 ul of acidified ethanol (87.5% v/v of ethanol, 12.5% 2 N HCl). Most serum proteins and antibodies were precipitated by the acid and IGF2 stayed soluble under acidic condition (Daughaday et al., 1980). After incubation at room temperature for at least 30 minutes, the samples were centrifuged at 1870 g for 30 min. A total of 185 ul supernatant was recovered, and the pellet discarded. To the recovered supernatant 38 ul of 1 M Tris, pH 9.0 was added to neutralize the acid. The sample was centrifuged in a speed vac for 45 min at alcohol mode to remove ethanol. The sample is now ready for testing with the capture ELISA method. For the standard curve, IGF2 diluted in MPBS with concentrations ranging from 40 nM to 0.625 nM went through the similar acid-ethanol treatment and was used as the reference standard. For capture ELISA, 100 ng/well of goat anti-human IGF2 polyclonal antibody (R and D Systems) was coated overnight. The acid/ethanol treated plasma samples were diluted 1:4 and 1:8 with MPBS and added to wells in duplicates. After 2 hours incubation at 37°C, human monoclonal antibody m610.27 was used to detect the bound IGF2. The binding of m610.27 was detected with goat anti-human Fc antibody conjugated with HRP.
Results
Design, generation, binding and stability of m67
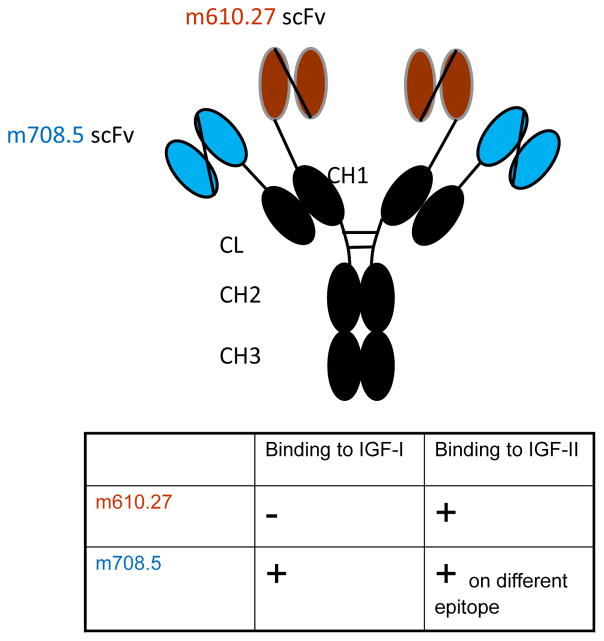
m67 was constructed by inserting m708.5 in scFv format to the N terminal of the kappa constant domain, and inserting m610.26 scFv to the N terminal of the CH1 domain. m708.5 is a cross-reactive IGF1/2 human monoclonal antibody, and m610.27 is the improved version of m610, which recognizes an epitope on IGF2 that is different from the epitope of m708.5 (Figure 1). The competition ELISA data (Figure 2) show that that m610.27 and m708.5 bind to different epitopes on IGF2.
Figure 1. Diagram of IGF2-bispecific antibody m67.
The two arms of m67 are m610.27 (recognizing long and mature IGF2) and m708.5 (cross-reactive to IGF1/2), both are fully human monoclonal antibodies. The two antibodies were constructed in single chain format and placed in front of heavy chain CH1 and kappa light chain constant domain. The specificity and epitopes of the two antibodies are indicated.
Figure 2. Competition of m610 with m708.5 in binding to IGF2.
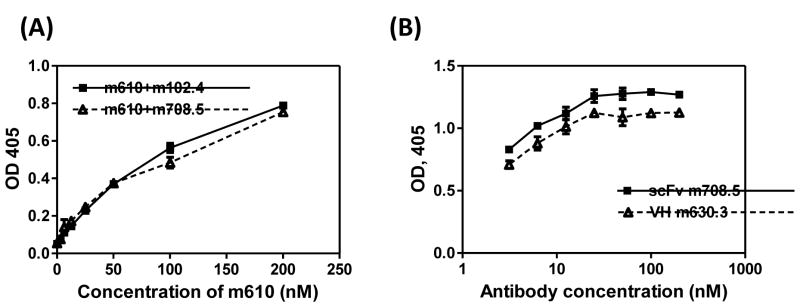
(A) IGF2 was directly coated on the ELISA plate. Bound scFv m610 was detected by HRP-conjugated anti-Flag antibody in the presence of fixed concentration of antibody competitors (IgG1 m708.5 or IgG1 m102.4. (B) IgG1 m610.27 was coated on the ELISA plate and IGF2 was captured by coated IgG1 m610.27. Bound scFv m708.5 or VH m630.3 was detected by HRP-conjugated anti-Flag tag antibody.
Some reported bispecific antibodies tend to be unstable for various reasons. m67 was incubated with human serum from healthy donor for up to nine days at 37°C. The binding ability of the incubated m67 was tested with ELISA. Our results indicated that incubation with serum for up to 9 days did not diminish the binding ability of m67 (Figure 3), implying it is stable under these conditions, and it is not degraded by proteases in serum.
Figure 3. Stability of m67 in human sera.
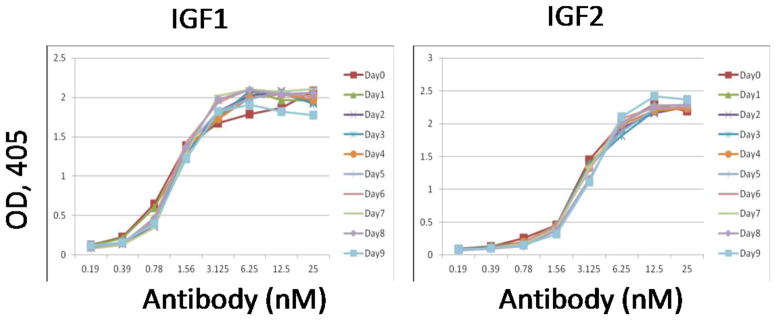
m67 was incubated with human sera at 37°C for 9 days and then tested for binding to IGF1 and IGF2 by ELISA.
m67 inhibited binding of IGFs to receptors and abolished the ligand-induced phosphorylation of receptors
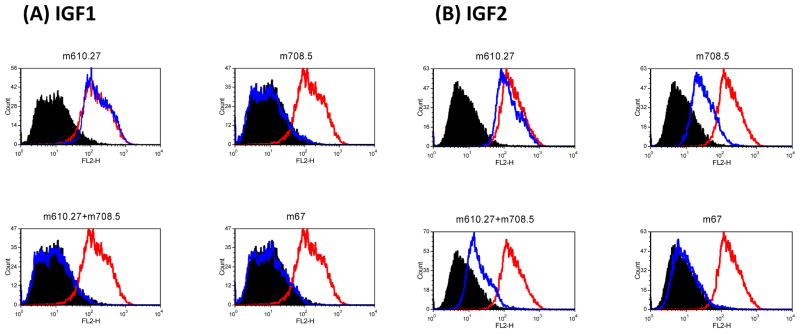
Because both its parental antibodies are inhibitory antibody to the binding to IGF-1R, m67 should have similar or higher inhibitory effect. This was tested by incubating individual antibody with MCF-7 cells and biotinylated ligand, IGF1 or 2. It was found that IGF1 binding to MCF-7 cells was greatly reduced when m67 was added. m67 also reduced biotinylated-IGF2 binding (Figure 4). The reduction by m67 on IGF1 and 2 binding to MCF-7 cells was greater than any of the single antibody at the equal concentration.
Figure 4. m67 inhibited bindings of IGF1 (A) and IGF2 (B) to MCF7 cells.
MCF-7 cells were incubated with 5 nM biotinylated IGF1 or 1 nM biotinylated IGF2 in the absence or presence of antibodies. Bound biotinylated IGF1 or 2 was detected by streptavidin-PE. Cells incubated with streptavidin-PE conjugate only as the background control are in black. Cells incubated with biotin-IGF1 or 2 are in red. Those for biotin-IGF1 or 2 with antibodies are in blue.
On the activity of receptors, the parental antibody of m67, m710.27, could inhibit the IGF2 induced-phosphorylation of insulin receptor (IR) and IGF-1R, and m708.5 could inhibit both IGF1 and 2 induced phosphorylation of the two receptors (Figure 5). m67 was compared with the single antibodies at similar concentrations for its inhibitory potency. It was found that m67 was at least as effective as each of the parental antibodies in inhibiting ligands-induced phosphorylation of the receptors.
Figure 5. Inhibition of IGF1R and IR phosphorylation.
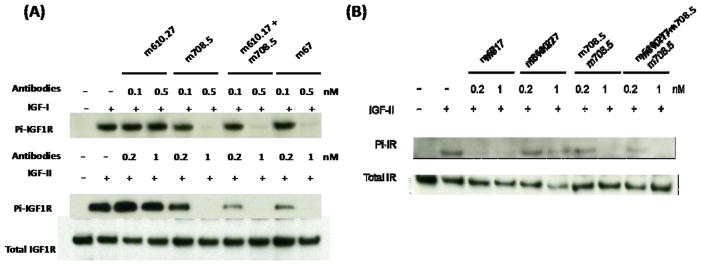
MCF-7 cells were starved in serum free medium for 5 h first, followed by addition of treatment medium with 1 nM IGF1 or 5 nM IGF2 with indicated concentrations of antibodies. Thirty minutes later cells were chilled and lysed. (A) IGF1R was immunoprecipitated and the phosphorylated IGF1R was detected with a phosphor-tyrosine specific antibody. The total amount of IGF1R were detected by the same polyclonal antibody used for the immunoprecipitation. (B) IR was immunoprecipitated and detected as following above methods.
m67 formed multi-molecular complex with IGF1 & 2
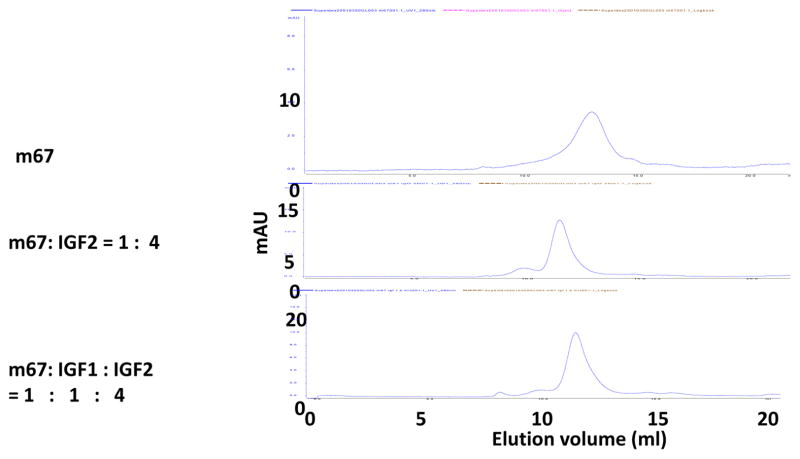
The bispecific antibody m67 has two binding sites of each parental antibody, with a total of four binding sites for IGF2. Therefore, they could form large complexes. We tested this possibility by performing size exclusion chromatography analysis of m67 and IGF2 mixture. Results indicated that in the presence of IGF2, they did form complexes larger than the m67 itself. IGF1 also helped form complexes but only when IGF2 was present (Figure 6). No free IGFs were detected at the ratio used in the study. Neither m610.27 nor m708.5 alone formed such large complex with IGF2 (data not shown).
Figure 6. Size-exclusion chromatography analysis of m67/IGFs complex.<.
br>m67, the mixture of m67 and IGF2, or the mixture of m67/IGF2 plus IGF1 was analyzed by Superdex 200 10/300G column.
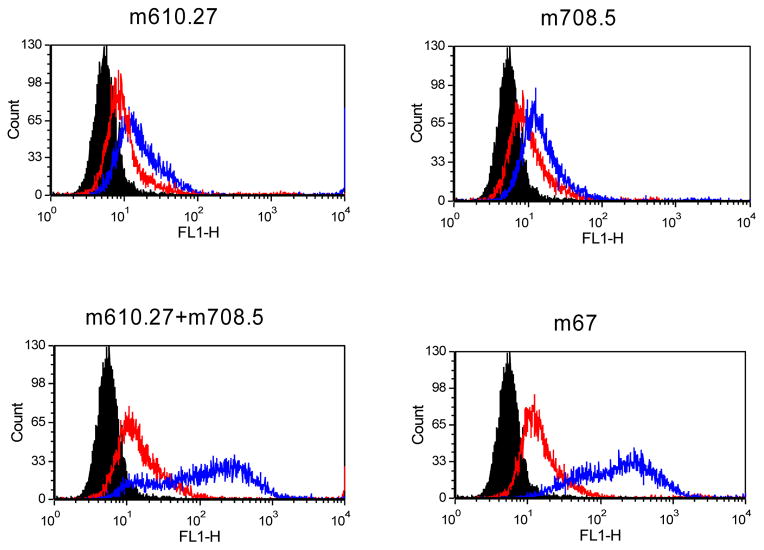
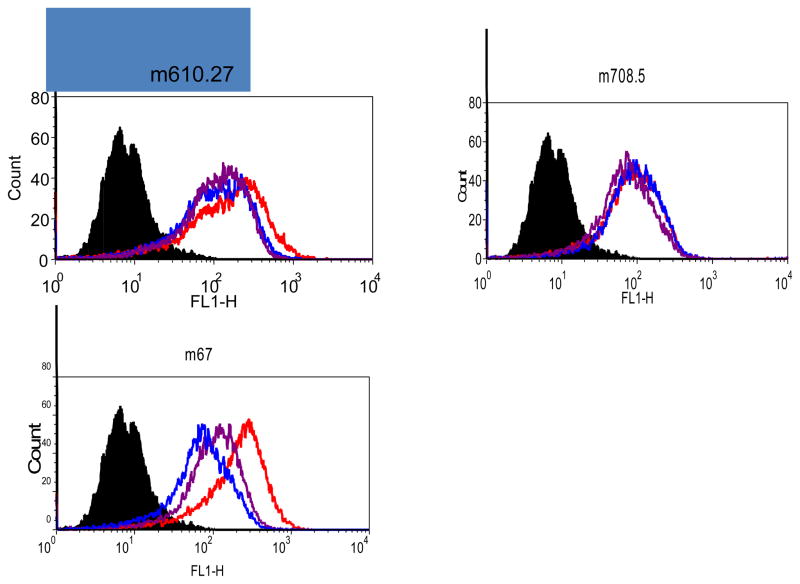
Next we tested whether such complexes, with multiple molecules of IgG, may be able to bind to the low affinity Fcgamma receptor II (CD32) through the avidity effect from multiple Fc molecules in the complex. BJAB cells, a B cell lymphoma cell line, express FcγRII, but not appreciable levels of IGF-1R. The free m67 did not bind to BJAB cells (data not shown). When m67and IGF2 complex or the mixture of its two parental antibodies with IGF2 were incubated to BJAB cells, there was significant binding to these cells (Figure 7). By contrast, the individual parental antibodies with IGF2 could not bind to the FcγRII on BJAB cells.
Figure 7. Binding of antibodies to BJAB Cells in the presence of IGF2.
Bound biotinylated IGF1 or 2 was detected by Streptavidin-PE. Cells incubated with Streptavidin-PE conjugate are shown in black as the background control. Cells incubated with IGF2 only are in red. Those for IGF2 with antibodies are in blue.
In the circulation, when macromolecular complex with multiple Fcs are presented to cells such as macrophages and neutrophils, these cells will endocytose/phagocytose the complex and degrade it. The endocytosis is one of the important mechanism to remove microorganisms, malfunctional proteins and cell debris. This process is believed to rely on the cytoskeleton, F-actin. We tested this possibility using U937 cells that have been induced to differentiate into macrophage-like cells by treatment with PMA. Our results indicated that U937 cells were able to internalize bound m67/IGF2 complex, whereas the single specific antibodies were not internalized, and the process is somewhat interfered by an inhibitor of F-actin, cytochalasin D (Figure 8), which interferes with the endocytosis in general.
Figure 8. Endocytosis of m67-IGF2 by macrophage-like U937 Cells.
Bound antibodies were detected by FITC-conjugated goat F(ab′)2 anti-human Fc IgG antibody. The black histograms are the blank cells incubated with the secondary antibody only. The histograms for cells incubated with antibody alone, the mixture of antibody and IGF2, and the mixture of the antibody, IGF2 and Cytochalasin D are in red, blue and purple, respectively.
Pharmacokinetics of m67 in cynomolgus macaques
In order to estimate the half-life of fully human antibody to human IGF2, cynomolgus macaques were chosen because they are close relatives to humans, both phylogenetically and physiologically. Not only does their immune system tolerate human antibodies, they also have detectible levels of mature IGF2, similar to human adults. Three female macaques were injected with a single dose of m67 at 2 mg/kg dose and plasma samples were collected before and after injection for monitoring the m67 levels throughout the study.
Adult macaques are known to have detectible levels of IGF2, and they also have binding proteins of IGF2. We measured the m67 concentration by an ELISA method. IGF2 was coated on ELISA wells, known amount of m67 in serial concentrations were added to MPBS as standards. The test plasmas were diluted so that the readout will fall in the linear range of the standard curve. The ELISA method to test m67 concentration was first verified by comparing spiking m67 in plasma collected from a healthy cynomolgus monkey or MPBS. When known concentration of m67 was spiked in the plasma and MPBS, respectively, and added to IGF2-coated ELISA wells, the detected m67 level in plasma and MPBS was similar (data not shown), indicating that monkey plasma proteins did not interfere with the assay.
Immediately after a single intravenous injection of m67 at the 2 mg/kg dose, the plasma concentration of m67 reached 100–120 nM, and remained at that level for several hours (Figure 9). It was then reduced rapidly to low levels of 20 nM in 24 hours. Although the highest concentration was relatively low compared with other PK study results, the data fit the two compartment model, and the trend of the distribution phase is similar to reported human monoclonal antibody PK studies in monkeys (Yeung et al., 2010). After 24 hours the concentrations of m67 stayed as low as 0.2 nM, but were always at a detectible level. The estimated elimination phase half-life was 6.4±0.6 days.
Figure 9. Plasma concentration-time course plot of m67.
A single injection of m67 at 2 mg/kg dose was administered. The result shown was the average of data from 3 animals. The inset shows the plot at within 24 hours after m67 injection.
Total IGF2 levels in animals remained unchanged throughout the study
Antibodies in general are stable proteins due to their affinity to the neonatal receptor, whereas free IGF2 would have very short half-life (minutes) due to its low molecular weight. It is known that majority of human IGF2 in plasma exists as a high molecular weight complex with a copy of IGF-binding proteins (IGFBPs) and the acid labile subunit (ALS). It is postulated that when free IGF2 binds to an antibody molecule, the IGF2/Ab complex behaves in a similar way to the IGF2/IGFBPs/ALS complex, and renders it a longer half-life.
Because there is sufficient m67 in the plasma to bind IGF2, it is expected that there was very little free IGF2 left, and the vast majority of IGF2 should stay as complex with either binding proteins or m67. For the study, it is important to know whether the total amount of IGF2, rather than free IGF2, undergoes any change during the study.
To free IGF2 from complexes, the plasma was treated with acidified alcohol, which denatures most of plasma proteins whereas the IGF2 is stable under acid condition. This process dissociates IGF2 from its binding proteins and antibody. The method was verified by adding known amount of IGF2 to mouse serum, which has no detectible amount of IGF2 but has IGF2 binding proteins. The result was within 90~95% recovery of added IGF2 amount (data not shown). Therefore the method was applied to test the total IGF2 levels in the monkey plasma.
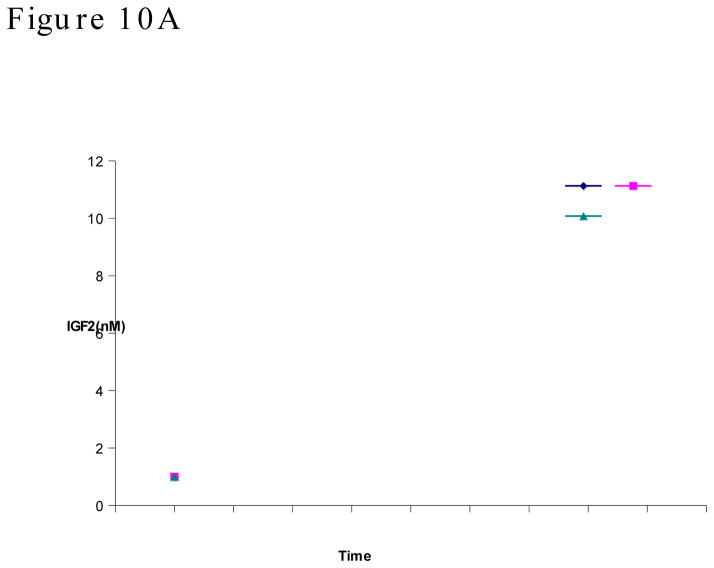
We found that the three macaques have slightly different levels of total IGF2 (18 to 36 nM) at the beginning of the study (Figure 10A). However, within the experimental errors, the IGF2 level in each animal largely maintained unchanged. In another word, the administering of m67 did not change the IGF2 levels in the animals. It is noticeable that the long term m67 concentration fell below the concentration of total IGF2. This may partially be the reason that m67 did not reduce the IGF2 levels.
Figure 10. A Plasma concentration-time course plot of total IGF2.
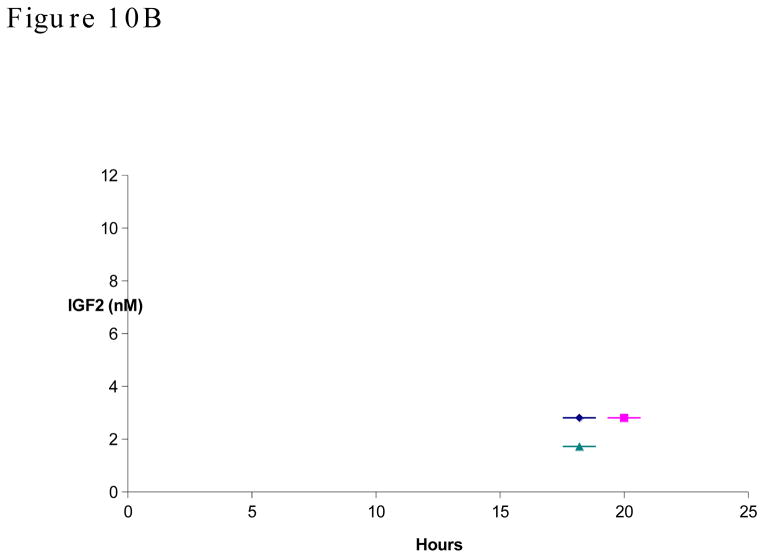
IGF2 was dissociated from binding proteins and antibody with acid/ethanol treatment, then detected with capture ELISA. Concentrations from three animals were shown individually. B, Plasma concentration-time course plot of total IGF2 within 24 hours after m67 injection.
Because during the first 24 hours after m67 injection, the antibody was at 120 to 20 nM range, which is higher than or close to total IGF2, we monitored the total IGF2 levels during this window (Figure 10B). It was found that during the first 24 hours, even though there were relatively high concentrations of m67, the IGF2 levels still did not diminish. These results suggest that either it takes long time for m67/IGF2 complex to be degraded or the affinity between m67 and IGF2 is weaker than the affinity between IGF2 and its binding proteins.
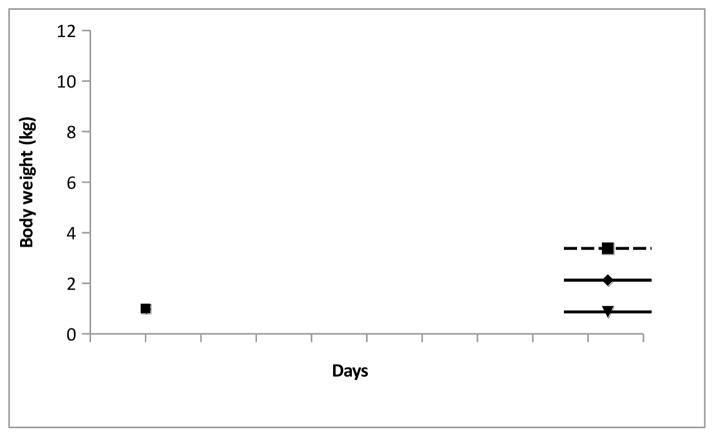
The antibody was well tolerated. Other than some redness at the injection sites during the first week after injection, no abnormalities were observed with the three animals. The body weight of the three monkeys maintained the pre-injection levels (Figure 11), and no sign of irritation were observed. All three animals were returned to stock after the study.
Figure 11. Body weights of animals during the study remained steady.
Body weight of the monkeys were taken two days before the injection of m67 and weekly after the injection.
Discussion
In this study we have constructed and characterized a bispecific IGF1 & 2 antibody m67 that is able to bind to IGF2 on two epitopes. This has proved to be an important feature of this unique bispecific antibody. Through the bivalent binding of m67, it formed multi-molecular complex, which bind to the low affinity FcgRII and in turn triggers endocytosis by macrophages. We further tested the pharmacokinetics of m67 in cynomolgus macaques and found that m67 had a half-life of approximately 6.5 days, when 2 mg/kg dose was given to macaques. In addition, a single dosing of m67 in monkeys did not change total IGF2 in the circulation.
IGFs are small proteins (79 amino acids for IGF1 and 67 amino acids for IGF2). It is challenging to identify high affinity antibodies that have different epitopes on such small protein. Previously, we reported another bispecific antibody, composed of m610.27 and m630.3 (Chen et al., 2012). m630.3 was identified from a domain antibody library, therefore m630 is a VH binder. We speculate VH may have different conformation on its antigen contacting surface and bind to different epitopes on IGF2. In fact, m630 itself is non-inhibitory to IGF2 binding of IGF-1R. The other arm of current bispecific antibody, m708.5 binds to both IGF1 & 2 (Zhao et al., 2011), therefore, the cross binding nature of the antibody determines that the epitope is slightly different from m610.27, which binds to IGF2 only.
In some cases, bispecific antibodies are somewhat unstable and tend to aggregate under long term storage. In this study, it was found that m67 was stable when incubated with human serum. Therefore, m67 has integral stable nature for further development. More characterizations regarding its physical and chemical features are needed to understand its developability. To prove the degradation of IGF2 by m67 and macrophages, it will require an animal model in which the fully human antibody will be tolerated, and endogenous IGF2 is at detectible levels. Because, adult mice do not have detectible levels of IGF2 (Feng and Dimitrov, 2008a), we sought to perform such test in two in vitro cell systems. In BJAB cells, there are sufficient FcγRII (CD32), which have low affinity to IgG and normally do not bind to soluble IgG. We showed that the single specific antibody either m610.27 or m708.5 even with IGF2 did not bind to BJAB cells. Only m67/IGF2 multi-molecular complex (or m610.27+m708.5 with IGF2) were able to BJAB cells at significant levels (Figure 7, panel 3). This part of in vitro result proved our hypothesis that multiple copies of Fc molecules bind to FcγRII through the avidity effect. In the activated U937 cells, the macrophage like endocytosis function was restored and these cells were used to test engulfing of m67/IGF2 complex. It was found that although single specific antibodies m610.27 or m708.5 bound to activated U937 cells, adding IGF2 to these samples did not triggered endocytosis. Only m67/IGF2 complex was endocytosed by activated U937 cells, indicating that multi-IgGs on the complex are required for the endocytosis (Figure 8, panel 3). Disrupting the endocytosis with F-actin inhibitor cytochalasin D partially restored the surface level of m67/IGF2 complex, supporting the notion that the reduction in blue graph is due to endocytosis. Therefore, the results from these two in vitro experiments strongly support our hypothesis. Ultimately, the hypothesis will have to be tested in an animal model.
Within certain ranges, the pharmacokinetics of protein is dose-dependent. It has been reported that for the same Ab, e.g. Figituzumab, a single injection at 10 or 20 mg/kg dose gives 21 or 28 days of half-life in healthy humans (Yin et al., 2012). Similarly, AMG386, a peptide-Fc fusion protein, given at 0.3 and 30 mg/kg dose in humans had half-life of 3.1 or 6.3 days, respectively (Herbst et al., 2009). The dose we gave to monkeys in this study was very low, in comparison to these reported studies. We speculate that higher doses of m67 in monkeys should give reasonably long half-life.
In conclusion, the IGF2 bispecific antibody m67 form multi-molecular complexes with IGF1 and IGF2. The large complex activated the endocytosis process of macrophages and had potential to be cleared from the circulation. In cynomolgus monkeys, m67 was found to have ~6.4 days of half-life after a single injection at 2 mg/kg dose. The relatively short half-life observed in the study is likely due to the low dose given to the monkeys. Throughout the study, the total IGF2 levels did not change significantly. Although these results do not support additional mechanism of (irreversible) removal of IGF2 from the circulation under the conditions of our experiments, the m67 efficacy and good drugability suggest a potential as a candidate therapeutic of IGF2-related cancers.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Footnotes
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bid HK, et al. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol Cancer Ther. 2012;11:649–59. doi: 10.1158/1535-7163.MCT-11-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Human monoclonal antibodies targeting nonoverlapping epitopes on insulin-like growth factor II as a novel type of candidate cancer therapeutics. Mol Cancer Ther. 2012;11:1400–10. doi: 10.1158/1535-7163.MCT-12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvaia N, et al. Insulin-like growth factor receptor type I as a target for cancer therapy. Front Biosci (Schol Ed) 2013;5:439–50. doi: 10.2741/s382. [DOI] [PubMed] [Google Scholar]
- Daughaday WH, et al. Inhibition of access of bound somatomedin to membrane receptor and immunobinding sites: a comparison of radioreceptor and radioimmunoassay of somatomedin in native and acid-ethanol-extracted serum. J Clin Endocrinol Metab. 1980;51:781–8. doi: 10.1210/jcem-51-4-781. [DOI] [PubMed] [Google Scholar]
- Feng Y, Dimitrov DS. Insulin-like Growth Factors as Targets for Cancer Treatment. In: Malloy AH, Carson EC, editors. Oncogene Proteins: New Research. Nova Science Publishers; Hauppauge, New York: 2008a. pp. 161–176. [Google Scholar]
- Feng Y, Dimitrov DS. Monoclonal antibodies against components of the IGF system for cancer treatment. Curr Opin Drug Discov Devel. 2008b;11:178–85. [PubMed] [Google Scholar]
- Feng Y, et al. Novel human monoclonal antibodies to insulin-like growth factor (IGF)-II that potently inhibit the IGF receptor type I signal transduction function. Mol Cancer Ther. 2006;5:114–20. doi: 10.1158/1535-7163.MCT-05-0252. [DOI] [PubMed] [Google Scholar]
- Herbst RS, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;27:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- Kimura T, et al. Targeting of bone-derived insulin-like growth factor-II by a human neutralizing antibody suppresses the growth of prostate cancer cells in a human bone environment. Clin Cancer Res. 2010;16:121–9. doi: 10.1158/1078-0432.CCR-09-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer CJ, et al. Randomized, phase III trial of first-line figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin alone in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2014;32:2059–66. doi: 10.1200/JCO.2013.54.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasota J, et al. Expression of the receptor for type i insulin-like growth factor (IGF1R) in gastrointestinal stromal tumors: an immunohistochemical study of 1078 cases with diagnostic and therapeutic implications. Am J Surg Pathol. 2013;37:114–9. doi: 10.1097/PAS.0b013e3182613c86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen M, et al. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol. 2013;37:234–40. doi: 10.1097/PAS.0b013e3182671178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mireuta M, et al. Quantification of binding of IGF-1 to BI 836845, a candidate therapeutic antibody against IGF-1 and IGF-2, and effects of this antibody on IGF-1:IGFBP-3 complexes in vitro and in male C57BL/6 mice. Endocrinology. 2014;155:703–15. doi: 10.1210/en.2013-1791. [DOI] [PubMed] [Google Scholar]
- Nowakowski A, et al. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci U S A. 2002;99:11346–50. doi: 10.1073/pnas.172229899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- Rajan A, et al. Cixutumumab for patients with recurrent or refractory advanced thymic epithelial tumours: a multicentre, open-label, phase 2 trial. Lancet Oncol. 2014;15:191–200. doi: 10.1016/S1470-2045(13)70596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JF, et al. Ganitumab with either exemestane or fulvestrant for postmenopausal women with advanced, hormone-receptor-positive breast cancer: a randomised, controlled, double-blind, phase 2 trial. Lancet Oncol. 2013;14:228–35. doi: 10.1016/S1470-2045(13)70026-3. [DOI] [PubMed] [Google Scholar]
- Visser M, et al. Allelotype of pediatric rhabdomyosarcoma. Oncogene. 1997;15:1309–14. doi: 10.1038/sj.onc.1201302. [DOI] [PubMed] [Google Scholar]
- Yang JC, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–34. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung YA, et al. A therapeutic anti-VEGF antibody with increased potency independent of pharmacokinetic half-life. Cancer Res. 2010;70:3269–77. doi: 10.1158/0008-5472.CAN-09-4580. [DOI] [PubMed] [Google Scholar]
- Yin D, et al. Pharmacokinetics and pharmacodynamics of figitumumab, a monoclonal antibody targeting the insulin-like growth factor 1 receptor, in healthy participants. J Clin Pharmacol. 2012;53:21–8. doi: 10.1177/0091270011432934. [DOI] [PubMed] [Google Scholar]
- Zhao Q, et al. Human monoclonal antibody fragments binding to insulin-like growth factors I and II with picomolar affinity. Mol Cancer Ther. 2011;10:1677–85. doi: 10.1158/1535-7163.MCT-11-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]