Abstract
Dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDD) is an aryl hydrocarbon receptor (AHR) agonist, an endocrine disruptor, and a potent global pollutant. TCDD exposure is associated with diseases of almost every organ system, and its toxicity is highly conserved across vertebrates. While the acute developmental effects of dioxin exposure have been extensively studied, the ability of early sublethal exposure to produce toxicity in adulthood or subsequent generations is poorly understood. This type of question is difficult to study because of the time frame of the effects. With human subjects, such a study could span more than a lifetime. We have chosen zebrafish (Danio rerio) as a model because they are vertebrates with short generation times and consistent genetic backgrounds. Zebrafish have very modest housing needs, facilitating single and multigenerational studies with minimal time and expense. We have used this model to identify transgenerational effects of TCDD on skeletal development, sex ratio, and male-mediated decreases in reproductive capacity. Here we compare these findings with transgenerational effects described in laboratory rodent species. We propose that the zebrafish is a cost-effective model system for evaluating the transgenerational effects of toxic chemicals and their role in the fetal basis of adult disease.
Keywords: TCDD, AHR, zebrafish, rodent, transgenerational, epigenetic
Introduction
Mounting evidence suggests that environmental factors can alter developmental programming, resulting in the adult onset of latent diseases, including but not restricted to cancer, diabetes, cardiovascular disease and reproductive disorders (Gluckman and Hanson, 2004; Lau and Rogers, 2004; Heindel, 2005; Marczylo et al., 2012; Veenendaal et al., 2013). The etiology of some diseases is now linked to tissue- and developmental stage-specific epigenetic alterations in gene expression, resulting from nutritional deficits or exposure to contaminants in utero. Exposure to endocrine disruptors is of concern due to the roles that hormones play in regulating transient and irreversible developmental processes. Evidence is mounting that developmental exposure to chemicals, including endocrine disruptors, results in adult disease (Heindel, 2008; Corrales et al., 2014b).
TCDD is a toxic environmental contaminant that impacts growth and development in vertebrates and is associated with several diseases. It is the prototypical member of a family of dioxin-like compounds (DLCs), and is generally produced as a byproduct of industrial processes and waste incineration. TCDD is stable in the environment, highly lipophilic and bioaccumulative, and human exposure comes mostly through dietary sources. TCDD acts primarily through activation of the AHR/ARNT transcriptional regulator to alter gene expression, but cross talk with other signal transduction systems is suspected (Poland and Bradfield, 1992; Swanson and Bradfield, 1993; Schmidt and Bradfield, 1996; Puga et al., 2009). AHR activation by TCDD leads to altered expression of hormone receptors, receptor activators and repressors, metabolic enzymes needed for metabolism of xenobiotics and hormone synthesis and degradation, and other gene products required for normal development and endocrine function (Abbott et al., 1994; Gierthy et al., 1996; Safe et al., 1998; Massaad et al., 2002; Beischlag et al., 2008)
Diseases in humans that have been associated with exposure to TCDD include cancer as well as chloracne, porphyria, and defects in the cardiovascular, skeletal, immune, central nervous system, hepatic and reproductive systems (Eskenazi et al., 2000; Guo et al., 2000; Pelclova et al., 2006; Warner et al., 2007; NAS-IOM, 2011; Warner et al., 2011). Recent epidemiologic evaluation following a major industrial release of TCDD revealed that exposure to TCDD in utero leads to reduced sperm quality, feminized sex ratio, and altered thyroid function in the offspring (Mocarelli et al., 2000; Baccarelli et al., 2008; Mocarelli et al., 2011).
Laboratory studies confirm the potential for TCDD to cause disease later in life. Direct exposure to TCDD leads to infertility in many vertebrate species, including humans, and is associated with down-regulation of enzymes in the estrogen synthesis pathway, decreased egg release, increased number of atretic ovarian follicles, and decreased fertilization success (DeVito and Birnbaum, 1994; King-Heiden et al., 2006; Yoshizawa et al., 2009; King-Heiden et al., 2012; Baker et al., 2013). Toxicity in adults following TCDD exposure during early development suggests that physiologic systems are being mis-programmed and that exposure to TCDD can potentially initiate irreversible and permanent modifications in gene expression and cell lineages. However, the molecular mechanisms that underlie latent and transgenerational disease caused by developmental exposure to TCDD are not well understood.
Our recent work has focused on studying the latent and transgenerational effects of TCDD exposure during critical periods of development, using zebrafish (Danio rerio) as a model system. In this review, we compare our findings with effects observed in rodent studies to highlight the usefulness of this model system for evaluating the potential for chemicals to cause disease in adults and subsequent generations.
Zebrafish as a Model for Multigenerational Studies
To study transgenerational effects, we need a vertebrate model that has a short time to sexual maturity so that we can study successive generations. From this perspective, humans are not ideal subjects for study (Heindel, 2007; Skogen and Overland, 2012). In addition, the diverse genetics of the human population, confounded by individual variations in exposures make studies with human subjects difficult. The zebrafish is well established as a model for investigating human disease, especially as it pertains to altered development. Attributes that make the zebrafish outstanding in this arena are: short time to sexual maturity (about 3–4 months), transparent embryos that allow observation of organ development without disturbing the embryo, the ability to obtain large groups of synchronously developing embryos, low cost for exposure chemicals since volumes are small, and the ease of housing multiple generations of fish. This last point means that one can expose the first F0 generation and maintain offspring across many generations inexpensively and compactly.
While small rodent models are more common than small fish models for studying human disease, rodents have a number of disadvantages for studying the fetal basis of adult disease. Rats and mice for example have far fewer offspring per pair, and maintenance costs are considerably greater. While zebrafish reach sexual maturity in a similar timeframe to some rodents, their small size allows for the ability to house and maintain large groups of synchronously developing fish over multiple generations inexpensively and compactly. Similar to human populations, laboratory zebrafish are less isogenic than laboratory rodent strains, which decreases inbreeding effects when studying changes in the zebrafish genome/epigenome. Zebrafish developmental processes are well characterized, and many organs and cell types have been marked with fluorescent reporters in transgenic lines. Due to complete sequencing of the zebrafish genome, technologies that include specific antibodies, genetic/epigenetic markers, and high throughput sequencing also can be readily utilized. MicroRNAs may be involved in the transgenerational inheritance of disease (Wagner et al., 2008; Grandjean et al., 2009) and there is a rapidly growing microRNA literature in zebrafish. Finally, developing zebrafish are very small and transparent, so development can be readily followed with microscopy and automated screening techniques (Kaufman et al., 2009; Wittmann et al., 2012; Westhoff et al., 2013).
Even though zebrafish are oviparous, the reproductive system of fish and mammals is similar. The testis and ovary in zebrafish contain the same germ cells that are found in mammals, and hormonal regulation of spermatogenesis and oogenesis is highly conserved across vertebrates, occurring via the hypothalamic-pituitary-gonadal axis (Segner, 2009; Liu et al., 2011; Lohr and Hammerschmidt, 2011).
Defining Transgenerational Toxicity: Zebrafish vs. Rodents
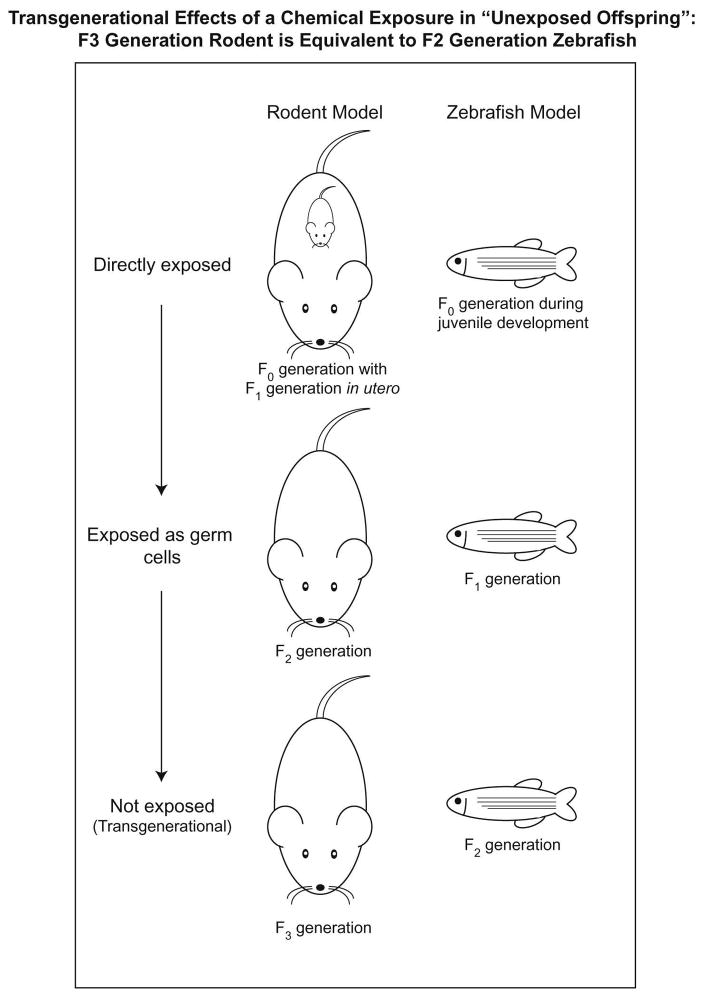
Chemical exposures that affect subsequent generations are now well documented. An epigenetic mechanism is likely for cases of multigenerational disease, in which neither the parent nor the offspring have been directly exposed. This is a transgenerational effect because there is no direct connection to chemical exposure (Skinner, 2008). In rodent models, exposure during early development requires prenatal exposure in an F0 generation mother during pregnancy (Figure 1, left column). This leads to F1 offspring that developed in an exposed environment. The F2 offspring then develop in parents that were exposed in utero, so only effects in the F3 generation can be due to epigenetic alterations in gametes.
Figure 1. Comparison of the experimental design of a transgenerational study in the laboratory rodent and zebrafish.
In the rodent model, in utero exposure to a chemical leads to direct exposure of the F0, F1 and F2 generations and the F3 generation is not exposed. In the zebrafish model, juvenile developmental exposure leads to direct exposure of the F0 and F1 generations to the chemical and the F2 generation is not exposed.
In contrast, zebrafish eggs are fertilized in water, embryos develop externally, and are subsequently exposed at the juvenile stage of development (Figure 1, right column). Thus, F0 fish are equivalent to F1 mice because they develop in an exposed environment. The F1 zebrafish generation originates from gametes produced by exposed fish, similar to the F2 mouse generation. The gametes producing the F2 zebrafish generation have not been exposed so the effects seen in F2 zebrafish are transgenerational. Thus, the F2 zebrafish is equivalent to the exposure-free F3 mouse.
Using Zebrafish to Identify Transgenerational Effects of TCDD
Sublethal TCDD exposure in utero and in early development leads to adverse health effects in adulthood and subsequent generations. Adverse effects have included increased congenital abnormalities, decreased survival, differences in sex ratios of offspring, and decreased reproductive function and fertility in both males and females (Wolf et al., 1999; Nomura et al., 2004; Ikeda et al., 2005a and b; King-Heiden et al., 2009; Ding et al., 2011). Transgenerational effects of TCDD exposure have now been observed in mice, rats and zebrafish (Bruner-Tran and Osteen, 2011; Manikkam et al., 2012a and b; Nilsson et al., 2012; Baker et al., 2014). The TCDD-induced transgenerational defects identified in these species involve skeletal development, sex ratio, ovary, and reproductive success and are summarized in Table 1.
Table 1.
Sex-Specific, Transgenerational Effects of TCDD in Rodents and Zebrafish
| Species | Sex | Transgenerational Effect | TCDD Exposure of F0 Generation | Reference |
|---|---|---|---|---|
| Mice | Female | Decreased pregnancy rate Increased preterm birth |
10 μg/kg, po, at E15.5 | Bruner-Tran and Osteen, 2011 |
| Rats | Female | Decreased follicle number Decreased primordial follicles Early puberty Increased small cysts in ovary |
100 ng/kg/day, ip, E8 to E14 |
Nilsson et al., 2012 Manikkam et al., 2012a,b |
| Male | Increased kidney disease | |||
| Zebrafish | Female and Male | Changed in sex ratio Increased skeletal malformations |
50 pg/ml, 1 hr, static waterborne at 3 and 7 wpfa | Baker et al., 2014 |
| Maleb | Decreased egg release Decreased egg fertilization |
Weeks post fertilization
Decreased egg release and fertilization is observed in control, female zebrafish mated with TCDD lineage F2 males. It does not occur when TCDD lineage F2 females are mated with control males. Thus, the transgenerational effect of TCDD, in decreasing reproductive capability in zebrafish, is male-mediated.
Skeletal Development
Direct TCDD exposure causes skeletal, cartilage, and bone abnormalities in several animal models (Peterson et al., 1993; Hornung et al., 1999; Xiong et al., 2008; Bursian et al., 2013). Spina bifida, a developmental abnormality that is caused by incomplete closing of the neural tube and malformed vertebrae, occurs in human offspring following exposure to Agent Orange, a TCDD-contaminated herbicide (NAS-IOM, 2011). In mink, skeletal abnormalities were observed in F1 offspring of TCDD-exposed adults (Bursian et al., 2013). TCDD exposure during development altered craniofacial structures in adult zebrafish, and produced scoliosis-like kinks in the axial skeletons of adult F0 parents as well as in F1 and F2 offspring (Table 1; King-Heiden et al., 2009; Baker et al., 2013; Baker et al., 2014). Transgenerational skeletal abnormalities have not yet been reported in mammals. This response might be idiopathic to fish, but the effects may also be easier to observe in zebrafish. It is also possible that zebrafish are more sensitive to skeletal toxicity compared to mammals. If so, a zebrafish model may be useful in developing screens for transgenerational skeletal effects of chemical exposure.
Sex Ratio
One effect of human exposure to TCDD is a change in the sex ratio towards a higher percentage of girls born to parents exposed during an industrial accident in Seveso, Italy (Clapp and Ozonoff, 2000; Eskanazi et al., 2004; Mocarelli et al., 2000 and 2008). Some studies in rats and zebrafish have also produced a shift in the sex ratio of offspring toward females (Ikeda et al., 2005b; Baker et al., 2013). The shift towards females was also observed in the F1 and F2 offspring of F0 zebrafish exposed to TCDD during sexual differentiation and maturation (Table 1; Baker et al., 2014). While not as well understood in fish as in mammals, sex determination in zebrafish is primarily genetic, with environmental factors influencing sex secondarily (Liew and Orban, 2014). Although, zebrafish would be useful in screening for the ability of environmental contaminants to influence sex ratios, zebrafish do not have a pair of highly differentiated sex chromosomes. Until we know more about what determines male and female sex in zebrafish, mechanistic interpretation will be difficult.
Ovary
While sex determination is not well understood in zebrafish, the pathways and genes involved in gonad differentiation are conserved between zebrafish and other vertebrates (Wilkins, 1995; Marshall-Graves and Peichel, 2010). Zebrafish (F0) exposed to TCDD as embryos or adults show similar ovarian toxicities (King-Heiden et al., 2005 and 2006; Hutz et al., 2006; Daouk et al., 2011; Baker et al., 2013). A group of studies from Skinner and colleagues reported several transgenerational phenotypic changes in F3 female rats following in utero exposure of the F1 generation to TCDD, including: early onset of puberty, reduced numbers of total follicles, reduced numbers of primordial follicles, and increased numbers of small cysts within the ovary (Table 1; Manikkam et al., 2012a and b; Nilsson et al., 2012). As in the rodent studies, F1 female zebrafish had abnormal ovarian structure, with atretic follicles (King-Heiden et al., 2009; Baker et al., 2014). However, this effect diminished with time, and was not statistically significant by the F2 generation. This steady diminution of effects over time may help provide insight into the mechanism of transgenerational effects, and will be an important part of assessing the long-term impact of developmental exposure on human populations.
Reproductive Success
Studies with mice have demonstrated transgenerational effects of TCDD exposure on fertility. Offspring of TCDD exposed mice were less likely than control to become pregnant, and those that did become pregnant were less likely than control to deliver at full term (Table 1, Bruner-Tran and Osteen, 2011). We observed similar results with F0 zebrafish exposed to TCDD during sexual development in the F0 generation. Both F1 and F2 offspring showed decreased reproductive capacity, with significantly decreased egg release and reduced percentage of eggs fertilized (Table 1; Baker et al., 2013 and 2014). A simple explanation for decreased egg release could be adverse effects on ovarian development, but interestingly, transgenerational reduction in egg release was linked to males rather than females. Egg release during spawning involves both a female releasing the eggs and a male eliciting egg release. In spawnings of TCDD-lineage F0, F1, and F2 zebrafish males with control females, fewer eggs were produced compared to controls. Similar results have been reported with control females that avoid mating with vinclozolin-lineage F3 male rats (Crews et al., 2007). In fact, decreased reproductive capacity was observed in spawnings of TCDD-lineage F1 and F2 zebrafish males with control females (Table 1; Baker et al., 2014), suggesting that transgenerational reduction in fertility can be attributed to effects on males in the lineage. These findings indicate that some transgenerational effects can be sex-specific. Decreased sperm release could explain these fertility deficits, but as with rodent studies, the testes of TCDD-lineage zebrafish appeared normal on histological examination (Manikkam et al., 2012a and b; Baker et al., 2014). Exposure to TCDD or PCBs in utero also decreased masculine, while increasing feminine sexual behavior in rats (Mably et al., 1992; Colciago et al., 2009). Thus, TCDD does something transgenerationally to alter reproductive success in the male; however at present we are limited to trying to link what is known about AHR and what we know about reproductive function. Further studies are needed to elucidate whether alterations in male zebrafish spawning behavior, pheromone production, and/or other aspects of male reproductive biology are causing decreased fertility and what mechanisms are responsible.
Epigenetic Effects of Chemical Exposure
As we search for the mechanisms that allow a chemical exposure to have effects that persist through multiple generations, epigenetic changes via covalent DNA and chromatin modification come to the forefront. Epigenetic modifications can be carried in the gametes, ultimately modifying gene expression to produce phenotypic changes. Heritable natural epigenetic changes producing a phenotype have been documented in plants, worms and insects (Cubas et al., 1999; Manning et al., 2006; Ruden and Lu, 2008; Greer et al., 2011). Kuroki and colleagues (2013) discovered that mice lacking the H3K9 demethylase, regulating histone function in the chromatin, were subject to male to female sex reversal, demonstrating that changes in chromatin can play a pivotal role in sex determination.
Several attempts have been made to identify epigenetic changes in DNA and chromatin in individuals displaying transgenerational effects of toxic chemicals. Skinner and colleagues have shown that DNA methylation is altered in many places throughout the genome in the affected generations compared to controls (Anway et al., 2005; Manikkam et al., 2012a and b). In other cases this group has also focused on altered gene expression patterns as biomarkers of the exposure (Nilsson et al., 2012). Dolinoy and colleagues (2006) showed an effect of genistein on coat color in mice that was associated with altered methylation upstream of the agouti gene, a regulator of coat color.
Studies on transgenerational effects of AHR agonists in zebrafish have just begun. Although no specific epigenetic change has been shown to produce the transgenerational effects caused by a toxicant, it appears likely that epigenetic changes play a role in producing and transmitting these effects through generations. Changes in DNA methylation and gene expression patterns have been identified in F0 generation zebrafish following exposure to benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene (Mirbahai et al., 2011; Fang et al., 2013; Corrales et al., 2014a and b). The transgenerational phenotypic effects identified in TCDD lineage zebrafish (Table 1, Baker et al., 2014) are similar to TCDD effects observed in the F0 generation. Whether the transgenerationally altered reproductive and skeletal phenotypes are due to epigenetic modifications in the regulation of AHR-ARNT signaling in these tissues will require further research.
In addition to clarifying mechanism, it will be important to assess the stability of the toxic effects across generations. While the effects on egg release persisted through the F2 generation in our zebrafish experiments, the effects on ovarian structure waned with each generation such that it was no longer observed in the F2 generation. The mechanism for such waning effects may be similar to the evolved multi-generational resistance to dioxin-like compound toxicity in wild fish populations (Wirgin et al., 2011). How long these effects last are vitally important. In the past we have been concerned about the persistence and chemical stability of the environmental contaminants themselves. However, if contaminants are capable of producing adverse effects that can be passed across generations, we also will want to know if these effects are reversible and how many generations will be affected.
Conclusion
Transgenerational toxicity due to TCDD exposure has been observed in mice, rats and zebrafish (Bruner-Tran and Osteen, 2011; Manikkam et al., 2012a and b; Baker et al., 2014). Remarkably, several of the phenotypic effects are similar across vertebrate classes, especially the reduction of reproductive capacity in unexposed TCDD-lineages. In zebrafish, unexposed TCDD-lineage F2 offspring have reproductive, skeletal, and sex ratio abnormalities. More specifically, the decrease in fertility and egg release in control female zebrafish is due to the unexposed, TCDD-lineage F2 male zebrafish. Thus, ancestral TCDD exposure reduces reproductive success of male zebrafish across multiple generations. This is most likely an epigenetic effect since TCDD has been shown not to be mutagenic (Poland and Glover, 1979). Epigenetic changes provide an avenue for better understanding how these heritable changes occur. Zebrafish are a promising model because many generations can be produced and studied in relatively little time and space. Also, reproduction and development are easy to assess in zebrafish, and this model is broadly available.
Highlights.
We review transgenerational effects of dioxin in fish and other vertebrate species
Zebrafish model is ideal for investigating multigenerational effects of chemicals
Dioxin induces transgenerational skeletal and reproductive phenoytpes in zebrafish
Acknowledgments
Funding
National Institutes of Health (NIH) (R01 ES012716) from the National Institute of Environmental Health Sciences (NIEHS) (to W.H. and R.E.P.); the University of Wisconsin Sea Grant Institute, National Sea Grant College Program, National Oceanic and Atmospheric Administration, U.S. Department of Commerce (NA 16RG2257), Sea Grant Project R/BT-22 and 25 (to W.H. and R.E.P.) and NIEHS support from T32 ES007015 and NCATS support from K01 OD010462 (to T.B.).
We would like to thank Dorothy Nesbit, Carrie Zellmer, and Bridget Baker for technical assistance, and advice. The contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institute of Environmental Health Sciences, National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- TCDD or dioxin
2,3,7,8-tetrachlorodibenzo-p-dioxin
- DLC
dioxin-like compound
- AHR
aryl hydrocarbon receptor
- ARNT
aryl hydrocarbon receptor nuclear translocator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott BD, Perdew GH, Buckalew AR, Birnbaum LS. Interactive regulation of Ah and glucocorticoid receptors in the synergistic induction of cleft palate by 2,3,7,8-tetrachlorodibenzo-p-dioxin and hydrocortisone. Toxicol Appl Pharmacol. 1994;128:138–150. doi: 10.1006/taap.1994.1191. [DOI] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Giacomini SM, Corbetta C, Landi MT, Bonzini M, Consonni D, Grillo P, Patterson DG, Pesatori AC, Bertazzi PA. Neonatal thyroid function in Seveso 25 years after maternal exposure to dioxin. PLoS Med. 2008;5:e161. doi: 10.1371/journal.pmed.0050161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TR, Peterson RE, Heideman W. Early dioxin exposure causes toxic effects in adult zebrafish. Toxicol Sci. 2013;135:241–250. doi: 10.1093/toxsci/kft144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TR, Peterson RE, Heideman W. Using zebrafish as a model system for studying the transgenerational effects of dioxin. Toxicol Sci. 2014;138:403–411. doi: 10.1093/toxsci/kfu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beischlag TV, Luis Morales J, Hollingshead BD, Perdew GH. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursian SJ, Kern J, Remington RE, Link JE, Fitzgerald SD. Dietary exposure of mink (Mustela vison) to fish from the upper Hudson River, New York, USA: Effects on organ mass and pathology. Environ Toxicol Chem. 2013;32:794–801. doi: 10.1002/etc.2114. [DOI] [PubMed] [Google Scholar]
- Clapp R, Ozonoff D. Where the boys aren’t: dioxin and the sex ratio. Lancet. 2000;355:1838–1839. doi: 10.1016/S0140-6736(00)02280-7. [DOI] [PubMed] [Google Scholar]
- Colciago A, Casati L, Mornati O, Vergoni AV, Santagostino A, Celotti F, Negri-Cesi P. Chronic treatment with polychlorinated biphenyls (PCB) during pregnancy and lactation in the rat Part 2: Effects on reproductive parameters, on sex behavior, on memory retention and on hypothalamic expression of aromatase and 5alpha-reductases in the offspring. Toxicol Appl Pharmacol. 2009;239:46–54. doi: 10.1016/j.taap.2009.04.023. [DOI] [PubMed] [Google Scholar]
- Corrales J, Fang X, Thornton C, Mei W, Barbazuk WB, Duke M, Scheffler BE, Willett KL. Effects on specific promoter DNA methylation in zebrafish embryos and larvae following benzo[a]pyrene exposure. Comp Biochem Physiol C Toxicol Pharmacol. 2014a;163:37–46. doi: 10.1016/j.cbpc.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales J, Thornton C, White M, Willett KL. Multigenerational effects of benzo[a]pyrene exposure on survival and developmental deformities in zebrafish larvae. Aquat Toxicol. 2014b;148:16–26. doi: 10.1016/j.aquatox.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci USA. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–161. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- Daouk T, Larcher T, Roupsard F, Lyphout L, Rigaud C, Ledevin M, Loizeau V, Cousin X. Long-term food-exposure of zebrafish to PCB mixtures mimicking some environmental situations induces ovary pathology and impairs reproduction ability. Aquat Toxicol. 2011;105:270–278. doi: 10.1016/j.aquatox.2011.06.021. [DOI] [PubMed] [Google Scholar]
- De Vito M, Birnbaum L. Toxicology of dioxins and related chemicals. In: Schecter A, editor. Dioxins and Health. Plenum Press; New York, NY: 1994. pp. 139–162. [Google Scholar]
- Ding TB, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Repro Toxicol. 2011;31:351–358. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Samuels S, Vercellini P, Olive D, Needham L, Patterson D, Brambilla P. Seveso Women’s Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40:1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Needham L, Patterson DG, Jr, Samuels S, Turner W, Gerthoux PM, Brambilla P. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112:22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Thornton C, Scheffler BE, Willett KL. Benzo[a]pyrene decreases global and gene specific DNA methylation during zebrafish development. Environ Toxicol Pharmacol. 2013;36:40–50. doi: 10.1016/j.etap.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierthy JF, Spink BC, Figge HL, Pentecost BT, Spink DC. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin, 12-O-tetradecanoylphorbol-13-acetate and 17 beta-estradiol on estrogen receptor regulation in MCF-7 human breast cancer cells. J Cell Biochem. 1996;60:173–184. doi: 10.1002/(sici)1097-4644(19960201)60:2<173::aid-jcb2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004;56:311–317. doi: 10.1203/01.PDR.0000135998.08025.FB. [DOI] [PubMed] [Google Scholar]
- Grandjean V, Gounon P, Wagner N, Martin L, Bernex F, Cuzin F, Rassoulzadegan M. The miR-124-Sox9 paramutation: RNA-mediated epigenetic control of embryonic and adult growth. Development. 2009;136:3647–3655. doi: 10.1242/dev.041061. [DOI] [PubMed] [Google Scholar]
- Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, Brunet A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature. 2011;479:365–371. doi: 10.1038/nature10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YL, Hsu PC, Hsu CC, Lambert GH. Semen quality after prenatal exposure to polychlorinated biphenyls and dibenzofurans. Lancet. 2000;356:1240–1241. doi: 10.1016/S0140-6736(00)02792-6. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. The fetal basis of adult disease: Role of environmental exposures--introduction. Birth Defects Res A Clin Mol Teratol. 2005;73:131–132. doi: 10.1002/bdra.20119. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. Role of exposure to environmental chemicals in the developmental basis of disease and dysfunction. Reprod Toxicol. 2007;23:257–259. doi: 10.1016/j.reprotox.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Heindel JJ. Animal models for probing the developmental basis of disease and dysfunction paradigm. Basic Clin Pharmacol Toxicol. 2008;102:76–81. doi: 10.1111/j.1742-7843.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Spitsbergen JM, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin alters cardiovascular and craniofacial development and function in sac fry of rainbow trout (Oncorhynchus mykiss) Toxicol Sci. 1999;47:40–51. doi: 10.1093/toxsci/47.1.40. [DOI] [PubMed] [Google Scholar]
- Hutz RJ, Carvan MJ, Baldridge MG, Conley LK, King-Heiden TC. Environmental toxicants and effects on female reproductive function. Tren Reprod Bio. 2006;2:1–11. doi: 10.1901/jaba.2006.2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Mitsui T, Setani K, Tamura M, Kakeyama M, Sone H, Tohyama C, Tomita T. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats disrupts brain sexual differentiation. Toxicol Appl Pharmacol. 2005a;205:98–105. doi: 10.1016/j.taap.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Tamura M, Yamashita J, Suzuki C, Tomita T. Repeated in utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure affects male gonads in offspring, leading to sex ratio changes in F2 progeny. Toxicol Appl Pharmacol. 2005b;206:351–355. doi: 10.1016/j.taap.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Kaufman CK, White RM, Zon L. Chemical genetic screening in the zebrafish embryo. Nat Protoc. 2009;4:1422–1432. doi: 10.1038/nprot.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden TC, Hutz RJ, Carvan MJ., 3rd Accumulation, tissue distribution, and maternal transfer of dietary 2,3,7,8,-tetrachlorodibenzo-p-dioxin: impacts on reproductive success of zebrafish. Toxicol Sci. 2005;87:497–507. doi: 10.1093/toxsci/kfi201. [DOI] [PubMed] [Google Scholar]
- King-Heiden TC, Carvan MJ, 3rd, Hutz RJ. Inhibition of follicular development, vitellogenesis, and serum 17beta-estradiol concentrations in zebrafish following chronic, sublethal dietary exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2006;90:490–499. doi: 10.1093/toxsci/kfj085. [DOI] [PubMed] [Google Scholar]
- King-Heiden TC, Spitsbergen J, Heideman W, Peterson RE. Persistent adverse effects on health and reproduction caused by exposure of zebrafish to 2,3,7,8-tetrachlorodibenzo-p-dioxin during early development and gonad differentiation. Toxicol Sci. 2009;109:75–87. doi: 10.1093/toxsci/kfp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Heiden TC, Mehta V, Xiong KM, Lanham KA, Antkiewicz DS, Ganser A, Heideman W, Peterson RE. Reproductive and developmental toxicity of dioxin in fish. Mol Cell Endocrinol. 2012;354:121–138. doi: 10.1016/j.mce.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki S, Matoba S, Akiyoshi M, Matsumura Y, Miyachi H, Mise N, Abe K, Ogura A, Wilhelm D, Koopman P, Nozaki M, Kanai Y, Shinkai Y, Tachibana M. Epigenetic regulation of mouse sex determination by the histone demethylase Jmjd1a. Science. 2013;341:1106–1109. doi: 10.1126/science.1239864. [DOI] [PubMed] [Google Scholar]
- Lau C, Rogers JM. Embryonic and fetal programming of physiological disorders in adulthood. Birth Defects Res C Embryo Today. 2004;72:300–312. doi: 10.1002/bdrc.20029. [DOI] [PubMed] [Google Scholar]
- Liew WC, Orban L. Zebrafish sex: a complicated affair. Brief Funct Genomics. 2014;13:172–187. doi: 10.1093/bfgp/elt041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chang J, Zhao Y, Zhu G. Changes of thyroid hormone levels and related gene expression in zebrafish on early life stage exposure to triadimefon. Environ Toxicol Pharmacol. 2011;32:472–477. doi: 10.1016/j.etap.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Lohr H, Hammerschmidt M. Zebrafish in endocrine systems: recent advances and implications for human disease. Ann Rev Physiol. 2011;73:183–211. doi: 10.1146/annurev-physiol-012110-142320. [DOI] [PubMed] [Google Scholar]
- Mably TA, Moore RW, Goy RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin. 2 Effects on sexual behavior and the regulation of luteinizing hormone secretion in adulthood. Toxicol Appl Pharmacol. 1992;114:108–117. doi: 10.1016/0041-008x(92)90102-x. [DOI] [PubMed] [Google Scholar]
- Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012a;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One. 2012b;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K, Tor M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet. 2006;38:948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- Marczylo EL, Amoako AA, Konje JC, Gant TW, Marczylo TH. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7:432–439. doi: 10.4161/epi.19794. [DOI] [PubMed] [Google Scholar]
- Marshall-Graves JA, Peichel CL. Are homologies in vertebrate sex determination due to shared ancestry or to limited options? Genome Biol. 2010;11:e205. doi: 10.1186/gb-2010-11-4-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaad C, Entezami F, Massade L, Benahmed M, Olivennes F, Barouki R, Hamamah S. How can chemical compounds alter human fertility? Eur J Obstet Gynecol Reprod Biol. 2002;100:127–137. doi: 10.1016/s0301-2115(01)00441-9. [DOI] [PubMed] [Google Scholar]
- Mirbahai L, Williams TD, Zhan H, Gong Z, Chipman JK. Comprehensive profiling of zebrafish hepatic proximal promoter CpG island methylation and its modification during chemical carcinogenesis. BMC Genomics. 2011;12:e3. doi: 10.1186/1471-2164-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Ferrari E, Patterson DG, Jr, Kieszak SM, Brambilla P, Vincoli N, Signorini S, Tramacere P, Carreri V, Sampson EJ, Turner WE, Needham LL. Paternal concentrations of dioxin and sex ratio of offspring. Lancet. 2000;355:1858–1863. doi: 10.1016/S0140-6736(00)02290-X. [DOI] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Patterson DG, Jr, Milani S, Limonta G, Bertona M, Signorini S, Tramacere P, Colombo L, Crespi C, Brambilla P, Sarto C, Carreri V, Sampson EJ, Turner WE, Needham LL. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ Health Perspect. 2008;116:70–77. doi: 10.1289/ehp.10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarelli P, Gerthoux PM, Needham LL, Patterson DG, Jr, Limonta G, Falbo R, Signorini S, Bertona M, Crespi C, Sarto C, Scott PK, Turner WE, Brambilla P. Perinatal exposure to low doses of dioxin can permanently impair human semen quality. Environ Health Perspect. 2011;119:713–718. doi: 10.1289/ehp.1002134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAS-IOM. Veterans and Agent Orange: Update 2010. The National Academies Press; 2011. [Google Scholar]
- Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7:e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Nakajima H, Ryo H, Li LY, Fukudome Y, Adachi S, Gotoh H, Tanaka H. Transgenerational transmission of radiation- and chemically induced tumors and congenital anomalies in mice: studies of their possible relationship to induced chromosomal and molecular changes. Cyto Gen Res. 2004;104:252–260. doi: 10.1159/000077499. [DOI] [PubMed] [Google Scholar]
- Pelclova D, Urban P, Preiss J, Lukas E, Fenclova Z, Navratil T, Dubska Z, Senholdova Z. Adverse health effects in humans exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Rev Environ Health. 2006;21:119–138. doi: 10.1515/reveh.2006.21.2.119. [DOI] [PubMed] [Google Scholar]
- Peterson RE, Theobald MG, Kimmel GL. Developmental and reproductive toxicity of dioxins and related compounds: cross-species comparisons. Crit Rev Toxicol. 1993;23:283–335. doi: 10.3109/10408449309105013. [DOI] [PubMed] [Google Scholar]
- Poland A, Bradfield C. A brief review of the Ah locus. Tohoku J Exp Med. 1992;168:83–87. doi: 10.1620/tjem.168.83. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E. An estimate of the maximum in vivo covalent binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to rat liver protein, ribosomal RNA, and DNA. Cancer Res. 1979;39:3341–3344. [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruden DM, Lu X. Hsp90 affecting chromatin remodeling might explain transgenerational epigenetic inheritance in Drosophila. Curr Genomics. 2008;9:500–508. doi: 10.2174/138920208786241207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S, Wang F, Porter W, Duan R, McDougal A. Ah receptor agonists as endocrine disruptors: antiestrogenic activity and mechanisms. Toxicol Lett. 1998;102–103:343–347. doi: 10.1016/s0378-4274(98)00331-2. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Ann Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Segner H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:187–195. doi: 10.1016/j.cbpc.2008.10.099. [DOI] [PubMed] [Google Scholar]
- Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Repro Toxicol (Elmsford, N Y) 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skogen JC, Overland S. The fetal origins of adult disease: a narrative review of the epidemiological literature. J R Soc Med Short Reports. 2012;3:e59. doi: 10.1258/shorts.2012.012048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson HI, Bradfield CA. The AH-receptor: genetics, structure and function. Pharmacogenetics. 1993;3:213–230. doi: 10.1097/00008571-199310000-00001. [DOI] [PubMed] [Google Scholar]
- Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD, Hanson MA, Roseboom TJ. Transgenerational effects of prenatal exposure to the 1944–45 Dutch famine. BJOG. 2013;120:548–553. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- Wagner KD, Wagner L, Ghanbarian H, Grandjean V, Gounon P, Cuzin F, Rassoulzadegan M. RNA induction and inheritance of epigenetic cardiac hypertrophy in the mouse. Dev Cell. 2008;14:962–969. doi: 10.1016/j.devcel.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Warner M, Eskenazi B, Olive DL, Samuels S, Quick-Miles S, Vercellini P, Gerthoux PM, Needham L, Patterson DG, Mocarelli P. Serum dioxin concentrations and quality of ovarian function in women of Seveso. Environ Health Perspect. 2007;115:336–340. doi: 10.1289/ehp.9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M, Mocarelli P, Samuels S, Needham L, Brambilla P, Eskenazi B. Dioxin exposure and cancer risk in the Seveso Women’s Health Study. Environ Health Perspect. 2011;119:1700–1705. doi: 10.1289/ehp.1103720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhoff JH, Giselbrecht S, Schmidts M, Schindler S, Beales PL, Tonshoff B, Liebel U, Gehrig J. Development of an automated imaging pipeline for the analysis of the zebrafish larval kidney. PLoS One. 2013;8:e82137. doi: 10.1371/journal.pone.0082137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins AS. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. BioEssays. 1995;17:71–77. doi: 10.1002/bies.950170113. [DOI] [PubMed] [Google Scholar]
- Wirgin I, Roy NK, Loftus M, Chambers RC, Franks DG, Hahn ME. Mechanistic basis of resistance to PCBs in Atlantic Tomcod from Hudson River. Science. 2011;331:1322–1325. doi: 10.1126/science.1197296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann C, Reischl M, Shah AH, Mikut R, Liebel U, Grabher C. Facilitating drug discovery: an automated high-content inflammation assay in zebrafish. J Vis Exp. 2012;65:e4203. doi: 10.3791/4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf CJ, Ostby JS, Gray LE., Jr Gestational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) severely alters reproductive function of female hamster offspring. Toxicol Sci. 1999;51:259–264. doi: 10.1093/toxsci/51.2.259. [DOI] [PubMed] [Google Scholar]
- Xiong KM, Peterson RE, Heideman W. AHR-mediated downregulation of Sox9b causes jaw malformation in zebrafish embryos. Mol Pharmacol. 2008;74:1544–1553. doi: 10.1124/mol.108.050435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa K, Brix AE, Sells DM, Jokinen MP, Wyde M, Orzech DP, Kissling GE, Walker NJ, Nyska A. Reproductive lesions in female Harlan Sprague-Dawley rats following two-year oral treatment with dioxin and dioxin-like compounds. Toxicol Pathol. 2009;37:921–937. doi: 10.1177/0192623309351721. [DOI] [PubMed] [Google Scholar]