Abstract
Background
Circulating interleukin-6 levels increase with advancing age and are a risk factor for various diseases and mortality. The characterization of gene expression profiles associated with interleukin-6 levels might suggest important molecular events underlying its regulation.
Methods and Results
We studied the association of transcriptional profiles with interleukin-6 levels in 2422 participants from Framingham Heart Study Offspring Cohort using Affymetrix Human Exon 1.0 ST Array. We identified 4139 genes that were significantly associated with interleukin-6 levels (FDR<0.05) after adjusting for age, sex and blood cell components. We then replicated 807 genes in the InCHIANTI study with 694 participants. Many of the top genes are involved in inflammation-related pathways or erythrocyte function, including JAK/Stat signaling pathway and interleukin-10 signaling pathway.
Conclusion
We identified and replicated 807 genes that were associated with circulating interleukin-6 levels. Future characterization of interleukin-6 regulation networks may facilitate the identification of additional potential targets for treating inflammation-related diseases.
Keywords: Inflammation, gene expression, interleukin-6, epidemiology
Introduction
Human interleukin-6 is a multifunctional pro-inflammatory cytokine produced by many cell types including immune cells [1], vascular smooth muscle cells [2], adipocytes and skeletal muscle [3]. Interleukin-6 has both an acute and chronic inflammatory role [4]. It mediates downstream inflammatory cascades, including the production of C-reactive protein and fibrinogen [5, 6]. The protein also provides various signals to regulate cell growth [1], immune responses [7], and acute phase protein secretion [6], Interleukin-6 is an important regulator of immune response, inflammation and hematopoiesis.
The circulating level of interleukin-6 is usually low in healthy individuals but increases in older adults, often for unclear reasons [8]. Interleukin-6 is associated with increased risk for mortality even after accounting for cardiovascular disease and its risk factors [9]. Various age-related diseases have been associated with elevated level of interleukin-6 including cardiovascular disease [10], cancer [11], and diabetes [12, 13]. The level of interleukin-6 has been recognized as an important inflammatory biomarker for the severity and risk of progression of many diseases, such as atherosclerosis [6], systemic lupus erythematosus [14], impaired hematopoiesis [15], and coronary heart disease [16]. In addition, elevated levels of interleukin-6 have been associated with cognitive decline [17], physical disability, and frailty in older adults [18–23].
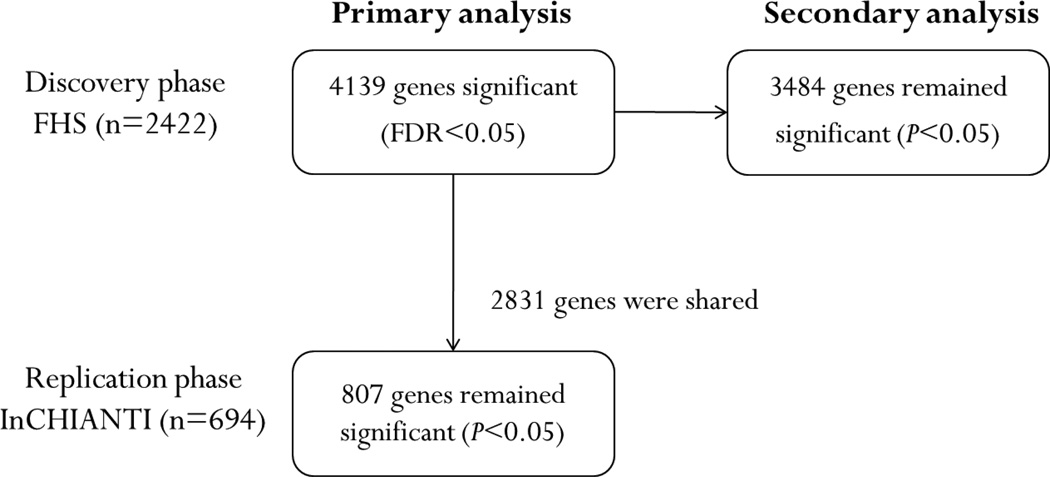
The expression of many genes has been associated with the concentration of circulating interleukin-6 levels [24–26]. Meanwhile, the expression of interleukin-6 gene is also regulated by a number of genes [27, 28]. However, prior studies typically focused on a few candidate genes using a small group of selected samples, which might limit their application to the general population. The objective of the present study was to assess the association of genome-wide gene expression with interleukin-6 levels in participants from the Framingham Heart Study, a large community-based cohort. Our results were further validated in an independent cohort, the InCHIANTI Study. The overall study design is shown in Figure 1.
Figure 1. Study design.
Primary analyses were adjusted for age and sex. Secondary analyses were adjusted for age, sex, and additional clinical covariates. A total of 4139 genes were found significantly associated with interleukin-6 levels (FDR<0.05), which were then tested in the replication cohort. Eight hundred and ninety-seven of them were replicated (P<0.05).
Methods
Study Samples
The Framingham Heart Study (FHS) is a longitudinal study aiming to investigate cardiovascular disease and its risk factors in the community. Three generations of participants have been enrolled since 1948 [29–31]. Every 2–8 years participants undergo a physical examination and assessment of cardiovascular disease risk factors, 12-lead electrocardiogram, along with lifestyle and medical history interview. Study samples for the current project were collected from the Offspring cohort enrolled in 1971, who are the children of the Original cohort as well as spouses of the offspring [30, 31]. Our study was limited to the participants who attended the eighth Offspring examination (2005–2008) and provided a blood sample for RNA collection. All participants gave written informed consent, and the study was approved by the Review Boards at the National Human Genome Research Institute and Boston University Medical Center.
Interleukin-6 Measurement
Fasting blood samples were obtained during the routine clinic visit, and the samples were frozen at −80°C. The interleukin-6 concentration was assayed by the quantitative enzyme-linked immunosorbent assay according to the manufacturers’ protocols (R&D Systems, Minneapolis, MN, USA). Ten percent of measures were run in duplicate. The minimum detectable concentration was 0.039 pg/ml. The mean intra-assay coefficient of variation was 4.0%[32]. More details are available at http://www.framinghamheartstudy.org/researchers/description-data/vascular-manuals/offspring_exam8_omni1_exam3_marker_manual.pdf.
Gene Expression Profiling
The gene expression profiling has been described in detail by Joehanes et al [33]. In brief, total RNA was isolated from frozen PAXgene blood tubes (PreAnalytiX, Hombrechtikon, Switzerland) and amplified using the WT-Ovation Pico RNA Amplification System (NuGEN, San Carlos, CA) according to the manufacturers’ standard operating procedures. The obtained cDNA was hybridized to the Affymetrix Human Exon 1.0 ST Array (Affymetrix, Inc., Santa Clara, CA). The raw data were quantile-normalized and log2 transformed, followed by summarization using Robust Multi-array Average [34]. The gene annotations were obtained from Affymetrix NetAffx Analysis Center (version 31). We excluded transcript clusters that were not mapped to RefSeq transcripts, resulting 17,873 distinct transcripts (17,324 distinct genes) for downstream analysis. Using partial least square method, we imputed the white blood cell and platelet counts and the percentage of lymphocytes, monocytes, eosinophils and basophils from gene expression data on measured cell counts in 2284 participants from FHS Third Generation Cohort. Given that the gene expression and interleukin-6 was not measured in the same examination for the FHS Third Generation Cohort, we did not include them in the analysis. The percentages of each imputed cell type were then normalized, where the negative predicted values were set to 0 and the sum of the percentages for all cell types were set 100%. Cross-validated estimates of prediction accuracy (R2) were 0.61, 0.41, 0.25, 0.83, 0.83, 0.81, 0.89, 0.25, for white blood cell counts, red blood cell counts, platelet counts, neutrophil percent, lymphocyte percent, monocyte percent, eosinophil percent, and basophil percent, respectively [33].
Statistical Analyses
Our primary analyses tested the association between interleukin-6 levels and gene expression. Gene expression was treated as the dependent measure and the loge of interleukin-6 concentration was treated as the exposure variable. The association was evaluated by the linear mixed effect models, in which clinical covariates, including sex, age, and differential cell counts, were cast as fixed factors, the technical covariates were cast as a random factor, and familial relatedness as random variance-covariance matrix.
The secondary analyses were adjusted for additional clinical covariates that might affect interleukin-6 levels [35]. Clinical covariates included current smoking status, systolic and diastolic blood pressures, hypertension treatment, body mass index, waist circumference, total/high-density lipoprotein cholesterol, triglycerides, lipid-lowering medication, glucose, diabetes, aspirin treatment (≥3 days per week), hormone replacement therapy, and prevalent cardiovascular disease.
We used false discovery rate (FDR)[36] to correct for multiple testing, which estimates the number of incorrectly rejected hypotheses divided by the total number of rejected hypotheses. Genes with FDR<0.05 were considered statistically significant.
All the analyses were performed using the R software package (www.r-project.org/). The linear mixed effect models were implemented in the “lme4” package, and "pedigreemm" package was used to account for pedigree information.
Replication Phase
We performed our replication on samples from the InCHIANTI study. InCHIANTI is a prospective population-based study of older adults that aims to identify risk factors for late-life disability [37, 38]. More than 1000 participants 65 years or older who lived in the Chianti area of Italy were enrolled between 1998 and 2000. RNA was collected using PAXgene technology during the participants 4th visit (2007/2008), and the Illumina Human HT12-v3 gene expression array quantified transcript expression levels. Details about the InCHIANTI interleukin-6 assay and gene expression profiling are available in the Supplemental Materials, and have been published previously [39]. Genes with nominal P<0.05 were considered as replicated. All participants gave written informed consent and the study was approved by the Italian National Institute on Research and Care of Aging (INRCA) Ethical Committee.
Results
Association of interleukin-6 with Gene Expression in FHS
A total of 2422 eligible participants from the Framingham Offspring Cohort were enrolled in our study. The descriptive characteristics of the participants (mean age 66±9 years, 54.9% women) are provided in Table 1.
Table 1.
Clinical characteristics of the study samples
| Characteristics | FHS (n=2422) + | InCHIANTI (n=694) + |
|---|---|---|
| Women, n (%) | 1,329 (54.9%) | 381 (54.9%) |
| Age, year ± SD | 66.4 ± 9.0 | 72.2 ± 15.3 |
| Interleukin-6 levels, pg/ml | 2.65 ± 2.98 | 3.80 ± 2.99 |
| Smoker, n (%) | 203 (8.4%) | 71 (10.4%) |
| Body mass index, kg/m2 | 28.5 ± 5.4 | 27.1 ± 4.3 |
| Waist circumference, cm | 100 ± 14 | 95 ± 12 |
| Systolic blood pressure, mm Hg | 129 ± 17 | 132 ± 20 |
| Diastolic blood pressure, mm Hg | 73 ± 10 | 78 ± 11 |
| Hypertension treatment | 1,298 (53.6%) | 217 (31.3%) |
| Total cholesterol, mg/dL | 186 ± 37 | 205 ± 40 |
| HDL cholesterol,mg/dL | 57 ± 18 | 56 ± 15 |
| Triglycerides, mg/dL | 119 ± 71 | 124 ± 74 |
| Glucose, mg/dL | 107 ± 24 | 94 ± 24 |
| Prevalent diabetes mellitus, n (%) | 423 (17.5%) | 97 (14.3%) |
| Prevalent cardiovascular disease | 240 (9.9%) | 188 (27.7%) |
| Aspirin treatment (>=3 days per week) | 1,087 (44.9%) | 173 (25.4%) |
| Lipid-lowering medication | 1,061 (43.8%) | 90 (13.2%) |
| Hormone replacement therapy | 141 (5.8%) | 32 (4.7%) |
Characteristics are represented by mean ± SD or n (%)
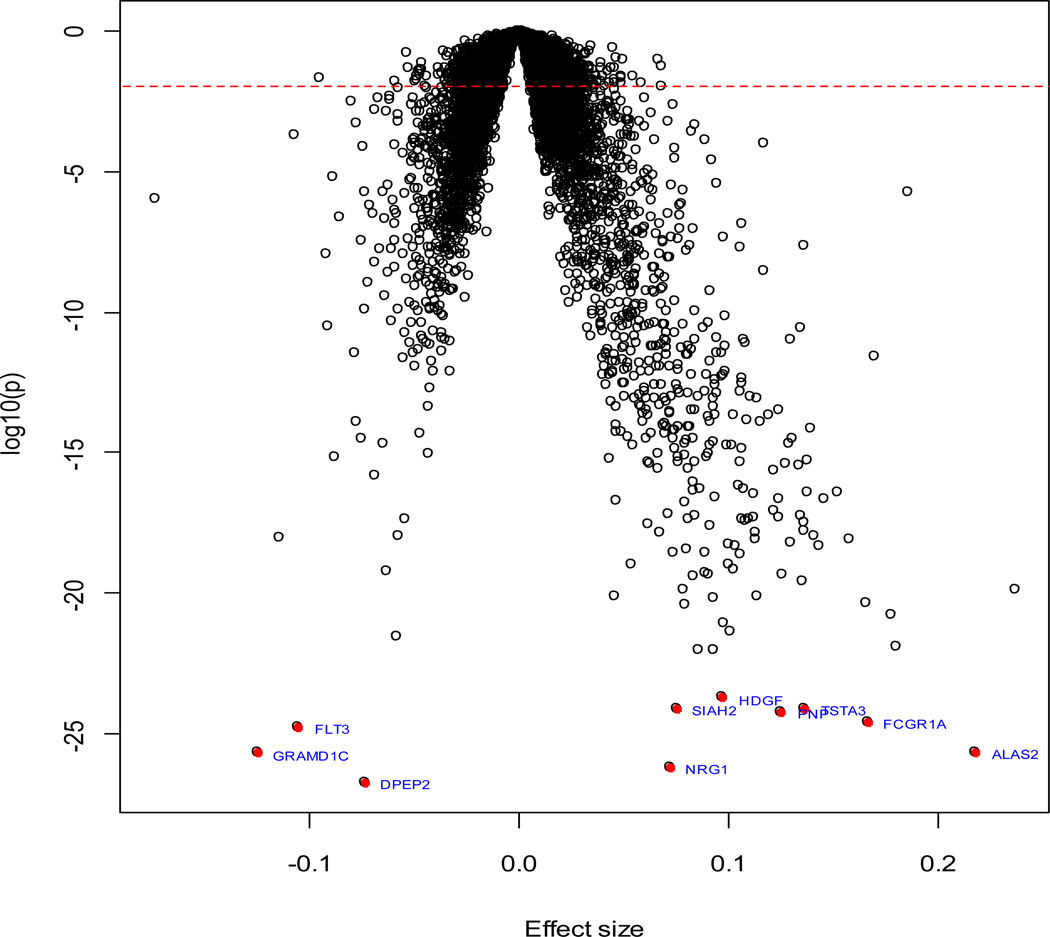
We identified 4139 genes that were significantly associated with interleukin-6 levels (FDR<5%). Among them, 1766 genes were negatively associated with interleukin-6 levels, whereas the remaining 2372 genes were positively associated. Figure 2 shows the volcano plot of all studied genes, and Table 2 shows the top associations (FDR<5.0×10−18). The most significant gene was DPEP2 (FDR=3.5×10−23), which encodes a dipeptidase that catalyzes various dipeptides including leukotriene D4 [40]. Many of the genes are involved in erythrocyte function (ALAS2, FLT3, SLC4A1, GLRX5, and STOM) or immune response (FCGR1A, PNP, and TSTA3).
Figure 2. Volcano plot of association results from primary analyses.
Each dot represents one gene. The x-axis represents the beta estimation (β) of each gene, whereas the y-axis represents the log10(P). Positive effects represent that the gene were positively associated with interleukin-6 levels, whereas negative effects represent that the genes were negatively associated with interleukin-6 levels. The red dash line indicates FDR<0.05.
Table 2.
Most significant genes associated with interleukin-6 levels from the primary analysis+
| Entrez Gene ID |
Gene | FHS | InCHIANTI‡ | ||||
|---|---|---|---|---|---|---|---|
| Effect size |
SE* | FDR$ | Effect size |
SE* | P value | ||
| 64174 | DPEP2 | −0.07 | 0.01 | 3.5×10−23 | −0.25 | 0.04 | 3.5×10−9 |
| 3084 | NRG1 | 0.07 | 0.01 | 5.6×10−23 | 0.14 | 0.05 | 4.0×10−3 |
| 54762 | GRAMD1C | −0.13 | 0.01 | 1.0×10−22 | |||
| 212 | ALAS2 | 0.22 | 0.02 | 1.0×10−22 | 0.18 | 0.05 | 1.2×10−4 |
| 2322 | FLT3 | −0.11 | 0.01 | 6.2×10−22 | |||
| 2209 | FCGR1A | 0.17 | 0.02 | 8.3×10−22 | 0.29 | 0.04 | 7.6×10−11 |
| 4860 | PNP | 0.13 | 0.01 | 1.5×10−21 | 0.15 | 0.05 | 1.5×10−3 |
| 6478 | SIAH2 | 0.07 | 0.01 | 1.6×10−21 | 0.15 | 0.05 | 1.8×10−3 |
| 7264 | TSTA3 | 0.14 | 0.01 | 1.6×10−21 | 0.12 | 0.05 | 1.0×10−2 |
| 3068 | HDGF | 0.10 | 0.01 | 3.5×10−21 | 0.10 | 0.05 | 2.8×10−2 |
| 51218 | GLRX5 | 0.09 | 0.01 | 1.5×10−19 | 0.16 | 0.05 | 6.2×10−4 |
| 482 | ATP1B2 | 0.09 | 0.01 | 1.5×10−19 | −0.09 | 0.05 | 7.5×10−2 |
| 6521 | SLC4A1 | 0.18 | 0.02 | 1.6×10−19 | 0.10 | 0.05 | 2.3×10−2 |
| 23500 | DAAM2 | −0.06 | 0.01 | 3.6×10−19 | |||
| 2040 | STOM | 0.10 | 0.01 | 5.3×10−19 | 0.11 | 0.04 | 8.5×10−3 |
| 5305 | PIP4K2A | 0.10 | 0.01 | 9.7×10−19 | 0.14 | 0.05 | 3.8×10−3 |
| 8991 | SELENBP1 | 0.18 | 0.02 | 1.7×10−18 | 0.15 | 0.05 | 2.3×10−3 |
| 9829 | DNAJC6 | 0.08 | 0.01 | 3.8×10−18 | |||
| 2766 | GMPR | 0.17 | 0.02 | 4.4×10−18 | 0.13 | 0.05 | 8.3×10−3 |
Primary analyses were adjusted for age and sex
Some genes were not present in InCHIANTI assay
SE: standard error;
FDR: false discovery rate
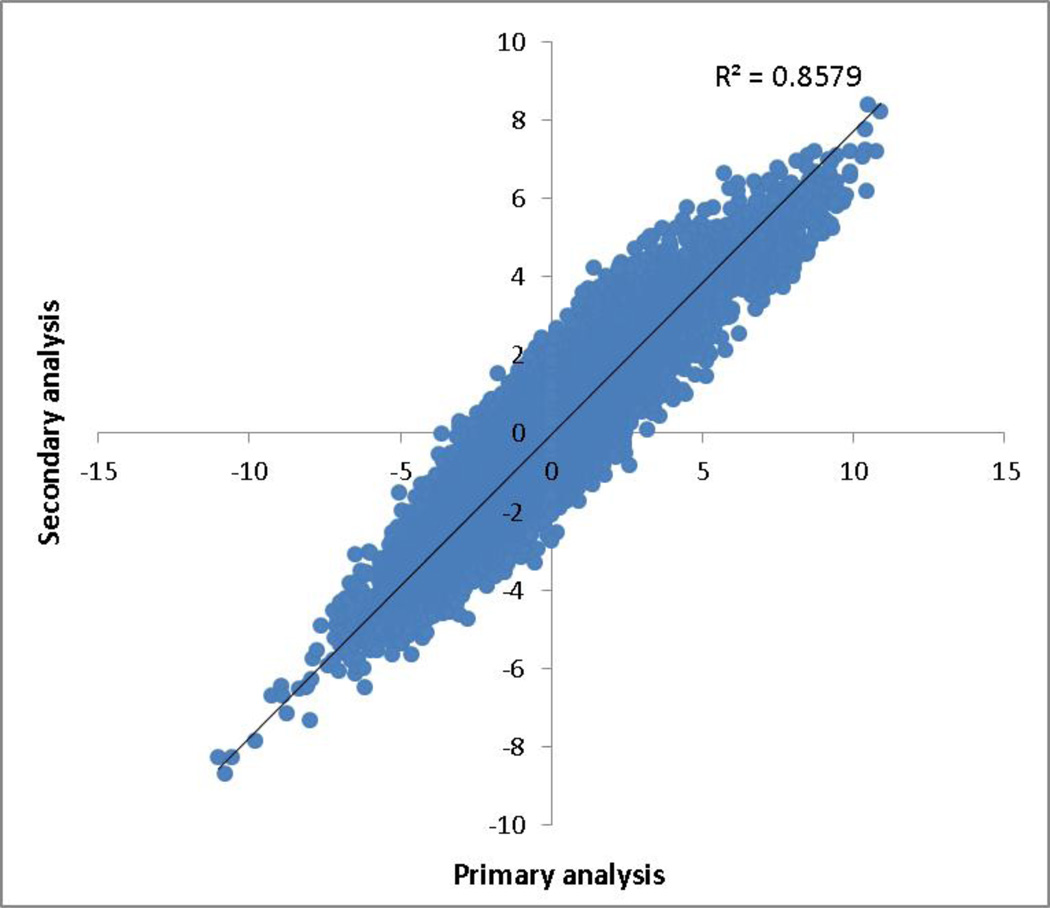
To further characterize the association of gene expression with interleukin-6 levels, we performed secondary analyses by adjusting for additional clinical covariates (see Methods). Figure 3 shows the comparison between primary and secondary analyses in terms of T-statistics. The results were highly correlated (R2=0.86). Among 4139 gene that were significant in the primary analysis, 3484 (84.2%) genes remained significant (P<0.05) in the secondary analysis.
Figure 3. Comparison of T-statistics between primary analyses and secondary analyses.
The x-axis represents the T-statistics of loge(interleukin-6) from the primary analysis and y-axis represents the T-statistics of loge(interleukin-6) from the secondary analysis. Each point represents one gene. The results were highly correlated (R2=0.86).
Given the heterogeneous nature of whole blood, we also examined the association of interleukin-6 levels with each cell type in the blood. As shown in Supplemental Table 1, the white blood cell counts, particularly lymphocytes and monocytes, were most significantly associated with interleukin-6 levels.
Replication in InCHIANTI
We tested our findings in InCHIANTI for replication. Table 1 shows the descriptive characteristics of the 694 eligible participants enrolled in the study (mean age 72.2±15.3 years, 54.9% women). Among the 4139 significant genes from FHS, 2831 genes also were measured in InCHIANTI, of which 807 were significant (P<0.05) and had the same direction of effects, including 43 highly significant genes (Bonferroni P<1.8×10−5). Among the top genes from FHS (FDR<5.0×10−18) that also were available in InCHIANTI (n=15 genes), all except one were replicated, suggesting the robustness of our results despite distinct transcriptional profiling platforms. The full list of replicated genes is provided in Supplemental Table 2. We also performed meta-analysis of both cohorts, and found that all the 807 replicated genes remained significant in the meta-analysis.
Pathway Analysis
We then examined the enrichment of interleukin-6 associated genes in canonical pathways by the Ingenuity Pathway Analysis (IPA) toolbox. Sixty-four canonical pathways were significantly enriched (FDR<0.05) with replicated interleukin-6 associated genes. Table 3 shows the top 10 enriched pathways, including well-known inflammation-related pathways like JAK/Stat signaling pathway (FDR=7.2×10−4, Supplemental Figure 1) and interleukin-10 signaling pathway (FDR=2.3×10−3, Supplemental Figure 2). We also performed pathway analysis separately for genes positively associated with interleukin-6 levels (Supplemental Table 3) and genes negatively associated with interleukin-6 levels (Supplemental Table 4). An enrichment analysis on gene ontology by Gene Set Enrichment Analysis [41] found 10 gene sets were significantly enriched with interleukin-6 associated genes (FDR<5%). These gene sets were listed in Supplemental Table 5.
Table 3.
Most significant canonical pathways enriched with genes associated with interleukin-6 levels
| Canonoical pathway |
P value | FDR | Ratio+ | Genes that were associated with interleukin-6 levels |
|---|---|---|---|---|
| JAK/Stat Signaling | 1.9×10−6 | 4.7×10−4 | 13/70 (0.186) | FOS, BCL2L1, SOCS3, RAF1, SOCS1, STAT6, AKT1, JAK1, RRAS, CISH, CDKN1A, TYK2, STAT1 |
| IL-10 Signaling | 2.2×10−6 | 4.7×10−4 | 13/72 (0.181) | IL1R2, CCR1, FOS, IKBKB, SOCS3, JAK1, IL1RN, BLVRA, TYK2, BLVRB, IL1B, MAP2K3, IL1RAP |
| Pancreatic Adenocarcinoma Signaling | 4.7×10−6 | 5.6×10−4 | 16/116 (0.138) | RAF1, E2F4, JAK1, PLD3, PA2G4, TFDP1, TYK2, TGFBR2, BCL2L1, AKT1, CDKN1A, PTGS2, CDKN1B, STAT1, NOTCH1, E2F2 |
| Glucocorticoid Receptor Signaling | 5.4×10−6 | 5.6×10−4 | 27/280 (0.0964) | TAF12, IL8, RAF1, JAK1, RRAS, SGK1, CDK7, TBP, PBX1, POLR2B, FCGR1A, EP300, TGFBR2, IL1R2, FOS, BCL2L1, IKBKB, AKT1, IL1RN, GTF2E1, CDKN1A, PRKAG2, IL1B, PTGS2, FKBP5, STAT1, PPP3CA |
| Heme Biosynthesis II | 2.0×10−5 | 1.7×10−3 | 5/10 (0.5) | UROD, UROS, FECH, ALAS2, HMBS |
| IGF-1 Signaling | 2.7×10−5 | 1.9×10−3 | 14/102 (0.137) | FOS, SOCS3, RAF1, SOCS1, NOV, AKT1, JAK1, RRAS, IGF1R, PRKAG2, PDPK1, IRS2, PRKCZ, PRKAR1A |
| NRF2-mediated Oxidative Stress Response | 3.2×10−5 | 1.9×10−3 | 20/190 (0.105) | RAF1, UBB, SOD1, RRAS, DNAJA4, HERPUD1, DNAJB2, GSTO1, PRKCZ, EP300, FOS, AKT1, MGST2, MAP2K3, CDC34, FKBP5, DNAJB5, MGST3, EPHX1, GSTK1 |
| Interferon Signaling | 4.2×10−5 | 2.2×10−3 | 8/34 (0.235) | IFIT3, SOCS1, OAS1, JAK1, TYK2, IFITM1, IFNGR1, STAT1 |
| IL-6 Signaling | 5.4×10−5 | 2.4×10−3 | 15/122 (0.123) | IL1R2, IKBKB, FOS, SOCS3, RAF1, SOCS1, IL8, AKT1, TNFAIP6, IL1RN, RRAS, IL6R, IL1B, MAP2K3, IL1RAP |
| NF-κB Signaling | 5.8×10−5 | 2.4×10−3 | 19/180 (0.106) | RAF1, RRAS, RELB, IGF2R, PRKCZ, EP300, TGFBR2, IL1R2, IKBKB, TNIP1, AKT1, ARAF, TLR5, IL1RN, PELI1, IGF1R, IL1B, CASP8, TNFSF13B |
| Glioma Signaling | 9.1×10−5 | 3.5×10−3 | 13/107 (0.121) | RAF1, E2F4, AKT1, PA2G4, CAMK1D, TFDP1, RRAS, CDKN1A, IGF1R, IGF2R, E2F2, PRKCZ, CAMK2G |
Ratio is the number of genes that were associated with interleukin-6 levels comparing to the total number of genes in the pathway
Discussion
Interleukin-6 is a pleiotropic cytokine that regulates a variety of inflammatory responses. In our study, we investigated the association of gene expression with interleukin-6 levels in 2422 participants from FHS. A total of 4139 genes were found to be significantly associated with interleukin-6 levels; 807 genes were successfully replicated in an additional 694 participants from InCHIANTI.
Many replicated genes are involved in inflammation-related pathways and red blood cell function. FLT3 encodes a receptor tyrosine kinase that regulates hematopoiesis and immune system [42]. STOM encodes a highly conserved stomatin located in the membrane of red blood cells. The deficiency or mutation of stomatin causes hereditary stomatocytosis [43]. SLC4A1 encodes an erythrocyte plasma membrane protein that is involved in carbon dioxide transport [44]. ALAS2 encodes an erythroid-specific mitochondrially located enzyme whose mutations play an important role in the development of sideroblastic anemia [45].
Interestingly, the expression of interleukin-6 receptor (IL6R) was found to be associated with interleukin-6 levels (Discovery P=8.8×10−4; Replication P=0.015). The binding of interleukin-6 to its receptor results in the activation of multiple signaling pathways [46–49], such as JAK/Stat signaling pathway and MAPK pathway, which regulate a variety of downstream biological activities. However, we did not observe an association between IL6 expression and interleukin-6 levels (P=0.12), suggesting that increased interleukin-6 concentration in serum is not being necessarily produced by the leukocytes, and the changes in expression that are seen in the serum might be the responses of the leukocytes to the interleukin-6 in serum.
The interleukin-10 signaling pathway is one of the most significant pathways enriched with interleukin-6 related genes. Participants of the Leiden 85-plus study with an impaired cytokine production to a stimulus were found to have increased mortality [50]. A significant association was detected with the IL10 gene promoter, suggesting the maladaptive immune response was under genetic control and in turn resulted in frailty in old age. Frail older adults are known to have higher levels of interleukin-6 than non-frail older adults [51]. Genetically altered il10 mice compared to age- and sex-matched control mice develop the characteristics of human frailty including elevated interleukin-6 levels and decline in muscle strength [52]. Subsequent work demonstrated that in addition to low-grade elevation of interleukin-6, the il10 frail mice develop cardiac and vascular dysfunction with advancing age [53] and have higher mortality [54]. A better understanding of the biologic mechanisms leading to elevated interleukin-6 levels and chronic inflammation with older age may result in therapies to ameliorate age-related multi-system decline.
It is estimated that some of the inter-individual variations of interleukin-6 levels are attributable to heritability [35, 55]. Several genetic loci, such as IL6R [56] and ABO [57], have already been identified to be associated with interleukin-6 levels. Yet much of the variability in interleukin-6 levels still remains unexplained. Our study identified hundreds of interleukin-6 associated genes, which, in combination with genetic variations, may provide new insights into the regulation of interleukin-6 levels.
The gene expression in this study was measured from the whole blood, which contains a variety of cell types. Since each cell type could have specific cell responses and may result in false discovery [58–60], we thereby accounted for the relative abundance of each cell type in our analyses. To further reduce the possibility of false discovery, we applied two different platforms for gene expression profiling: Affymetrix Human Exon 1.0 ST Array for discovery and Illumina Human HT-12 v3 Array for replication. We expect that many non-replicated genes were simply due to the difference in microarray platforms and sample size (2422 vs. 694). Future increases in sample size and improvement of gene expression profiling platforms may further increase the power to identify significant genes [61, 62].
Our study has certain limitations. All participants included in this study were exclusively middle age to older adults of European descent, thus the generalizability of our findings to younger individuals or other races/ethnicities is unclear. We only measured interleukin-6 together with expression levels from the blood collected during one physical examination, but the interleukin-6 concentration may fluctuate over time [63]. Therefore our study cannot comment on longitudinal variation in the relations between gene expression and circulating interleukin-6 levels. Lastly, this study was largely limited to the association analyses, and we cannot infer causality between interleukin-6 levels and gene expression.
In conclusion, we studied the association of gene expression with interleukin-6 levels in a large community-based cohort and replicated it in another cohort. We successfully identified and replicated 807 genes that were significantly associated with interleukin-6 levels. Future characterization of interleukin-6 regulation network would enable the identification of additional potential therapeutic targets for inflammation treatment.
Supplementary Material
Highlights.
We studied the association of gene expression with interleukin-6 levels in 2422 participants from Framingham Heart Study Offspring Cohort, and validated the result in 694 participants from InCHIANTI study
We identified and replicated 807 genes that were associated with circulating interleukin-6 levels
Many of the interleukin-6 associated genes are involved in inflammation-related pathways or erythrocyte function
Acknowledgements
FHS gene expression profiling was funded through the Division of Intramural Research (Principal Investigator, Daniel Levy), National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Measurement of interleukin-6 was funded through R01 HL 064753 and R01 HL076784. This work is supported by NIH grants 1R01 HL64753 (Benjamin), R01AG028321 (Benjamin), R01AG029451 (Murabito). This study was supported in part by the Intramural Research Program, National Institute on Aging (Ferrucci). UK based work was supported by a Wellcome Trust grant to the University of Exeter, plus internal medical school funding (Melzer). The Framingham Heart Study is supported by National Heart, Lung, and Blood Institute contract N01-HC-25195.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors declare no commercial conflicts of interest.
Contributor Information
Honghuang Lin, Section of Computational Biomedicine, Department of Medicine, Boston University School of Medicine, Boston, MA, USA; National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA.
Roby Joehanes, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Mathematical and Statistical Computing Laboratory, Center for Information Technology, National Institute of Health, Bethesda, MD, USA; Population Sciences Branch, National Heart, Lung, and Blood Institute, Bethesda, MD, USA.
Luke C. Pilling, Epidemiology and Public Health, Medical School, University of Exeter, Exeter EX1 2LU, UK.
Josée Dupuis, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
Kathryn L. Lunetta, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA.
Sai-Xia Ying, Mathematical and Statistical Computing Laboratory, Center for Information Technology, National Institute of Health, Bethesda, MD, USA.
Emelia J. Benjamin, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Section of Cardiovascular Medicine and Preventive Medicine, Department of Medicine, Boston University School of Medicine, Boston, MA, USA; Department of Epidemiology, Boston University School of Public Health, Boston, MA, USA.
Dena Hernandez, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA.
Andrew Singleton, Laboratory of Neurogenetics, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA.
David Melzer, Epidemiology and Public Health, Medical School, University of Exeter, Exeter EX1 2LU, UK.
Peter J. Munson, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Mathematical and Statistical Computing Laboratory, Center for Information Technology, National Institute of Health, Bethesda, MD, USA; Population Sciences Branch, National Heart, Lung, and Blood Institute, Bethesda, MD, USA.
Daniel Levy, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Population Sciences Branch, National Heart, Lung, and Blood Institute, Bethesda, MD, USA.
Luigi Ferrucci, Clinical Research Branch, National Institute on Aging, Baltimore, MD, USA.
Joanne M. Murabito, National Heart Lung and Blood Institute’s and Boston University’s Framingham Heart Study, Framingham, MA, USA; Section of General Internal Medicine, Department of Medicine, Boston University School of Medicine, Boston, MA, USA.
References
- 1.Kishimoto T. The biology of interleukin-6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 2.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clinical chemistry. 2008;54:24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 3.Raschke S, Eckel J. Adipo-myokines: two sides of the same coin--mediators of inflammation and mediators of exercise. Mediators of inflammation. 2013;2013:320724. doi: 10.1155/2013/320724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feghali CA, Wright TM. Cytokines in acute and chronic inflammation. Frontiers in bioscience : a journal and virtual library. 1997;2:d12–d26. doi: 10.2741/a171. [DOI] [PubMed] [Google Scholar]
- 5.Abeywardena MY, Leifert WR, Warnes KE, Varghese JN, Head RJ. Cardiovascular biology of interleukin-6. Curr Pharm Des. 2009;15:1809–1821. doi: 10.2174/138161209788186290. [DOI] [PubMed] [Google Scholar]
- 6.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thromb Haemost. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 8.Brubaker AL, Palmer JL, Kovacs EJ. Age-related Dysregulation of Inflammation and Innate Immunity: Lessons Learned from Rodent Models. Aging and disease. 2011;2:346–360. [PMC free article] [PubMed] [Google Scholar]
- 9.Schnabel RB, Yin X, Larson MG, Yamamoto JF, Fontes JD, Kathiresan S, Rong J, Levy D, Keaney JF, Jr, Wang TJ, Murabito JM, Vasan RS, Benjamin EJ. Multiple inflammatory biomarkers in relation to cardiovascular events and mortality in the community. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1728–1733. doi: 10.1161/ATVBAHA.112.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB, Framingham S. Heart, Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 11.Weidle UH, Klostermann S, Eggle D, Kruger A. Interleukin 6/interleukin 6 receptor interaction and its role as a therapeutic target for treatment of cachexia and cancer. Cancer genomics & proteomics. 2010;7:287–302. [PubMed] [Google Scholar]
- 12.Bertoni AG, Burke GL, Owusu JA, Carnethon MR, Vaidya D, Barr RG, Jenny NS, Ouyang P, Rotter JI. Inflammation and the incidence of type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2010;33:804–810. doi: 10.2337/dc09-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 14.Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, Kim SH, Park HS, Suh CH. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. Journal of clinical immunology. 2007;27:461–466. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 15.Porta C, De Amici M, Quaglini S, Paglino C, Tagliani F, Boncimino A, Moratti R, Corazza GR. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 16.Luc G, Bard JM, Juhan-Vague I, Ferrieres J, Evans A, Amouyel P, Arveiler D, Fruchart JC, Ducimetiere P, Group PS. C-reactive protein, interleukin-6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2003;23:1255–1261. doi: 10.1161/01.ATV.0000079512.66448.1D. [DOI] [PubMed] [Google Scholar]
- 17.Weaver JD, Huang MH, Albert M, Harris T, Rowe JW, Seeman TE. Interleukin-6 and risk of cognitive decline: MacArthur studies of successful aging. Neurology. 2002;59:371–378. doi: 10.1212/wnl.59.3.371. [DOI] [PubMed] [Google Scholar]
- 18.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am. J. Med. 2006;119:526–517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys WG, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 20.Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 21.Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab. 2003;88:2019–2025. doi: 10.1210/jc.2002-021694. [DOI] [PubMed] [Google Scholar]
- 22.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 23.Figaro MK, Kritchevsky SB, Resnick HE, Shorr RI, Butler J, Shintani A, Penninx BW, Simonsick EM, Goodpaster BH, Newman AB, Schwartz AV, Harris TB. Diabetes, inflammation, and functional decline in older adults: findings from the Health, Aging and Body Composition (ABC) study. Diabetes Care. 2006;29:2039–2045. doi: 10.2337/dc06-0245. [DOI] [PubMed] [Google Scholar]
- 24.Cheng CC, Hsueh CM, Chen CY, Chen TH, Hsu SL. Interleukin-6 upregulates paraoxonase 1 gene expression via an AKT/NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2013;437:55–61. doi: 10.1016/j.bbrc.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Lai HS, Lin WH, Lai SL, Lin HY, Hsu WM, Chou CH, Lee PH. Interleukin-6 Mediates Angiotensinogen Gene Expression during Liver Regeneration. PLoS ONE. 2013;8:e67868. doi: 10.1371/journal.pone.0067868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108:3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grassl C, Luckow B, Schlondorff D, Dendorfer U. Transcriptional regulation of the interleukin-6 gene in mesangial cells. Journal of the American Society of Nephrology : JASN. 1999;10:1466–1477. doi: 10.1681/ASN.V1071466. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari SL, Ahn-Luong L, Garnero P, Humphries SE, Greenspan SL. Two promoter polymorphisms regulating interleukin-6 gene expression are associated with circulating levels of C-reactive protein and markers of bone resorption in postmenopausal women. The Journal of clinical endocrinology and metabolism. 2003;88:255–259. doi: 10.1210/jc.2002-020092. [DOI] [PubMed] [Google Scholar]
- 29.Dawber TR, Kannel WB, Lyell LP. An approach to longitudinal studies in a community: the Framingham Study. Ann N Y Acad Sci. 1963;107:539–556. doi: 10.1111/j.1749-6632.1963.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 30.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP The Framingham Offspring Study. Design and preliminary data. Preventive medicine. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 31.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 32.Fontes JD, Yamamoto JF, Larson MG, Wang N, Dallmeier D, Rienstra M, Schnabel RB, Vasan RS, Keaney JF, Jr, Benjamin EJ. Clinical correlates of change in inflammatory biomarkers: The Framingham Heart Study. Atherosclerosis. 2013;228:217–223. doi: 10.1016/j.atherosclerosis.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joehanes R, Ying S, Huan T, Johnson AD, Raghavachari N, Wang R, Liu P, Woodhouse KA, Sen SK, Tanriverdi K, Courchesne P, Freedman JE, O'Donnell CJ, Levy D, Munson PJ. Gene expression signatures of coronary heart disease. Arterioscler Thromb Vasc Biol. 2013;33:1418–1426. doi: 10.1161/ATVBAHA.112.301169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics (Oxford, England) 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 35.Schnabel RB, Lunetta KL, Larson MG, Dupuis J, Lipinska I, Rong J, Chen MH, Zhao Z, Yamamoto JF, Meigs JB, Nicaud V, Perret C, Zeller T, Blankenberg S, Tiret L, Keaney JF, Jr, Vasan RS, Benjamin EJ. The relation of genetic and environmental factors to systemic inflammatory biomarker concentrations. Circ Cardiovasc Genet. 2009;2:229–237. doi: 10.1161/CIRCGENETICS.108.804245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 37.Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. The journals of gerontology. Series A, Biological sciences and medical sciences. 2004;59:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 38.Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, Guralnik JM. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. Journal of the American Geriatrics Society. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 39.Harries LW, Hernandez D, Henley W, Wood AR, Holly AC, Bradley-Smith RM, Yaghootkar H, Dutta A, Murray A, Frayling TM, Guralnik JM, Bandinelli S, Singleton A, Ferrucci L, Melzer D. Human aging is characterized by focused changes in gene expression and deregulation of alternative splicing. Aging cell. 2011;10:868–878. doi: 10.1111/j.1474-9726.2011.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Habib GM, Shi ZZ, Cuevas AA, Lieberman MW. Identification of two additional members of the membrane-bound dipeptidase family. Faseb J. 2003;17:1313–1315. doi: 10.1096/fj.02-0899fje. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gilliland DG, Griffin JD. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. doi: 10.1182/blood-2002-02-0492. [DOI] [PubMed] [Google Scholar]
- 43.Fricke B, Jarvis HG, Reid CD, Aguilar-Martinez P, Robert A, Quittet P, Chetty M, Pizzey A, Cynober T, Lande WF, Mentzer WC, During M, Winter S, Delaunay J, Stewart GW. Four new cases of stomatin-deficient hereditary stomatocytosis syndrome: association of the stomatin-deficient cryohydrocytosis variant with neurological dysfunction. British journal of haematology. 2004;125:796–803. doi: 10.1111/j.1365-2141.2004.04965.x. [DOI] [PubMed] [Google Scholar]
- 44.Figueroa D. The Diego blood group system: a review. Immunohematology/American Red Cross. 2013;29:73–81. [PubMed] [Google Scholar]
- 45.Bishop DF, Tchaikovskii V, Hoffbrand AV, Fraser ME, Margolis S. X-linked sideroblastic anemia due to carboxyl-terminal ALAS2 mutations that cause loss of binding to the beta-subunit of succinyl-CoA synthetase (SUCLA2) J Biol Chem. 2012;287:28943–28955. doi: 10.1074/jbc.M111.306423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishimura R, Moriyama K, Yasukawa K, Mundy GR, Yoneda T. Combination of interleukin-6 and soluble interleukin-6 receptors induces differentiation and activation of JAK-STAT and MAP kinase pathways in MG-63 human osteoblastic cells. J Bone Miner Res. 1998;13:777–785. doi: 10.1359/jbmr.1998.13.5.777. [DOI] [PubMed] [Google Scholar]
- 47.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer research. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 48.Ueda T, Bruchovsky N, Sadar MD. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J Biol Chem. 2002;277:7076–7085. doi: 10.1074/jbc.M108255200. [DOI] [PubMed] [Google Scholar]
- 49.Yang L, Wang L, Lin HK, Kan PY, Xie S, Tsai MY, Wang PH, Chen YT, Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462–469. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- 50.van den Biggelaar AH, Huizinga TW, de Craen AJ, Gussekloo J, Heijmans BT, Frolich M, Westendorp RG. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Experimental gerontology. 2004;39:1407–1414. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Leng SX, Yang H, Walston JD. Decreased cell proliferation and altered cytokine production in frail older adults. Aging clinical and experimental research. 2004;16:249–252. doi: 10.1007/BF03327392. [DOI] [PubMed] [Google Scholar]
- 52.Walston J, Fedarko N, Yang H, Leng S, Beamer B, Espinoza S, Lipton A, Zheng H, Becker K. The physical and biological characterization of a frail mouse model. J Gerontol A Biol Sci Med Sci. 2008;63:391–398. doi: 10.1093/gerona/63.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sikka G, Miller KL, Steppan J, Pandey D, Jung SM, Fraser CD, 3rd, Ellis C, Ross D, Vandegaer K, Bedja D, Gabrielson K, Walston JD, Berkowitz DE, Barouch LA. Interleukin 10 knockout frail mice develop cardiac and vascular dysfunction with increased age. Experimental gerontology. 2013;48:128–135. doi: 10.1016/j.exger.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ko F, Yu Q, Xue QL, Yao W, Brayton C, Yang H, Fedarko N, Walston J. Inflammation and mortality in a frail mouse model. Age (Dordr.) 2012;34:705–715. doi: 10.1007/s11357-011-9269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Worns MA, Victor A, Galle PR, Hohler T. Genetic and environmental contributions to plasma C-reactive protein and interleukin-6 levels--a study in twins. Genes Immun. 2006;7:600–605. doi: 10.1038/sj.gene.6364330. [DOI] [PubMed] [Google Scholar]
- 56.Reich D, Patterson N, Ramesh V, De Jager PL, McDonald GJ, Tandon A, Choy E, Hu D, Tamraz B, Pawlikowska L, Wassel-Fyr C, Huntsman S, Waliszewska A, Rossin E, Li R, Garcia M, Reiner A, Ferrell R, Cummings S, Kwok PY, Harris T, Zmuda JM, Ziv E, Health A Body Composition S. Admixture mapping of an allele affecting interleukin 6 soluble receptor and interleukin 6 levels. American journal of human genetics. 2007;80:716–726. doi: 10.1086/513206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naitza S, Porcu E, Steri M, Taub DD, Mulas A, Xiao X, Strait J, Dei M, Lai S, Busonero F, Maschio A, Usala G, Zoledziewska M, Sidore C, Zara I, Pitzalis M, Loi A, Virdis F, Piras R, Deidda F, Whalen MB, Crisponi L, Concas A, Podda C, Uzzau S, Scheet P, Longo DL, Lakatta E, Abecasis GR, Cao A, Schlessinger D, Uda M, Sanna S, Cucca F. A genome-wide association scan on the levels of markers of inflammation in Sardinians reveals associations that underpin its complex regulation. PLoS Genet. 2012;8:e1002480. doi: 10.1371/journal.pgen.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palmer C, Diehn M, Alizadeh AA, Brown PO. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC genomics. 2006;7:115. doi: 10.1186/1471-2164-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feezor RJ, Baker HV, Mindrinos M, Hayden D, Tannahill CL, Brownstein BH, Fay A, MacMillan S, Laramie J, Xiao W, Moldawer LL, Cobb JP, Laudanski K, Miller-Graziano CL, Maier RV, Schoenfeld D, Davis RW, Tompkins RG Inflammation, L.-S.C.R.P. Host Response to Injury, Whole blood and leukocyte RNA isolation for gene expression analyses. Physiol Genomics. 2004;19:247–254. doi: 10.1152/physiolgenomics.00020.2004. [DOI] [PubMed] [Google Scholar]
- 60.Shen-Orr SS, Tibshirani R, Khatri P, Bodian DL, Staedtler F, Perry NM, Hastie T, Sarwal MM, Davis MM, Butte AJ. Cell type-specific gene expression differences in complex tissues. Nature methods. 2010;7:287–289. doi: 10.1038/nmeth.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adam O, Lavall D, Theobald K, Hohl M, Grube M, Ameling S, Sussman MA, Rosenkranz S, Kroemer HK, Schafers HJ, Bohm M, Laufs U. Rac1-induced connective tissue growth factor regulates connexin 43 and N-cadherin expression in atrial fibrillation. J Am Coll Cardiol. 2010;55:469–480. doi: 10.1016/j.jacc.2009.08.064. [DOI] [PubMed] [Google Scholar]
- 62.Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., 2nd Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Galphaq. Circ Res. 2010;106:1459–1467. doi: 10.1161/CIRCRESAHA.110.217513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Danesh J, Kaptoge S, Mann AG, Sarwar N, Wood A, Angleman SB, Wensley F, Higgins JP, Lennon L, Eiriksdottir G, Rumley A, Whincup PH, Lowe GD, Gudnason V. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS medicine. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.