Abstract
The age of pubertal onset for girls has declined over past decades. Research suggests that endocrine disrupting chemicals (EDCs) may play a role but exposure at multiple stages of development has not been considered. We examined in utero and peripubertal exposure to bisphenol-A (BPA) and phthalates in relation to serum hormones and sexual maturation among females in a Mexico City birth cohort. We measured phthalate metabolite and BPA concentrations in urine collected from mothers during their third trimester (n=116) and from their female children at ages 8–13 years (n=129). Among girls, we measured concurrent serum hormone concentrations, Tanner stages for breast and pubic hair development, and collected information on menarche onset. We used linear and logistic regression to model associations between in utero and peripubertal measures of exposure with hormones and sexual maturation, respectively, controlling for covariates. An interquartile range (IQR) increase in in utero urinary mono-2-ethylhexyl phthalate (MEHP) was positively associated with 29% (95% CI:9.2–52.6%) higher dehydroepiandrosterone sulfate (DHEA-S), an early indicator of adrenarche, and 5.3 (95% CI:1.13–24.9) times higher odds of a Tanner stage >1 for pubic hair development. Similar relationships were observed with other in utero but not peripubertal di-2-ethylhexyl phthalate (DEHP) metabolites. IQR increases in in utero monobenzyl phthalate (MBzP) and monoethyl phthalate (MEP) were associated with 29% and 25% higher serum testosterone concentrations (95% CIs:4.3–59.3; 2.1–54.1), respectively. In addition, we observed suggestive associations between in utero and peripubertal MEP concentrations and increased odds of having undergone menarche, and between peripubertal MnBP concentrations and increased odds of having a Tanner stage >1 for both breast and pubic hair development. BPA was not associated with in utero or peripubertal serum hormones or sexual maturation.
Our findings suggest in utero phthalate exposure may impact hormone concentrations during peripubescence and timing of sexual maturation. Efforts to control phthalate exposure during pregnancy should be of high priority. 3
Keywords: adrenarche, bisphenol A, in utero exposure, phthalates, sexual maturation
1. Introduction
Population trends since the mid-1900s show shifts in the age of onset and progression of puberty in both boys and girls (Aksglaede, et al. 2009, Euling, et al. 2008a). There is concern that in utero and early life exposure to endocrine disrupting compounds (EDCs), such as bisphenol A (BPA) and phthalates, could play a role in this shift (Euling, et al. 2008b, Meeker 2012). Early onset of puberty is an important public health issue since it is associated with increased risk of psychological and social issues during adolescence (Short and Rosenthal 2008), as well as metabolic (Frontini, et al. 2003), cardiovascular (Lakshman, et al. 2009), and endocrine-related diseases or cancers (Apter, et al. 1989, Gail, et al. 1989, Lacey, et al. 2009) in adulthood. Exposure to phthalates and BPA has been hypothesized to be associated with earlier puberty in both boys and girls (Buck Louis, et al. 2008, Mouritsen, et al. 2010, Schoeters, et al. 2008).
Phthalates are used in a wide range of consumer products, resulting in ubiquitous exposure (Silva, et al. 2004, Teitelbaum, et al. 2008). In humans, studies of adults have linked phthalate exposure with altered reproductive steroid (Meeker, et al. 2009, Pan, et al. 2006, Sathyanarayana, et al. 2013) and thyroid (Meeker, et al. 2007, Meeker and Ferguson 2011) hormone concentrations, although most studies to date have focused on men. Findings from previous studies evaluating exposure to various phthalates and sexual maturation in girls have not been consistent. In a cross-sectional evaluation of sexual development among 1,151 U.S. girls at 6–8 years of age, exposure to low molecular weight phthalates (sum of monoethyl phthalate (MEP), mono-n-butyl phthalate (MnBP), and mono-isobutyl phthalate (MiBP)) was weakly associated with a higher prevalence of breast and pubic hair development (thelarche and pubarche, respectively), while exposure to high molecular weight phthalates (sum of mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), mono-2-ethylhexyl phthalate (MEHP), monobenzyl phthalate (MBzP), and mono-3-carboxypropyl phthalate (MCPP)) was associated with lower prevalence of pubarche (Wolff, et al. 2010). In a recent 7-year follow-up of these participants, high molecular weight phthalate metabolites measured at enrollment were again associated with later pubic hair development (Wolff, et al. 2014). In a cross sectional study of 725 Danish girls aged 5–19 years, girls in the highest quartiles of urinary MBzP and MnBP plus MiBP concentrations entered pubarche at significantly later ages compared to girls in the lowest quartiles (Frederiksen, et al. 2012). Conversely, in a study evaluating in utero exposure among 121 Australian girls, di-2-ethylhexyl phthalate (DEHP) metabolite concentrations in maternal serum during pregnancy were associated with earlier age of menarche in female offspring (Hart, et al. 2013). Animal and in vitro studies have demonstrated that several phthalates and/or their metabolites are anti-androgenic (Autian 1973, Borch, et al. 2006), estrogenic (Chen, et al. 2014, Harris, et al. 1997), and associated with adverse reproductive and developmental effects in males (Foster 2006), but studies examining exposure related effects in females are limited. Recently, a study of female rats found that both neonatal and prepubertal dibutyl phthalate (DBP) exposure was associated with earlier pubertal onset (Hu, et al. 2013).
BPA is a high production chemical used in the manufacture of polycarbonate plastics, epoxy resins, and thermal paper, and can be measured in the urine of almost everyone tested (Calafat, et al. 2008). Human studies evaluating adult exposure to BPA have reported associations between exposure, serum estradiol concentrations, and oocyte number among women undergoing in-vitro fertilization (Ehrlich, et al. 2012, Mok-Lin, et al. 2010). However, no human studies have evaluated associations between early life exposure to BPA and sexual maturation. In animals, in utero and perinatal BPA exposure has been linked to early puberty onset (Adewale, et al. 2009, Maffini, et al. 2006).
To date there have been limited human studies to assess associations between EDC exposure and sexual maturation. In addition, previous studies have been primarily cross-sectional, although in utero exposure may play an important role in later development. Among studies that have looked at in utero exposure to EDCs, few have examined the impacts of exposure on the tempo of sexual maturation in girls. We examine phthalate and BPA exposure during two potentially sensitive developmental time points – in utero and peripubertal – in relation to serum hormone concentrations and physical measures of puberty, defined by breast development, and pubarche, defined by pubic hair development, in girls.
2. Materials and Methods
2.1 Study Population
Our study population comprises a subset of participants from the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) project, a longitudinal cohort study of pregnant women recruited from maternity hospitals in Mexico City and their offspring. Our analysis includes women from the second and third of three sequentially enrolled cohorts who were recruited from 1997 to 2004 during their first trimester. Mothers were followed throughout pregnancy, and both mothers and offspring were followed through postnatal visits as previously described (Lewis, et al. 2013). During their third trimester, mothers provided a urine sample and completed interview-based questionnaires. In 2010, a subset of male and female child participants, who were now 8 to 13 years of age, were re-contacted to participate in follow-up studies (n=250), and subsequently provided spot urine samples, serum samples, anthropometry, and completed an interview-based questionnaire. In the present analysis we included 132 female children who had urinary phthalate metabolite and BPA measurements from their urine sample collected at follow-up (n=129) and/or their mother’s urine sample collected during her third trimester (n=116). Urine samples were available from both in utero and peripubertal time points for 113 mother/female child pairs. One participant who did not have serum hormone measurements, was only included in the sexual maturation analyses. Research protocols were approved by the ethics and research committees of the Mexico National Institute of Public Health and the University of Michigan, and all participants provided informed consent prior to enrollment.
2.2 Urinary Phthalate Metabolites and BPA
Each mother provided a spot urine sample during her third trimester of pregnancy study visit as a measure of in utero exposure to the child, and each female child provided a spot urine sample during a follow-up study visit at 8–13 years of age as a measure of peripubertal exposure. Samples were collected in commercially available urine containers, frozen at −80°C, and analyzed at NSF International (Ann Arbor, MI). Total (free + glucuronidated) BPA and nine phthalate metabolites, comprising MEP, MnBP, MiBP, MBzP, MCPP, MEHP, MEHHP, MEOHP, and MECPP were measured in urine using isotope dilution– liquid chromatography–tandem mass spectrometry (ID–LC–MS/MS) as previously described (Lewis, et al. 2013). Specific gravity (SG) was measured using a handheld digital refractometer (Atago Co., Ltd., Tokyo, Japan) at the time of sample analysis. Values below the limit of detection (LOD) were replaced with the LOD/√2 (Hornung and Reed 1990).
2.3 Hormones
We measured total estradiol, total testosterone, inhibin B, and sex hormone-binding globulin (SHBG) as biomarkers of puberty and breast development, and dehydroepiandrosterone sulfate (DHEA-S) as a biomarker of adrenarche and pubic hair development. We collected fasting serum samples from children during follow-up visits at 8–13 years of age. Samples were sent to the Clinical Ligand Assay Service Satellite (CLASS) Laboratory at the University of Michigan (Ann Arbor, MI) for hormone analysis. DHEA-S, estradiol, SHBG, and total testosterone (Total T) were measured using an automated chemiluminescent immunoassay (Bayer Diagnostics ACS:180). Active inhibin B was assayed using Gen II ELISA (Beckman Coulter, Webster, TX). Values below the limit of detection (LOD) were replaced with the LOD/√2.
2.4 Sexual Maturation
We used Tanner staging as a measure of sexual maturation, where stage 1 indicates no development and stage 5 indicates full development (Marshall and Tanner 1969). Each child was examined according to a standardized protocol by a trained pediatrician, who assessed Tanner stages in girls for puberty, as defined by breast development, and pubarche, as defined by pubic hair growth. We also asked girls if they had started having their period (menarche), using the interview-based questionnaire.
2.5 Covariates
Trained staff measured each child’s height and weight according to standard protocols (Lohman, et al. 1988), and we calculated age-specific BMI z-scores based on the World Health Organization child reference curves for age and sex (WHO 2007). We collected information on child age and demographics via questionnaire.
2.6 Statistical Methods
We calculated Spearman rank correlations among serum hormone concentrations to account for outliers in non-transformed data. Serum hormone and urinary phthalate metabolites and BPA were log normally distributed, and were ln-transformed prior to regression analyses.
We used linear regression to assess associations between ln-transformed serum hormone concentrations and ln-transformed urinary phthalate metabolite and BPA concentrations. Age and BMI Z-score were included in all models as potential confounders, while urinary specific gravity was included to adjust for urine dilution. We also created exposure categories by first correcting urinary phthalate metabolite and BPA values for specific gravity using the following equation: where Pc is the SG-corrected phthalate metabolite or BPA concentration (ng/mL), P is the measured phthalate metabolite or BPA concentration, SGp is the median urinary specific gravity (mothers = 1.013, girls = 1.017), and SGi is the individual’s urinary specific gravity. SG-corrected concentrations were then divided into tertiles and entered into models as categorical predictors of ln-transformed serum hormone concentrations. Results are presented as the percent change in hormone (95% confidence interval) per interquartile range (IQR) increase for continuous predictors or compared to the reference group for categorical predictors. We calculated p for trend by entering phthalate or BPA tertile variables into models as continuous predictors. As a sensitivity analysis, we restricted the above analyses to girls that had not yet undergone menarche.
We used logistic regression to examine associations between urinary phthalate metabolite or BPA concentrations and the odds of pubertal onset, defined by having a Tanner stage >1 for breast development or self-report of having undergone menarche, and the odds of pubarche, defined by having a Tanner stage >1 for pubic hair development. Natural log-transformed metabolite concentrations were entered into models as continuous predictors while controlling for age, BMI Z-score, and urinary specific gravity. Results are presented as odds ratios (OR, 95% confidence interval), which express the change in odds of pubertal onset per IQR increase in markers of exposure. All analyses were performed using SAS version 9.3 (Cary, NC). Due to our relatively small sample size and the exploratory nature of this analysis, we considered findings as statistically significant at p≤0.05 and suggestive at p≤0.10.
3. Results
3.1 Distributions and Correlations
Distributions of serum hormone concentrations are presented in Supplementary Table S1; geometric mean concentrations were all within the normal range for this age group (Gardner and Shoback, 2011). Consistent with typical hormonal changes during puberty, girls who had undergone menarche (n=30) had higher serum estradiol, testosterone, DHEA-S, and inhibin B concentrations, and lower serum SHBG concentrations, compared to girls who had not (n=101). Similarly, serum hormone concentrations were correlated with one another in patterns that varied by menarche status (Supplementary Table S2). Particularly among participants that had not yet undergone menarche, estradiol, inhibin B, and DHEA-S tended to be moderately and positively correlated with one another, while SHBG was negatively correlated with DHEA-S. With the exception of MEP, geometric mean concentrations of urinary phthalate metabolites and BPA in maternal urine samples were lower than concentrations in peripubertal urine samples (Table 1).
Table 1.
Distribution of maternal (in utero exposure) and peripubertal urinary phthalate metabolites and BPA (μg/L, not adjusted for urine dilution)
| LOD | GM | 5th | 25th | 50th | 75th | 95th | |
|---|---|---|---|---|---|---|---|
| In utero (n=116) | |||||||
| MEHP | 1.0 | 5.0 | <1.0 | 2.52 | 5.63 | 9.50 | 30.1 |
| MEHHP | 0.1 | 19.1 | 3.49 | 9.13 | 22.7 | 37.5 | 96.7 |
| MEOHP | 0.1 | 11.6 | 2.45 | 5.80 | 13.25 | 24.7 | 56.1 |
| MECPP | 0.2 | 30.9 | 6.12 | 15.1 | 35.4 | 58.1 | 138 |
| MBzP | 0.2 | 4.1 | 0.95 | 2.22 | 3.67 | 7.04 | 29.6 |
| MnBP | 0.5 | 54.3 | 7.06 | 26.4 | 54.2 | 119 | 606 |
| MiBP | 0.2 | 2.0 | 0.34 | 0.96 | 2.01 | 3.62 | 12.6 |
| MCPP | 0.2 | 1.0 | <0.2 | 0.50 | 1.07 | 1.93 | 6.55 |
| MEP | 1.0 | 114 | 9.12 | 40.8 | 116 | 242 | 1900 |
| BPA | 0.4 | 0.78 | <0.4 | <0.4 | 0.70 | 1.42 | 4.06 |
| Peripubertal (n=129) | |||||||
| MEHP | 1.0 | 5.8 | <1.0 | 3.55 | 5.49 | 11.4 | 19.4 |
| MEHHP | 0.1 | 45.4 | 11.4 | 23.9 | 46.1 | 86.6 | 189 |
| MEOHP | 0.1 | 20.0 | 4.78 | 11.6 | 20.3 | 39 | 84.5 |
| MECPP | 0.2 | 61.8 | 14.8 | 36 | 67.1 | 111 | 213 |
| MBzP | 0.2 | 5.8 | 1.21 | 3.36 | 5.82 | 10.8 | 23.5 |
| MnBP | 0.5 | 108 | 18.3 | 59.4 | 109 | 224 | 615 |
| MiBP | 0.2 | 10.9 | 2.85 | 5.93 | 11.7 | 19.1 | 45 |
| MCPP | 0.2 | 2.2 | 0.44 | 1.06 | 2.45 | 3.93 | 13.7 |
| MEP | 1.0 | 97.5 | 15 | 32.2 | 82.9 | 243 | 1230 |
| BPA | 0.4 | 1.2 | <0.4 | 0.583 | 1.3 | 2.57 | 5.82 |
LOD, limit of detection; GM, geometric mean
3.2 Covariates
Age was positively associated with serum estradiol, testosterone, DHEA-S, and inhibin B concentrations (Supplementary Table S3), as well as increased odds of having a Tanner stage >1 for either breast or pubic hair development or having undergone menarche (Supplementary Table S4). BMI z-score and age were both negatively associated with serum SHBG concentrations, while BMI z-score was also negatively associated with inhibin B concentrations (Supplementary Table S3).
3.3 In utero phthalates, BPA and peripubertal hormones
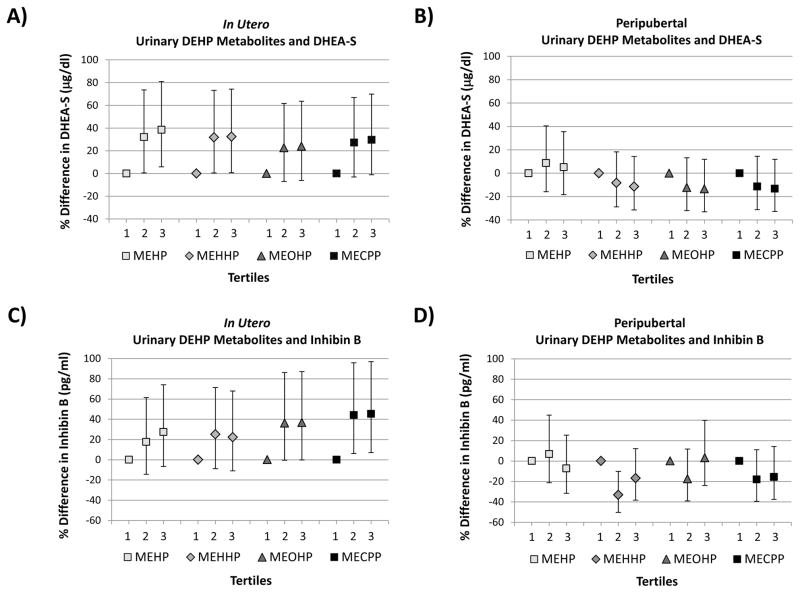
An IQR increase in in utero, ln-transformed urinary MEHP concentration was associated with a 29% (95% CI: 9%, 53%) increase in DHEA-S concentration measured at 8–13 years, adjusting for age, BMI Z-score, and urinary specific gravity (Table 2). Similar associations were observed between in utero measurements of other DEHP metabolites (MECPP, MEHHP, and MEOHP) and peripubertal levels of DHEA-S. When we considered tertiles of SG-corrected in utero urinary DEHP metabolites, we observed similar positive associations with peripubertal DHEA-S (Figure 1). In utero MEHP and MECPP concentrations were also both positively associated with serum inhibin B concentrations (Table 2, Figure 1).
Table 2.
Percent change in ln-transformed hormone per IQR increase in urinary phthalate metabolite or BPA, adjusted for age, BMI Z-score, and specific gravity
| In utero Measures of Urinary BPA and Phthalate Metabolites (n=115) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ln Estradiol | ln Testosterone | ln SHBG | ln DHEA-S | ln Inhibin B | |||||||||||
| % Change | 95%CI | p-value | % Change | 95%CI | p-value | % Change | 95%CI | p-value | % Change | 95%CI | p-value | % Change | 95%CI | p-value | |
|
|
|
|
|
|
|||||||||||
| ln MEHP | 10.0 | −4.5, 26.6 | 0.19 | −7.2 | −28.1, 19.9 | 0.57 | −5.4 | −15.7, 6.0 | 0.33 | 29.1 | 9.2, 52.6 | 0.003 | 22.5 | 0.9, 48.7 | 0.04 |
| ln MEHHP | 8.3 | −6.1, 24.9 | 0.27 | 3.3 | −20.2, 33.8 | 0.80 | −3.2 | −13.8, 8.7 | 0.58 | 19.2 | 0.3, 41.6 | 0.05 | 17.3 | −3.7, 42.8 | 0.11 |
| ln MEOHP | 8.6 | −6.4, 25.9 | 0.28 | 4.6 | −20.1, 36.9 | 0.74 | −4.8 | −15.6, 7.4 | 0.42 | 23.5 | 3.3, 47.6 | 0.02 | 18.4 | −3.6, 45.3 | 0.11 |
| ln MECPP | 11.8 | −3.4, 29.3 | 0.13 | 0.4 | −23.0, 30.9 | 0.98 | −4.5 | −15.1, 7.5 | 0.44 | 24.1 | 4.2, 47.9 | 0.02 | 22.5 | 0.3, 49.7 | 0.05 |
| ln MBzP | −2.7 | −13.8, 9.7 | 0.65 | 28.9 | 4.3, 59.3 | 0.02 | −1.7 | −10.8, 8.4 | 0.73 | 0.0 | −13.7, 15.9 | 1.00 | 0.4 | −15.1, 18.6 | 0.97 |
| ln MnBP | 0.6 | −12.0, 15.0 | 0.93 | 12.4 | −11.6, 42.8 | 0.34 | −1.4 | −11.4, 9.9 | 0.80 | 18.8 | 1.2, 39.4 | 0.04 | −1.7 | −18.4, 18.3 | 0.85 |
| ln MiBP | 6.9 | −7.0, 23.0 | 0.34 | 17.4 | −8.6, 50.8 | 0.21 | −0.4 | −11.0, 11.5 | 0.95 | 19.5 | 1.0, 41.3 | 0.04 | 4.5 | −13.9, 26.9 | 0.65 |
| ln MCPP | 3.6 | −11.0, 20.6 | 0.65 | 7.0 | −18.6, 40.7 | 0.62 | −3.2 | −14.4, 9.4 | 0.60 | 21.5 | 1.2, 45.8 | 0.04 | 12.1 | −9.1, 38.3 | 0.28 |
| ln MEP | 1.1 | −10.0, 13.6 | 0.85 | 25.5 | 2.1, 54.1 | 0.03 | −4.1 | −12.6, 5.4 | 0.38 | 10.8 | −3.8, 27.6 | 0.15 | 7.9 | −8.1, 26.8 | 0.35 |
| ln BPA | −4.4 | −26.0, 23.5 | 0.73 | 2.0 | −35.7, 61.8 | 0.93 | −7.1 | −24.4, 14.2 | 0.48 | 20.9 | −11.4, 65.1 | 0.23 | 6.5 | −25.3, 52.0 | 0.72 |
| Peripubertal Measures of Urinary BPA and Phthalate Metabolites (n=128) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ln Estradiol | ln Testosterone | ln SHBG | ln DHEA-S | ln Inhibin B | |||||||||||
| % Change | 95%CI | p-value | % Change | 95%CI | p-value | % Change | 95%CI | p-value | % Change | 95%CI | p-value | % Change | 95%CI | p-value | |
|
|
|
|
|
|
|||||||||||
| ln MEHP | −0.3 | −13.3, 14.6 | 0.96 | −4.1 | −24.2, 21.4 | 0.73 | 2.7 | −8.4, 15.3 | 0.64 | −1.8 | −16.6, 15.7 | 0.83 | −0.7 | −18.3, 20.7 | 0.94 |
| ln MEHHP | 4.5 | −10.9, 22.5 | 0.59 | 4.8 | −19.9, 37.2 | 0.73 | 2.6 | −10.1, 17.0 | 0.70 | −11.2 | −26.3, 6.9 | 0.21 | 2.4 | −18.1, 28.0 | 0.83 |
| ln MEOHP | 6.2 | −8.7, 23.6 | 0.43 | 5.0 | −18.7, 35.5 | 0.71 | 4.3 | −7.9, 18.2 | 0.50 | −10.1 | −24.6, 7.3 | 0.24 | 8.6 | −12.1, 34.2 | 0.44 |
| ln MECPP | 5.9 | −8.7, 22.9 | 0.45 | −10.0 | −30.0, 15.7 | 0.41 | 3.6 | −8.4, 17.1 | 0.57 | −11.9 | −25.9, 4.8 | 0.15 | 1.6 | −17.5, 25.2 | 0.88 |
| ln MBzP | 3.3 | −9.8, 18.2 | 0.64 | 15.7 | −7.8, 45.2 | 0.21 | 14.4 | 2.6, 27.6 | 0.02 | −10.6 | −23.6, 4.7 | 0.16 | 1.4 | −16.1, 22.5 | 0.89 |
| ln MnBP | 2.6 | −10.5, 17.7 | 0.71 | 0.8 | −20.0, 26.9 | 0.95 | 4.2 | −6.9, 16.7 | 0.47 | −3.3 | −17.7, 13.5 | 0.68 | −7.7 | −23.7, 11.8 | 0.41 |
| ln MiBP | 14.2 | −1.3, 32.2 | 0.07 | 7.5 | −16.2, 37.9 | 0.57 | 4.9 | −7.1, 18.5 | 0.44 | −2.2 | −17.7, 16.4 | 0.80 | −2.7 | −20.9, 19.7 | 0.79 |
| ln MCPP | −2.5 | −15.0, 11.8 | 0.71 | −10.1 | −28.6, 13.2 | 0.36 | 9.2 | −2.3, 22.1 | 0.12 | −7.2 | −20.9, 8.9 | 0.36 | −7.0 | −23.2, 12.6 | 0.45 |
| ln MEP | −3.8 | −16.0, 10.1 | 0.57 | 17.0 | −6.6, 46.7 | 0.17 | 3.8 | −7.1, 16.0 | 0.50 | −8.8 | −22.1, 6.8 | 0.25 | −14.0 | −28.6, 3.7 | 0.11 |
| ln BPA | −5.0 | −19.6, 12.2 | 0.54 | 10.7 | −16.4, 46.7 | 0.47 | −3.2 | −15.6, 11.1 | 0.65 | −1.9 | −19.3, 19.3 | 0.85 | 15.9 | −8.1, 46.2 | 0.21 |
CI, confidence interval
Figure 1.
Percent differences in serum hormone concentrations within the 2nd and 3rd tertiles of SG-corrected urinary DEHP metabolites compared to the 1st tertile adjusted for age and age-specific BMI z-score: A) in utero DEHP metabolites and peripubertal DHEA-S; B) peripubertal DEHP metabolites and DHEA-S C) in utero DEHP metabolites and peripubertal inhibin B; D) peripubertal DEHP metabolites and inhibin B.
p for trend:
Panel A: MEHP = 0.018; MEHHP = 0.044; MEOHP = 0.13; MECPP = 0.059
Panel B: MEHP = 0.69; MEHHP = 0.35; MEOHP = 0.27; MECPP = 0.27
Panel C: MEHP = 0.12; MEHHP = 0.21; MEOHP = 0.051; MECPP = 0.016
Panel D: MEHP = 0.62; MEHHP = 0.22; MEOHP = 0.84; MECPP = 0.27
In utero MBzP and MEP concentrations were positively associated with testosterone after adjustment for covariates. For example, an IQR increase in in utero MBzP was associated with a 29% (95% CI: 4%, 59%) increase in serum testosterone concentrations, and categorical analyses showed a monotonic increase in testosterone by tertile of urinary MBzP (p for trend = 0.007) (Figure 2).
Figure 2.
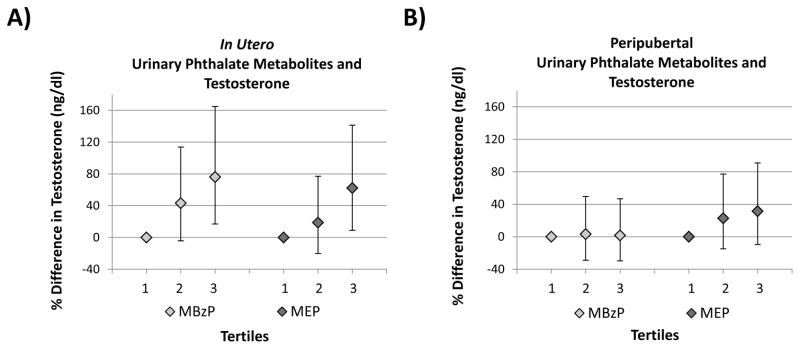
Percent differences in serum hormone concentrations within the 2nd and 3rd tertiles of SG-corrected urinary MBzP and MEP concentrations compared to the 1st tertile adjusted for age and age-specific BMI z-score: A) in utero MBzP or MEP and peripubertal testosterone; B) peripubertal MBzP or MEP and testosterone.
P for trend:
Panel A: MBzP = 0.007; MEP = 0.018
Panel B: MBzP = 0.93, MEP = 0.15
Urinary phthalate metabolite concentrations measured during the mother’s third trimester were not associated with peripubertal measures of serum estradiol or SHBG. In utero BPA measurements were not associated with any peripubertal hormone concentrations.
3.4 Cross-sectional associations between peripubertal phthalates, BPA and hormones
An IQR increase in urinary MBzP concentrations measured at 8–13 years of age was associated with 14% (95% CI: 3%, 28%) higher concurrently measured serum SHBG concentrations (Table 2). We found a suggestive, but not statistically significant, positive relationship between peripubertal MiBP and estradiol concentrations (Table 2). Peripubertal measures of urinary phthalate metabolites were not associated with concurrent serum testosterone, DHEA-S, or inhibin B concentrations, and peripubertal BPA concentrations were not associated with any concurrent serum hormone measurements.
3.5 Sensitivity Analyses
Twenty of the 115 participants with in utero phthalate metabolite and BPA measurements (17%) and 30 of the 128 participants with peripubertal phthalate metabolite and BPA measurements (23%) had undergone menarche. In sensitivity analyses we restricted the hormone analysis to girls that had not yet undergone menarche, and our results did not materially change (Supplementary Table S5).
3.6 Phthalates and BPA as predictors of sexual maturation
Forty-five girls had a Tanner stage >1 for breast development, while 34 had a Tanner stage >1 for pubic hair development. An IQR increase in in utero MEHP concentration was associated with 5.3 (95% CI: 1.13, 24.9) times higher odds of having a Tanner stage >1 for pubic hair development after adjustment for age, BMI Z-score, and specific gravity (Table 3).
Table 3.
Change in the odds of having a Tanner stage >1 or having undergone menarche per IQR increase in ln-transformed urinary phthalate metabolite or BPA concentrations adjusted for age, BMI Z-score, and specific gravity.
| In utero Urinary Metabolite Measurements (n=116) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Menarche | Breast Development | Pubic Hair Development | |||||||
| OR | 95%CI | p-value | OR | 95%CI | p-value | OR | 95%CI | p-value | |
|
|
|
|
|||||||
| lnMEHP | 0.91 | 0.31,2.73 | 0.87 | 0.86 | 0.34,2.19 | 0.75 | 5.30 | 1.13,24.95 | 0.03 |
| lnMEHHP | 1.62 | 0.54,4.81 | 0.39 | 0.66 | 0.29,1.54 | 0.34 | 2.66 | 0.80,8.82 | 0.11 |
| lnMEOHP | 1.55 | 0.52,4.63 | 0.43 | 0.73 | 0.31,1.74 | 0.48 | 2.31 | 0.73,7.31 | 0.16 |
| lnMECPP | 1.54 | 0.52,4.57 | 0.43 | 0.76 | 0.32,1.82 | 0.54 | 2.25 | 0.70,7.17 | 0.17 |
| lnMBzP | 0.89 | 0.33,2.38 | 0.82 | 1.88 | 0.82,4.30 | 0.13 | 1.60 | 0.50,5.06 | 0.43 |
| lnMnBP | 0.92 | 0.32,2.62 | 0.87 | 0.97 | 0.42,2.25 | 0.94 | 1.92 | 0.61,6.04 | 0.26 |
| lnMiBP | 0.42 | 0.12,1.50 | 0.18 | 0.70 | 0.25,1.95 | 0.49 | 1.52 | 0.33,6.89 | 0.59 |
| lnMCPP | 1.07 | 0.31,3.68 | 0.91 | 1.26 | 0.46,3.47 | 0.66 | 2.05 | 0.53,7.92 | 0.30 |
| lnMEP | 2.66 | 0.91,7.76 | 0.07 | 1.06 | 0.48,2.35 | 0.89 | 2.02 | 0.72,5.65 | 0.18 |
| lnBPA | 0.35 | 0.06,2.18 | 0.26 | 2.04 | 0.39,10.79 | 0.40 | 1.15 | 0.13,10.38 | 0.90 |
| Peripubertal Measures Urinary Metabolites (n=129) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Menarche | Breast Development | Pubic Hair Development | |||||||
| OR | 95%CI | p-value | OR | 95%CI | p-value | OR | 95%CI | p-value | |
|
|
|
|
|||||||
| lnMEHP | 0.52 | 0.20,1.34 | 0.18 | 1.37 | 0.55,3.41 | 0.51 | 1.13 | 0.38,3.37 | 0.83 |
| lnMEHHP | 1.32 | 0.45,3.85 | 0.61 | 1.74 | 0.63,4.85 | 0.29 | 1.41 | 0.36,5.47 | 0.62 |
| lnMEOHP | 1.48 | 0.52,4.20 | 0.46 | 1.63 | 0.63,4.24 | 0.32 | 1.39 | 0.39,4.89 | 0.61 |
| lnMECPP | 1.22 | 0.44,3.33 | 0.70 | 1.45 | 0.57,3.69 | 0.43 | 1.22 | 0.35,4.23 | 0.76 |
| lnMBzP | 2.80 | 0.86,9.13 | 0.09 | 1.58 | 0.61,4.06 | 0.35 | 3.13 | 0.70,13.97 | 0.14 |
| lnMnBP | 1.21 | 0.47,3.16 | 0.69 | 2.41 | 0.95,6.06 | 0.06 | 2.64 | 0.85,8.23 | 0.09 |
| lnMiBP | 1.31 | 0.37,4.57 | 0.67 | 1.74 | 0.62,4.83 | 0.29 | 2.45 | 0.57,10.40 | 0.23 |
| lnMCPP | 0.95 | 0.37,2.42 | 0.91 | 1.48 | 0.69,3.18 | 0.31 | 1.63 | 0.62,4.31 | 0.32 |
| lnMEP | 2.58 | 0.90,7.39 | 0.08 | 1.55 | 0.60,3.97 | 0.36 | 1.91 | 0.59,6.21 | 0.28 |
| lnBPA | 0.44 | 0.14,1.36 | 0.16 | 0.99 | 0.33,2.96 | 0.99 | 0.68 | 0.20,2.33 | 0.54 |
OR, odds ratio; CI, confidence interval
We observed suggestive associations between both in utero and peripubertal MEP concentrations and increased odds of having undergone menarche (Table 3). Suggestive associations were also observed between peripubertal MnBP concentrations and increased odds of having a Tanner stage >1 for both breast and pubic hair development (Table 3). In utero BPA, peripubertal BPA, and peripubertal DEHP metabolite measurements were not associated with menarche status, breast development, or pubic hair development.
4. Discussion
To our knowledge, this is the first study to evaluate associations of urinary phthalate metabolite and BPA concentrations measured both during in utero development and peripubescence, with hormone levels and measures of sexual maturation in girls. We found that in utero measures of DEHP exposure were associated with increased DHEA-S concentrations and increased odds of pubic hair development, or pubarche, among girls 8 to 13 years of age. DHEA-S is an adrenal hormone and an important indirect precursor to both testosterone and estrogen. The rise in DHEA-S and other androgens (adrenarche) is a precursor to pubarche, and both processes are controlled by the hypothalamic-pituitary-adrenal axis. Although puberty, defined by breast development and reproductive maturity, is controlled by the distinctly separate hypothalamic-pituitary-gonadal axis, adrenarche usually occurs approximately 1–3 years prior to pubertal onset. We also found an association between in utero measures of MBzP and increased peripubertal testosterone, an indication of pubertal onset. In contrast, we observed a positive association between concurrent measures of MBzP and SHBG, which typically decreases during puberty. Despite changing hormone profiles during peripubescence, associations between markers of exposure and hormone concentrations were unchanged when we restricted analyses to girls that had not yet undergone menarche. Overall, these findings suggest that in utero exposure to DEHP and possibly butylbenzyl phthalate (BBzP, MBzP parent compound) may lead to earlier onset of adrenarche and puberty, relationships that may not be detected when measuring concurrent exposure.
4.1 Comparisons with other studies
Overall, in utero and peripubertal metabolite concentrations in our study population were similar to those previously reported in a subset of our participants (Lewis, et al. 2013). Comparisons of urinary phthalate metabolite and BPA concentrations found in this population have already been made with participants in the National Health and Nutrition Examination Survey (NHANES) and other similar populations (Lewis, et al. 2013). Briefly, MEHP and MnBP concentrations were higher in maternal urine samples from our population (in utero exposure) than among adult female NHANES participants during the same time period (1999–2004), while MBzP, MiBP, and MCPP concentrations were lower (CDC 2013). Maternal DEHP metabolite and MCPP concentrations were similar to those reported in pregnant women in Israel, although women in the current study had higher MnBP and lower MiBP and MEP concentrations (Berman, et al. 2009). Maternal urinary BPA concentrations were lower than those reported in pregnant Spanish women (Casas, et al. 2011), and adult female NHANES participants (CDC 2013). Compared to boys and girls ages 6–11 years participating in NHANES 2009–2010, female child participants in the current study had higher urinary concentrations of DEHP metabolites, MnBP, and MEP, but lower concentrations of MBzP and BPA (CDC 2013).
In the only prior study to evaluate associations between in utero phthalate exposure and sexual maturation in girls, investigators reported that DEHP metabolite concentrations in maternal serum during pregnancy were associated with earlier age of menarche in female offspring reported at 14–16 years of age (Hart, et al. 2013). Although we did not observe an association between in utero phthalate exposure and earlier age of menarche, we found that DEHP metabolites were associated with earlier age of adrenarche and pubarche. This difference may be explained by the age of participants included in each study, since our study population was 8–13 years of age, while the population studied by Hart et al. was older and all participants had already undergone menarche.
In a large cross-sectional study in the U.S., Wolff et al. investigated associations between EDC exposures and sexual maturation among 1,151 girls aged 6–8 years with phthalate exposures similar to those reported here (Wolff, et al. 2010). The authors reported suggestive associations between the sum of urinary low molecular weight phthalate metabolites (MEP, MnBP, and MiBP) and earlier breast and pubic hair development measured one year later. This finding is similar to the suggestive associations we observed between peripubertal MEP and menarche, as well as peripubertal MnBP and breast and pubic hair development, indicating concurrent exposure to these compounds may be related to earlier pubertal onset and adrenarche. Conversely, Wolff et al. also reported a significant negative association between high molecular weight phthalate metabolites (MECPP, MEHHP, MEOHP, MEHP, MBzP, and MCPP) and prevalence of pubic hair development, suggesting delayed adrenarche. In a recent follow-up of these participants, high molecular weight phthalate metabolites measured at baseline (age 6–8 years) were again associated with later pubic hair development over 7 years of observation (Wolff, et al. 2014). However, urinary phthalate metabolites were only measured at one point in time during early childhood, limiting the authors’ ability to account for in utero or concurrent exposure. We did not observe similar associations between peripubertal DEHP metabolites (components of the high molecular weight summary measure in Wolff et al.) and either hormones or measures of sexual maturation in our study population.
A cross-sectional study of 725 Danish girls at ages ranging from 6–19 years also examined associations between phthalate exposure and sexual maturation (Frederiksen, et al. 2012). This study population had similar median DEHP metabolite concentrations, but higher MiBP and MBzP, and lower MEP and MnBP concentrations compared to our population. Researchers reported that girls in the highest MBP (MnBP + MiBP) and MBzP quartiles entered stage 2 of pubic hair development at a higher age compared to those in the lowest quartiles, suggesting that exposure is related to delayed adrenarche. However, they did not detect associations between phthalate exposure and serum hormones or breast development. This is in contrast to our findings, as we observed positive, albeit only suggestive, associations between peripubertal MnBP and the odds of having a Tanner stage >1 for both breast and pubic hair development adjusting for age, suggesting that exposure is related to earlier pubertal onset and adrenarche.
A subset of 84 participants from the Danish study, comprised of girls aged 6–13 years at first examination, was followed for five years to examine longitudinal relationships between phthalate exposure and sexual maturation (Mouritsen, et al. 2013). Investigators calculated geometric mean (GM) phthalate metabolite concentrations from urine samples repeatedly collected six months apart for each individual as well as GMs for the study population. They reported that girls whose individual MBP (MnBP + MiBP) GM was above the group GM had lower levels of DHEA-S at 13 years of age (n=33), but not at 10 years of age (n=47). Since increased DHEA-S is a precursor to pubic hair development, these findings are consistent with the larger Danish study in which the highest quartile of MBP was associated with delayed pubic hair development among girls (Frederiksen, et al. 2012).
However, since the associations with peripubertal exposure measured in the current study and reported by Wolff et al. and Frederiksen et al. are cross-sectional, reverse causation cannot be ruled out. For example, the observed association in the present study between peripubertal MEP concentrations and increased odds of having undergone menarche could instead be a result of increased use of personal care products that may contain DEP, the MEP parent compound, such as lotions, deodorants, cosmetics, or hair products, among girls that had already undergone menarche. In contrast, when we considered in utero phthalate exposure, we observed positive, much stronger associations, particularly between DEHP metabolites and both DHEA-S concentrations and pubic hair development.
4.2 Possible Mechanisms
During in utero development the fetus is especially susceptible to environmental exposures, which can potentially lead to adverse health effects during childhood, adolescence, and adulthood. The mechanism by which in utero phthalate exposure may specifically impact sexual maturation, including adrenarche, in girls is unknown. A recent study in male rats demonstrated that in utero DEHP exposure was associated with a number of changes in gene expression in the adrenal gland of prepubertal and adult rats (Martinez-Arguelles, et al. 2014) suggesting epigenetic changes. However, in a similar study aldosterone, an adrenal hormone, decreased among males and increased among females after in utero DEHP exposure, so it is uncertain whether effects seen in males are relevant to females (Martinez-Arguelles, et al. 2013).
Regarding peripubertal exposure, evidence from a recent cross-sectional study suggests that phthalate exposure may affect secretion of kisspeptin (Chen, et al. 2013), a neuropeptide thought to initiate puberty. The authors reported that urinary MMP, MBzP, and MnBP concentrations were associated with serum kisspeptin concentrations, which was also positively associated with increasing developmental phases of central precocious puberty among girls 2.5 to 11.5 years of age. These findings are consistent with observations from the current study, including suggestive associations between increased odds of having undergone menarche and concurrently measured MBzP, and between increased odds of breast of pubic hair development and concurrently measured MnBP. These findings suggest that future studies should further investigate potential links between phthalate exposure during sensitive developmental time points, kisspeptin secretion, and early onset of puberty.
Comparison of our findings to the animal literature is difficult as there are conflicting findings regarding phthalate exposure and sexual maturation. In a study of prepubertal rats, researchers observed accelerated puberty, as determined by early vaginal opening and first estrous cycle, and significantly increased cholesterol, luteinizing hormone (LH) and estradiol after exposure to DEHP via inhalation (Ma, et al. 2006). The authors suggested that DEHP may induce LH secretion by the pituitary, altering pubertal development. Interestingly, kisspeptin secretion is thought to induce LH release, suggesting a potential link with the mechanism from the human study discussed above. However, Ma et al. did not evaluate in utero exposure, and in a study of female rats with in utero and lactational DEHP exposure via gavage, researchers observed delayed vaginal opening and first estrous (Grande, et al. 2006). This contrast highlights the potential importance of the timing, as well as other aspects of exposure such as route, concentration, and dose regimen.
4.3 Strengths and Limitations
A limitation of this study is that we only measured in utero phthalate metabolite and BPA concentrations at one time point in pregnancy, during the third trimester. Because urinary phthalates metabolites and BPA can vary significantly during pregnancy (Braun, et al. 2012, Cantonwine, et al. 2013, Meeker, et al. 2013), one spot urine may not adequately reflect in utero exposure. This is particularly important if sensitive windows of exposure for reproductive development occur earlier in pregnancy. In addition, the urine containers used to collect maternal samples in this study were not specified as phthalate or BPA free so sample contamination cannot be ruled out, although typical urine containers have not been found to contain these compounds. The peripubertal urine samples were collected using the same polypropylene tubes used by the CDC NHANES, which have been tested for phthalate and BPA contamination. We also measured oxidative phthalate metabolites (MEHHP, MEOHP, and MECPP) which can only be formed by enzymatic reactions and are not potential contaminants.
Hormone concentrations among post-menarcheal girls were also likely influenced by menstrual cycle phase at the time of sample collection, potentially leading to outcome misclassification among this group. However, when we restricted our analyses to pre-menarcheal girls our associations between markers of exposure and serum hormone concentrations did not materially change. We also measured serum hormones using standardized immunoassays rather than the gold standard LC/MS-MS due to budget constraints. In addition, we measured total hormone concentrations rather than the free hormone fractions which may be more biologically relevant. Relationships between free hormone concentrations and female sexual maturation should be considered in future studies.
We did not have information on the girls’ diet, a potential confounder since diet may be associated with BPA and phthalate exposure as well as timing of pubertal onset. The cross-sectional design of our peripubertal analysis is also a limitation, since multiple measurements during the pubertal transition to assess longitudinal associations would be ideal. As a result of the exploratory nature of these analyses, we also made a large number of comparisons, increasing the likelihood of chance significant findings. An additional limitation is our relatively small sample size, which resulted in substantial imprecision in some instances. Only 30 girls in our study population had undergone menarche, limiting our ability to assess associations within this subgroup. Our future work is designed to address some of these limitations to build on the compelling findings presented here.
The present study also has a number of strengths. We measured exposure both in utero and during peripubescence, and as a result were able to compare changes in sexual maturation related to exposure during the two developmental time points. To our knowledge this is the first study to assess sexual maturation in relation to both in utero and peripubertal phthalate and BPA exposure, as most previous studies were either limited to cross-sectional analyses or were not able to examine in utero exposure. Our findings suggest that in utero exposure to phthalates may be an important factor in female sexual maturation and that more research is necessary to identify sensitive windows of exposure during pregnancy.
4.4 Conclusion
Our findings suggest that exposure to phthalates during in utero development may impact circulating levels of sex hormones during peripubescence as well as the timing of sexual maturation in girls. We did not observe similar associations when looking at concurrent measures of exposure, suggesting that EDC exposure during sensitive windows of in utero development may have differential impacts on female sexual maturation. Our work also indicates that although efforts have been made to reduce phthalate and BPA exposure among infants and children, efforts to control phthalate exposure during pregnancy should also be of high priority.
Supplementary Material
Highlights.
We examined in utero and peripubertal EDC exposure in relation to sexual development.
In utero MEHP was positively associated with peripubertal DHEA-S and pubarche.
In utero MBzP and MEP were positively associated with peripubertal testosterone.
Peripubertal DEHP metabolites were not associated with hormones, sexual maturation.
Timing of EDC exposure plays a crucial role in their effects on sexual development.
Acknowledgments
Funding Sources
This work is supported by grants P20ES018171, P42ES017198, R01ES018872, R01ES021446, P30ES017885, 1P01ES022844, and T32ES007062, from the National Institute of Environmental Health Sciences (NIEHS) and RD834800 and RD835436 from the US Environmental Protection Agency (USEPA).
We thank Kurtis Kneen, Scott Clipper, Gerry Pace, David Weller, and Jennifer Bell of NSF International in Ann Arbor, MI, USA for urine analysis and Dan McConnell of the UM CLASS lab for serum hormone analysis.
Abbreviations
- BBzP
butylbenzyl phthalate
- BMI-Z
body mass index Z score for age and sex
- BPA
bisphenol A
- CDC
Centers for Disease Control and Prevention
- CLASS
Clinical Ligand Assay Service Satellite
- DEHP
di-2-ethylhexyl phthalate
- DHEA-S
dehydroepiandrosterone sulfate
- EDC
endocrine disrupting compound
- ELEMENT
Early Life Exposure in Mexico to Environmental Toxicants
- GM
geometric mean
- ID–LC–MS/MS
isotope dilution–liquid chromatography–tandem mass spectrometry
- IQR
interquartile range
- LH
luteinizing hormone
- LOD
limit of detection
- MBzP
monobenzyl phthalate
- MCPP
mono-3-carboxypropyl phthalate
- MECPP
mono-2-ethyl-5-carboxypentyl phthalate
- MEHHP
mono-2-ethyl-5-hydroxyhexyl phthalate
- MEHP
mono-2-ethylhexyl phthalate
- MEOHP
mono-2-ethyl-5-oxohexyl phthalate
- MEP
monoethyl phthalate
- MiBP
mono-isobutyl phthalate
- MnBP
mono-n-butyl phthalate
- NHANES
National Health and Nutrition Examination Survey
- OR
odds ratio
- PCO
polycystic ovaries
- PCOS
polycystic ovary syndrome
- SES
socioeconomic status
- SG
specific gravity
- SHBG
sex hormone-binding globulin
Footnotes
Research Ethics
Research protocols were approved by the ethics and research committees of the Mexico National Institute of Public Health and the University of Michigan, and all participants provided informed consent prior to enrollment.
Declaration of Interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biology of Reproduction. 2009;81:690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksglaede L, Juul A, Olsen LW, Sorensen TI. Age at puberty and the emerging obesity epidemic. PloS one. 2009;4:e8450. doi: 10.1371/journal.pone.0008450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apter D, Reinila M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast-cancer, are preserved into adulthood. International Journal of Cancer. 1989;44:783–787. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- Autian J. Toxicity and health threats of phthalate esters: review of the literature. Environmental Health Perspectives. 1973;4:3–26. doi: 10.1289/ehp.73043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman T, Hochner-Celnikier D, Calafat AM, Needham LL, Amitai Y, Wormser U, Richter E. Phthalate exposure among pregnant women in Jerusalem, Israel: results of a pilot study. Environment International. 2009;35:353–357. doi: 10.1016/j.envint.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223:144–155. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environmental Health Perspectives. 2012;120:739–745. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE, Jr, Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, Sippell W, Abbott DH, Soto A, Tyl RW, Bourguignon JP, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics. 2008;121(Suppl 3):S192–207. doi: 10.1542/peds.1813E. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environmental Health Perspectives. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine DE, Cordero JF, Rivera-Gonzalez LO, Anzalota Del Toro LV, Ferguson KK, Mukherjee B, Calafat AM, Crespo N, Jimenez-Velez B, Padilla IY, et al. Urinary phthalate metabolite concentrations among pregnant women in Northern Puerto Rico: Distribution, temporal variability, and predictors. Environment International. 2014;62C:1–11. doi: 10.1016/j.envint.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas L, Fernandez MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodriguez LS, Riano I, Tardon A, et al. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environment International. 2011;37:858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables; September 2013; Atlanta, GA. 2013. [Google Scholar]
- Chen C-Y, Chou Y-Y, Wu Y-M, Lin C-C, Lin S-J, Lee C-C. Phthalates may promote female puberty by increasing kisspeptin activity. Human Reproduction. 2013;28:2765–2773. doi: 10.1093/humrep/det325. [DOI] [PubMed] [Google Scholar]
- Chen X, Xu S, Tan T, Lee ST, Cheng SH, Lee FW, Xu SJ, Ho KC. Toxicity and estrogenic endocrine disrupting activity of phthalates and their mixtures. International Journal of Environmental Research and Public Health. 2014;11:3156–3168. doi: 10.3390/ijerph110303156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye XY, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Human Reproduction. 2012;27:3583–3592. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sorensen TI, Dunkel L, Himes JH, Teilmann G, Swan SH. Examination of US puberty-timing data from 1940 to 1994 for secular trends: panel findings. Pediatrics. 2008a;121(Suppl 3):S172–191. doi: 10.1542/peds.2007-1813D. [DOI] [PubMed] [Google Scholar]
- Euling SY, Selevan SG, Pescovitz OH, Skakkebaek NE. Role of environmental factors in the timing of puberty. Pediatrics. 2008b;121(Suppl 3):S167–171. doi: 10.1542/peds.2007-1813C. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. International Journal of Andrology. 2006;29:140–147. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-145. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Sorensen K, Mouritsen A, Aksglaede L, Hagen CP, Petersen JH, Skakkebaek NE, Andersson AM, Juul A. High urinary phthalate concentration associated with delayed pubarche in girls. International Journal of Andrology. 2012;35:216–226. doi: 10.1111/j.1365-2605.2012.01260.x. [DOI] [PubMed] [Google Scholar]
- Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. International Journal of Obesity and Related Metabolic Disorders. 2003;27:1398–1404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. Journal of the National Cancer Institute. 1989;81:1879–86. doi: 10.1093/jnci/81.24.1879. [DOI] [PubMed] [Google Scholar]
- Gardner DG, Shoback D, editors. Appendix: Normal Hormone Reference Ranges. [Accessed April 17, 2014];Greenspan’s Basic & Clinical Endocrinology. (9). 2011 http://accessmedicine.mhmedical.com/content.aspx?bookid=380&Sectionid=39744067.
- Grande SW, Andrade AJ, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di(2-ethylhexyl)phthalate: effects on female rat reproductive development. Toxicological Sciences. 2006;91:247–254. doi: 10.1093/toxsci/kfj128. [DOI] [PubMed] [Google Scholar]
- Harris CA, Henttu P, Parker MG, Sumpter JP. The estrogenic activity of phthalate esters in vitro. Environmental Health Perspectives. 1997;105:802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart R, Doherty DA, Frederiksen H, Keelan JA, Hickey M, Sloboda D, Pennell CE, Newnham JP, Skakkebaek NE, Main KM. The influence of antenatal exposure to phthalates on subsequent female reproductive development in adolescence: a pilot study. Reproduction. 2013 doi: 10.1530/REP-13-0331. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5:46–51. [Google Scholar]
- Hu J, Du G, Zhang W, Huang H, Chen D, Wu D, Wang X. Short-term neonatal/prepubertal exposure of dibutyl phthalate (DBP) advanced pubertal timing and affected hypothalamic kisspeptin/GPR54 expression differently in female rats. Toxicology. 2013;314:65–75. doi: 10.1016/j.tox.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Lacey JV, Jr, Kreimer AR, Buys SS, Marcus PM, Chang SC, Leitzmann MF, Hoover RN, Prorok PC, Berg CD, Hartge P, et al. Breast cancer epidemiology according to recognized breast cancer risk factors in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial Cohort. BMC Cancer. 2009:9–84. doi: 10.1186/1471-2407-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. Journal of Clinical Endocrinology and Metabolism. 2009;94:4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, Tellez-Rojo MM. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93:2390–2398. doi: 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman TG, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- Ma M, Kondo T, Ban S, Umemura T, Kurahashi N, Takeda M, Kishi R. Exposure of prepubertal female rats to inhaled di(2-ethylhexyl)phthalate affects the onset of puberty and postpubertal reproductive functions. Toxicological Sciences. 2006;93:164–171. doi: 10.1093/toxsci/kfl036. [DOI] [PubMed] [Google Scholar]
- Maffini MV, Rubin BS, Sonnenschein C, Soto AM. Endocrine disruptors and reproductive health: the case of bisphenol-A. Molecular and Cellular Endocrinology. 2006;254–255:179–186. doi: 10.1016/j.mce.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arguelles D, Campioli E, Lienhart C, Fan J, Culty M, Zirkin B, Papadopoulos V. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate induces long-term changes in gene expression in the adult male adrenal gland. Endocrinology. 2014 doi: 10.1210/en.2013. [DOI] [PubMed] [Google Scholar]
- Martinez-Arguelles DB, Campioli E, Culty M, Zirkin BR, Papadopoulos V. Fetal origin of endocrine dysfunction in the adult: the phthalate model. Journal of Steroid Biochemistry and Molecular Biology. 2013;137:5–17. doi: 10.1016/j.jsbmb.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Meeker JD. Exposure to environmental endocrine disruptors and child development. Archives of Pediatrics & Adolescent Medicine. 2012;166:952–958. [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environmental Health Perspectives. 2007;115:1029–1034. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Urinary metabolites of di(2-ethylhexyl) phthalate are associated with decreased steroid hormone levels in adult men. Journal of Andrology. 2009;30:287–297. doi: 10.2164/jandrol.108.006403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Cantonwine DE, Rivera-Gonzalez LO, Ferguson KK, Mukherjee B, Calafat AM, Ye X, Anzalota Del Toro LV, Crespo-Hernandez N, Jimenez-Velez B, et al. Distribution, variability, and predictors of urinary concentrations of phenols and parabens among pregnant women in Puerto Rico. Environmental Science & Technology. 2013;47:3439–3447. doi: 10.1021/es400510g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in US adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Environmental Health Perspectives. 2011;119:1396–1402. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. International Journal of Andrology. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouritsen A, Aksglaede L, Sorensen K, Mogensen SS, Leffers H, Main KM, Frederiksen H, Andersson AM, Skakkebaek NE, Juul A. Hypothesis: exposure to endocrine-disrupting chemicals may interfere with timing of puberty. International Journal of Andrology. 2010;33:346–359. doi: 10.1111/j.1365-2605.2010.01051.x. [DOI] [PubMed] [Google Scholar]
- Mouritsen A, Frederiksen H, Sorensen K, Aksglaede L, Hagen C, Skakkebaek NE, Main KM, Andersson AM, Juul A. Urinary phthalates From 168 girls and boys measured twice a year during a 5-Year period: associations with adrenal androgen levels and puberty. Journal of Clinical Endocrinology & Metabolism. 2013;98:3755–3764. doi: 10.1210/jc.2013-1284. [DOI] [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environmental Health Perspectives. 2006;114:1643–1648. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyanarayana S, Barrett E, Butts S, Wang CW, Swan S. Phthalate exposure and reproductive hormone concentrations in pregnancy. Reproduction. 2013 doi: 10.1530/REP-13-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeters G, Den Hond E, Dhooge W, Van Larebeke N, Leijs M. Endocrine disruptors and abnormalities of pubertal development. Basic & Clinical Pharmacology & Toxicology. 2008;102:168–175. doi: 10.1111/j.1742-7843.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Short MB, Rosenthal SL. Psychosocial development and puberty. Annals of the New York Academy of Sciences. 2008;1135:36–42. doi: 10.1196/annals.1429.011. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the US population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environmental Health Perspectives. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research. 2008;106:257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Growth reference data for 5–19 years. World Health Organization; 2007. [Online.] [Google Scholar]
- Wolff MS, Teitelbaum SL, McGovern K, Windham GC, Pinney SM, Galvez M, Calafat AM, Kushi LH, Biro FM Breast Cancer Environment Research Program. Phthalate exposure and pubertal development in a longitudinal study of US girls. Human Reproduction. 2014;29:1558–1566. doi: 10.1093/humrep/deu081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, Kushi LH, Erdmann C, Hiatt RA, Rybak ME, et al. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environmental Health Perspectives. 2010;118:1039–1046. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.