Abstract
Objective
The correlation between intestinal cholesterol absorption values and plasma low-density lipoprotein-cholesterol (LDL-C) levels remains controversial. Niemann-Pick-C1-Like 1 (NPC1L1) is essential for intestinal cholesterol absorption, and is the target of ezetimibe, a cholesterol absorption inhibitor. However, studies with NPC1L1 knockout mice or ezetimibe cannot definitively clarify this correlation because NPC1L1 expression is not restricted to intestine in humans and mice. In this study we sought to genetically address this issue.
Methods and results
We developed a mouse model that lacks endogenous (NPC1L1) and LDL receptor (LDLR) (DKO), but transgenically expresses human NPC1L1 in gastrointestinal tract only (DKO/L1IntOnly mice). Our novel model eliminated potential effects of non-intestinal NPC1L1 on cholesterol homeostasis. We found that human NPC1L1 was localized at the intestinal brush border membrane of DKO/L1IntOnly mice. Cholesterol feeding induced formation of NPC1L1-positive vesicles beneath this membrane in an ezetimibe-sensitive manner. Compared to DKO mice, DKO/L1IntOnly mice showed significant increases in cholesterol absorption and blood/hepatic/biliary cholesterol. Increased blood cholesterol was restricted to very low-density lipoprotein (VLDL) and LDL fractions, which was associated with increased secretion and plasma levels of apolipoproteins B100 and B48. Additionally, DKO/L1IntOnly mice displayed decreased fecal cholesterol excretion and hepatic/intestinal expression of cholesterologenic genes. Ezetimibe treatment virtually reversed all of the transgene-related phenotypes in DKO/L1IntOnly mice.
Conclusion
Our findings from DKO/L1IntOnly mice clearly demonstrate that NPC1L1-mediated cholesterol absorption is a major determinant of blood levels of apolipoprotein B-containing atherogenic lipoproteins, at least in mice.
Keywords: apolipoprotein B, chylomicron remnant, cholesterol absorption, fecal neutral sterol excretion, low-density lipoprotein receptor, Niemann-Pick-C1-Like 1
1. Introduction
Cholesterol is a major structural component of mammalian cell membranes. It traffics through the circulation in the form of lipoprotein particles that are composed of apolipoproteins (Apo) and various lipids including cholesterol. There are two major lipoprotein particles in human blood: low-density lipoprotein (LDL) and high-density lipoprotein (HDL). High blood LDL-cholesterol (LDL-C) concentration is the major independent risk factor for atherosclerosis (1), the underlying pathologic process of coronary heart disease. Whole-body cholesterol homeostasis is balanced primarily by de novo biosynthesis, intestinal absorption, and biliary excretion (2), though the relative contribution of each pathway remains elusive due to the crosstalk among these intertwined metabolic and physiological processes.
Ezetimibe is a potent intestinal cholesterol absorption inhibitor (3) and the first new drug approved for LDL-C lowering after statins that inhibit 3-hydroxy-3-methyl-glutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of cholesterol biosynthesis (4). Randomized clinical trials and other studies have shown that either ezetimibe monotherapy or coadministration with a statin effectively lowers plasma total cholesterol and LDL-C in primary hypercholesterolemic subjects (5). Niemann-Pick C1-Like 1 (NPC1L1) (6) was identified as the molecular target of ezetimibe (7–9). NPC1L1 is highly expressed at the brush border membrane of enterocytes (7) and at the apical side of cultured polarized cells (10). The tissue distribution of NPC1L1 expression differs among species. In humans, NPC1L1 is expressed in intestine, liver and other tissues, but in mice it is almost exclusively expressed in small intestine (6, 7, 11). Despite this, low-levels of NPC1L1 mRNA were seen in extra-intestinal tissues of mice, such as gallbladder, stomach, and liver (7). NPC1L1 protein is expressed in the gallbladder epithelium of wild-type mice (see results). We have previously observed that the hepatic level of NPC1L1 mRNA is robustly induced in lovastatin-treated mice overexpressing ATP-binding cassette transporter G5 and G8 (ABCG5/ABCG8) (12), though the significance of this observation has yet to be elucidated. Nonetheless, NPC1L1 knockout (L1-KO) mice are defective in intestinal cholesterol absorption and insensitive to ezetimibe (7). These animals are protected against diet-induced hypercholesterolemia and several other metabolic disorders (13–17). Strikingly, NPC1L1 disruption almost completely prevents atherogenesis in apoE-deficient mice (18).
Although these animal studies with ezetimibe and genetic disruption of NPC1L1 together imply a positive correlation between intestinal cholesterol absorption and blood atherogenic LDL-C levels, which is consistent with reports in Finish men (19) and in individuals in the Dallas Heart Study (20), low-levels of NPC1L1 expression in non-intestinal cell types in mice interfere with definitive establishment of intestinal NPC1L1 as a major player in determining blood LDL-C levels. Ezetimibe inhibits NPC1L1 function in liver (11) and perhaps in other tissues such as gallbladder, which may alter cholesterol homeostasis independent of intestinal cholesterol absorption. Additionally, the positive correlation in humans does not exist in all human populations investigated. For example, it has been reported that cholesterol absorption values range widely from 29% to 80% in normal men and women consuming a moderately low cholesterol diet, yet these values do not correlate with plasma LDL-C levels in this population (21) and in other subjects (22). These observations in humans appeared to suggest a minor role of intestinal cholesterol absorption in determining net blood LDL-C levels.
In this study, we created a mouse model expressing no endogenous NPC1L1, but human NPC1L1 in gastrointestinal tract only (L1IntOnly mice) to directly address how intestinal NPC1L1-mediated cholesterol absorption regulates cholesterol metabolism and blood LDL-C levels. This model limited potential effects of low-levels of endogenous NPC1L1 expression in non-intestinal cell types on cholesterol homeostasis, and avoided variations of transcriptional activity of endogenous NPC1L1 gene under different nutritional and pathophysiological conditions. Considering NPC1L1 as an uptake transporter of free cholesterol, we hypothesized that intestinal NPC1L1 may predominantly act on the production side of lipoproteins. To eliminate the major LDL-C clearance pathway so that we can focus on lipoprotein production, and to create a human-like plasma lipoprotein-cholesterol profile in mice, we crossed L1IntOnly mice to LDLR knockout mice, and generated mice lacking both endogenous NPC1L1 and LDLR, but expressing human NPC1L1 in gastrointestinal tract only (DKO/L1IntOnly mice). Our findings from this novel genetic model clearly demonstrate that intestinal NPC1L1-mediated cholesterol absorption is a major determinant of plasma atherogenic apoB-containing lipoprotein levels.
2. Materials and methods
2.1. Creation of intestine-specific human NPC1L1 transgenic mice
A full-length human NPC1L1 cDNA (GeneBank accession no. AY436875) was cloned into pcDNA.3 plasmid as described previously (10). The resultant plasmid was designated as pcDNA3.L1. The pcDNA3.L1 was cut by XbaI. The ends of resultant DNA fragment were blunted by T4 DNA polymerase and then cut by KpnI. The NPC1L1-containing fragment was subcloned into KpnI and blunted SalI sites of pBluescript(+) plasmid. The resultant plasmid was named pBS.L1. The pBS.L1 was cut with KpnI, blunted by T4 DNA polymerase, and then cut by ClaI. The NPC1L1-containing fragment was cloned into SmaI and ClaI sites of pBSII-12.4kbVill plasmid containing 12.4kb mouse villin promoter (23) (kindly provided by Dr. Deborah L. Gumucio in the Department of Cell and Developmental Biology at the University of Michigan Medical School). The resultant plasmid was designated as pBSII-12.4kbVill-L1, which was cut with PmeI to remove the vector sequence. The villin-NPC1L1-containing DNA fragment was then purified and microinjected into fertilized embryos obtained from C57BL/6 mice and B6D2 mice using the standard transgenic technology in the Transgenic Mouse Core Facility at Wake Forest University Health Sciences. Several transgenic founders were obtained from both C57BL/6 embryos and B6D2 embryos. One founder derived from a B6D2 embryo was able to stably transmit the transgene into next generations after crossing with B6D2 mice and thus used for establishment of an intestine-specific human NPC1L1 transgenic (L1-TgInt) mouse line.
2.2. Generation of DKO/L1IntOnly mice
L1-KO mice were generated by using C57BL/6 mouse embryonic stem cells and thus have pure C57BL/6 genetic background (13). Homozygous L1-KO mice were crossed with L1-TgInt mice. The resultant mice heterozygous for NPC1L1 and hemizygous for villin-NPC1L1 transgene were crossed with C57BL/6 L1-KO mice to generate L1-KO mice with villin-NPC1L1 transgene, which was then crossed with C57BL/6 LDLR knockout mice to produce mice heterozygous for both endogenous NPC1L1 and LDLR alleles with or without the villin-NPC1L1 transgene. These mice were then crossed to each other to produce mice lacking both endogenous NPC1L1 and LDLR (DKO) with or without the villin-NPC1L1 transgene. The DKO mice with the transgene were then crossed to the DKO mice without the transgene to produce DKO mice and DKO/L1IntOnly mice for all experiments in this study. These mice had 93.75% C57BL/6 genetic background.
2.3. Animal housing and diets
All mice were housed in a specific pathogen-free animal facility in plastic cages at 22°C, with a daylight cycle from 6AM to 6P M. The mice were provided with water and a standard chow diet (Prolab RMH 3000; LabDiet, Brentwood, MO) ad libitum, unless stated otherwise. All animal procedures were approved by the Institutional Animal Care and Use Committees at Wake Forest University Health Sciences and at University of Maryland.
At 6 weeks of age, male mice were fed a synthetic diet containing 10% energy from palm oil and 0.2% (w/w) cholesterol with or without ezetimibe (0.005% w/w) for 3 weeks prior to sacrifice during the daylight cycle after a 4h fast. Bile, blood, liver, and five equal segments of small intestine were collected from each mouse.
2.4. Immunofluorescence histochemistry
Intestinal tissues were washed in PBS and fixed in 4% paraformaldehyde at 4°C overnight. The tissues were then embedded in to O.C.T. blocks and cut into 10μm cryo-sections. Before antibody staining, the sections were subjected to antigen retrieval in boiled Buffer A [10 mM citrate buffer (pH 6.0)]. Sections were blocked and permeabilized in Buffer B [PBS plus 0.1% Triton X-100 and 2% bovine serum albumin (BSA)]. The rabbit anti-human NPC1L1 antibody (11) was applied for overnight at 4°C in Buffer B. After 3 times of wash with PBS, Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (Invitrogen) was sequentially added and incubated in Buffer B for 1 h at room temperature. After immunostaining, sections were incubated with 300 nM DAPI in PBS for 10 min before mounting. All fluorescence pictures were taken under deconvolution Leica CTR6500 microscope.
For quantification of intracellular NPC1L1-positive vesicles, 10–12 frames of fluorescence pictures were randomly taken. Alexa Fluor 488-labeled NPC1L1 vesicles and DAPI-labeled nucleus were counted using ImageJ 1.47 software. The final numbers of vesicles were normalized with cell numbers per frame.
2.5. Measurements of fecal neutral sterol excretion and intestinal cholesterol absorption
The six-week-old male mice were fed the synthetic diet with or without ezetimibe for 14 days, and then housed individually. Feces were collected for 3 days from these individually housed mice. Fecal neutral sterol excretion was determined by gas-liquid chromatography as described previously (11). On Day 17 of the diet feeding, each mouse was administered by gavage 100μl soybean oil containing 0.2μCi [3H]-sitostanol and 0.1μCi [14C]-cholesterol (American Radiolabeled Chemical, Inc.). Feces were then collected for 3 days for the determination of fractional intestinal cholesterol absorption as described previously (11).
2.6. Lipid analyses in plasma, liver and bile
Plasma concentrations of total cholesterol, free cholesterol, and triglyceride were analyzed by enzymatic assays as described previously (11). For analysis of hepatic lipid contents, the lipids were extracted from ~80 mg of liver tissues and measured enzymatically as described previously (11). Biliary concentrations of free cholesterol, phospholipids and bile acids were determined as described previously (11).
2.7. Determination of plasma lipoprotein-cholesterol profile
An equal amount of plasma sample from each mouse in each group was pooled. The pooled sample was analyzed for the plasma lipoprotein-cholesterol profile by the fast phase liquid chromatography (FPLC) method (24) using a Superose 6 10/300 GL column (GE Healthcare) and a LaChrom Elite HPLC system (Hitachi High Technologies). Briefly, a 50μl pooled plasma sample from each group was diluted with PBS (0.05M phosphate, 0.9% sodium chloride, 0.01% EDTA, and 0.01% sodium azide) to 400μl total volume and then injected onto the FPLC system with online mixing of enzymatic reagents (Cholesterol Liquid Reagent Set, Pointe Scientific, Inc.) with effluent from the column at a flow rate of 0.4 ml/min. The lipoprotein-cholesterol distribution was monitored by a computer.
2.8. Determination of in vivo apoB secretion
In vivo apoB secretion was performed according to the two publications (25, 26). Both DKO and DKO/L1IntOnly female mice were fed the synthetic diet described above for 3 weeks. The mice were then fasted for 4 h prior to retro-orbital injection of 150 μl of saline containing Triton WR 1339 at 500 mg/Kg BW and [35S]-Methionine (NEG009C005MC, PerkinElmer) at 5 mCi/kg BW. Blood samples were collected at 60 and 120 min after injection. A total of 10 μl of plasma was subjected to 8% SDS-PAGE. The gel was dried, exposed to a FUJIFILM imaging plate (BAS-IP MS 2040), and read with a Phosphorimager (Fuji).
2.9. Quantitative real-time PCR (qPCR) analysis
Total RNAs were extracted from liver and intestine using Trizol reagents. The qPCR was performed on individual samples (n = 5) as described previously (12).
2.10. Statistical analysis
Data are presented as Means ± SEM (Standard Error of Mean). Significance of differences was determined for each group of values by One-way ANOVA (Tukey-Kramer honestly significant difference) except two group comparisons and quantification of vesicles on fluorescence images, which were done using Student-t-test. Values of P < 0.05 were considered significant. All analyses were performed using GraphPad Prism Software Version 5.
3. Results
3.1. Intestinal expression of human NPC1L1 in mice lacking endogenous NPC1L1 restores cholesterol absorption and reduces fecal neutral sterol excretion
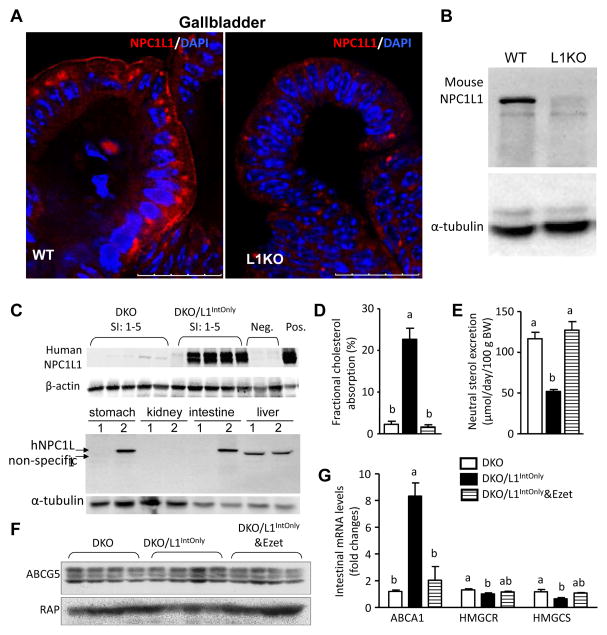
It was believed that mice express NPC1L1 exclusively in the small intestine (6, 7, 11). However, during the survey of tissue distribution of NPC1L1 protein expression in wild-type mice while using L1-KO mice as controls, we found that NPC1L1 protein is also expressed in the gallbladder epithelium of wild-type mice (Fig. 1A–B). To get rid of the effects of gallbladder NPC1L1 and perhaps low levels of NPC1L1 in other tissues on cholesterol trafficking in mice, and to specifically study the role of intestinal NPC1L1 in regulating cholesterol homeostasis, we used the transgenic approach to express human NPC1L1 in the intestine of L1-KO mice. To determine if NPC1L1 transgene is expressed at the desired location, we measured human NPC1L1 protein expression in the five equally divided segments of the entire small intestine from L1-KO mice and DKO mice by immunoblotting using an anti-human NPC1L1 antibody (11). Consistent with the mouse villin promoter activity (23), human NPC1L1 protein is expressed in all segments from DKO/L1IntOnly mice (Fig. 1C, top panel), although the expression level was substantially lower in the most proximal segment (SI-1) relative to other segments. As expected, L1-KO and DKO mice expressed no endogenous NPC1L1 when an anti-mouse NPC1L1 antibody (27) was used (data not shown). Since villin promoter activity was observed in stomach and kidney in a transgenic mouse line (28), we immunoblotted these tissues for human NPC1L1, and found that human NPC1L1 transgene is expressed in the stomach, but not kidney and liver (Fig. 1C, bottom panel).
Fig. 1.
A) Immunofluorescence staining of mouse NPC1L1 in the gallbladder of wild-type (WT) and L1KO mice on regular chow diet using a rabbit Anti-NPC1L1 Antibody (aa1000–1100) IHC-plus LS-B88 (LifeSpan BioSciences, Inc., Seattle, WA). White arrows denote the luminal surface of gallbladder epithelium. B) Immunoblots of mouse NPC1L1 in the 100μg of gallbladder homogenates of WT and L1KO mice using the above antibody. C) Immunoblots of human NPC1L1 in the homogenates (30μg protein each) of the 5 equal segments of small intestine (SI: 1–5). Two L1-KO mouse livers as negative controls (Neg., 20μg). The L1KO liver transgenically overexpressing human NPC1L1 as a positive control (Pos., 20μg). In the bottom panel, 50μg homogenate proteins were used for stomach, kidney and liver, and 20μg for intestine (jejunum). 1, DKO; 2, DKO/IntOnly. D) Intestinal cholesterol absorption (n = 8). E) Fecal neutral sterol excretion (n = 8–12). F) Immunoblots of ABCG5 and receptor-associated protein (RAP) (as a loading control) in the jejunal membrane preparations. G) Relative mRNA levels of cholesterol-sensitive genes in the jejuna (n = 5). Groups not sharing a common superscript letter are significantly different (P < 0.05). Ezet, ezetimibe; HMGCR, HMG-CoA reductase; HMGCS, HMG-CoA synthase.
To determine if transgenically expressed NPC1L1 is functional, we measured intestinal cholesterol absorption and fecal neutral sterol excretion in our mice. As shown in Fig. 1D, intestinal expression of human NPC1L1 substantially increased cholesterol absorption from 2.27% in DKO mice to 22.68% in DKO/L1IntOnly mice, and this increase was completely abolished by ezetimibe treatment (down to 1.64%). As a result of increased intestinal cholesterol absorption, fecal neutral sterol excretion was significantly reduced in DKO/L1IntOnly (51.93 μmol/day/100g BW) versus DKO (116.68 μmol/day/100 g BW) mice, and this reduction was fully rescued by ezetimibe (Fig. 1E). These changes in intestinal absorption and fecal excretion of cholesterol were not associated with significant alterations in body weight [20.5 ± 0.71, 22.1 ± 0.59, and 23.4 ± 0.79 grams (Mean ± SEM, n = 8–12) at necropsy (9-week-old) for DKO, DKO/L1IntOnly, and DKO/L1IntOnly treated with ezetimibe groups, respectively]. The reduced fecal neutral sterol excretion was unlikely a result of reduced secretion of sterols via intestinal heterodimeric sterol exporter ABCG5/ABCGG8, because ABCG5 protein levels remained similar among three groups (Fig. 1F). The NPC1L1-mediated increase in intestinal cholesterol absorption caused a significant up-regulation in intestinal expression of the mRNA for ABCA1 (Fig. 1G), which is a target gene of the nuclear receptor liver x receptor (LXR) that senses cellular cholesterol content (29). This observation suggests an elevation of cholesterol in enterocytes from DKO/L1IntOnly mice relative to DKO mice. Consistently, intestinal mRNAs levels of two cholesterologenic genes HMG-CoA reductase and HMG-CoA synthase were decreased due to feedback regulation. Ezetimibe treatment virtually abolished the effect of intestinal NPC1L1 on ABCA1 expression.
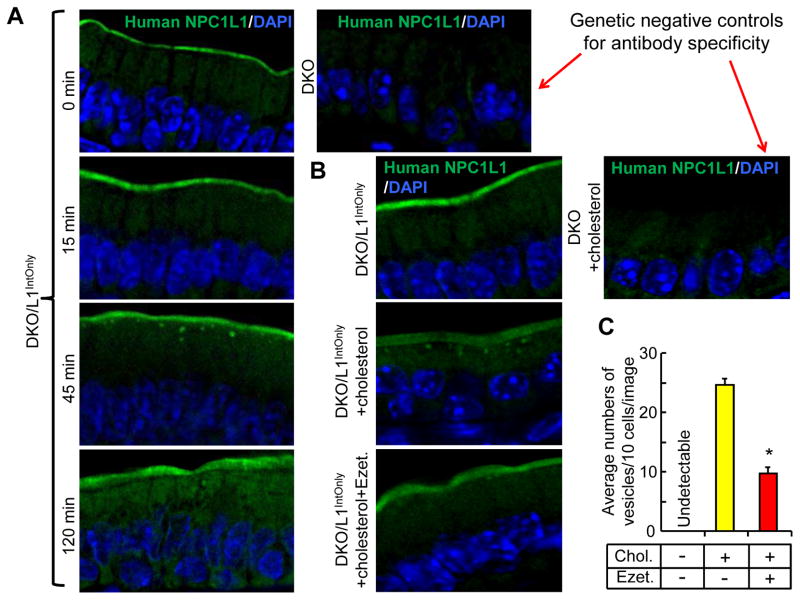
3.2. Ezetimibe inhibits cholesterol-induced vesicular translocation of brush border membrane-localized human NPC1L1 in the small intestine of mice
To identify the subcellular localization of the transgene-derived human NPC1L1 protein, we performed immunofluorescence studies with the jejunal sections. Consistently with the subcellular localization of endogenous NPC1L1 proteins (7, 14), we found that human NPC1L1 protein in our transgenic mice was almost exclusively localized at the brush border membrane (Fig. 2). We have previously shown that cholesterol depletion induces NPC1L1 translocation from the endocytic recycling compartment to the plasma membrane in McArdle RH7777 rat hepatoma cells and this cholesterol-induced NPC1L1 translocation is coupled to NPC1L1’s ability to transport free cholesterol into the cells in an ezetimibe-sensitive manner (10). To examine whether cholesterol feeding alters the subcellular localization of human NPC1L1 in vivo, we fed the overnight-fasted mice with a synthetic diet containing 0.2% cholesterol (11) and harvested jejuna for immunofluorescence studies at different time points after diet feeding (Fig. 2A). While no NPC1L1-positive vesicles were observed at 0 and 15 min, many NPC1L1-positive vesicles appeared beneath the brush border membrane at 45 min. These vesicles were greatly reduced at 120 min. To determine whether ezetimibe inhibits formation of NPC1L1-positive vesicles, we administered the mice with ezetimibe by gastric gavage and found that ezetimibe treatment for only 3 days substantially reduced the cholesterol-induced formation of NPC1L1-positive vesicles beneath the brush border membrane (Fig. 2B and 2C).
Fig. 2.
Ezetimibe blocks cholesterol-induced vesicular trafficking of human NPC1L1 in DKO/L1IntOnly mice. A) Cholesterol feeding induces vesicular trafficking of intestinal NPC1L1. Two-month-old male mice were fasted overnight, and then fed with a synthetic diet containing 0.2% (w/w) cholesterol. The mice were sacrificed at 15, 45 and 120 min, and the jejuna taken for immunofluorescence studies. B) Ezetimibe (Ezet.) inhibits cholesterol-induced NPC1L1 trafficking. Two-month-old male mice were pretreated with or without ezetimibe (10 mg/kg/day) by gavage for 3 days. On day 4, the overnight-fasted mice were treated with ezetimibe or vehicle. After 30 min, the mice were administered by gavage with 150 μl medium-chain triglycerides (MCT) oil or MCT oil containing 40mg/ml cholesterol. The mice were sacrificed 30 min post MCT gavage, and the jejuna taken for immunofluorescence staining. C) Average numbers of NPC1L1-positive vesicles beneath the brush border membrane of the jejunum. Multiple images (n = 10–12) from (B) were taken and counted. Chol., cholesterol. *P = 3.85E-10 (Student-t-test).
3.3. Intestinal expression of human NPC1L1 in mice lacking endogenous NPC1L1 increases plasma atherogenic lipoproteins and apoB
To define how intestinal NPC1L1 influences cholesterol homeostasis, we measured plasma concentrations of total cholesterol, free cholesterol and cholesterol ester (Table 1). In mice lacking endogenous NPC1L1 (L1-KO mice), intestinal expression of human NPC1L1 significantly increased plasma total cholesterol and cholesterol ester without altering free cholesterol and triglyceride concentrations. Ezetimibe treatment completely reversed these changes. In mice lacking both endogenous NPC1L1 and LDLR, the transgenic expression of human NPC1L1 substantially raised plasma concentrations of total cholesterol, free cholesterol, cholesterol ester, and triglyceride. Ezetimibe treatment completely reversed the rise of cholesterol in these DKO animals that lack the major cholesterol clearance pathway due to genetic deletion of LDLR.
Table 1.
Plasma lipid concentrations (mg/dL) (Mean ± SEM)
| TC | FC | CE | TG | |
|---|---|---|---|---|
| L1-KO | 120 ± 3b | 32 ± 1ab | 147 ± 4b | 69 ± 5a |
| L1IntOnly | 141 ± 7a | 36 ± 2a | 176 ± 10a | 65 ± 4a |
| L1IntOnly&Ezet | 113 ± 9b | 29 ± 2b | 140 ± 12b | 68 ± 12a |
| DKO | 306 ± 15b | 87 ± 6b | 365 ± 20b | 78 ± 4b |
| DKO/L1IntOnly | 478 ± 5a | 191 ± 15a | 479 ± 33a | 119 ± 15a |
| DKO/L1IntOnly&Ezet | 319 ± 15b | 83 ± 6b | 395 ± 27b | 99 ± 10ab |
Mice were fasted for 4 h during the daytime cycle prior to collection of blood samples for analyses of plasma concentrations of total cholesterol (TC), free cholesterol (FC), and triglyceride (TG). The amount of cholesterol ester was calculated by subtracting free cholesterol from total cholesterol and multiplying by 1.67 to convert to cholesterol ester mass. For each parameter, values among single L1-KO background groups [L1-KO (n = 11), L1IntOnly (n = 8), and L1IntOnly&Ezet (n = 7)] or among DKO background groups [DKO (n = 10), DKO/L1IntOnly (n = 8), and DKO/L1IntOnly&Ezet (n = 7)] not sharing a common superscript letter are significantly different (P < 0.05). Ezet, ezetimibe
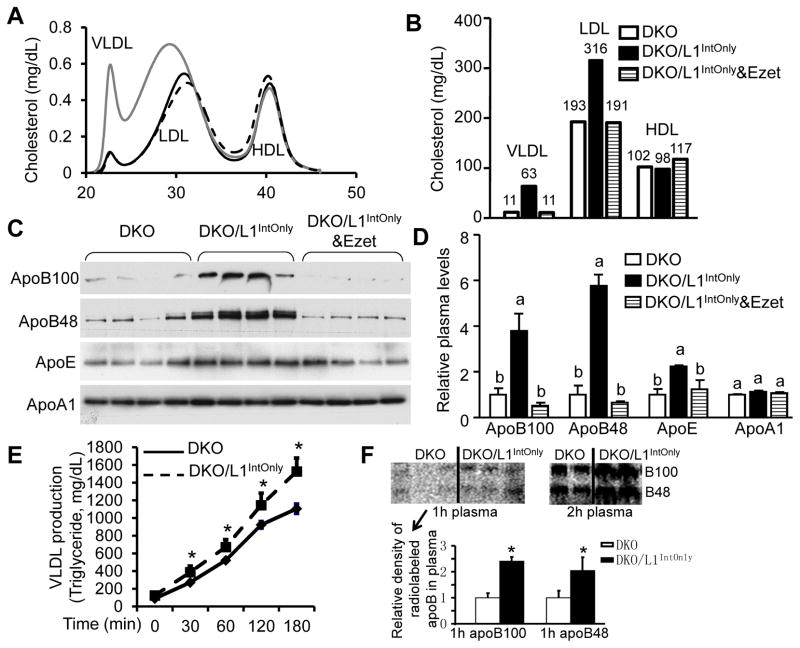
To determine in which lipoprotein particles the increased cholesterol was distributed, we measured lipoprotein-cholesterol profile (Fig. 3A). In the absence of LDLR, cholesterol was elevated only in the fractions of very low-density lipoproteins (VLDLs) and LDLs, but not high-density lipoproteins (HDLs). When cholesterol mass in each lipoprotein fraction was calculated based on the total cholesterol concentration in the sample, similar results were obtained (Fig. 3B). Treating DKO/L1IntOnly mice with ezetimibe brought their VLDL and LDL cholesterol levels back to those observed in the DKO mice.
Fig. 3.
Ezetimibe blocks intestinal NPC1L1-mediated increases in plasma atherogenic lipoproteins in DKO/L1IntOnly mice. A) Plasma lipoprotein-cholesterol profile of pooled plasma samples (n = 5). B) Calculated cholesterol mass in each lipoprotein subclass based on the lipoprotein profile and total plasma cholesterol concentrations. C) Immunoblots of plasma apolipoproteins. Mice were fasted for 4 h prior to blood collection. Plasma samples were diluted by 1:200 with saline. A total of 14μl of diluted plasma sample from each mouse was separated on a 4–15% gradient SDS-polyacrylamide gel for immunoblotting. The same membrane was used for all the apolipoproteins selected. D) Densitometry quantification of (C). Groups not sharing a common superscript letter are significantly different (P < 0.05). E) VLDL production (plasma triglyceride concentrations at different time points) after retro-orbital injections of Triton WR-1339 at 500mg/kg body weight in overnight-fasted male mice (n = 4). * P < 0.05. F) ApoB secretion in 4h-fasted mice. Densitometry was done using ImageJ software for the radiolabeled apoB bands in plasma samples collected 1h post Triton WR-1339 and [35S]methionine injection. * P < 0.05.
Increased plasma cholesterol levels in VLDL and LDL particles may result from increased particle size and/or numbers. While the lipoprotein-cholesterol profile (Fig. 3A) indicates that there was an increase in LDL particle size in DKO/L1IntOnly mice, we also found that plasma levels of apoB100 and apoB48 were significantly increased in DKO/L1IntOnly mice (Fig. 3C and 3D). Given that each of these atherogenic lipoprotein particles contains only one apoB molecule, our finding indicates that VLDL and LDL particle numbers were also increased in these transgenic animals. Ezetimibe treatment completely abolished these increases. Plasma apoE levels were also elevated in DKO/L1IntOnly mice, consistent with the presence of this apolipoprotein in almost all lipoproteins in mice (30). Plasma apoA1 levels remained unaltered, consistent with unchanged HDL-C levels.
To determine if increased plasma cholesterol is a result of increased lipoprotein production, we measured lipoprotein and apoB production using the triton block method in the fasted mice. DKOIntOnly mice had a significant increase in the production of VLDL-TG (Fig. 3E) and apoB (Fig. 3F). These data, together with LDLR-null background, suggest that elevated plasma cholesterol in DKOIntOnly mice was likely resulted from increased production of apoB-containing lipoproteins.
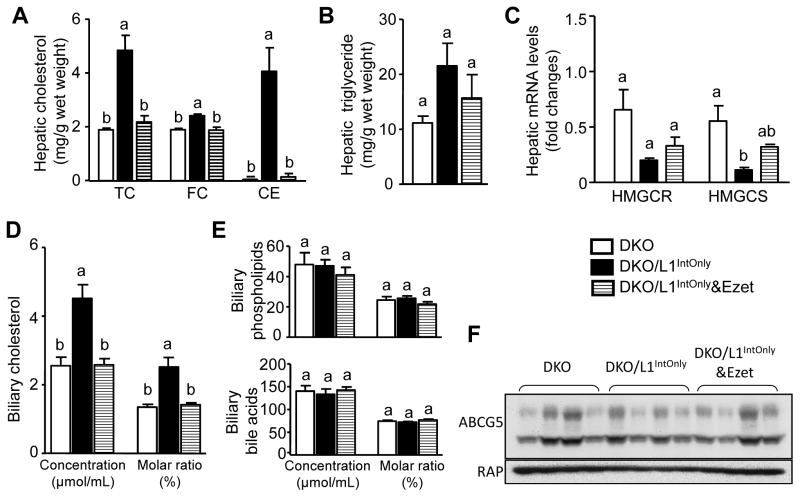
3.4. Intestinal expression of human NPC1L1 in DKO mice increases hepatic cholesterol level
Cholesterol absorbed from intestine and carried in chylomicrons is delivered to liver via hepatic uptake of chylomicron remnants (31). In agreement with this trafficking route of intestinal cholesterol, intestinal expression of human NPC1L1 in DKO mice significantly increased hepatic total cholesterol, free cholesterol and cholesterol ester (Fig. 4A), without altering hepatic triglyceride (Fig. 4B). Ezetimibe completely reversed the transgene effect on hepatic cholesterol content. Likely as a compensatory mechanism, hepatic expression levels of HMG-CoA synthase were suppressed in DKO/L1IntOnly mice (Fig. 4C).
Fig. 4.
Ezetimibe blocks intestinal NPC1L1-mediated increases in hepatic and biliary cholesterol. A) Total cholesterol (TC), free cholesterol (FC) and cholesterol ester (CE). The amount of cholesterol ester was calculated by subtracting free cholesterol from total cholesterol and multiplying by 1.67 to convert to cholesterol ester mass. B) Triglycerides (n = 8–12). C) Relative hepatic mRNA levels of cholesterol-regulated genes (n = 5). HMGCR, HMG-CoA reductase; HMGCS, HMG-CoA synthase. D), E) Biliary concentrations and molar ratios of cholesterol, phospholipids and bile acids (n = 8–12). F) Immunoblots of ABCG5 and receptor-associated protein (RAP) (as an internal control) in the liver membrane preparations. Groups not sharing a common superscript letter are significantly different (P < 0.05).
3.5. Intestinal expression of human NPC1L1 in DKO mice stimulates biliary cholesterol secretion
After receiving excess cholesterol, liver may directly secrete cholesterol into bile via the heterodimer of ABCG5/ABCG8 (32–34), in addition to producing more VLDL-cholesterol and inhibiting LDLR-mediated uptake, Consistently, we found that biliary cholesterol concentrations and molar ratios were significantly increased in DKO/L1IntOnly mice compared to DKO mice (Fig. 4D), and biliary concentrations and molar ratios of phospholipids and bile acids remained unaffected (Fig. 4E). Ezetimibe completely reversed the effect of intestinal NPC1L1 on biliary cholesterol excretion. Increased biliary cholesterol may reflect increased availability of free cholesterol for biliary secretion, rather than elevated expression of the cholesterol exporter because hepatic ABCG5 protein levels were comparable among all groups (Fig. 4F).
4. Discussion
Using genetically engineered mouse models, the present study establishes an important role of intestinal NPC1L1-dependent cholesterol absorption in promoting an atherogenic blood lipoprotein profile. Our data from this novel genetic model demonstrate that intestinal NPC1L1 significantly contributes to production of apoB-containing lipoproteins. Despite increased cholesterol secretion into circulation and bile, and despite suppressed expression of HMG-CoA synthase, liver still accumulates cholesterol derived from intestinal NPC1L1, demonstrating a predominant role of intestinal cholesterol absorption in inducing hepatic cholesterol accumulation. It should be pointed out that NPC1L1 transgene is also expressed in the stomach of our transgenic mice. Although we believe that the phenotypes seen in these animals are attributable to intestinal NPC1L1, we cannot exclude potential effects of stomach NPC1L1 on cholesterol homeostasis.
Blood apoB-containing LDL-cholesterol is an independent risk factor of atherosclerosis. Our current findings suggest that cholesterol-lowering effects of ezetimibe or NPC1L1 deletion are largely LDLR-independent, thus ezetimibe may be prescribed to hypercholesterolemic subjects with LDLR pathway deficiency to lower an independent atherosclerosis risk factor. Consistently, plasma LDL-cholesterol and atherosclerosis are substantially attenuated in apoE-deficient mice when NPC1L1 function is inhibited by ezetimibe or genetically disrupted (18, 35). Ezetimibe treatment also reduces LDL-cholesterol and atherosclerosis in LDLR/apoE double knockout mice (36), prevents cholesterol diet-induced hypercholesterolemia in LDLR knockout mice (37), and reduces LDL-cholesterol in familial hypercholesterolemic patients with homozygous LDLR mutations (38).
It was noticed that the fractional intestinal cholesterol absorption was about 23% in our transgenic mice, which is considered low given that intestinal cholesterol absorption is about 50% on average in mice on regular chow diet (39). While the low cholesterol absorption efficiency in our mice may be due to the expression level of the transgene, it is also possible that we only measured cholesterol absorption in mice on a Western-type diet, which contained 0.2% cholesterol (w/w), ten times higher than that normally present in a chow diet (~0.015%). This high amount of dietary non-labeled “cold” cholesterol has the potential to compete with the radiolabeled “hot” cholesterol for incorporation into the mixed micelles in the intestinal lumen, thereby resulting reduced absorption of “hot” cholesterol. Alternatively, human NPC1L1 protein may not function efficiently in the mouse intestine. Nonetheless, in humans, intestinal cholesterol absorption rates range from 29% to 80% (21), and the intestinal cholesterol absorption value in our transgenic mice is close to the low end of human values. This low fractional cholesterol absorption already had a profound effect on whole-body cholesterol homeostasis in our transgenic mice.
In normal animals, the bulk of cholesterol in the gut lumen is taken up by enterocytes primarily in the proximal proportion of small intestine. The appearance of NPC1L1-positive vesicles beneath the brush border membrane (Fig. 2) suggests that NPC1L1-dependent cholesterol absorption is likely a vesicle-mediated endocytic process, which is consistent with a previous study on mouse endogenous NPC1L1 (40). After entering enterocytes, the majority of free cholesterol is esterified by acyl CoA:cholesterol acyltransferase 2 (ACAT2) (41) and assembled into chylomicrons for secretion into lymphatic system. Chylomicrons then flow from lymphatics to blood circulation where they are catabolized/modified and converted to apoE-containing chylomicron remnants. The bulk of cholesterol absorbed from intestine is delivered to liver via hepatic uptake of chylomicron remnants. The majority of these remnant particles are cleared from circulation via hepatic LDLR. This may explain why intestinal NPC1L1-mediated cholesterol absorption exerts a profound effect on hepatic cholesterol homeostasis.
The delivery of intestine-derived cholesterol to liver results in suppression of hepatic HMG-CoA synthase (Fig. 4C). However, this transcriptional feedback regulation of cholesterol synthesis is not sufficient to cope with the cholesterol derived from intestinal NPC1L1. As a result, other adaptive mechanisms are also initiated in the liver of DKO/L1IntOnly mice to prevent over-accumulation of free cholesterol, including augmented conversion of free cholesterol to cholesterol ester (Fig. 4A) and increased secretion of free cholesterol into bile (Fig. 4D). It has been shown that cholesterol ester formation drives hepatic VLDL-cholesterol secretion (42). Consistently, we observed that DKO/L1IntOnly mice relative to DKO mice display increased plasma levels of apoB100 and VLDL-cholesterol (Fig. 3). Since apoB100 is exclusively produced by liver, our data in mice lacking the major clearance pathway of apoB-containing lipoproteins indicate that there is increased hepatic VLDL production in DKO/L1IntOnly mice, which are consistent with previous studies showing that ezetimibe may have a predominant impact on hepatic production of VLDL-cholesterol (37, 43). Indeed, we did observe increased hepatic VLDL production and apoB100 secretion in DKO/L1IntOnly mice (Fig. 3).
It should be emphasized that apoB48 secretion and plasma apoB48 levels are also elevated in DKO/L1IntOnly mice (Fig. 3). Since both liver and intestine can synthesize apoB48 in mice (44), accumulated apoB48 may come from VLDL produced by liver and chylomicrons secreted by intestine. Although fasting plasma samples were analyzed for apoB48 and VLDL production in the current study, it is believed that chylomicrons are continuously secreted by intestine (45). Intestinal NPC1L1 may play an important role in maintaining continuous production of chylomicrons in the fasting state in addition to mediating chylomicron-cholesterol production during postprandial period. Several major health problems such as insulin resistance, diabetes and obesity are associated with increased postprandial production of chylomicrons (46–49). Chylomicron remnants contribute significantly to the risk of atherosclerosis (45). These previous observations together with our current findings suggest that inhibiting intestinal NPC1L1 might be an attractive strategy in reducing atherosclerosis risk factors in subjects with insulin resistance, type 2 diabetes and obesity.
HIGHLIGHTS.
It was unclear if intestinal cholesterol absorption correlates with blood LDL-C.
We express human NPC1L1 in intestine of mice lacking NPC1L1 and LDL receptor.
Intestinal cholesterol absorption is a major determinant of blood LDL-C levels.
Intestinal cholesterol absorption is a major determinant of hepatic cholesterol.
Acknowledgments
The authors thank Dr. Harry R. Davis for critical reading of the manuscript and Drs. Yiannis A. Ioannou and Joanna P. Davies at Mount Sinai School of Medicine in New York for providing L1-KO mice. The authors also thank Dr. Feng Guo and Tanya Paschke in the Transgenic Core Facility of Wake Forest University Health Sciences for their excellent technical assistance. This work was supported in part by a research grant from the Investigator-Initiated Studies Program of MSP Pharmaceuticals, Inc. (to L.Y.), by Award Numbers R01DK085176 (to L.Y.), R01HL107500 (B.X.) and R01DK084172 (H.S.) from the National Institute of Health (to L.Y.), and by a Scientist Development Grant 0635261N from the American Heart Association (to L.Y.).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institute of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson PW, Garrison RJ, Castelli WP, Feinleib M, McNamara PM, Kannel WB. Prevalence of coronary heart disease in the Framingham Offspring Study: role of lipoprotein cholesterols. The American journal of cardiology. 1980;46(4):649–54. doi: 10.1016/0002-9149(80)90516-0. [DOI] [PubMed] [Google Scholar]
- 2.Dietschy JM, Turley SD, Spady DK. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. Journal of lipid research. 1993;34(10):1637–59. [PubMed] [Google Scholar]
- 3.Van Heek M, France CF, Compton DS, McLeod RL, Yumibe NP, Alton KB, Sybertz EJ, Davis HR., Jr In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J Pharmacol Exp Ther. 1997;283(1):157–63. [PubMed] [Google Scholar]
- 4.Brown MS, Faust JR, Goldstein JL. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. The Journal of biological chemistry. 1978;253(4):1121–8. [PubMed] [Google Scholar]
- 5.Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, Knopp RH, Lipka LJ, Lebeaut AP, Yang B, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: pooled analysis of two phase II studies. Clin Ther. 2001;23(8):1209–30. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 6.Davies JP, Levy B, Ioannou YA. Evidence for a Niemann-pick C (NPC) gene family: identification and characterization of NPC1L1. Genomics. 2000;65(2):137–45. doi: 10.1006/geno.2000.6151. [DOI] [PubMed] [Google Scholar]
- 7.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, et al. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303(5661):1201–4. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR, Jr, Dean DC, Detmers PA, et al. The target of ezetimibe is Niemann-Pick C1-Like 1 (NPC1L1) Proceedings of the National Academy of Sciences of the United States of America. 2005;102(23):8132–7. doi: 10.1073/pnas.0500269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinglass AB, Kohler M, Schulte U, Liu J, Nketiah EO, Thomas A, Schmalhofer W, Williams B, Bildl W, McMasters DR, et al. Extracellular loop C of NPC1L1 is important for binding to ezetimibe. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(32):11140–5. doi: 10.1073/pnas.0800936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu L, Bharadwaj S, Brown JM, Ma Y, Du W, Davis MA, Michaely P, Liu P, Willingham MC, Rudel LL. Cholesterol-regulated translocation of NPC1L1 to the cell surface facilitates free cholesterol uptake. The Journal of biological chemistry. 2006;281(10):6616–24. doi: 10.1074/jbc.M511123200. [DOI] [PubMed] [Google Scholar]
- 11.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. The Journal of clinical investigation. 2007;117(7):1968–78. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang W, Ma Y, Yu L. Plasma cholesterol is hyperresponsive to statin in ABCG5/ABCG8 transgenic mice. Hepatology. 2006;44(5):1259–66. doi: 10.1002/hep.21380. [DOI] [PubMed] [Google Scholar]
- 13.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. The Journal of biological chemistry. 2005;280(13):12710–20. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 14.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, et al. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. The Journal of biological chemistry. 2004;279(32):33586–92. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 15.Jia L, Ma Y, Rong S, Betters JL, Xie P, Chung S, Wang N, Tang W, Yu L. Niemann-Pick C1-Like 1 deletion in mice prevents high-fat diet-induced fatty liver by reducing lipogenesis. Journal of lipid research. 2010;51(11):3135–44. doi: 10.1194/jlr.M006353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jia L, Ma Y, Liu G, Yu L. Dietary cholesterol reverses resistance to diet-induced weight gain in mice lacking Niemann-Pick C1-Like 1. Journal of lipid research. 2010;51(10):3024–33. doi: 10.1194/jlr.M008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2008;295(4):G776–83. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis HR, Jr, Hoos LM, Tetzloff G, Maguire M, Zhu LJ, Graziano MP, Altmann SW. Deficiency of Niemann-Pick C1 Like 1 Prevents Atherosclerosis in ApoE−/− Mice. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(4):841–49. doi: 10.1161/01.ATV.0000257627.40486.46. [DOI] [PubMed] [Google Scholar]
- 19.Kesaniemi YA, Miettinen TA. Cholesterol absorption efficiency regulates plasma cholesterol level in the Finnish population. European journal of clinical investigation. 1987;17(5):391–5. doi: 10.1111/j.1365-2362.1987.tb01132.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, Hobbs HH. Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipoprotein levels. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(6):1810–5. doi: 10.1073/pnas.0508483103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosner MS, Lange LG, Stenson WF, Ostlund RE., Jr Percent cholesterol absorption in normal women and men quantified with dual stable isotopic tracers and negative ion mass spectrometry. Journal of lipid research. 1999;40(2):302–8. [PubMed] [Google Scholar]
- 22.Sehayek E, Nath C, Heinemann T, McGee M, Seidman CE, Samuel P, Breslow JL. U-shape relationship between change in dietary cholesterol absorption and plasma lipoprotein responsiveness and evidence for extreme interindividual variation in dietary cholesterol absorption in humans. Journal of lipid research. 1998;39(12):2415–22. [PubMed] [Google Scholar]
- 23.Madison BB, Dunbar L, Qiao XT, Braunstein K, Braunstein E, Gumucio DL. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. The Journal of biological chemistry. 2002;277(36):33275–83. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 24.Garber DW, Kulkarni KR, Anantharamaiah GM. A sensitive and convenient method for lipoprotein profile analysis of individual mouse plasma samples. Journal of lipid research. 2000;41(6):1020–6. [PubMed] [Google Scholar]
- 25.Siri P, Candela N, Zhang YL, Ko C, Eusufzai S, Ginsberg HN, Huang LS. Post-transcriptional stimulation of the assembly and secretion of triglyceride-rich apolipoprotein B lipoproteins in a mouse with selective deficiency of brown adipose tissue, obesity, and insulin resistance. The Journal of biological chemistry. 2001;276(49):46064–72. doi: 10.1074/jbc.M108909200. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Hernandez-Ono A, Crooke RM, Graham MJ, Ginsberg HN. Effects of antisense-mediated inhibition of 11beta-hydroxysteroid dehydrogenase type 1 on hepatic lipid metabolism. Journal of lipid research. 2011;52(5):971–81. doi: 10.1194/jlr.M013748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valasek MA, Weng J, Shaul PW, Anderson RG, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. The Journal of biological chemistry. 2005;280(30):28103–9. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- 28.Roth S, Franken P, van Veelen W, Blonden L, Raghoebir L, Beverloo B, van Drunen E, Kuipers EJ, Rottier R, Fodde R, et al. Generation of a tightly regulated doxycycline-inducible model for studying mouse intestinal biology. Genesis. 2009;47(1):7–13. doi: 10.1002/dvg.20446. [DOI] [PubMed] [Google Scholar]
- 29.Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289(5484):1524–9. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- 30.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(15):5374–9. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grundy SM. Absorption and metabolism of dietary cholesterol. Annu Rev Nutr. 1983;3:71–96. doi: 10.1146/annurev.nu.03.070183.000443. [DOI] [PubMed] [Google Scholar]
- 32.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(25):16237–42. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. The Journal of clinical investigation. 2002;110(5):671–80. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graf GA, Li WP, Gerard RD, Gelissen I, White A, Cohen JC, Hobbs HH. Coexpression of ATP-binding cassette proteins ABCG5 and ABCG8 permits their transport to the apical surface. The Journal of clinical investigation. 2002;110(5):659–69. doi: 10.1172/JCI16000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis HR, Jr, Compton DS, Hoos L, Tetzloff G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2001;21(12):2032–8. doi: 10.1161/hq1201.100260. [DOI] [PubMed] [Google Scholar]
- 36.Davis HR, Jr, Lowe RS, Neff DR. Effects of ezetimibe on atherosclerosis in preclinical models. Atherosclerosis. 2011;215(2):266–78. doi: 10.1016/j.atherosclerosis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. Journal of lipid research. 2005;46(4):779–89. doi: 10.1194/jlr.M400475-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105(21):2469–75. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 39.Wang DQ. Regulation of intestinal cholesterol absorption. Annual review of physiology. 2007;69:221–48. doi: 10.1146/annurev.physiol.69.031905.160725. [DOI] [PubMed] [Google Scholar]
- 40.Xie C, Zhou ZS, Li N, Bian Y, Wang YJ, Wang LJ, Li BL, Song BL. Ezetimibe blocks the internalization of NPC1L1 and cholesterol in mouse small intestine. Journal of lipid research. 2012;53(10):2092–101. doi: 10.1194/jlr.M027359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Current opinion in lipidology. 2001;12(2):121–7. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Xie C, Woollett LA, Turley SD, Dietschy JM. Fatty acids differentially regulate hepatic cholesteryl ester formation and incorporation into lipoproteins in the liver of the mouse. Journal of lipid research. 2002;43(9):1508–19. doi: 10.1194/jlr.m200146-jlr200. [DOI] [PubMed] [Google Scholar]
- 43.Telford DE, Sutherland BG, Edwards JY, Andrews JD, Barrett PH, Huff MW. The molecular mechanisms underlying the reduction of LDL apoB-100 by ezetimibe plus simvastatin. Journal of lipid research. 2007;48(3):699–708. doi: 10.1194/jlr.M600439-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Greeve J, Altkemper I, Dieterich JH, Greten H, Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. Journal of lipid research. 1993;34(8):1367–83. [PubMed] [Google Scholar]
- 45.Warnakula S, Hsieh J, Adeli K, Hussain MM, Tso P, Proctor SD. New insights into how the intestine can regulate lipid homeostasis and impact vascular disease: frontiers for new pharmaceutical therapies to lower cardiovascular disease risk. Can J Cardiol. 2011;27(2):183–91. doi: 10.1016/j.cjca.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 46.Phillips C, Murugasu G, Owens D, Collins P, Johnson A, Tomkin GH. Improved metabolic control reduces the number of postprandial apolipoprotein B-48-containing particles in type 2 diabetes. Atherosclerosis. 2000;148(2):283–91. doi: 10.1016/s0021-9150(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 47.Mamo JC, Watts GF, Barrett PH, Smith D, James AP, Pal S. Postprandial dyslipidemia in men with visceral obesity: an effect of reduced LDL receptor expression? American journal of physiology Endocrinology and metabolism. 2001;281(3):E626–32. doi: 10.1152/ajpendo.2001.281.3.E626. [DOI] [PubMed] [Google Scholar]
- 48.Adeli K, Lewis GF. Intestinal lipoprotein overproduction in insulin-resistant states. Current opinion in lipidology. 2008;19(3):221–8. doi: 10.1097/MOL.0b013e3282ffaf82. [DOI] [PubMed] [Google Scholar]
- 49.Su JW, Lambert JE, Clandinin MT, Proctor SD. Impaired postprandial metabolism of apolipoprotein B48-containing remnant particles in normolipidemic subjects with brittle type 1 diabetes. Diabetes Care. 2009;32(2):e21. doi: 10.2337/dc08-1573. [DOI] [PubMed] [Google Scholar]