Abstract
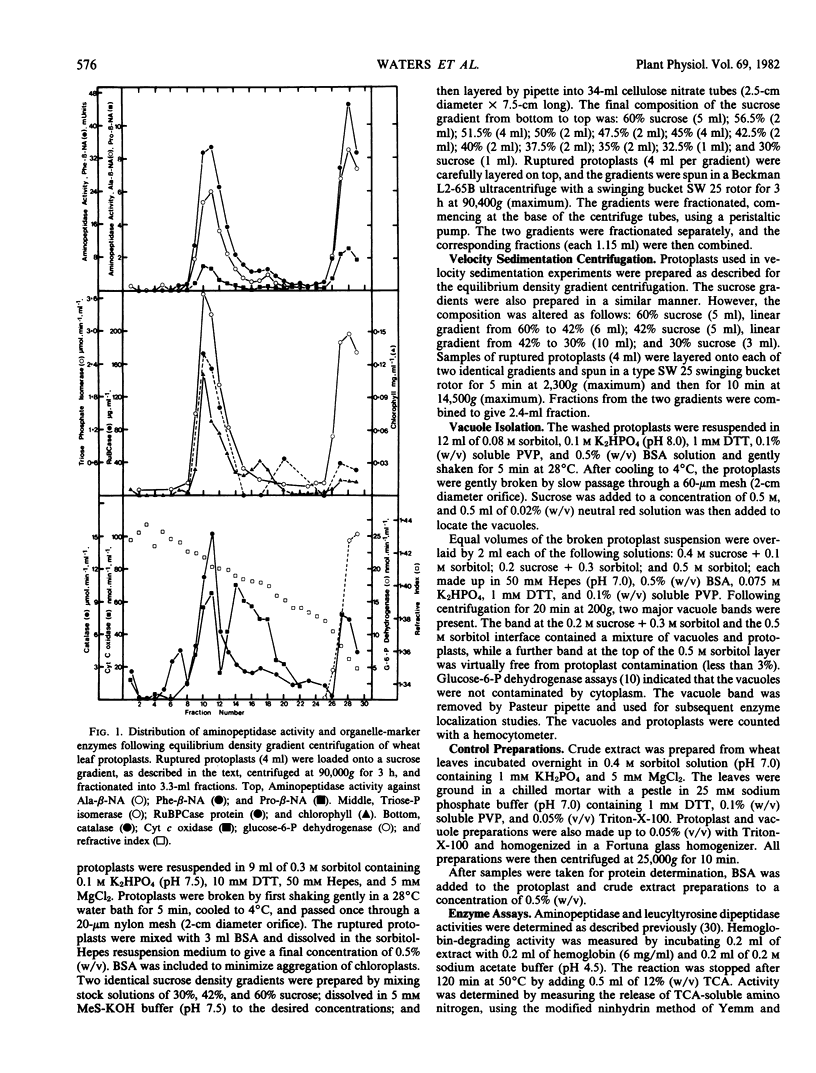
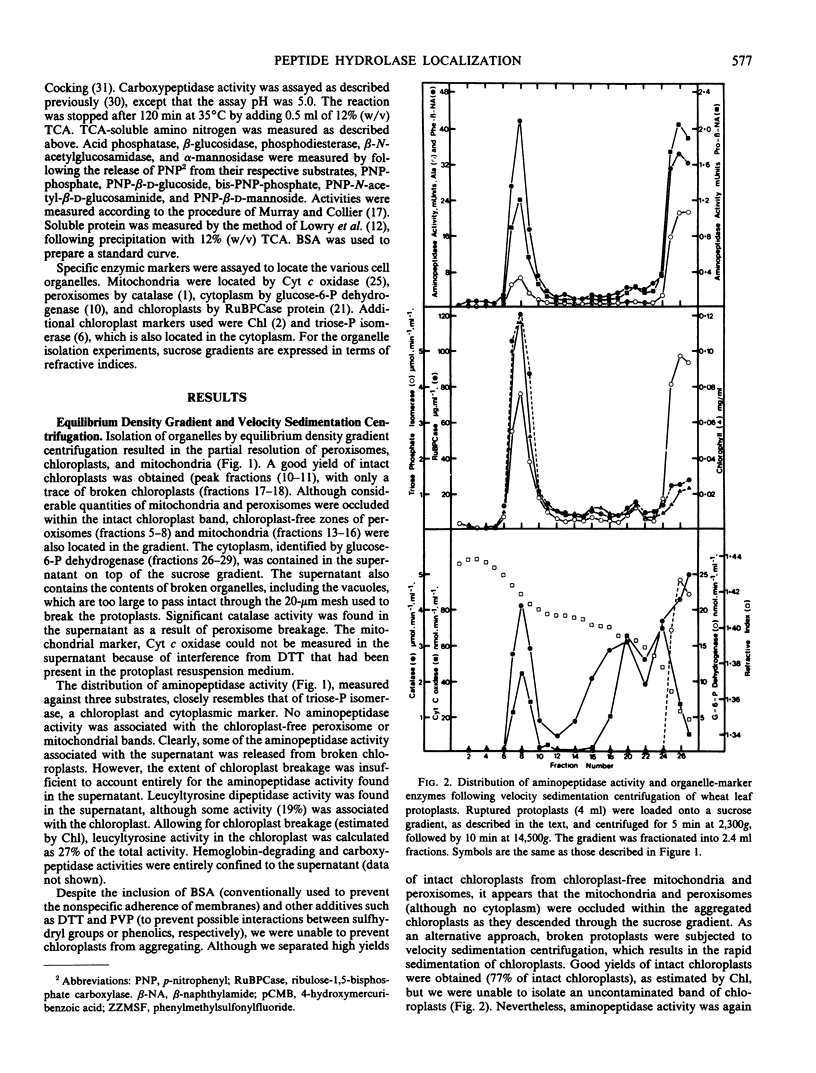
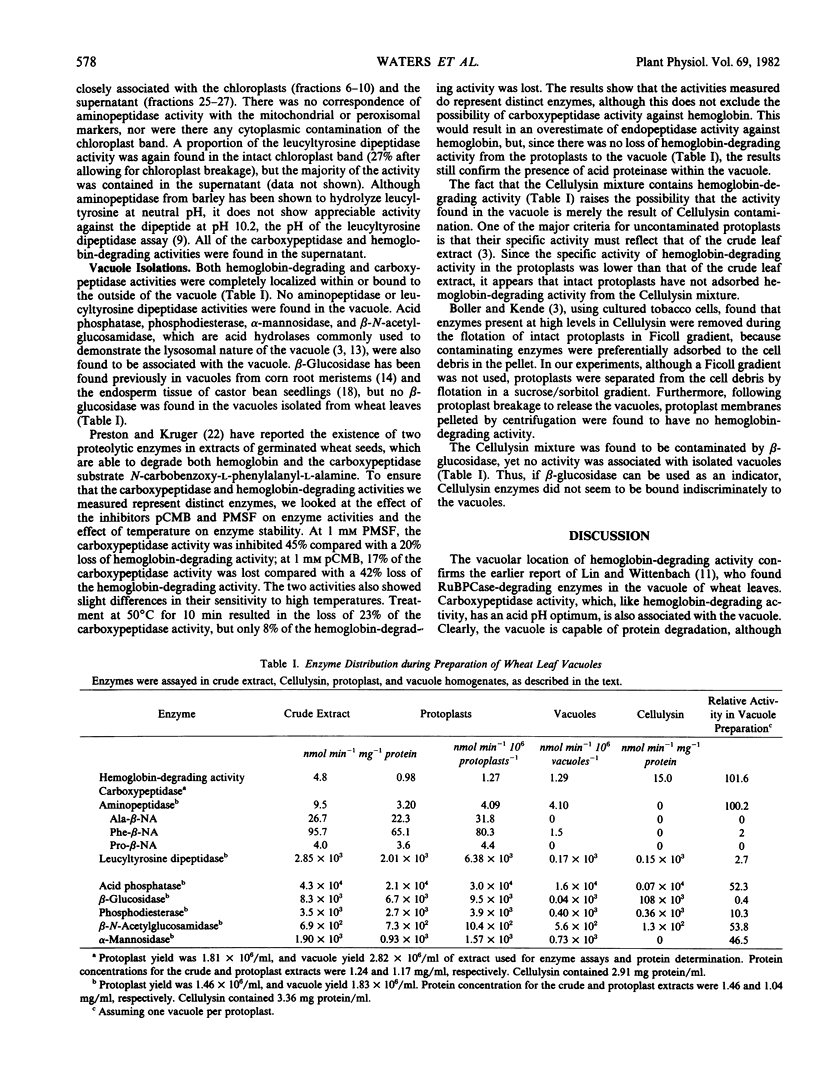
Protoplasts from 8- to 9-day-old wheat (Triticum aestivum L.) leaves were used to isolate organelles which were examined for their contents of peptide hydrolase enzymes and, in the case of vacuoles, other acid hydrolases. High yields of intact chloroplasts were obtained using both equilibrium density gradient centrifugation and velocity sedimentation centrifugation on sucrose-sorbitol gradients. Aminopeptidase activity was found to be distributed, in approximately equal proportions, between the chloroplasts and cytoplasm. Leucyltyrosine dipeptidase was mainly found in the cytoplasm, although about 27% was associated with the chloroplasts. Vacuoles shown to be free from Cellulysin contamination contained all of the protoplast carboxypeptidase and hemoglobin-degrading activities. The acid hydrolases, phosphodiesterase, acid phosphatase, α-mannosidase, and β-N-acetylglucosamidase were found in the vacuole to varying degrees, but no β-glucosidase was localized in the vacuole.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey J. W., De Duve C. Digestive activity of lysosomes. I. The digestion of proteins by extracts of rat liver lysosomes. J Biol Chem. 1968 Jun 25;243(12):3255–3263. [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolehmainen L., Mikola J. Partial purification and enzymatic properties of an aminopeptidase from barley. Arch Biochem Biophys. 1971 Aug;145(2):633–642. doi: 10.1016/s0003-9861(71)80023-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W., Wittenbach V. A. Subcellular localization of proteases in wheat and corn mesophyll protoplasts. Plant Physiol. 1981 May;67(5):969–972. doi: 10.1104/pp.67.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura M., Beevers H. Hydrolases in vacuoles from castor bean endosperm. Plant Physiol. 1978 Jul;62(1):44–48. doi: 10.1104/pp.62.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston K. R., Kruger J. E. Purification and properties of two proteolytic enzymes with carboxypeptidase activity in germinated wheat. Plant Physiol. 1976 Oct;58(4):516–520. doi: 10.1104/pp.58.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragster L. E., Chrispeels M. J. Autodigestion in crude extracts of soybean leaves and isolated chloroplasts as a measure of proteolytic activity. Plant Physiol. 1981 Jan;67(1):104–109. doi: 10.1104/pp.67.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnarrenberger C., Oeser A., Tolbert N. E. Development of Microbodies in Sunflower Cotyledons and Castor Bean Endosperm during Germination. Plant Physiol. 1971 Nov;48(5):566–574. doi: 10.1104/pp.48.5.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Wilden W., Herman E. M., Chrispeels M. J. Protein bodies of mung bean cotyledons as autophagic organelles. Proc Natl Acad Sci U S A. 1980 Jan;77(1):428–432. doi: 10.1073/pnas.77.1.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]


