Abstract
Environmental exposures such as toxicants, nutrition and stress have been shown to promote the epigenetic transgenerational inheritance of disease susceptibility. Endocrine disruptors are one of the largest groups of specific toxicants shown to promote this form of epigenetic inheritance. These environmental compounds that interfere with normal endocrine signaling are one of the largest classes of toxicants we are exposed to on a daily level. The ability of ancestral exposures to promote disease susceptibility significantly increases the potential biohazards of these toxicants. Therefore, what your great-grandmother was exposed to during pregnancy may influence your disease development, even in the absence of any exposure, and you are going to pass this on to your grandchildren. This non-genetic form of inheritance significantly impacts our understanding of biology from the origins of disease to evolutionary biology. The current review will describe the previous studies and endocrine disruptors shown to promote the epigenetic transgenerational inheritance of disease.
Keywords: Toxicants, Epimutations, DNA Methylation, Ancestral, Exposure, Environment, Evolutionary Biology, Review
INTRODUCTION
If genetic (DNA sequence) mutations are the cause of disease susceptibility, how come identical twins generally have different disease conditions? How come if someone moves early in life from one region of the world to another that they generally develop the prominent disease conditions of the place they move rather than from where they were born? How come hundreds of environmental toxicants that are associated with disease do not induce DNA sequence mutations? These and other observations suggest environment has a significant impact on disease development [1] (Table 1), and classic genetic mechanisms have difficulty explaining these observations.
Table 1.
Environmental Epigenetic Impacts on Biology & Disease
|
One of the most predominant paradigms in the biological sciences today is “Genetic Determinism”. The concept is that the DNA sequence alone is the building block for biology and that mutations in this sequence are the primary causal factors for most biological phenomenon from disease development to evolutionary biology. This paradigm is the basis for most of our current education programs and theories in biology. The problem is that many phenomenon, Table 1, can not be easily explained with classic genetics or DNA sequence mutation mechanisms alone. An example is the numerous genome wide association studies (GWAS) that have generally shown less than 1% of a specific disease population has a correlated DNA sequence mutation [2,3]. Could it be that an additional mechanism may be involved that we have not seriously considered in the past? It is not that genetics and the DNA sequence are not absolutely critical for biology, it is simply not the whole story.
The additional molecular factor to be considered is “Epigenetics”. Although more traditional definitions exist [4,5], in considering the new science regarding mechanism “Epigenetics” is defined as:
“Molecular factors/processes around the DNA that regulate genome activity independent of DNA sequence, and these processes are mitotically stable”.
The term epigenetics was coined by Dr. Conrad Waddington, University of Edinborough, in the 1940’s to describe gene-environment observations that could not be explained with classic genetics [6], Table 2. In the 1970’s the first epigenetic molecular mark was identified as DNA methylation in which a small (methyl) chemical group is attached to DNA at primarily the cytosine base in animals [7,8]. In the 1990’s the histone proteins DNA is wrapped around were found to also be chemically modified to alter gene expression. In the 2000’s non-coding RNA molecules were identified that can act as epigenetic factors [9]. The coiling, looping and general structure of DNA, termed chromatin structure, is also an epigenetic factor [10]. Therefore, the currently known epigenetic molecular processes are DNA methylation, histone modifications, functional non-coding RNA and chromatin structure, [1] Table 2. All these epigenetic processes are important and have distinct roles in the regulation of how genes are expressed in the genome, independent of DNA sequence. New epigenetic marks and processes will also likely be identified in the future.
Table 2.
History of Epigenetics
| 1940s | Conrad Waddington coined the term epigenetics as environment-gene interaction induced phenotype |
| 1975 | Holliday and Pugh/ Riggs identify DNA methylation |
| 1988 | X- chromosome inactivation and DNA methylation |
| 1990s | Imprinted genes, allelic expression and DNA methylation |
| 1995s | Histone modifications and chromatin structure |
| 2000s | Functional non-coding RNAs |
| 2005s | Epigenome mapping |
The ultimate control of genome activity (i.e. gene expression) will be the combined and cooperative actions of both epigenetic and genetic mechanisms. Two of the most studied epigenetic processes are X-chromosome inactivation and imprinted genes [11,12], Table 2. The female has two X-chromosomes and requires one to be inactivated for normal biology and this has been shown to involve DNA methylation and non-coding RNA. Imprinted genes are a small set of genes that are expressed from either the mother’s (maternal) or father’s (paternal) contributed DNA (allele), but not both. Imprinting has also has been shown to involve DNA methylation and non-coding RNA to control this parent of origin gene expression [11,12]. These are good examples of how epigenetics and genetics cooperate to control genome activity and normal biology.
Environmental Epigenetics
The vast majority of environmental factors and toxicants do not have the ability to alter DNA sequence or promote genetic mutations [13]. In contrast, the environment can dramatically influence epigenetic processes to alter gene expression and development. Therefore, epigenetics provides a molecular mechanism for the environment to directly alter the biology of an organism [1]. “Environmental Epigenetics” is defined as the ability of an environmental factor to directly act and alter epigenetic processes to promote gene expression and phenotype (physiological characteristics) alterations. The altered epigenetic mark(s) at a specific DNA site in response to an environmental factor to influence gene expression is termed an “Epimutation” [5]. Therefore, DNA sequence changes are genetic mutations, while environmentally altered epigenetic sites that influence genome activity are epimutations [5].
There are a number of environmental epigenetic models where direct exposures to environmental factors promote disease development or altered physiological characteristics (i.e. phenotypes). One of the best examples of an animal model is the Agouti mouse where a gestating female is exposed to abnormal nutrition or toxicants that influence a specific DNA methylation site to alter the coat/hair color of the offspring from yellow to brown [14,15]. One of the best examples of a human model is in the late 1950’s and early 1960’s when women in the late stages of pregnancy were exposed to the pharmaceutical diethylstilbesterol (DES) which was shown to promote abnormal uterine and cervical development in the female offspring and grand-offspring [16,17]. Subsequently the phenotypes were found to be due to abnormal epigenetic programming of these organs and critical genes [18,19]. A large number of more recent studies have demonstrated direct exposure to toxicants or abnormal nutrition (caloric restriction or high fat diets) promotes specific epigenetic alterations to influence disease development or physiological phenotypes [20], Table 3.
Table 3.
Direct (Multigenerational) Exposure Epigenetic Phenotypes
| Exposure | Model | Generation | Phenotype |
|---|---|---|---|
| Flutamide (Anti-androgenic Pharmaceutical) | Rat | F1, F2 | Testis Defect |
| Diethylstilbesterol (DES) (Pharmaceutical Estrogen) | Mouse | F1, F2 | Female Reproductive Tract Abnormalities |
| High Fat Diet (Nutrition) | Mouse | F1 | Metabolic Disease |
| Caloric Restriction (Nutrition) | Human | F1, F2 | Metabolic Disease |
| Alcohol (Toxicant) | Mouse | F1 | Skull and Facial Abnormalities |
| Bisphenol A (BPA) (Toxicant) | Agouti Mouse | F1 | Coat Color Change |
| Genistein (Estrogenic Plant Compound) | Agouti Mouse | F1 | Coat Color Change and Obesity |
These direct exposures to environmental factors include nutrition, stress, temperatures, pharmaceuticals, synthetic chemicals and environmental toxicants. Epigenetic effects have been observed in nearly all organisms studied from plants to humans. Generally exposures at critical windows of early development (fetal, birth, puberty) have the most dramatic impact on later life disease development or abnormal physiology. This developmental concept is referred to as the developmental origins of health and disease [21]. Since epigenetics and genetics cooperate in regulating genome activity (gene expression), a cascade of genetic and epigenetic events are required to achieve normal adult development (differentiation) [4], Figure 1. The direct environmental exposure at a critical window of early development can alter the epigenetic programming that subsequently influences genetic programming and gene expression. The result is an environmentally modified versus normal adult differentiated (mature) state that has an altered epigenome and transcriptome which later in life promotes the susceptibility to develop disease or abnormal physiology, Figure 1. Epigenetics provides a molecular process to allow the environment to cooperate with genetic processes to influence the phenotypes and biology of the individual. This is a normal component of biology that can be altered by abnormal environmental conditions during development.
Figure 1.
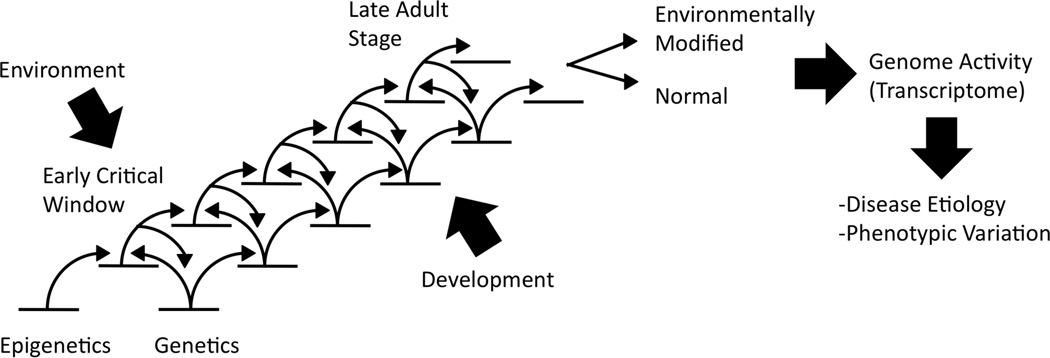
Epigenetic and genetic cascade of events involved in development. Modified from [4].
Epigenetic Transgenerational Inheritance
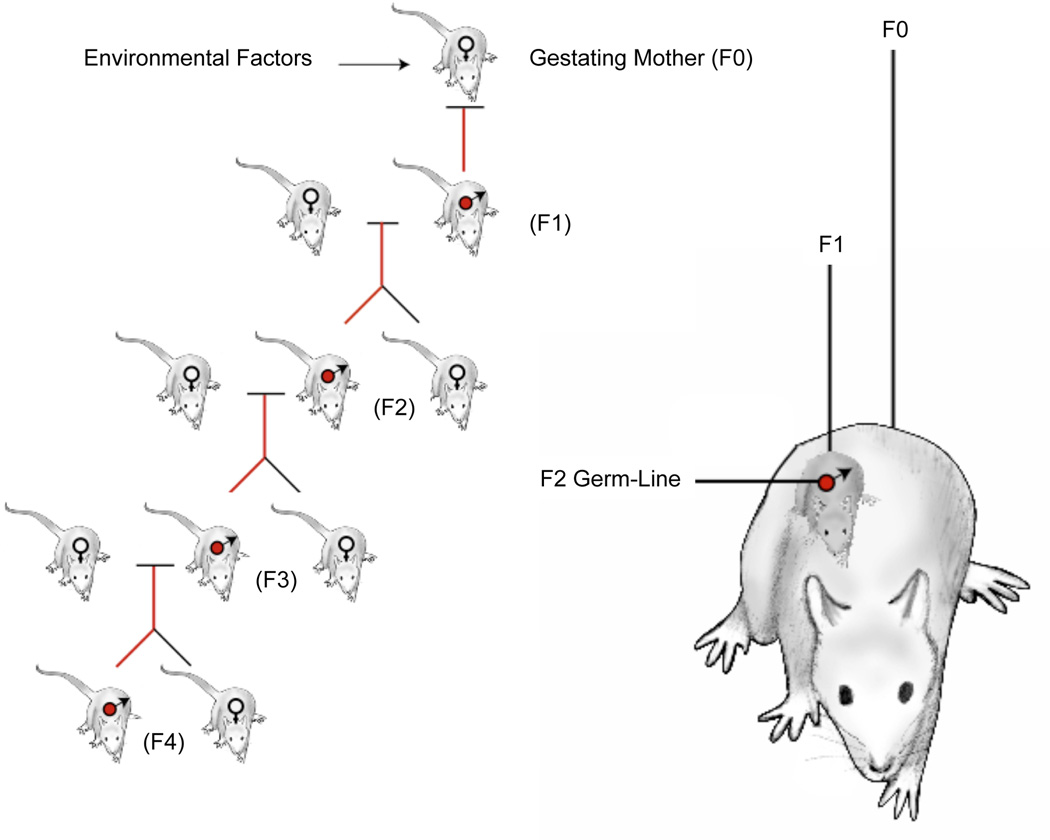
During an investigation of the actions of two environmental toxicants, endocrine disruptors, on the process of gonadal (testis and ovary) development in the fetus, effects on the adult following this fetal exposure were identified [22]. The F1 generation adult males developed a testis abnormality. When the F1 generation offspring was bred to generate the F2 generation (grand-offspring) the F2 generation adult males were found to have the same testis defects as the F1 generation. When the animals were bred to the fourth generation the adult testis defect continued in over 90% of all male progeny [22]. As the animals aged, both males and females developed disease in a variety of organs [23]. The frequency of the abnormality did not decline at each generation, but stayed high suggesting a non-Mendelian phenomenon not following classic genetics. When the male vinclozolin lineage animal was outcrossed to a wildtype female the transgenerational phenotype was maintained at the same frequency, but a reverse outcross of a vinclozolin lineage female to a wildtype male resulted in loss of the phenotype [22]. Therefore, a transgenerational phenomenon was observed that was found to be transmitted through the male germline (sperm), Figure 2. Later experiments with other toxicant exposures have shown transmission through the female germline predominantly [13], such that the transgenerational phenotype is transmitted in a parent of origin allelic manner, similar to imprinted genes.
Figure 2.
Environmentally induced epigenetic transgenerational inheritance through male germline. Exposure of the F0 generation gestating female, F1 generation fetus, and germline within the F1 generation fetus that will generate the F2 generation. Therefore, the F3 generation is the first transgenerational generation not directly exposed. Modified from [24].
In considering transgenerational phenomenon it is essential to distinguish between direct exposure effects versus germline (sperm or egg) mediated transgenerational events. When a gestating female is exposed the F0 generation female, the F1 generation fetus and the germ cell (sperm or egg) that is inside the fetus that will produce the F2 generation are directly exposed, Figure 2. Any effects in the F0, F1 and F2 generations are primarily due to direct exposure toxicity or environmental epigenetics as discussed above. The F3 generation (great grand-offspring) is minimally needed to assess transgenerational phenomenon, since direct exposure effects are not involved [24], Figure 2. In contrast, in the event an adult male or non-pregnant female are exposed the F0 generation adult and germ cell that will generate the F1 generation are directly exposed, such that examination of the F2 generation (grand-offspring) is required to demonstrate a transgenerational phenomenon [24]. When multiple generations are directly exposed, Figure 2, this is referred to as a multigenerational exposure which has been shown with a variety of exposures [5,24], Table 3. The ability to transmit information from one generation to the next requires the sperm or egg such that transgenerational events are germ cell mediated [5].
In considering the development of the germ cell (sperm or egg) there are several critical stages of development where dramatic epigenetic programming occurs [25,26]. The first is when the stem cells (precursor cells) for the germ cells called primordial germ cells develop during fetal development prior to and during the time of testis and ovary development. The DNA methylation of these cells is predominantly erased and then re-methylation occurs during testis and ovary maturation. The second is when the sperm and egg come together at fertilization and the DNA contributed by the sperm and egg again are demethylated to create the embryonic stem cells [25,26]. This epigenetic programming allows a cell to develop pluripotency. Interestingly, when the exposures to toxicants or abnormal nutrition occurs during fetal testis or ovary development the epigenetic programming or DNA methylation of the germ cell can become reprogrammed and transmit the altered epigenetic information to the next generation [5,22]. A set of imprinted gene sites have been shown to be protected from DNA methylation erasure at fertilization in a sex specific manner [27] such that they transmit the epigenetic information to all subsequent generations [5]. When these normal sperm and egg epigenetic programming events are altered [28] they have the ability to transmit this epigenetic information transgenerationally.
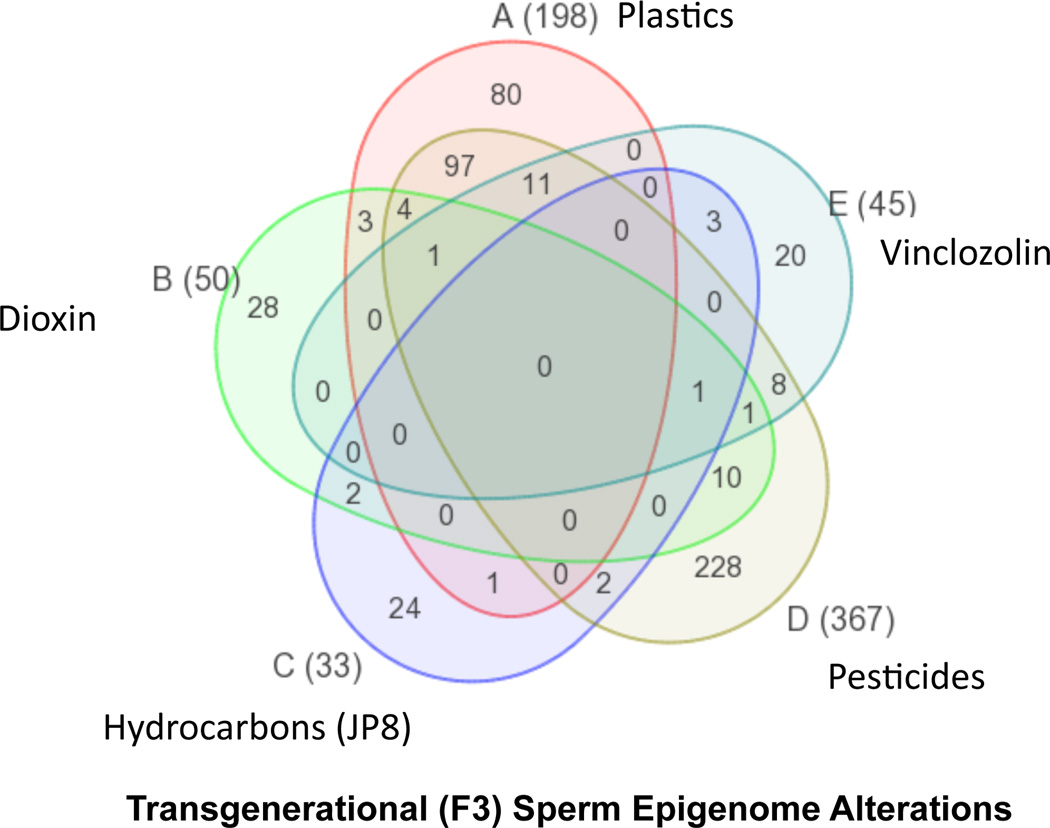
If the germ cell (sperm or egg) is transmitting epigenetic information transgenerationally then altered epigenetic marks (epimutations) should be observed. Analysis of the F3 generation (great-grand-offspring) sperm from environmental toxicant lineage versus control lineage males were found to have epimutations with altered DNA methylation [29,30]. Interestingly, a variety of different environmental toxicants shown to promote transgenerational disease were each found to promote a unique signature or pattern of epimutations in the F3 generation sperm [30], Figure 3. This figure shows a fungicide vinclozolin with 45 epimutations, plastics (BPA and phthalate) derived compounds with 198 epimutations, pesticides with 367 epimutations, hydrocarbons (jet fuel) with 33 epimutations, and dioxin with 50 epimutations. The inner circle shows 0 overlap between these epimutations and the outer portion of each exposure circle has the majority of epimutations unique to the exposure [30], Figure 3. Therefore, these various environmental toxicants promote the epigenetic transgenerational inheritance of exposure specific sperm epimutations. These exposure specific epimutation signatures may be used in the future as biomarkers/diagnostics for your ancestral exposure and future disease susceptibility.
Figure 3.
Ancestral exposure specific epimutation biomarkers. Transgenerational F3 generation sperm differential DNA methylation regions (epimutations) with the total listed next to exposure in brackets and Venn diagram showing overlap between the exposure epimutation. Modified from [30].
The “Epigenetic Transgenerational Inheritance” is defined as [5]:
“Germline (sperm or egg) transmission of epigenetic information between generations in the absence of any direct exposures or genetic manipulations”.
A number of different environmental toxicants (e.g. endocrine disruptors) have been shown to promote the epigenetic transgenerational inheritance of disease or abnormal phenotypes [31], Table 4. These toxicants range from fungicides [22,23], pesticides [32,33], industrial contaminants [34], plastics [35–39], to hydrocarbons [40]. In addition to environmental toxicants, nutrition also can promote the epigenetic transgenerational inheritance of disease and abnormal physiologies [41–43]. This can include high fat diets and caloric restriction. Good examples are famine human populations in Sweden [44] and Holland [45] that have been shown to have generational disease phenotypes. Another environmental factor shown to promote transgenerational inheritance is stress [46–48]. In addition, individuals stress responses can be altered by ancestral exposures in the transgenerational generations [49]. In plants, cold temperature and drought have been shown to promote epigenetic transgenerational inheritance of flowering and growth characteristics [50,51]. A wide variety of environmental factors have been shown to induce the phenomenon, Table 4. Epigenetic transgenerational inheritance phenomenon have been shown in plants [50–52], worms [53,54], flies [55,56], fish [57], rodents [22,58], pigs [59] and humans [44,45].
Table 4.
Exposure Induced Epigenetic Transgenerational Inheritance
| Endocrine Disruptor Exposures | |
| Vinclozolin (Agricultural Fungicide) | Anway, et al. 2005; Anway, et al. 2006 [22,23] |
| Methoxychlor (Agricultural Pesticide) | Anway, et al. 2005; Manikkam, et al. 2014 [22,91] |
| TCDD/Dioxin (Industrial Contaminant) | Manikkam, et al. 2012; Bruner-Tran, 2011 [34,77] |
| Plastics (Bisphenol-A, Phthalate-DEHP & DBP) | Manikkam, et al. 2013; Manikkam, et al. 2012 [30,36] |
| Jet Fuel [JP8] (Hydrocarbon Mixture) | Tracey, et al. 2013 [40] |
| Permethrin & DEET Pesticide & Insect Repellent | Manikkam, et al. 2012 [32] |
| DDT (Pesticide) | Skinner, et al. 2013 [60] |
| Bisphenol A (BPA) (Plastic Toxicant) | Salian, et al. 2009; Wolstenholme, et al. 2012 [35,38] |
| Phthalates (Plastic Toxicant) | Doyle, et al. 2013 [39] |
| Tributyltin (Industrial Toxicant) | Chamorro-Garcia, et al. 2013 [79] |
| Other Types Exposures | |
| Folate (Nutrition) | Padmanabhan, et al. 2013 [92] |
| High Fat Diet (Nutrition) | Dunn, et al. 2011; Burdge, et al. 2011 [42,93] |
| Caloric Restriction (Nutrition) | Burdge, et al. 2007;Pembrey, et al. 2006; Veenendaal, et al. 2013; Painter, et al. 2008 [41,44,45,94] |
| Temperature & Drought (Plant Flowering and Health) | Song, et al. 2013; Norouzitallab, et al. 2014; Zheng, et al. 2013; Suter, et al. 2013 [50,51,95,96] |
| Stress (Behavioral) | Gapp, 2014; Dias, 2014 [46,47] |
| Smoking (Health) | Pembrey, 2010; Rehan, 2013 [97,98] |
| Alcohol (Health) | Govorko, 2012 [99] |
A variety of different disease and abnormal physiological conditions can be induced transgenerationally [22,23,30,60–63], Table 5. The frequency of these phenotypes range from 10% to greater than 90% depending on the disease, environmental factor involved, and male or female sex. Interestingly, with many of the exposures the vast majority of females develop ovarian disease such as polycystic ovaries [64], which is one of the most common female reproductive diseases in women [65]. Testis abnormalities and sperm cell defects are also very common among the different exposures [66]. The primary tumors developed in either male or female are mammary gland / breast tumors [23,61]. Behavioral effects in regards to anxiety or social recognition are also observed [63,67]. Recently we found the pesticide DDT promotes the susceptibility to develop obesity in the transgenerational F3 generation in greater than 50% of the males and females, but had no effect on the F1 generation obesity frequency [60]. The environmentally induced epigenetic transgenerational inheritance of disease and abnormal physiologies suggest ancestral exposures may have an important role in why the majority of disease conditions in our population have dramatically increased over the past several decades.
Table 5.
Transgenerational Disease and Abnormality Examples
| Disease & Abnormalities | Frequency |
|---|---|
| • Spermatogenic Defect | (>90%) |
| • Male infertility | (Complete ~10%, Severe 20%) |
| • Kidney disease | (~30–40%) |
| • Prostate disease | (~50%) |
| • Increase in mammary tumor formation | (~10–20%) |
| • Behavior (Mate Preference, Anxiety & Stress) | (>90%) |
| • Pre-eclampsia-like during late pregnancy | (~10%) |
| • Premature Ovarian Failure POF/ POI | (>90%) |
| • Polycystic Ovarian Disease | (>90%) |
| • Female Premature Pubertal Onset | (>30%) |
| • Obesity | (~10–50%) |
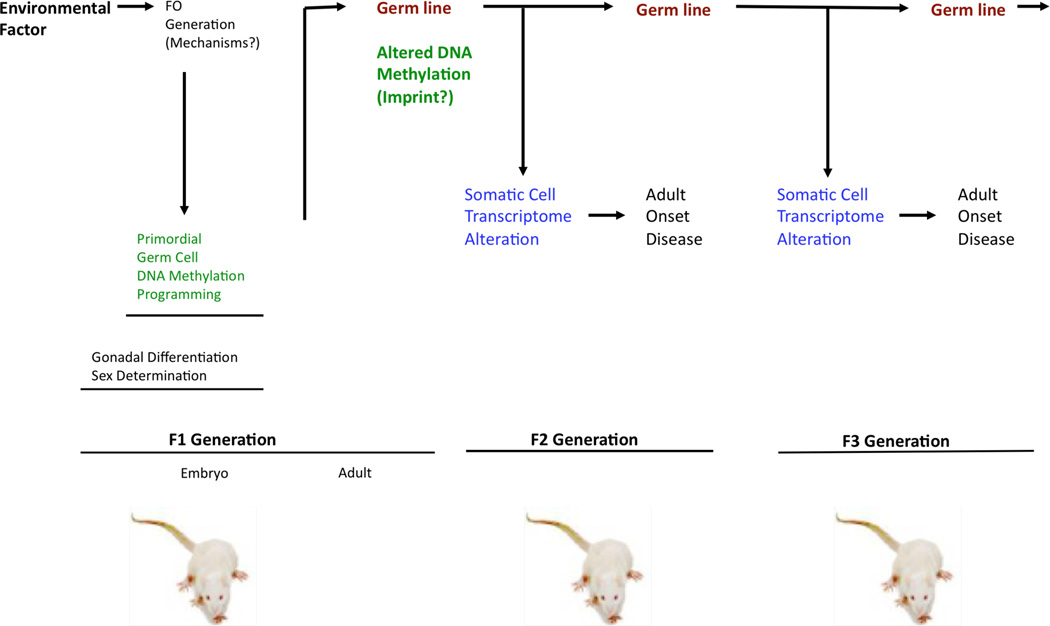
The basic mechanism involved in environmentally induced epigenetic transgenerational inheritance of disease or abnormal physiologies is presented in Figure 4 [5]. The exposure of a gestating female at the critical window of gonadal (testis or ovary) sex determination modifies the epigenetic (e.g. DNA methylation) programming of the germ cell that the F1 generation adult animal will transmit to the F2 generation. All cell types and tissues derived from the developing embryo will have an altered epigenome and transcriptome such that those tissues sensitive to an altered gene expression profile will develop disease or abnormalities as the individual ages. This adult F2 generation individual will then transmit the same germ cell epimutations to the next F3 generation (great-offspring) and the same mechanism and process occur in all subsequent generations, Figure 4. The transgenerational germ cell (sperm) epigenome changes [29,30] and altered transgenerational tissue and cell transcriptomes [64,68,69] have been confirmed. Therefore, environmental exposures can promote this form of non-genetic inheritance through this epigenetic transgenerational inheritance mechanism to promote disease and altered phenotypes. The potential role of this mechanism in our understanding of disease etiology needs to be considered.
Figure 4.
Role of germline in epigenetic transgenerational inheritance. Summary of environmentally induced epigenetic reprogramming of primordial germ cells that leads to the germline transmission of epimutations resulting in all somatic cells having an altered transcriptome that results in disease susceptibility. Modified from [5].
Endocrine Disruptors
As discussed above, a number of environmental toxicants can promote the epigenetic transgenerational inheritance of disease, Table 4. Many of these chemicals act as endocrine disruptors. Endocrine disruptors are defined as environmental chemicals that can interfere with endocrine hormone signaling (e.g. hormone receptor actions) to alter cellular function and health [70–74]. When a chemical can bind to a hormone receptor and act as an agonist or antagonist, or alter the downstream signaling transduction of the hormone, the compound is considered an endocrine disruptor. Some of these compounds are natural substances obtained from our diet. A good example of this is genestein which is a compound found in soy that has estrogenic activity and is a strong estrogen signaling agonist [75]. However, the majority of the endocrine disruptors studied are man made chemicals used in the environment. Nearly all known signal transduction systems are linked to hormone signaling so can be altered by endocrine disruptors. The categorizing of endocrine disruptors often is associated with the specific class of hormones. For example, many compounds have estrogenic activity or block estrogenic hormone action, such as bisphenol A (BPA), DES, and genestein. Others bind to broader spectrum receptors such as the aryl hydrocarbon receptor (AhR) and PPAR/RXR receptors that binds a variety of organic compounds such as dioxin, organophosphates and hydrocarbons. The major endocrine disruptors that have been shown to promote the epigenetic transgenerational inheritance of disease are presented in Table 4.
The initial endocrine disruptor found to promote the epigenetic transgenerational inheritance of disease was the anti-androgenic compound vinclozolin, which is one of the most commonly used agricultural fungicides world wide [22,23,76]. The pesticide methoxychlor is a mixed estrogenic, antiestrogenic and anti-androgenic endocrine disruptor shown to promote transgenerational disease [22,33,76]. The industrial contaminant dioxin binds the AhR receptor and promotes transgenerational disease [34,77]. The plastic derived estrogenic compound BPA has been shown to promote transgenerational disease [30,35,36] and behavioral abnormalities [37,38,78]. The plastic derived phthalates also promotes transgenerational disease [30,36,39]. The hydrocarbon mixture of jet fuel (JP8) which can associate with AhR also promotes transgenerational disease [40]. A common pesticide and insect repellent (permethrin and DEET) also promotes transgenerational disease [32]. The pesticide DDT is an estrogenic endocrine disruptor and promotes transgenerational disease such as obesity [60]. The industrial biocide tributylin that influence the PPAR/RXR receptors was found to promote transgenerational obesity and metabolic disorder [79]. The studies discussed above, Table 4, are transgenerational studies that demonstrate the germline transmission of phenotypes in absence of direct exposure or genetic manipulation. A number of studies reported in the literature are incorrectly referred to as transgenerational and instead are due to direct environmental exposures [24]. It is anticipated a variety of different endocrine disruptors and other exposures will also promote transgenerational phenotypes when sufficient generations are considered.
Interestingly, the majority of the endocrine disruptors found to promote the epigenetic transgenerational inheritance of disease listed in Table 4 generally promoted similar disease or abnormalities. Testis and ovary disease was the most common, along with kidney and prostate disease [22,23,30,33,34,60]. Those diseases listed in Table 5 have more similarities than differences between the various endocrine disruptor exposures. Although some disease differences occur, such as DDT, jet fuel, and plastics promoting obesity, but not vinclozolin or dioxin, the transgenerational disease phenotypes were often similar. Therefore, exposure specific or signal transduction specific effects were not generally observed.
As shown in Figure 4, since the germline is transmitting an altered epigenome to the embryonic stem cell, all adult cell types that develop will have an altered epigenome and transcriptome [63,68,69]. Although there are exposure specific germline epimutation signatures [30], and tissue and cell specific transgenerational transcriptomes [69], the disease etiology and biology is generally similar between the various exposures. The hypothesis is proposed that in the event a large number of epimutation and gene expression changes are present in tissues that are sensitive to disease, the tissues will develop the disease independent of the specific environmental exposure and transgenerational epigenetic signature. Therefore, if you effect the expression of hundreds of genes in certain cell types, independent of the specific set of genes, a disease susceptibility will exist. This is a more system biology consideration versus a reductionist view involving specific genes or epimutations. Understanding this phenomena and molecular mechanisms involved will significantly enhance our future ability to therapeutically treat, prevent and improve health.
Epigenetics and Evolution
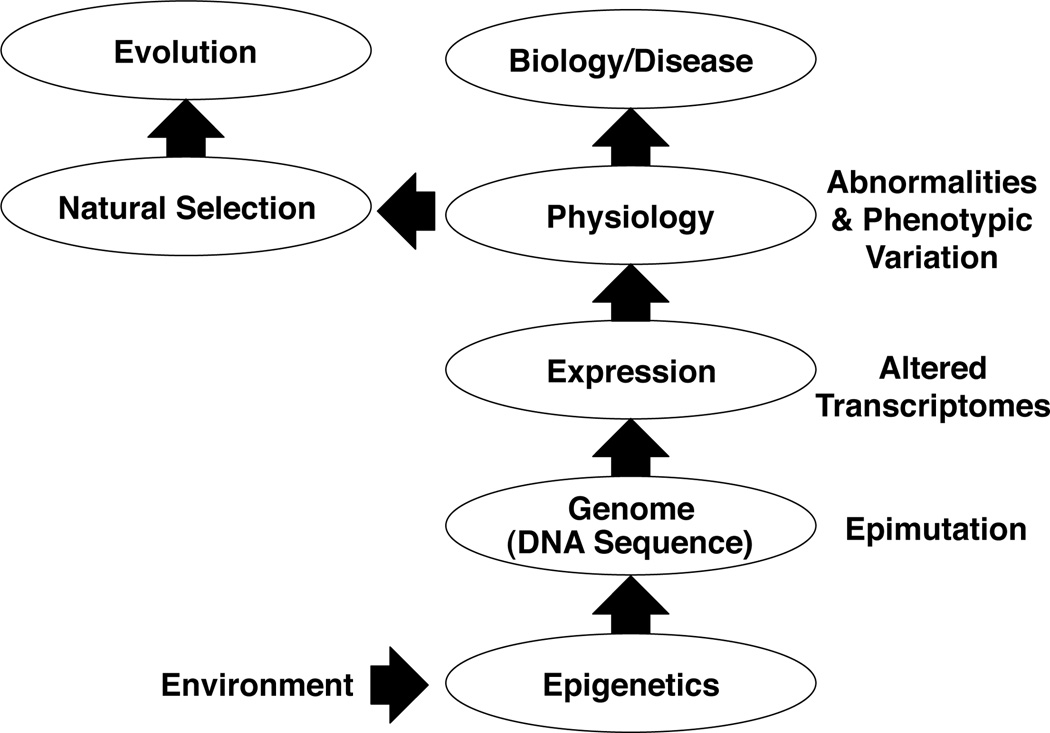
The current molecular mechanism considered in evolutionary biology involves random DNA sequence mutations and other genetic mechanisms such as genetic drift to facilitate neo-Darwinian natural selection events. Nearly all the current models for evolution involve genetic mechanisms. The environment is considered important for the natural selection process, but has not been considered to alter the molecular processes of evolution. The problem with this theory is that the frequency of genetic mutations is extremely rare such that the speed of evolution is difficult to explain. In fact the probability of a random mutation is over 1000 times less than the anticipated frequency of an epigenetic change [80]. Environmental epigenetics and particularly environmentally induced epigenetic transgenerational inheritance may provide a molecular mechanism to enhance the genetic mechanisms currently considered. As discussed, environmental induction of epigenetic change can dramatically increase phenotypic variation that can facilitate natural selection, and epigenetic transgenerational inheritance can allow the continued presence of adapted phenotypes. Therefore, several recent reviews have suggested a role for epigenetics in evolution [81–84].
A previous example provided was that the F3 generation toxicant exposure lineage animals when compared to control lineage animals had different mate preference characteristics [67]. Recently the gene bionetworks in specific regions of the brain have been identified and correlated to the epigenetic transgenerational inheritance of mate preference differences [85]. Mate preference involving biological parameters such as bird feather color and song is a critical component of sexual selection, which Charles Darwin proposed as a major determinant in evolutionary biology [86]. More recently we examined the epimutations and genetic mutations in a number of different species of Darwin’s Finches and found a large number of epimutations that significantly correlated with the phenotypic relatedness (family tree) of the finches [87]. Therefore, data is starting to be obtained that supports an important role for environmental epigenetics and epigenetic transgenerational inheritance in facilitating natural selection and evolution. In addition to a role in disease development, epigenetics will have a role in other areas of biology such as evolution, Figure 5.
Figure 5.
Schematic of environmental effects of epigenetics promoting disease etiology or phenotypic variation to influence evolution.
Conclusions
Scientific observations over the past 200 years have demonstrated a significant impact of environmental exposures on all aspects of biology, but genetics alone can not easily explain many of these observations. Although “Genetic Determinism” has helped elucidate many aspects of biology, such that the DNA sequence and genetics are critical for all of biology, genetics has limitations in its ability to explain major factors such as disease development and evolution, Table 1. Epigenetics provides solutions for these failures of genetic determinism, Figure 5. The current concept for inheritance involves primarily genetics in that your DNA sequence is considered your destiny. The environmentally induced epigenetic transgenerational inheritance discussed provides a form of non-genetic inheritance which we previously did not appreciate [5,88,89]. This significantly impacts how we think about who we are and how our environment may be a significant factor in our health and evolution.
The current concept in science today for disease etiology involves DNA sequence mutations and abnormalities as the primary causal factor. However, environmentally induced epigenetic inheritance of disease will likely be an equally important consideration. Although the concept that our ancestors exposures and the epimutations inherited affects our health has an element of doom and gloom, the simple realization that this mechanism exists and epimutations are present can be used to address the issue. The epimutations can potentially be used to develop diagnostics to assess what your ancestral exposures potentially were and what disease you may be susceptible to develop. These diagnostics could be used to predict a disease development, before the disease develops. Therapeutics could then be potentially created to prevent the disease from developing, which may be more efficient than trying to treat the disease after it has developed. This is termed “preventative medicine” and we have not been able to do this well because we did not have these early stage diagnostics. These epimutations may act as diagnostics to provide the ability to facilitate preventative medicine in the future.
The current concept in evolutionary biology is that random DNA sequence mutations and classic genetic mechanisms promote phenotypic variation that natural selection acts upon to facilitate Darwinian evolution. Although environment is a critical component for natural selection, the ability of environmental factors to promote directly phenotypic variation through epigenetic transgenerational inheritance is a novel concept for evolution. Lamarck proposed in 1800 the ability of environment to promote phenotypic change [90]. Therefore, environmentally induced epigenetic transgenerational inheritance provides a neo-Lamarckian concept that facilitates Darwinian evolution. Epigenetics now needs to be seriously considered as an additional molecular component of evolution.
Consideration of environmentally induced epigenetic transgenerational inheritance is anticipated to have a significant role in all areas of biology. This phenomenon significantly extends our current genetic determinism focus. Environmental epigenetics and epigenetic inheritance will help to better understand how environment (e.g. endocrine disruptors) influences our health and disease.
HIGHLIGHTS.
Epigenetic transgenerational inheritance is a non-genetic form of inheritance.
A variety of endocrine disruptors promote the epigenetic transgenerational inheritance of disease.
Environmental epigenetics provides a molecular mechanism for disease susceptibility and etiology.
Acknowledgement
Acknowledgement of Dr. Eric Nilsson for critical review of the manuscript and Ms. Heather Johnson for assistance in preparation of the manuscript. This research was supported by an NIH grant to MKS.
Glossary
- Epigenetics
Molecular factors/processes around the DNA that regulate genome activity independent of DNA sequence, and these processes are mitotically stable
- Genetic Determinism
The concept is that the DNA sequence alone is the building block for biology and that mutations in this sequence are the primary causal factors for most biological phenomenon from disease development to evolutionary biology.
- Environmental Epigenetics
The ability of an environmental factor to directly act and alter epigenetic processes to promote gene expression and phenotypic change (physiological characteristics).
- Epimutation
The altered epigenetic mark(s) at a specific DNA site in response to an environmental factor that influence gene expression.
- Epigenetic Transgenerational Inheritance
Germline (sperm or egg) transmission of epigenetic information between generations in the absence of any direct exposures or genetic manipulations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao H, Chen ZJ. Genetic association studies in female reproduction: from candidate-gene approaches to genome-wide mapping. Mol Hum Reprod. 2013;19:644–654. doi: 10.1093/molehr/gat040. [DOI] [PubMed] [Google Scholar]
- 4.Skinner MK. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics. 2011;6:838–842. doi: 10.4161/epi.6.7.16537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. 2010;21:214–222. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waddington CH. The epigenotype. Endeavour. 1942;1:18–20. [Google Scholar]
- 7.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–232. [PubMed] [Google Scholar]
- 8.Singer J, Roberts-Ems J, Riggs AD. Methylation of mouse liver DNA studied by means of the restriction enzymes msp I and hpa II. Science. 1979;203:1019–1021. doi: 10.1126/science.424726. [DOI] [PubMed] [Google Scholar]
- 9.Kornfeld JW, Bruning JC. Regulation of metabolism by long, non-coding RNAs. Front Genet. 2014;5:57. doi: 10.3389/fgene.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaniv M. Chromatin remodeling: from transcription to cancer. Cancer Genet. 2014 doi: 10.1016/j.cancergen.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Henckel A, Chebli K, Kota SK, Arnaud P, Feil R. Transcription and histone methylation changes correlate with imprint acquisition in male germ cells. EMBO J. 2012;31:606–615. doi: 10.1038/emboj.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarrey JR. The epigenome as a target for heritable environmental disruptions of cellular function. Mol Cell Endocrinol. 2012;354:9–15. doi: 10.1016/j.mce.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Blewitt M, Whitelaw E. The use of mouse models to study epigenetics. Cold Spring Harb Perspect Biol. 2013;5:a017939. doi: 10.1101/cshperspect.a017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal AJ, Jirtle RL. Epigenomic disruption: the effects of early developmental exposures. Birth Defects Res A Clin Mol Teratol. 2010;88:938–944. doi: 10.1002/bdra.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Kalfa N, Paris F, Soyer-Gobillard MO, Daures JP, Sultan C. Prevalence of hypospadias in grandsons of women exposed to diethylstilbestrol during pregnancy: a multigenerational national cohort study. Fertil Steril. 2011;95:2574–2577. doi: 10.1016/j.fertnstert.2011.02.047. [DOI] [PubMed] [Google Scholar]
- 18.Pistek VL, Furst RW, Kliem H, Bauersachs S, Meyer HH, Ulbrich SE. HOXA10 mRNA expression and promoter DNA methylation in female pig offspring after in utero estradiol-17beta exposure. J Steroid Biochem Mol Biol. 2013;138:435–444. doi: 10.1016/j.jsbmb.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 19.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–3382. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albert O, Jegou B. A critical assessment of the endocrine susceptibility of the human testis to phthalates from fetal life to adulthood. Hum Reprod Update. 2014;20:231–249. doi: 10.1093/humupd/dmt050. [DOI] [PubMed] [Google Scholar]
- 21.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 22.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–5523. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng S, Jacobsen SE, Reik W. Epigenetic reprogramming in plant and animal development. Science. 2010;330:622–627. doi: 10.1126/science.1190614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Messerschmidt DM, Knowles BB, Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014;28:812–828. doi: 10.1101/gad.234294.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seisenberger S, Peat JR, Reik W. Conceptual links between DNA methylation reprogramming in the early embryo and primordial germ cells. Curr Opin Cell Biol. 2013;25:281–288. doi: 10.1016/j.ceb.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Skinner M, Guerrero-Bosagna C, Haque MM, Nilsson E, Bhandari R, McCarrey J. Environmentally Induced Transgenerational Epigenetic Reprogramming of Primordial Germ Cells and Subsequent Germline. PLoS ONE. 2013;8:e66318. doi: 10.1371/journal.pone.0066318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrero-Bosagna C, Settles M, Lucker B, Skinner M. Epigenetic transgenerational actions of vinclozolin on promoter regions of the sperm epigenome. PLoS ONE. 2010;5:e13100. doi: 10.1371/journal.pone.0013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational Actions of Environmental Compounds on Reproductive Disease and Epigenetic Biomarkers of Ancestral Exposures. PLoS ONE. 2012;7:e31901. doi: 10.1371/journal.pone.0031901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of endocrine disruptors. Reprod Toxicol. 2011;31:337–343. doi: 10.1016/j.reprotox.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner M. Pesticide and Insect Repellent Mixture (Permethrin and DEET) Induces Epigenetic Transgenerational Inheritance of Disease and Sperm Epimutations. Reproductive Toxicology. 2012;34:708–719. doi: 10.1016/j.reprotox.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manikkam M, M HM, Guerrero-Bosagna C, Nilsson E, Skinner M. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult onset disease through the female germline. PLoS ONE. 2014;9:e102091. doi: 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS ONE. 2012;7:e46249. doi: 10.1371/journal.pone.0046249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 2009;85:11–18. doi: 10.1016/j.lfs.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner M. Plastics Derived Endocrine Disruptors (BPA, DEHP and DBP) Induce Epigenetic Transgenerational Inheritance of Adult-Onset Disease and Sperm Epimutations. PLoS ONE. 2013;8:e55387. doi: 10.1371/journal.pone.0055387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–839. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolstenholme JT, Edwards M, Shetty SR, Gatewood JD, Taylor JA, Rissman EF, Connelly JJ. Gestational Exposure to Bisphenol A Produces Transgenerational Changes in Behaviors and Gene Expression. Endocrinology. 2012;153:3828–3838. doi: 10.1210/en.2012-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational Effects of Di-(2-ethylhexyl) Phthalate on Testicular Germ Cell Associations and Spermatogonial Stem Cells in Mice. Biol Reprod. 2013;88:112. doi: 10.1095/biolreprod.112.106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tracey R, Manikkam M, Guerrero-Bosagna C, Skinner M. Hydrocarbon (Jet Fuel JP-8) Induces Epigenetic Transgenerational Inheritance of Adult-Onset Disease and Sperm Epimutations. Reproductive Toxicology. 2013;36:104–116. doi: 10.1016/j.reprotox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr. 2007;97:435–439. doi: 10.1017/S0007114507352392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Waterland RA. Epigenetic Mechanisms Affecting Regulation of Energy Balance: Many Questions, Few Answers. Annu Rev Nutr, [Epub ahead of print] 2013 doi: 10.1146/annurev-nutr-071813-105315. [DOI] [PubMed] [Google Scholar]
- 44.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 45.Veenendaal MV, Painter RC, de Rooij SR, Bossuyt PM, van der Post JA, Gluckman PD, Hanson MA, Roseboom TJ. Transgenerational effects of prenatal exposure to the 1944–45 Dutch famine. BJOG. 2013;120:548–553. doi: 10.1111/1471-0528.12136. [DOI] [PubMed] [Google Scholar]
- 46.Dias BG, Ressler KJ. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat Neurosci. 2014;17:89–96. doi: 10.1038/nn.3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gapp K, Jawaid A, Sarkies P, Bohacek J, Pelczar P, Prados J, Farinelli L, Miska E, Mansuy IM. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat Neurosci. 2014;17:667–669. doi: 10.1038/nn.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matthews SG, Phillips DI. Transgenerational inheritance of stress pathology. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 49.Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci U S A. 2012;109:9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song J, Irwin J, Dean C. Remembering the prolonged cold of winter. Curr Biol. 2013;23:R807–R811. doi: 10.1016/j.cub.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Norouzitallab P, Baruah K, Vandegehuchte M, Van Stappen G, Catania F, Vanden Bussche J, Vanhaecke L, Sorgeloos P, Bossier P. Environmental heat stress induces epigenetic transgenerational inheritance of robustness in parthenogenetic Artemia model. FASEB J, (Epub ahead of print) 2014 doi: 10.1096/fj.14-252049. [DOI] [PubMed] [Google Scholar]
- 52.Hauser MT, Aufsatz W, Jonak C, Luschnig C. Transgenerational epigenetic inheritance in plants. Biochim Biophys Acta. 2011;1809:459–468. doi: 10.1016/j.bbagrm.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelly WG. Transgenerational epigenetics in the germline cycle of Caenorhabditis elegans. Epigenetics Chromatin. 2014;7:6. doi: 10.1186/1756-8935-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benayoun BA, Brunet A. Epigenetic memory of longevity in Caenorhabditis elegans. Worm. 2012;1:77–81. doi: 10.4161/worm.19157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buescher JL, Musselman LP, Wilson CA, Lang T, Keleher M, Baranski TJ, Duncan JG. Evidence for transgenerational metabolic programming in Drosophila. Dis Model Mech. 2013;6:1123–1132. doi: 10.1242/dmm.011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grentzinger T, Armenise C, Brun C, Mugat B, Serrano V, Pelisson A, Chambeyron S. piRNA-mediated transgenerational inheritance of an acquired trait. Genome Res. 2012;22:1877–1888. doi: 10.1101/gr.136614.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baker TR, Peterson RE, Heideman W. Using Zebrafish as a Model System for Studying the Transgenerational Effects of Dioxin. Toxicol Sci. 2014;138:403–411. doi: 10.1093/toxsci/kfu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guerrero-Bosagna C, Covert T, Haque MM, Settles M, Nilsson EE, Anway MD, Skinner MK. Epigenetic Transgenerational Inheritance of Vinclozolin Induced Mouse Adult Onset Disease and Associated Sperm Epigenome Biomarkers. Reproductive Toxicology. 2012;34:694–707. doi: 10.1016/j.reprotox.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braunschweig M, Jagannathan V, Gutzwiller A, Bee G. Investigations on transgenerational epigenetic response down the male line in F2 pigs. PLoS ONE. 2012;7:e30583. doi: 10.1371/journal.pone.0030583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skinner MK, Manikkam M, Tracey R, Nilsson E, Haque MM, Guerrero-Bosagna C. Ancestral DDT Exposures Promote Epigenetic Transgenerational Inheritance of Obesity BMC Medicine. 2013;11:228. doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson EE, Anway MD, Stanfield J, Skinner MK. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction. 2008;135:713–721. doi: 10.1530/REP-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anway MD, Skinner MK. Transgenerational effects of the endocrine disruptor vinclozolin on the prostate transcriptome and adult onset disease. Prostate. 2008;68:517–29. doi: 10.1002/pros.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skinner MK, Anway, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova M, Skinner M. Environmentally Induced Epigenetic Transgenerational Inheritance of Ovarian Disease. PLoS ONE. 2012;7:e36129. doi: 10.1371/journal.pone.0036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barthelmess EK, Naz RK. Polycystic ovary syndrome: current status and future perspective. Front Biosci (Elite Ed) 2014;6:104–119. doi: 10.2741/e695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J Androl. 2006;27:868–879. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–5946. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerrero-Bosagna C, Savenkova M, Haque MM, Sadler-Riggleman I, Skinner MK. Environmentally Induced Epigenetic Transgenerational Inheritance of Altered Sertoli Cell Transcriptome and Epigenome: Molecular Etiology of Male Infertility. PLoS ONE. 2013;8:e59922. doi: 10.1371/journal.pone.0059922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Skinner MK, Manikkam M, Haque MM, Zhang B, Savenkova M. Epigenetic Transgenerational Inheritance of Somatic Transcriptomes and Epigenetic Control Regions. Genome Biol. 2012;13:R91. doi: 10.1186/gb-2012-13-10-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bergman A, Heindel JJ, Kasten T, Kidd KA, Jobling S, Neira M, Zoeller RT, Becher G, Bjerregaard P, Bornman R, Brandt I, Kortenkamp A, Muir D, Drisse MN, Ochieng R, Skakkebaek NE, Bylehn AS, Iguchi T, Toppari J, Woodruff TJ. The impact of endocrine disruption: a consensus statement on the state of the science. Environ Health Perspect. 2013;121:A104–A106. doi: 10.1289/ehp.1205448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P, Fischer B, Schaedlich K, Schmidt JS, Amezaga MR, Bhattacharya S, Rhind SM, O’Shaughnessy PJ. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 72.Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, Xu J, Matikainen T, Benito Hernandez A, Nunez G, Casper RF. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest. 2007;117:3971–3978. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Brevik A, Lindeman B, Brunborg G, Duale N. Paternal Benzo[a]pyrene Exposure Modulates MicroRNA Expression Patterns in the Developing Mouse Embryo. Int J Cell Biol. 2012;2012:407431. doi: 10.1155/2012/407431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zama AM, Uzumcu M. Epigenetic effects of endocrine-disrupting chemicals on female reproduction: an ovarian perspective. Front Neuroendocrinol. 2010;31:420–439. doi: 10.1016/j.yfrne.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the female reproductive system by the phytoestrogen genistein. Reprod Toxicol. 2007;23:308–316. doi: 10.1016/j.reprotox.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Paoloni-Giacobino A. Epigenetic effects of methoxychlor and vinclozolin on male gametes. Vitam Horm. 2014;94:211–227. doi: 10.1016/B978-0-12-800095-3.00008-0. [DOI] [PubMed] [Google Scholar]
- 77.Bruner-Tran KL, Osteen KG. Developmental exposure to TCDD reduces fertility and negatively affects pregnancy outcomes across multiple generations. Reprod Toxicol. 2011;31:344–350. doi: 10.1016/j.reprotox.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jang YJ, Park HR, Kim TH, Yang WJ, Lee JJ, Choi SY, Oh SB, Lee E, Park JH, Kim HP, Kim HS, Lee J. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology. 2012;296:73–82. doi: 10.1016/j.tox.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Chamorro-Garcia R, Sahu M, Abbey RJ, Laude J, Pham N, Blumberg B. Transgenerational inheritance of increased fat depot size, stem cell reprogramming, and hepatic steatosis elicited by prenatal exposure to the obesogen tributyltin in mice. Environ Health Perspect. 2013;121:359–366. doi: 10.1289/ehp.1205701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmitz RJ, Schultz MD, Urich MA, Nery JR, Pelizzola M, Libiger O, Alix A, McCosh RB, Chen H, Schork NJ, Ecker JR. Patterns of population epigenomic diversity. Nature. 2013;495:193–198. doi: 10.1038/nature11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Day T, Bonduriansky R. A unified approach to the evolutionary consequences of genetic and nongenetic inheritance. Am Nat. 2011;178:E18–E36. doi: 10.1086/660911. [DOI] [PubMed] [Google Scholar]
- 82.Geoghegan JL, Spencer HG. Exploring epiallele stability in a population-epigenetic model. Theor Popul Biol. 2013;83:136–144. doi: 10.1016/j.tpb.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 83.Guerrero-Bosagna C, Sabat P, Valladares L. Environmental signaling and evolutionary change: can exposure of pregnant mammals to environmental estrogens lead to epigenetically induced evolutionary changes in embryos? Evol Dev. 2005;7:341–350. doi: 10.1111/j.1525-142X.2005.05033.x. [DOI] [PubMed] [Google Scholar]
- 84.Klironomos FD, Berg J, Collins S. How epigenetic mutations can affect genetic evolution: model and mechanism. Bioessays. 2013;35:571–578. doi: 10.1002/bies.201200169. [DOI] [PubMed] [Google Scholar]
- 85.Skinner MK, Savenkova M, Zhang B, Crews D. Gene Bionetworks Involved in Epigenetic Transgenerational Inheritance of Altered Mate Preference: Environmental Epigenetics and Evolutionary Biology BMC Genomics. 2014;15 doi: 10.1186/1471-2164-15-377. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Darwin C. The Descent of Man, and Selection in Relation to Sex. London: T. Murray; 1871. [Google Scholar]
- 87.Skinner MK, Gurerrero-Bosagna C, Haque MM, Koop JAH, A KS, Clayton DH. Role of Epigenetics in the Speciation and Evolution of Darwin’s Finches Genome Biology & Evolution. 2014 doi: 10.1093/gbe/evu158. (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat Rev Genet. 2012;13:153–162. doi: 10.1038/nrg3188. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt CW. Uncertain inheritance transgenerational effects of environmental exposures. Environ Health Perspect. 2013;121:A298–A303. doi: 10.1289/ehp.121-A298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamarck J. Recherches sur l’organisation des corps vivans. Maillard, Paris: Chez L’auteur; 1802. [Google Scholar]
- 91.Manikkam M, M HM, Guerrero-Bosagna C, Nilsson E, Skinner M. Pesticide methoxychlor promotes the epigenetic transgenerational inheritance of adult onset disease through the female germline. PLoS ONE, (Pending) 2014 doi: 10.1371/journal.pone.0102091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Padmanabhan N, Watson ED. Lessons from the one-carbon metabolism: passing it along to the next generation. Reprod Biomed Online. 2013;27:637–643. doi: 10.1016/j.rbmo.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 93.Burdge GC, Hoile SP, Uller T, Thomas NA, Gluckman PD, Hanson MA, Lillycrop KA. Progressive, Transgenerational Changes in Offspring Phenotype and Epigenotype following Nutritional Transition. PLoS ONE. 2011;6:e28282. doi: 10.1371/journal.pone.0028282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, Roseboom TJ. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG. 2008;115:1243–1249. doi: 10.1111/j.1471-0528.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 95.Zheng X, Chen L, Li M, Lou Q, Xia H, Wang P, Li T, Liu H, Luo L. Transgenerational variations in DNA methylation induced by drought stress in two rice varieties with distinguished difference to drought resistance. PLoS ONE. 2013;8:e80253. doi: 10.1371/journal.pone.0080253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suter L, Widmer A. Environmental heat and salt stress induce transgenerational phenotypic changes in Arabidopsis thaliana. PLoS ONE. 2013;8:e60364. doi: 10.1371/journal.pone.0060364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pembrey ME. Male-line transgenerational responses in humans. Hum Fertil (Camb) 2010;13:268–271. doi: 10.3109/14647273.2010.524721. [DOI] [PubMed] [Google Scholar]
- 98.Rehan VK, Liu J, Sakurai R, Torday JS. Perinatal nicotine-induced transgenerational asthma. Am J Physiol Lung Cell Mol Physiol. 2013;305:L501–L507. doi: 10.1152/ajplung.00078.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Govorko D, Bekdash RA, Zhang C, Sarkar DK. Male germline transmits fetal alcohol adverse effect on hypothalamic proopiomelanocortin gene across generations. Biol Psychiatry. 2012;72:378–388. doi: 10.1016/j.biopsych.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]