Abstract
Background
Mercury is a global contaminant of concern though little is known about exposures in México.
Objectives
To characterize mercury levels in pregnant women, children, and commonly consumed seafood samples.
Methods
Use resources of the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) birth cohorts to measure total mercury levels in archived samples from 348 pregnant women (blood from three trimesters and cord blood), 825 offspring (blood, hair, urine) and their mothers (hair), and 91 seafood and canned tuna samples from Mexico City.
Results
Maternal blood mercury levels correlated across three trimesters and averaged 3.4μg/L. Cord blood mercury averaged 4.7μg/L and correlated with maternal blood from trimester 3 (but not trimesters 1 and 2). In children, blood, hair and urine mercury levels correlated and averaged 1.8μg/L, 0.6μg/g, and 0.9μg/L, respectively. Hair mercury was 0.5μg/g in mothers and correlated with child's hair. Mean consumption of canned tuna, fresh fish, canned sardine, and shellfish was 3.1, 2.2, 0.5, and 1.0 times per month respectively in pregnant women. Mean mercury content in 7 of 23 seafood species and 5 of 9 canned tuna brands purchased exceeded the U.S. EPA guidance value of 0.3 μg/g.
Conclusions
Mercury exposures in pregnant women and children from Mexico City, via biomarker studies, are generally 3-5 times greater than values reported in population surveys from the U.S., Canada, and elsewhere. In particular, mercury levels in 29-39% of the maternal participants exceeded the biomonitoring guideline associated with the U.S. EPA reference dose for mercury.
Keywords: mercury, biomarkers, exposure assessment, children, pregnant women
1.0 INTRODUCTION
Mercury is found in a number of items deemed important to human health, such as seafood, dental amalgams, and vaccines (ATSDR 1999). Public health messages concerning the safety of these items have been confusing. Challenges stem from the fact that mercury exists in a variety of chemical forms, and that these forms vary in their exposure routes, metabolism within the body, and target organ toxicity (Clarkson and Magos 2006). Inorganic mercury includes elemental metallic mercury (Hg0) and oxidized mercury salts (Hg+/2+), and these forms can affect the renal and nervous systems. Organic mercury is generally found as methylmercury, and exposures occur via ingestion (mainly contaminated seafood). Methylmercury can impair the nervous and cardiovascular systems. In addition, methylmercury can cross the placenta, and a number of epidemiological studies have established that methylmercury is a threat to neurodevelopment (Mergler et al. 2007). Much of the evidence concerning methylmercury's neurodevelopmental effects have been drawn from studies involving susceptible groups with high exposures (Karagas et al. 2012), and so it needs to be resolved how applicable such findings are to the general population in which exposures occur at a much lower levels.
Mercury is a global contaminant of concern. Many governments, including México, have recognized this by adopting the Minamata Convention on Mercury in 2013 (UNEP 2013). The ultimate goal of the Convention is to reduce human exposures to mercury. While certain geographic regions have tremendous biomonitoring programs and epidemiological datasets in which the efficacy of the Convention may be tracked, there are many areas in which such resources or information do not exist. One geographic region that lacks detailed information on mercury exposure is México. The few studies measuring mercury in Mexican populations have focused on susceptible groups. These include, for example, fish-eating populations (Guentzel et al. 2007; Trasande et al. 2010) or workers and residents of mining and waste disposal sites contaminated with inorganic mercury and other heavy metals (de Lourdes Sotos-Ríos et al. 2010; Costilla-Salazar et al. 2011). Little is known about mercury exposures amongst the general population of México, especially urban residents, women of childbearing age, and children.
The current study was pursued to increase understanding of mercury exposures amongst residents of Mexico City. Three biomarkers of mercury exposure were studied: urine (inorganic mercury), hair (mainly methylmercury) and blood (mainly methylmercury but can also contain some inorganic mercury) (Clarkson and Magos 2006). The first objective was to characterize mercury exposures in pregnant women by measuring mercury levels in maternal blood during three trimesters and in cord blood. The second objective was to report upon mercury exposures in children by measuring mercury in their blood, hair, and urine, as well as in the hair of their mothers. The third objective was to characterize mercury in commonly consumed seafood samples within Mexico City. These objectives capitalized upon the rich resources of a sequentially enrolled epidemiologic birth cohort series running since 1994 called the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) study (Téllez-Rojo et al. 2006; Afeiche et al. 2011).
2.0 METHODS
2.1 ELEMENT Cohort
The ELEMENT study was initially designed to research the influence of maternal lead exposure on offspring neurodevelopment. Pertinent details of ELEMENT, such as inclusion and exclusion criteria, collection methods, and demographics can be found elsewhere (Téllez-Rojo et al. 2006; Afeiche et al. 2011). In brief, Cohort 1 subjects were recruited 1994-1995, Cohort 2 subjects were recruited 1997-2001, Cohort 3 subjects were recruited 2001-2004, and in 2006 participants were recruited from all three cohorts (Afeiche et al. 2011). Mothers and children from the ELEMENT study population who had at least one biological sample available for mercury analysis were included in the present study thus leading to varying sample sizes and collection dates (Supplemental Table 1).
A validated food frequency questionnaire was utilized which included useful data on seafood consumption. Specifically, at each prenatal visit mothers were asked of their exposure to four items (canned tuna, fresh fish, canned sardine, and shellfish). Information was collected on their self-reported servings and average portion sizes over the past week.
The research protocol was approved by the ethics and research committees of the partnering institutions, including the National Institute of Public Health of Mexico, the Harvard School of Public Health, the Brigham and Women's Hospital, the University of Michigan School of Public Health, the University of Toronto, and the participating hospitals.
2.2 Human Biospecimens
Venous whole blood samples were collected into vials certified for trace metals analysis and stored at 4°C until analysis. Spot (second morning void) urine samples were collected and stored frozen until analysis. Scalp hair samples were obtained from each participant using stainless steel scissors and the proximal end was designated.
2.3 Seafood Samples
In 2011, edible samples (n=91) of seafood commonly sold in Mexico City were obtained from vendors (n=26 vendors, including 14 within La Nueva Viga which is the city's largest vendor). In addition, 35 cans of tuna, representing nine commonly purchased brands, were collected from three local supermarkets.
2.4 Mercury Analysis
Analytical measurement of all samples for total mercury content was carried out using a Direct Mercury Analyzer 80 (DMA-80, Milestone Inc., CT) as previously described (Paruchuri et al. 2010). Briefly, urine and blood samples were vortexed, and a portion was then placed into a quartz sampling boat. The specific gravity of urine was measured using a refractometer (PAL-10S, Atago). For hair, a 2 cm segment cut from the proximal end was washed with acetone, rinsed three times with Milli-Q water, dried overnight and ~2-5mg was placed into a nickel sampling boat. Seafood samples (>1 gram) were dried and analyzed as we have previously described (Nam and Basu 2011). Quality control measures included daily instrument calibration, procedural blanks, replicates, and several certified reference materials including CRM #13 for hair (National Institute for Environmental Studies, Japan), DOLT-4 (dogfish liver; National Research Council, Canada), and QMEQAS for blood and urine (Institut National de Santé Publique du Québec). Recoveries of the reference materials ranged from 80 to 110%. The analytical detection limit was less than 1 ng mercury per measure.
2.5 Statistical Analysis
All data were initially analyzed using univariate descriptive statistics and graphical displays. Outliers were detected using the generalized extreme studentized deviation method. Demographic characteristics of participants in this study and other ELEMENT study participants excluded due to unavailable mercury biomarkers were compared using appropriate two sample tests. Spearman correlations were used to assess association among trimester-specific blood mercury levels, and among mercury biomarkers in the children and their mothers. The intraclass correlation coefficient (ICC) was computed on log-transformed values of maternal trimester-specific blood mercury as a summary measure of the extent of correlation across trimesters. Bivariate analyses were used to relate mercury biomarker values with demographic characteristics and seafood consumption data. Data are reported as mean ± standard deviation, unless otherwise indicated. Data were analyzed using SAS 9.2 (SAS Institute Inc. Cary, NC).
3.0 RESULTS
3.1 Mercury Biomarkers in Pregnant Women
Prenatal mercury data was drawn from ELEMENT mothers (n=348) for whom we had at least one prenatal biomarker. Characteristics of these mothers (age: 26.8 ± 5.7 years; maternal education: 10.8 ± 2.8 years; 73.6% married) were not statistically different from mothers excluded due to not having a prenatal mercury biomarker measurement. Pregnant mothers were sampled at 13.2 (± 2.7; range 3-24; n=328), 24.7 (± 2.6, range 15-34; n=335), and 34.4 (± 2.1, range 25-40; n=317) weeks of pregnancy. The mean gestation age was 38.8 ± 1.7 weeks (range: 27-43) and delivery weight was 3.1 ± 0.5 kilograms (range: 1-4.5).
The number of participating mothers for whom we have prenatal mercury biomarker measurements from the 1st, 2nd, or 3rd trimester (n=217, 264, 248, respectively) varies. Similarly, there is variation in the number of participating mothers for whom we have a mercury biomarker measurement from one, two, or all three trimesters (n=68, 103, 154, respectively).. Of the 144 participants who provided cord blood mercury, 55 participants had mercury data from cord blood and all three trimesters.
Every blood sample investigated had detectable levels of mercury (Table 1). Total mercury in cord blood ranged from 0.8 to 16.9 μg/L. Approximately one-fifth of the cord blood samples had mercury levels that exceeded the biomonitoring guideline associated with the U.S. Environmental Protection Agency's reference dose for mercury of 5.8 μg/L. As discussed by Stern and Smith (2003), cord blood levels are on average 70% higher than maternal blood and thus the biomonitoring guideline for mercury in maternal blood should be 3.5 μg/L (Mahaffey et al., 2004). When applied to the current study, approximately one-third of the pregnant women had blood mercury levels in excess of the biomonitoring guideline (Table 1).
Table 1.
Total mercury (μg/L) in prenatal blood samples and cord blood of participating mothers in the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) cohort. The percent of individuals who have blood mercury levels that exceed the biomonitoring guideline associated with the U.S. Environmental Protection Agency's reference dose (RfD; maternal blood = 3.5 μg/L; cord blood = 5.8 μg/L; Mahaffey et al., 2004) are indicated.
| n | Mean | SD | Min | 10% | 25% | 50% | 75% | 90% | Max | % > Rfd | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st trimester | 217 | 3.33 | 2.05 | 0.43 | 1.32 | 1.87 | 2.88 | 3.70 | 5.69 | 11.89 | 28.6 |
| 2nd trimester | 264 | 3.11 | 1.88 | 0.38 | 1.17 | 1.73 | 2.75 | 3.85 | 5.15 | 13.47 | 33.0 |
| 3rd trimester | 248 | 3.70 | 3.49 | 0.29 | 1.19 | 1.59 | 2.79 | 4.67 | 6.62 | 31.15 | 39.2 |
| Cord blood | 144 | 4.66 | 2.75 | 0.76 | 1.90 | 2.58 | 4.07 | 5.71 | 7.61 | 16.90 | 21.1 |
Across the three trimesters, maternal blood mercury values were not significantly different (Table 1) and levels were significantly correlated with Spearman coefficients ranging from 0.29 to 0.43. As a summary measure of correlation across trimesters, the ICC was 0.37. The highest correlation was found between trimesters 2 and 3 (r = 0.43; p<0.001). When compared to cord blood mercury, the only trimester that was significantly associated was trimester 3 (r = 0.33; p = 0.002). The correlation with cord blood mercury weakened in a temporal manner from trimesters 3 to 2 (r = 0.21; p = 0.051) to 1 (r = 0.18; p = 0.15).
In bivariate analyses, parity and gestation length were not associated with blood mercury during any of the trimesters or in cord blood. Maternal age (years) was associated with blood mercury levels in trimesters 2 (β=0.015, p<0.05) and 3 (β =0.020, p<0.05), but not levels in trimester 1 or in cord blood. Maternal education (years of school) was associated with blood mercury levels in trimesters 1 (β =0.039, p<0.01) and 2 (β =0.035, p<0.01), but not levels in trimester 3 or in cord blood. The influence of seafood consumption on mercury biomarkers in these pregnant women is described later.
3.2 Mercury Biomarkers in Children and their Mothers
Children's data was drawn from a total of 825 individuals for whom we had at least one mercury biomarker measurement. The average children's age was 10.3 ± 2.6 years, and 50.6% are male. In some of the mothers of these children, hair mercury concentration was measured.
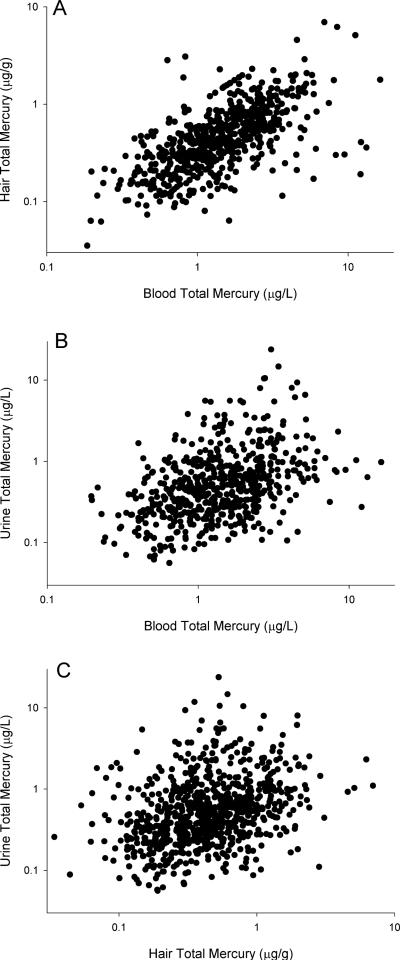
In children, blood, hair and urine mercury levels were 1.83 ± 1.67 μg/L, 0.56 ± 0.50 μg/g, and 0.90 ± 1.54 μg/L respectively (Table 2). In children's urine, the mean specific gravity was 1.017, and ranged from 1.000 to 1.033. The Spearman correlation between the unadjusted and specific gravity-adjusted urine mercury values was 0.85, and here we focus upon unadjusted urine mercury values to ease comparisons with other studies. Across children, mercury levels in blood, hair and urine were correlated (Figure 1). Blood and hair mercury levels of the same individuals were most correlated (r=0.68, p<0.001), while urine mercury levels were moderately correlated to both blood (r=0.41, p<0.001) and hair (r=0.34, p<0.001) mercury levels. Mother's hair mercury levels at the same visit were 0.53 ± 0.48 μg/g. Children's hair mercury levels correlated with that of their mother's (r=0.47, p<0.001).
Table 2.
Total mercury concentrations in biomarkers of participating children and their mothers in the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) cohort.
| Biomarker | n | Mean | SD | Min | 10% | 25% | 50% | 75% | 90% | Max | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Child | Blood Mercury (μg/L) | 601 | 1.83 | 1.67 | 0.19 | 0.53 | 0.83 | 1.37 | 2.33 | 3.39 | 16.33 |
| Hair Mercury (μg/g) | 796 | 0.56 | 0.50 | 0.03 | 0.16 | 0.26 | 0.43 | 0.73 | 1.12 | 6.22 | |
| Urine Mercury (μg/L) | 786 | 0.90 | 1.54 | 0.02 | 0.17 | 0.26 | 0.49 | 0.90 | 1.89 | 23.78 | |
| Mother | Hair Mercury (μg/g) | 371 | 0.53 | 0.48 | 0.03 | 0.15 | 0.24 | 0.39 | 0.67 | 1.02 | 4.19 |
Figure 1.
Scatterplots relating three mercury biomarkers of participating children in the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) cohort. All correlations were statistically staificant (A: blood and hair - r=0.68, p<0.001; B: blood and urine - r=0.41, p<0.001; C: urine and hair - r=0.34, p<0.001)
In addition to the data presented here, many of the children provided biological samples approximately 18 months following this visit (i.e., their average age was 12.6±2.6 years in this follow-up visit; Supplemental Tables 4 and 5). Mercury was measured in blood and hair in 61% of the samples collected during the 18 month follow-up visit. There was no difference in mercury levels between these two time points. The Spearman correlation between the two time points for blood (r=0.45; p<0.0001; n=288) and hair (r=0.43; p<0.0001; n=364) were significant.
In bivariate analyses, maternal age, marital status, and child sex were not associated with children's biomarker levels. Child's age was negatively associated with all three biomarkers (estimates for blood, hair and urine were -0.036, -0.053, and -0.039, respectively; all p<0.001).
3.3 Mercury Content in Seafood
To increase understanding of mercury levels in commercial food items, mercury was measured in 18 fresh fish species, 5 shellfish species, and 9 different brands of canned tuna (Table 3). Mean total mercury concentrations across the samples varied over 500-fold and ranged from 0.003 μg/g for crab, catfish and carp to 1.65 μg/g for shark.
Table 3.
Total mercury levels (μg/g, wet weight) in seafood and canned tuna collected from n=26 vendors in Mexico City in 2011. Many of the samples exceed the U.S. Environmental Protection Agency's safe consumption level of 0.3 μg/g wet weight.
| n | Mean | SD | Min | Median | Max | ||
|---|---|---|---|---|---|---|---|
| Seafood | Mojarra (Sunfish) | 17 | 0.027 | 0.029 | 0.001 | 0.019 | 0.085 |
| Cazón (School Shark) | 10 | 1.557 | 0.632 | 0.458 | 1.600 | 2.491 | |
| Huachinango (Red Snapper) | 10 | 0.449 | 0.482 | 0.028 | 0.199 | 1.319 | |
| Sierra (Spanish Mackerel) | 7 | 0.151 | 0.119 | 0.042 | 0.078 | 0.313 | |
| Tiburon (Shark) | 4 | 1.658 | 0.611 | 0.868 | 1.764 | 2.236 | |
| Robalo (Sea Bass) | 4 | 0.127 | 0.058 | 0.101 | 0.116 | 0.174 | |
| Basa oriental (Catfish) | 2 | 0.003 | 0.003 | 0.003 | |||
| Cintilla | 2 | 0.049 | 0.048 | 0.050 | |||
| Atun (Tuna) | 2 | 0.109 | 0.096 | 0.119 | |||
| Salmon | 2 | 0.017 | 0.012 | 0.022 | |||
| Mero (Grouper) | 2 | 0.764 | 0.703 | 0.825 | |||
| Lenguado (Flounder) | 2 | 0.062 | 0.026 | 0.099 | |||
| Esmedregal (Cobia) | 1 | 0.637 | |||||
| Jurel | 1 | 0.426 | |||||
| Bagre (Catfish) | 1 | 0.344 | |||||
| Bandera | 1 | 0.196 | |||||
| Carpa (Carp) | 1 | 0.003 | |||||
| Perdillo | 1 | 0.038 | |||||
| Calamar (Squid) | 5 | 0.044 | 0.054 | 0.006 | 0.030 | 0.138 | |
| Camaron (Shrimp) | 8 | 0.010 | 0.003 | 0.007 | 0.009 | 0.016 | |
| Jaiva (Crab) | 3 | 0.047 | 0.014 | 0.031 | 0.054 | 0.057 | |
| Rojo (Crab) | 3 | 0.003 | 0.001 | 0.003 | 0.004 | 0.004 | |
| Pulpo (Octopus) | 2 | 0.014 | 0.009 | 0.018 | |||
| Canned Tuna | Brand #1 | 5 | 0.405 | 0.089 | 0.252 | 0.400 | 0.520 |
| Brand #2 | 5 | 0.015 | 0.003 | 0.011 | 0.016 | 0.019 | |
| Brand #3 | 5 | 0.700 | 0.390 | 0.238 | 0.807 | 1.187 | |
| Brand #4 | 5 | 0.676 | 0.418 | 0.147 | 0.741 | 1.334 | |
| Brand #5 | 5 | 0.283 | 0.151 | 0.096 | 0.299 | 0.471 | |
| Brand #6 | 3 | 0.346 | 0.297 | 0.134 | 0.139 | 0.766 | |
| Brand #7 | 3 | 0.514 | 0.186 | 0.263 | 0.571 | 0.707 | |
| Brand #8 | 3 | 0.084 | 0.048 | 0.017 | 0.114 | 0.122 | |
| Brand #9 | 1 | 0.092 |
3.4 Seafood Consumption in Pregnant Women
Similar data were obtained from pregnant women concerning their seafood consumption across the three trimesters (Supplemental Tables 6 and 7) and here we describe data from women surveyed in trimester 3. Canned tuna, fresh fish, canned sardine, and shellfish were reported to have been consumed 3.0, 2.2, 0.4, and 1.0 times per month respectively in pregnant women. These particular items were consumed 6.6 times per month with 752.2 grams consumed per month. When the seafood consumption data (i.e., number of monthly servings of the four items) was related with blood mercury values, positive and significant (p<0.001) Spearman correlations were obtained for trimesters 2 (r=0.246) and 3 (r=0.173) but not for trimester 1 (r=0.012, p=0.85) (Supplemental Table 7).
4.0 DISCUSSION
Methylmercury is a proven neurodevelopmental toxicant (Karagas et al. 2012; Mergler et al. 2007). Given the challenges of assessing in utero mercury exposures, researchers use cord blood mercury as a proxy of in utero exposures. Here, the mercury in cord blood averaged 4.7 μg/L and the 90th percentile value was 7.6 μg/L. When compared to many groups worldwide (Stern and Smith 2003) these cord blood mercury levels from Mexico City are elevated though not quite as high as levels found in mercury-sensitive groups such as avid fish consumers.
Mercury in cord blood relates with concurrent methylmercury exposure in the mother (Stern and Smith 2003), and here we show a significant correlation between cord blood mercury and trimester 3 maternal blood. However, it is not clear how well mercury in cord blood reflects in utero exposures earlier in pregnancy. Pregnancy entails a number of physiological and metabolic changes (i.e., owing to factors such as hemodilution and transplacental transfer), and so we hypothesized that temporal change in mercury biomarkers would occur. Here we found that relationships between cord blood mercury and trimesters 2 and 1 were not statistically significant and weakened in a temporal manner. Such makes sense as the half-life of blood mercury is approximately 3-4 months, and thus early in utero exposures may not be well reflected in cord blood samples. Though, it should also be noted that mean maternal blood mercury values and seafood consumption was consistent throughout the three trimesters. Other studies have also explored for temporal changes in mercury biomarkers across pregnancy. A study from South Korea examined maternal blood samples from early (median = 3.9 μg/L) and late (median = 3.2 μg/L) pregnancy, and found no difference in total mercury levels (Lee et al. 2010). A study from Sweden found that maternal blood methylmercury decreased 23% when comparing women in early (median = 0.9 μg/L) and late (median = 0.7 μg/L) pregnancy (Vahter et al. 2000), and attributed this decrease to reduced fish consumption.
In the current study, average blood mercury levels in pregnant women ranged between 3 and 4 μg/L. Here we put this finding into perspective. First, an expert panel stated that a normal expected range of blood mercury in a population (i.e., not selected solely on the basis of high fish consumption) would be 1 to 5 μg/L (Mergler et al. 2007). In the current study, the 90th percentile blood mercury value across the trimesters was 5.2 – 6.6 μg/L, indicating that most fall within what this panel deemed expected. Second, the findings here show that exposures amongst pregnant mothers from Mexico City are higher than exposures in woman from the United States. Median blood mercury in women of reproductive age (16-49 years) from the U.S. National Health and Nutrition Examination Survey (NHANES) (1999-2002; date cycle that most closely overlaps the current study) was reported to be 0.86 μg/L (Jones et al. 2004). This NHANES value is slightly higher than the minimum values and lower than the 10th percentile values we report here. Notably, within the NHANES dataset, Mexican-American women had lower blood mercury (0.73 μg/L) than the average. The comparison between these two datasets suggest that pregnant women from Mexico City have blood mercury levels approximately 3-4 fold higher than their counterpart in the United States. Third, the results here can be compared to the biomonitoring guideline associated with the U.S. Environmental Protection Agency's reference dose of 5.8 μg/L in cord blood (Mahaffey et al. 2003) which equates to approximately 3.5 μg/L in maternal blood as discussed by Stern and Smith (2004). This guideline indicates an amount of mercury in blood that is associated with increased risk of learning disabilities in unborn children. From the 1999-2000 U.S. NHANES, approximately 15.7% of women of reproductive age had blood mercury levels above the 3.5 μg/L guideline (Mahaffey et al. 2003). In our study on pregnant women from Mexico City, 28.6-39.2% of participants exceeded this guideline.
In the children studied here we were able to obtain hair and urine samples as well as blood, and this permitted us to delve deeper into potential sources of mercury exposure. In general the mean mercury values in all three biomarkers from Mexico City children were approximately 3-5 times higher than what has previously been reported in the U.S. (CDC 2013; McDowell et al. 2004) and Canada (Wong and Lye 2008). Though, as expected and just like with the mothers, the ELEMENT children had lower exposures than children from populations which normally consume higher amounts of seafood, such as the Faroe Islands (mean blood mercury was 9.0 μg/L; ~7 years of age; (Debes et al. 2006)) and Seychelles (mean hair was 6.1 μg/g; ~9 years of age; (Myers et al. 2009)).
What are the sources of mercury in Mexico City? For organic methylmercury (largely reflected in hair and blood), seafood are considered to be the major source in most populations. We recognize that the ELEMENT seafood dietary consumption surveys were not initially designed to address issues of mercury exposure; however reasonable data was obtained. Seafood was consumed nearly 7 times per month (~750-800 grams consumed per month), and among the 4 items queried canned tuna was most popular. Similar to this work in Mexico City, Ruelas-Inzunza et al. (2011) reported individuals in coastal areas of Mexico consumed approximately 25 g/day of fish and 4 g/day of shrimp (~870 grams/month). These values from México are approximately 2-fold higher than the amount of seafood consumed by the average American woman of childbearing age (442 grams/month; (EPA 2002). In addition, we report here upon mercury content in commercially available seafood in Mexico City. When the results are compared to the U.S. EPA guidance level of 0.3 μg/g wet weight to protect public health (EPA 2001), the mean total mercury value in 7 species and 5 of the 9 canned tuna brands were in excess. Such data, when coupled with the seafood consumption information, may help explain why biomarkers of methylmercury exposure are higher in Mexico City than amongst the general U.S. population.
Urine is the preferred biomarker of inorganic mercury exposure. In the current study, urine was only examined in children and was not available from the mothers. The main source of urine mercury in the general population is personal dental amalgam. In México, the prevalence of mercury amalgams is not known, though dental caries are prevalent among Mexican children (>75%) (Casanova-Rosado et al. 2005). Another potential source of inorganic mercury may be skin-lightening creams, which have been shown to be used in México (Dickenson et al. 2013). New research is also questioning the original source of mercury that is found in urine. While present in an inorganic form, stable isotope work has revealed that the mercury in urine may be derived from organic mercury in fish that underwent internal demethylation (Sherman et al. 2013).
The data we reported upon here from the ELEMENT cohort may have an important role in the global effort to understand the public health risks of mercury, especially methylmercury exposure from seafood. To date, decisions have mainly relied upon data from two longitudinal birth cohort studies in the Faroe Islands and Seychelles which address neuropsychological outcomes in relation to current, early-life, and developmental exposures to methylmercury and fish consumption (Davidson et al. 2006; Debes et al. 2006; Myers et al. 2009). The studies differ in many regards (e.g., exposure biomarkers, neurodevelopmental tests, timing of tests, costressors, participant diet). The ELEMENT cohort may help overcome some of these limitations. Our cohort's exposure and sampling design allows us to investigate fish consumption, mercury exposure (organic and inorganic via three relevant biomarkers), and neurodevelopmental functioning (as well as other outcomes, such as cardiovascular disease) at multiple times along a “lifestage” conceptual framework including multiple pregnancy timepoints. While the mercury levels we report here are 3-5 times higher than the average citizen of the United States and many other countries, they are still much lower than what has been found in the Faroe Islands or Seychelles (i.e., 10-15 times higher). In addition, sources of methylmercury exposure (e.g., canned tuna) amongst ELEMENT participants may be more relevant than the aforementioned studies. ELEMENT has also been used to study a number of other environmental toxicants (e.g., lead and other metals) thus affording an opportunity to examine real-world mixtures. Accordingly, mercury exposures within the ELEMENT cohort potentially have broad applicability to many populations worldwide and thus capitalizing upon the strengths of this cohort may help increase our understanding of the risk and benefits of seafood consumption.
Despite a number of strengths, there are potential limitations of our study that need to be recognized. The study population was drawn from selected hospitals in Mexico City which limits our ability to generalize the findings to the at-large population. However, a robust sample size was achieved and all participants enrolled initially in a study concerning lead and thus are not expected to be biased in terms of their perceived exposures to mercury. The seafood dietary consumption surveys were not initially designed to address issues of mercury exposure, however reasonable data was obtained and similar to what others have found. The current work lays the foundation for future-planned research that will employ more detailed surveys of seafood consumption. For inorganic mercury exposure, the survey did not capture potential predictors (e.g., personal amalgam, skin lightening creams). For each of the biomarkers total levels of mercury were characterized and future work should examine particular chemical species to help better pinpoint sources and potential risks.
In summary, here we report upon several biomarkers of mercury exposure from residents of Mexico City (blood in pregnant mothers across three trimesters and at delivery via cord blood; blood, hair, and urine of children, and hair of their mothers; and commercial seafood commonly purchased). In general, the work shows for the first time that women and children living in Mexico City have greater exposures to both methylmercury and inorganic mercury when compared to general populations sampled from across North America and Europe. Though, exposures here are lower than mercury sensitive groups, such as avid fish consumers.
Supplementary Material
HIGHLIGHTS.
- mercury measured in 348 pregnant women, 825 offspring, and 91 seafood from Mexico
- exposures to organic and inorganic mercury are widespread
- exposures are 3-5 times higher than populations surveys elsewhere
ACKNOWLEDGEMENTS
We thank the mothers and children who participated in the Early Life Exposures in Mexico to Environmental Toxicants (ELEMENT) project and the American British Cowdray Hospital for the use of its research facilities. This study was supported by U.S. National Institute of Health (NIH) grants R01 ES021446, R01 ES007821, R01 ES014930, R01 ES013744, P30 ES00002, P42 ES05947, K01 ES014907, K01ES019909, K23 ES000381, T32 ES007062, T42 008455, and UL1RR024986. Additional funding was provided by Consejo Nacional de Ciencia y Tecnología (CONACyT) Grant 4150M9405 and by the Rackham School of Graduate Studies at the University of Michigan. Additional support for the interpretation of results and authorship of this publication was made possible by NIEHS P01 ES012874 and a STAR Research Assistance Agreement No. RD-83172501 awarded by the U.S. Environmental Protection Agency (EPA). We thank the anonymous reviewers for carefully reviewing the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afeiche M, Peterson KE, Sánchez BN, Cantonwine D, Lamadrid-Figueroa H, Schnaas L, Ettinger AS, Hernández-Avila M, Hu H, Téllez-Rojo MM. Prenatal lead exposure and weight of 0- to 5-year-old children in Mexico City. Environ Health Perspect. 2011;119:1436–41. doi: 10.1289/ehp.1003184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Mercury. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 1999. [Google Scholar]
- Casanova-Rosado AJ, Medina-Solís CE, Casanova-Rosado JF, Vallejos-Sánchez AA, Maupomé G, Avila-Burgos L. Dental caries and associated factors in Mexican schoolchildren aged 6-13 years. Acta Odontol Scand. 2005;63:245–51. doi: 10.1080/00016350510019865. [DOI] [PubMed] [Google Scholar]
- CDC . Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (September, 2013) U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2013. [April 10, 2014]. http://www.cdc.gov/exposurereport/ [Google Scholar]
- Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–62. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- Costilla-Salazar R, Trejo-Acevedo A, Rocha-Amador D, Gaspar-Ramírez O, Díaz-Barriga F, Pérez-Maldonado IN. Assessment of polychlorinated biphenyls and mercury levels in soil and biological samples from San Felipe, Nuevo Mercurio, Zacatecas, Mexico. Bull Environ Contam Toxicol. 2011;86:212–216. doi: 10.1007/s00128-010-0165-z. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Weiss B, Shamlaye CF, Cox C. Prenatal methyl mercury exposure from fish consumption and child development: a review of evidence and perspectives from the Seychelles Child Development Study. Neurotoxicology. 2006;27:1106–9. doi: 10.1016/j.neuro.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol. Teratol. 2006;28:363–75. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lourdes Soto-Ríos M, Rothenberg SJ, Juárez-Pérez CA, Aguilar-Madrid G. Variability of mercury in urine among Mexican women residing in a mining area. J Occup Environ Med. 2010;52:62–66. doi: 10.1097/JOM.0b013e3181c75469. [DOI] [PubMed] [Google Scholar]
- Dickenson CA, Woodruff TJ, Stotland NE, Dobraca D, Das R. Elevated mercury levels in pregnant woman linked to skin cream from Mexico. Am J Obstet Gynecol. 2013;209:e4–5. doi: 10.1016/j.ajog.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA . Estimated Per Capita Fish Consumption in the United States. U.S. Environmental Protection Agency; Washington DC: 2002. Report EPA-821-C-02-003. [Google Scholar]
- EPA . Water Quality Criterion for the Protection of Human Health: Methylmercury Final. U.S. Environmental Protection Agency; Washington DC: 2001. Report EPA-823-R-01-001. [Google Scholar]
- Guentzel JL, Portilla E, Keith KM, Keith EO. Mercury transport and bioaccumulation in riverbank communities of the Alvarado Lagoon System, Veracruz State, Mexico. Sci Total Environ. 2007;388(1-3):316–24. doi: 10.1016/j.scitotenv.2007.07.060. [DOI] [PubMed] [Google Scholar]
- Jones RL, Sinks T, Schober SE, Pickett M. Blood mercury levels in young children and childbearing-aged women--United States, 1999-2002. Morb Mortal Wkly Rep. 2004;53:1018–20. [PubMed] [Google Scholar]
- Karagas MR, Choi AL, Oken E, Horvat M, Schoeny R, Kamai E. Review Evidence on the Human Health Effects of Low-Level Methylmercury Exposure. Environ Health Perspect. 2012;120:799–806. doi: 10.1289/ehp.1104494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B-E, Hong Y-C, Park H, Ha M, Koo BS, Chang N, et al. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ Health Perspect. 2010;118:437–43. doi: 10.1289/ehp.0900731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC. Blood Organic Mercury and Dietary Mercury Intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2003;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell M, Dillon CF, Osterloh J, Bolger PM, Pellizzari E, Fernando R, Montes de Oca R, Schober SE, Sinks T, Jones RL, Mahaffey KR. Hair Mercury Levels in U.S. Children and Women of Childbearing Age: Reference Range Data from NHANES 1999-2000. Environ Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergler D, Anderson H, Chan LHM, Mahaffey KR, Murray M, Sakamoto M, Stern AH. Methylmercury exposure and health effects in humans: a worldwide concern. Ambio. 2007;36:3–11. doi: 10.1579/0044-7447(2007)36[3:meahei]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Myers GJ, Thurston SW, Pearson AT, Davidson PW, Cox C, Shamlaye CF, Cernichiari E, Clarkson TW. Postnatal exposure to methyl mercury from fish consumption: a review and new data from the Seychelles Child Development Study. Neurotoxicology. 2009;30:338–49. doi: 10.1016/j.neuro.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam D-H, Basu N. Rapid methods to detect organic mercury and total selenium in biological samples. Chem Cent J. 2011;5:3. doi: 10.1186/1752-153X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri Y, Siuniak A, Johnson N, Levin E, Mitchell K, Goodrich JM, Renne E, Basu N. Occupational and environmental mercury exposure among small-scale gold miners in the Talensi-Nabdam District of Ghana's Upper East region. Sci Total Environ. 2010;408:6079–85. doi: 10.1016/j.scitotenv.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruelas-Inzunza J, Páez-Osuna F, Ruiz-Fernández AC, Zamora-Arellano N. Health risk associated to dietary intake of mercury in selected coastal areas of Mexico. Bull Environ Contam Toxicol. 2011;86:180–188. doi: 10.1007/s00128-011-0189-z. [DOI] [PubMed] [Google Scholar]
- Sherman LS, Blum JD, Franzblau A, Basu N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ Sci Technol. 2013;47:3403–9. doi: 10.1021/es305250z. [DOI] [PubMed] [Google Scholar]
- Stern AH, Smith AE. An Assessment of the Cord Blood:Maternal Blood Methylmercury Ratio: Implications for Risk Assessment. Environ Health Perspect. 2003;111:1465–1470. doi: 10.1289/ehp.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Téllez-Rojo MM, Bellinger DC, Arroyo-Quiroz C, Lamadrid-Figueroa H, Mercado-García A, Schnaas-Arrieta L, Wright RO, Hernández-Avila M, Hu H. Longitudinal associations between blood lead concentrations lower than 10 microg/dL and neurobehavioral development in environmentally exposed children in Mexico City. Pediatrics. 2006;118:e323–30. doi: 10.1542/peds.2005-3123. [DOI] [PubMed] [Google Scholar]
- Trasande L, Cortes JE, Landrigan PJ, Abercrombie MI, Bopp RF, Cifuentes E. Methylmercury exposure in a subsistence fishing community in Lake Chapala, Mexico: an ecological approach. Environ Health. 2010;9:1. doi: 10.1186/1476-069X-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNEP [April 10, 2014];Minamata Conveontion on Mercury. 2013 http://mercuryconvention.org.
- Vahter M, Akesson A, Lind B, Björs U, Schütz A, Berglund M. Longitudinal Study of Methylmercury and Inorganic Mercury in Blood and Urine of Pregnant and Lactating Women, as Well as in Umbilical Cord Blood. Environ Res. 2000;84:186–94. doi: 10.1006/enrs.2000.4098. [DOI] [PubMed] [Google Scholar]
- Wong SL, Lye EJD. Lead, mercury and cadmium levels in Canadians. Health Reports. 2008;19:31–36. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.