Abstract
While it is well established that the axons of adult neurons have a lower capacity for regrowth, some regeneration of certain CNS populations after spinal cord injury (SCI) is possible if their axons are provided with a permissive substrate, such as an injured peripheral nerve. While some axons readily regenerate into a peripheral nerve graft (PNG), these axons almost always stall at the distal interface and fail to re-innervate spinal cord tissue. Treatment of the glial scar at the distal graft interface with chondroitinase ABC (ChABC) can improve regeneration, but most regenerated axons need further stimulation to extend beyond the interface. Previous studies demonstrate that pharmacologically inhibiting kinesin-5, a motor protein best known for its essential role in mitosis but also expressed in neurons, with the pharmacological agent monastrol increases axon growth on inhibitory substrates in vitro. We sought to determine if monastrol treatment after a SCI improves functional axon regeneration. Animals received complete thoracic level 7 (T7) transections and PNGs and were treated intrathecally with ChABC and either monastrol or DMSO vehicle. We found that combining ChABC with monastrol significantly enhanced axon regeneration. However, there were no further improvements in function or enhanced c-Fos induction upon stimulation of spinal cord rostral to the transection. This indicates that monastrol improves ChABC-mediated axon regeneration but that further treatments are needed to enhance the integration of these regrown axons.
Keywords: spinal cord injury, kinesin-5, monastrol, chondroitinase ABC, peripheral nerve grafting, axonal regeneration
Introduction
While it is well established that adult neurons have a lower capacity for regrowth, some regeneration of certain CNS populations after SCI is possible if their axons are provided with a permissive substrate, such as an injured peripheral nerve. While some axons readily regenerate into a PNG, coaxing these same axons to grow beyond the graft has proven to be a greater challenge. Axons almost always stall at the distal interface and fail to re-innervate spinal cord tissue. This is thought to be due at least partly to the inhibitory matrix associated with the glial scar at the graft-host interface. Cleavage of inhibitory sugar moieties of CSPG within the scar at the distal PNG-spinal cord interface with ChABC allows some axons to regenerate out of the graft and re-enter the spinal cord. The axonal regeneration resulting from this combinatorial approach is functional (Tom et al., 2009). Despite ChABC, the percentage of axons exiting the graft is small (<20% of total axons that regenerate into the graft). This suggests that most axons that extend into the PNG need further stimulation to grow beyond the interface. Thus, while modulating the inhibitory matrix of the glial scar promotes some axonal regeneration, developing additional strategies to improve the axons’ intrinsic abilities to regenerate is necessary to increase the potential for functional recovery after injury.
The cytoskeleton is essential to the differentiation and growth of the axon, the regeneration of injured axons, and the motility and navigation of the growth cone. Microtubules, one component of the axonal cytoskeleton, contribute to the structural integrity of axons and also to the intracellular transport of organelles through their interactions with motor proteins. Short microtubules move in the axon anterogradely and retrogradely, with the same forces imposed on the longer microtubules contributing to the structural integrity that regulates whether an axon grows or retracts (Baas et al., 2006). The anterograde transport of short MTs to the growing tip of the axon appears to be a major driving force in promoting axonal extension. Thus, augmenting the forces on microtubules that contribute to axon growth and oppose axon retraction is a potentially powerful strategy to enhance axon regeneration after injury.
Interestingly, it was recently discovered that the motor protein kinesin-5 (also known as Eg5, a motor protein best known for its essential role in mitosis) acts as a “brake” to immobilize short microtubules in juvenile axons (Myers and Baas, 2007). Furthermore, inhibition of kinesin-5 (either pharmacologically with the drug monastrol or with siRNA) markedly increases the frequency of short microtubule transport and diminishes bouts of axonal retraction, presumably due to the effects of the kinesin-5 on short and long microtubules respectively. Additionally, treatment with monastrol, a cell-permeable small molecule that prevents normal kinesin-5 ATPase activity, allows adult axons to partially overcome normally inhibitory cues and grow on substrates, such as CSPG, in vitro (Lin et al., 2011).
In this study, we sought to determine if these in vitro results translate to an in vivo setting to improve axonal regeneration beyond a PNG interface that is also treated with ChABC. Additionally, we wanted to assess if enhanced regeneration mediates functional recovery.
Methods and Materials
Spinal cord injuries
All procedures complied with Drexel University’s Institutional Animal Care and Use Committee and National Institutes of Health guidelines for experimentation with laboratory animals. Adult female Sprague-Dawley rats (Charles River, 225–250 grams) were deeply anesthetized with isoflurane. The dorsal surface of the of thoracic level 7 (T7) was exposed by laminectomy and the spinal cord was completely transected by aspiration. A heterologous tibial nerve was harvested from a donor rat that received a tibial nerve cut one week prior to harvest. Four segments of the harvested predegenerated tibial nerve were grafted into the fresh cavity so that they spanned the lesion. The dura was sutured shut using 9-0 sutures. Then, an intrathecal catheter (ReCathCo) attached to an osmotic minipump (Alzet, model 2002) was placed subdurally so that the end of the catheter was just rostral to the distal PNG-host interface. The minipump was filled with 20U/mL ChABC (Amsbio) thermostabilized in trehalose/PBS (Lee et al., 2010) and either 1mM monastrol (EMD Biosciences) or DMSO vehicle. To prevent rejection of the PNG, the recipient animals received daily subcutaneous injections of Cyclosporine A (10mg/kg, Sandimmune, Novartis Pharmaceuticals) starting 3 days before the injury and grafting for the duration of the experiment.
Behavioral testing
Hindlimb function for animals treated with ChABC+DMSO (n=8) and ChABC+monastrol (n=8) was evaluated in an open field measuring 2.5 × 3 feet using the Basso-Beattie-Bresnahan (BBB) rating scale by two individuals blinded to the treatment condition prior to injury and every other week for 13 weeks starting one week after the transection and grafting. The scores of the two groups were compared at each time point using a Mann-Whitney U test (SPSS).
Electrical stimulation of the PNG
Similar to previously methodology (Tom et al., 2009), at least 13 weeks after the transection and grafting, animals (n=5 per experimental group) were anesthetized with ketamine and xylazine and the T4 spinal cord was exposed by laminectomy. A multi-unit electrode (A4×4 3mm 100–125–177, NeuroNexus) was used to stimulate spinal cord rostral to the PNG for one hour (1 mA amplitude, 100 μs pulse duration, and 50 Hz frequency). Animals were killed and perfused one hour later with 4% PFA.
Labeling of regenerated axons in PNG
To trace axons that regenerated into the PNG, animals were deeply anesthetized. At least 13 weeks after the transection and grafting, the portion of the spinal column containing the lesion was exposed and 10% biotinylated dextran amine (BDA, Invitrogen) was injected into the PNG using a glass pipette to label regenerating axons [n=3 animals per group] (Tom et al., 2009)]. Animals were perfused with 4% PFA one week later.
Histology
Segments of spinal cord containing the PNGs were dissected and post-fixed overnight in 4% PFA at 4°C. The tissue then was cryoprotected in 30% sucrose before sectioning on a cryostat. To visualize BDA-labeled axons in the animals injected with BDA, horizontal sections (25 μm) were cut in a series of six sets. Sections were blocked in 5% normal goat serum, 1% bovine serum albumin, 0.1% Triton X-100 in PBS for 1 hour at room temperature and then incubated with anti-GFAP (Dako) overnight at 4°C. The sections were rinsed in PBS and incubated in streptavidin-Alexa 488 or goat anti-rabbit Alexa 594 (Invitrogen) overnight at 4°C. Sections were rinsed in PBS, mounted onto glass slides, and coverslipped using FluorSave (Calbiochem).
For animals that were electrically stimulated, transverse sections of spinal cord within 1.5mm caudal to the distal interface was sectioned in a series of six, blocked in the same blocking solution described above, and incubated with an antibody to c-Fos (Abcam, Cambridge, MA). The sections were rinsed and incubated with goat-anti rabbit Alexa 594 overnight at 4°C. Sections were rinsed in PBS, mounted onto glass slides, and coverslipped using FluorSave (Calbiochem).
All sections were imaged using an Olympus BX51 microscope.
Quantification
To quantify BDA+ regenerating axons that emerged from PNGs, horizontal sections (five sections per animal) containing a PNG were used for image analysis. The number of BDA+ axons located in the PNG just rostral to and just caudal to the distal interface was counted. The ratio of number of axons distal to the graft to axons caudal to the graft was calculated. The ratios of the ChABC+monastrol and ChABC+DMSO were compared statistically using a student’s t test (SPSS). Significance was determined if p < 0.05.
To quantify the number of c-Fos+ cells after stimulation of the rostral cord, four transverse sections immediately caudal to the PNG from each electrophysiologically stimulated animal were imaged. A predetermined saturation threshold level (MetaMorph, Molecular Devices, Sunnyvale, CA) was applied to each section to eliminate background or non-specific staining. All images were subject to the same saturation threshold. Cells with c-Fos+ nuclei above threshold were counted in gray matter ipsilateral and contralateral to the graft. The numbers of ipsilateral c-Fos+ nuclei were statistically compared a student’s t test (SPSS). Significance was determined if p< 0.05.
Results
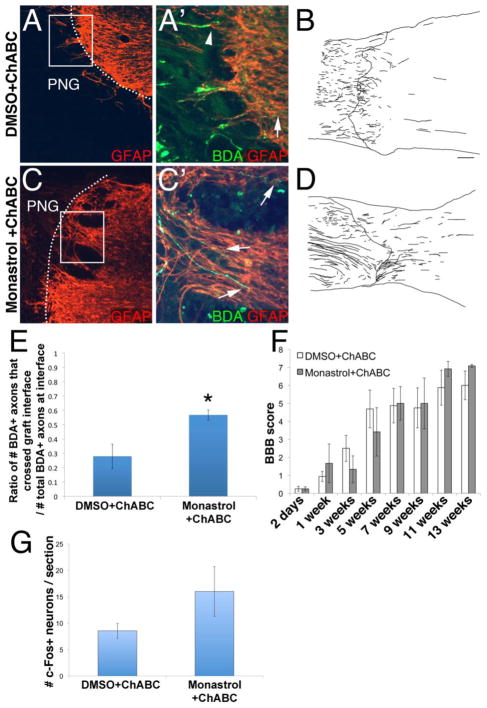
We wanted to determine if inhibiting kinesin-5 pharmacologically with monastrol improves the ability of axons to regenerate. To do this, a PN was grafted into T7 transection site. The distal interface was treated with ChABC and monastrol or DMSO vehicle. Three months later, axons that regenerated into the grafts were labeled with BDA. In animals treated with DMSO, some axons were able to cross the distal interface and extend into caudal spinal cord (Figure 1a′, arrow, b). However, many axons did not extend beyond this boundary (arrowheads in Figure 1a′), agreeing with previous data indicating that ChABC alone promotes limited regeneration. Animals treated with monastrol displayed significantly enhanced regeneration. Many more axons were observed crossing the distal interface to reinnervate spinal cord (arrows in Figure 1c, d). Approximately 57% ± 3.5% of axons present at the distal end of the PNG were able to extend into GFAP+ rich spinal cord in the monastrol-treated animals, whereas ~28% ± 8.4% were able to in the DMSO-treated animals (Figure 1e, p<0.05).
Figure 1. Monastrol treatment enhances regeneration beyond a peripheral nerve graft and through a chondroitinase-treated glial scar.
Axons that regenerated into the graft were labeled with BDA (green). GFAP+ host astrocytes are in red. Insets are high magnification images of boxed regions. While some axons regenerated through the ChABC-treated interface (d), most axons failed to extend into caudal spinal cord (arrows; a′). On the other hand, significantly more axons emerged from the graft (arrowheads, b′, d, e) to reinnervate spinal cord tissue following monastrol treatment. The functional relevance of the axons that regenerated out of the PNG in the ChABC and DMSO or monastrol-treated animals was examined. In the BBB test (f), the scores of both sets of animals improved over time after the injury. However, the scores between the two groups were not significantly different at any of the tested time points. Additionally, spinal cord rostral to the PNG was electrically stimulated. Though this resulted in the induction of c-Fos in neurons caudal to the injury in both groups, there was no significant difference in the number of c-Fos+ neurons (g). c: caudal; scale bar: 100um.; * = p<0.03
To determine if monastrol-enhanced regeneration had functional relevance and improved functional recovery, we tested the ability of these T7 transected rats to locomote in the open field. DMSO and monastrol-treated animals had similar BBB scores at all testing time points (Figure 1f). At 2 days after injury, BBB scores for DMSO animals were 0.25 ± 0.1 and monastrol animals were 0.25 ± 0.1. At one week, BBB scores improved to 0.9 ± 0.3 for DMSO animals and 1.67 ± 1.1 for monastrol animals. The BBB scores continued to improve during the testing period of 13 weeks: 3 weeks (DMSO: 2.5 ± 0.7; monastrol: 1.3 ± 0.7); 5 weeks (DMSO: 4.7 ± 1.0; monastrol: 3.4 ± 1.3); 7 weeks (DMSO: 4.9 ± 1.0; monastrol: 5.0 ± 0.9); 9 weeks (DMSO: 4.75 ± 1.1; monastrol: 5.0 ± 1.4); 11 weeks (DMSO: 5.9 ± 1.0; monastrol: 6.9 ± 0.4). At 13 weeks, scores for both groups (DMSO: 6 ± 0.8; monastrol: 7.1 ± 0.1) indicated that the animals were capable of extensive movement of the joints at the hip, knee, and ankle. Thus, both cohorts of ChABC-treated animals were better able to locomote in the open-field. However, the monastrol treatment did not further improve the ability of completely transected ChABC-treated animals to locomote.
Though improved regeneration did not enhance locomotor function, we wanted to further assess integration of the regenerated axons by examining the extent of c-Fos induction in spinal cord caudal to the PNG upon stimulation of spinal cord rostral to the PNG. We found that there was no significant difference in the numbers of c-Fos+ neurons (Fig. 1g). DMSO-treated animals had 8.5 ± 1.4 c-Fos+ neurons per section while monastrol-treated animals had 16.0 ± 4.7. This indicates that monastrol-mediated axon regeneration did not integrate and form functional synapses upon distal neurons.
Discussion
Although ChABC-mediated digestion of CSPG, a major component of the inhibitory matrix of the glial scar, improves the ability of axons to regenerate, the resulting regeneration is modest. Thus, more robust regeneration may be limited by the meager intrinsic ability of mature axons to extend. Because microtubules play a critical role in axon extension, we sought to determine if inhibiting kinesin-5 in injured axons pharmacologically with monastrol will help overcome this barrier to result in improved regeneration beyond a PNG-cord interface also treated with ChABC. We found that monastrol indeed improved anatomical regeneration. However, these axons did not appear to integrate into circuitry. The monastrol and DMSO treated animals had similar BBB scores. Furthermore, we did not observe any differences in c-Fos induction in neurons upon stimulation of spinal cord rostral to the graft.
Embryonic neurons are capable of significantly better axonal growth than more mature axons. This is probably due to a combination of changes that are extrinsic and intrinsic to the neuron. In particular, the expression of several proteins that help regulate protein synthesis, such as KLF (Krüppel-like factor) and mTOR (mammalian target of rapamycin), are developmentally regulated. Proteins associated with the axonal cytoskeleton are likely targets of this transcriptional control (Blackmore, 2012).
We chose to focus on monastrol to affect microtubules, one component of the axon cytoskeleton, because we were building upon previous work demonstrating that monastrol treatment to pharmacologically inhibit kinesin-5 activity in neurites in vitro improves growth, including on inhibitory CSPG substrates (Lin et al., 2011). One benefit of using monastrol, a pharmacological agent, is that we can more closely control how long we affect kinesin-5 than with other techniques, such as genetic knockdown. Moreover, pharmacologically inhibiting kinesin-5 activity is currently being tested as an anti-cancer treatment in several clinical trials. Thus, in the future, we may build upon the data presented in this manuscript to determine if other similarly-acting drugs are more effective than monastrol at promoting growth.
It is important to note that other drugs that affect microtubule behaviors have also been shown to promote axonal extension. The microtubule-stabilizer taxol (paclitaxel) has been shown to promote axonal regeneration (Hellal et al., 2011, Sengottuvel et al., 2011). NAP, a short, 8-amino acid peptide, is thought to protect microtubules from degradation. Interestingly, NAP has been demonstrated to increase axonal extension in culture (Sudo and Baas, 2011). F05 is a triazine that was shown to increase dynamic microtubules and increase axonal regeneration in vitro on inhibitory substrates and in vivo in dorsal column and optic nerve injury models (Hellal et al., 2011). While how each of these compounds interacts with microtubules is not completely understood, it is clear that they affect microtubule behaviors via different mechanisms. It is not yet known if promoting microtubule stability or some other, subtler effect on microtubule would best promote outgrowth.
Unlike drugs such as taxol or F05, monastrol would not be expected to add to or stabilize microtubule mass, but rather shift the balance of forces on existing microtubules. Given that kinesin-5 is important for growth cone navigation (Nadar et al., 2008), it is perhaps not surprising that the axons in our study could not reintegrate to elicit functional recovery. One possibility is that a better functional outcome could be achieved by optimizing the exposure of the injury site to monastrol for a more limited period of time.
Our focus was on affecting microtubules to enhance regrowth of injured axons, so we used a complete transection model, the best model to assess true regeneration. However, microtubules are not only important for axon extension; dynamic microtubules are also critical for the initiation of collateral branch points. In fact, inhibiting kinesin-5 activity increases neuritic branching in vitro (Myers and Baas, 2007). Interestingly, collateral sprouting has been associated with significant functional improvements. It would be interesting to determine if a microtubule-focused strategy that increases short microtubule transport (e.g. with monastrol) would also improve functionally-compensatory collateral sprouting in an incomplete injury model (i.e. contusion or hemisection).
An earlier concern about the use of kinesin-5 inhibitors to augment regeneration of injured adult axons was whether adult neurons express enough kinesin-5 for its inhibition to elicit a notable effect. Our results suggest that they do. It is also possible that monastrol affects microtubules in glia, although not much is known regarding kinesin-5 expression levels in astrocytes or Schwann cells. Microtubule stabilization via taxol administration diminishes astrocytic migration in vitro and has been suggested to decrease glial scarring after injury (Hellal et al., 2011, Sengottuvel et al., 2011). Furthermore, Schwann cells and astrocytes have “unfavorable” interactions upon their contacting each other, resulting in the formation of sharp boundaries between them that have been linked to regenerative failure (Deng et al., 2008). In our study, we did not notice any appreciable difference in the astrocytic response at the graft interface between monastrol and DMSO treated animals (note similar GFAP expression levels in Figures 1a and c). Thus, we do not believe that the enhanced regeneration observed in the monastrol-treated animals is mediated by altering or attenuating the gliotic response at the injury site.
Although multiple groups, including ours, have shown that an increase in the number of axons into a denervated region after injury can correlate with improved function, it is clear that promoting axon regeneration alone is not always sufficient to improve function. In both this and previous studies, the presence of more fibers does not always translate into improved function. Thus, additional strategies, such as the addition of exogenous factors to promote the integration of regenerated axons or rehabilitation to shape functional synapse formation on appropriate targets need to be utilized.
While we did not directly assess which population of fibers regenerate out of the graft, it is likely that they are of propriospinal and not supraspinal origin. It was previously demonstrated that the closer the injury is to the soma, the greater the increase in regeneration-associated gene expression. As such, supraspinal neurons, e.g. the reticular formation and the red nucleus, regenerate more readily into PNs grafted into a cervical injury site than a thoracic site; grafts placed into thoracic level injuries contain mainly regenerating axons from propriospinal neurons (Richardson et al., 1984). Thus, we can expect that in these experiments in which PNs were inserted into T7Tx sites, primarily propriospinal axons and not descending axons regenerate into the PNGs.
One thing to note is that we observed improved BBB scores over time in both the DMSO+ChABC and monastrol+ChABC groups. It is possible that the improvement is due to regeneration of injured fibers. It is also possible that the improvement is related to neurotrophins secreted from the Schwann cells in the PNG. Neurotrophins, such as brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), can promote some degree of locomotor recovery in the absence of any axon regeneration through a lesion site (Boyce et al., 2007). As we did not resect the spinal cord above the T7 transection site, it is not clear what the mechanism of this improvement is. We will begin to explore the sources of this recovery in future studies.
In conclusion, pharmacologically inhibiting kinesin-5 with monastrol significantly enhanced the ability of axons to regenerate out of a PNG to reinnervate caudal spinal cord after injury. However, this increased anatomical regeneration did not improve functional recovery. Thus, monastrol treatment alone is not sufficient to promote functional axon regeneration but has the potential to be a key component of a combinatorial strategy.
Acknowledgments
This work was funded by the Craig H. Neilsen Foundation (VJT) and NIH/NINDS R01 NS085426 (VJT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baas PW, Vidya Nadar C, Myers KA. Axonal transport of microtubules: the long and short of it. Traffic. 2006;7:490–498. doi: 10.1111/j.1600-0854.2006.00392.x. [DOI] [PubMed] [Google Scholar]
- Blackmore MG. Molecular control of axon growth: insights from comparative gene profiling and high-throughput screening. Int Rev Neurobiol. 2012;105:39–70. doi: 10.1016/B978-0-12-398309-1.00004-4. [DOI] [PubMed] [Google Scholar]
- Boyce VS, Tumolo M, Fischer I, Murray M, Lemay MA. Neurotrophic factors promote and enhance locomotor recovery in untrained spinalized cats. J Neurophysiol. 2007;98:1988–1996. doi: 10.1152/jn.00391.2007. [DOI] [PubMed] [Google Scholar]
- Deng LX, Hu J, Liu NK, Smith GM, Xu XM. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2008. GDNF modifies astrogliotic responses at graft-host interfaces allowing robust axonal regeneration into Schwann cell-seeded guidance channels grafted into hemisected adult rat spinal cords. 2008 Online, vol. Program No. 352.19. [Google Scholar]
- Hellal F, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, McKeon RJ, Bellamkonda RV. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3340–3345. doi: 10.1073/pnas.0905437106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Liu M, Son YJ, Timothy Himes B, Snow DM, Yu W, Baas PW. Inhibition of Kinesin-5, a microtubule-based motor protein, as a strategy for enhancing regeneration of adult axons. Traffic. 2011;12:269–286. doi: 10.1111/j.1600-0854.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KA, Baas PW. Kinesin-5 regulates the growth of the axon by acting as a brake on its microtubule array. The Journal of cell biology. 2007;178:1081–1091. doi: 10.1083/jcb.200702074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadar VC, Ketschek A, Myers KA, Gallo G, Baas PW. Kinesin-5 is essential for growth-cone turning. Curr Biol. 2008;18:1972–1977. doi: 10.1016/j.cub.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Issa VM, Aguayo AJ. Regeneration of long spinal axons in the rat. Journal of neurocytology. 1984;13:165–182. doi: 10.1007/BF01148324. [DOI] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D. Taxol Facilitates Axon Regeneration in the Mature CNS. Journal of Neuroscience. 2011;31:2688–2699. doi: 10.1523/JNEUROSCI.4885-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo H, Baas PW. Strategies for diminishing katanin-based loss of microtubules in tauopathic neurodegenerative diseases. Hum Mol Genet. 2011;20:763–778. doi: 10.1093/hmg/ddq521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom VJ, Sandrow-Feinberg HR, Miller K, Santi L, Connors T, Lemay MA, Houle JD. Combining peripheral nerve grafts and chondroitinase promotes functional axonal regeneration in the chronically injured spinal cord. Journal of Neuroscience. 2009;29:14881–14890. doi: 10.1523/JNEUROSCI.3641-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]