Abstract
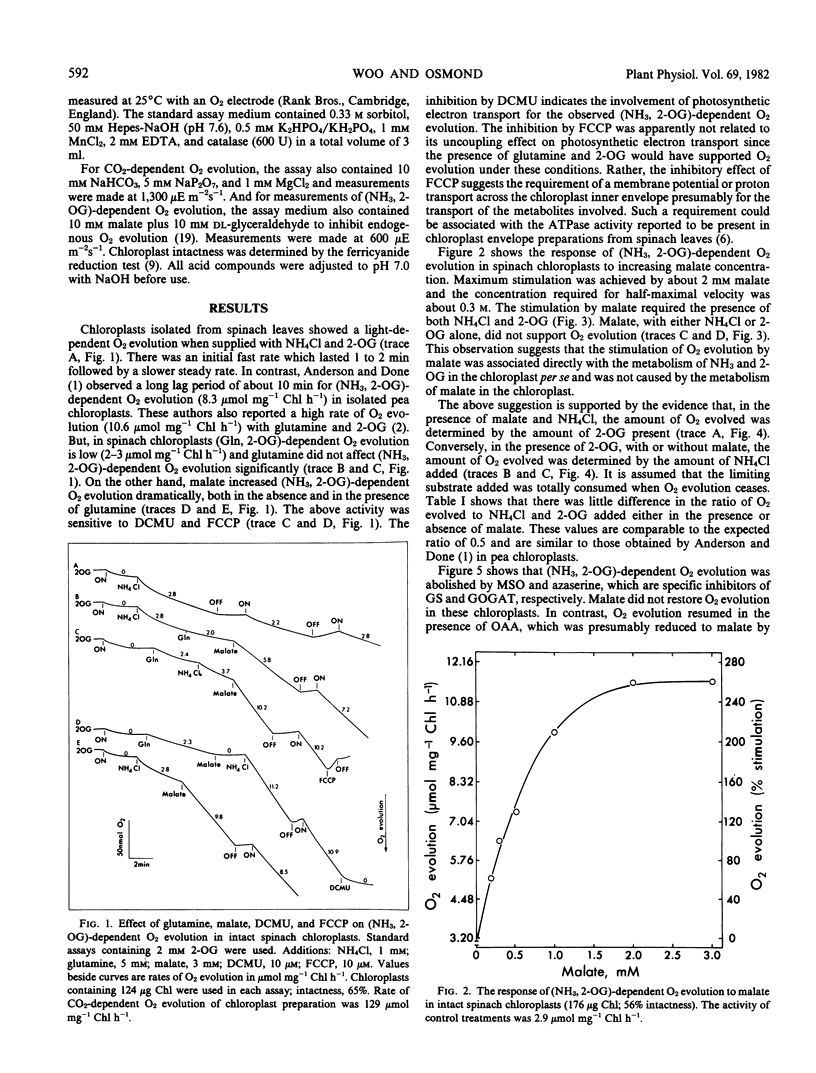
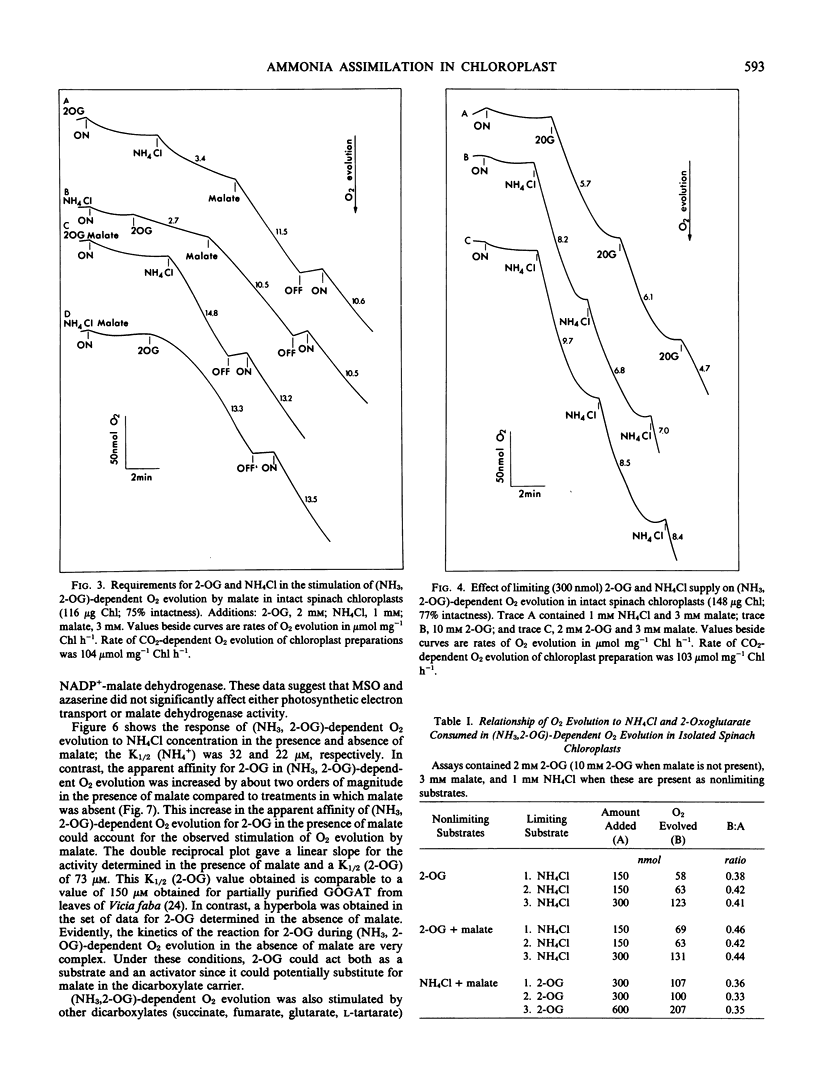
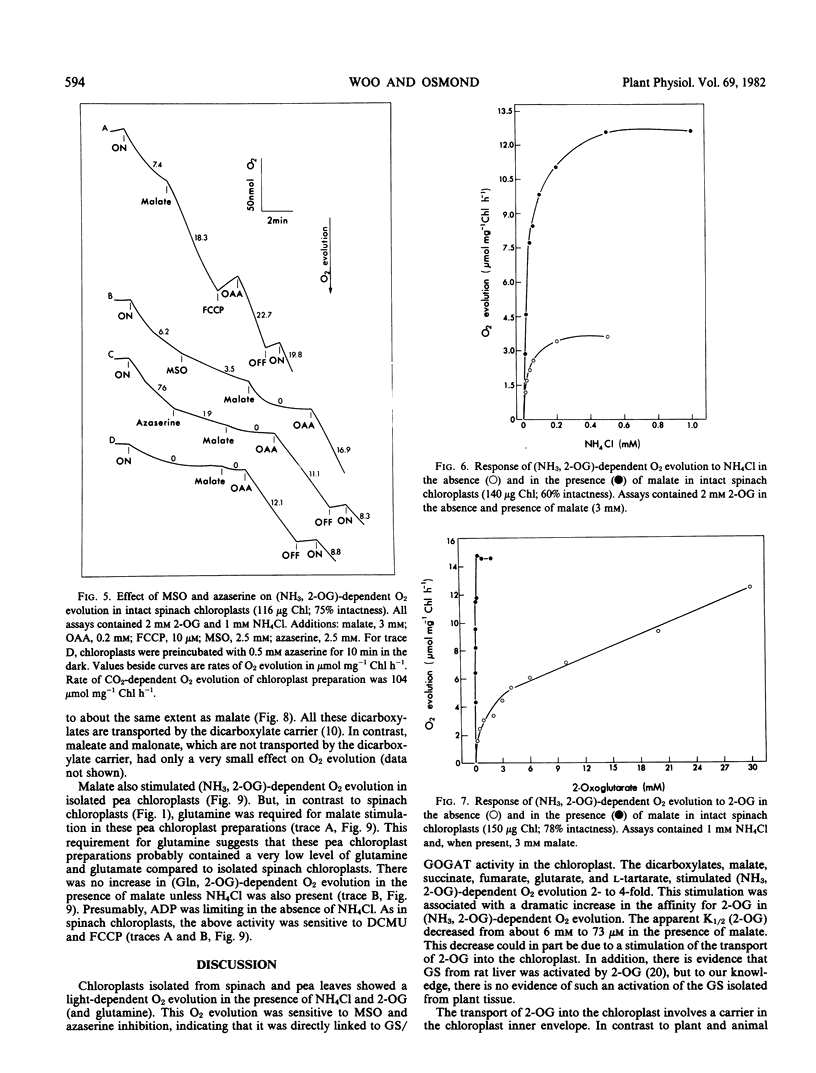
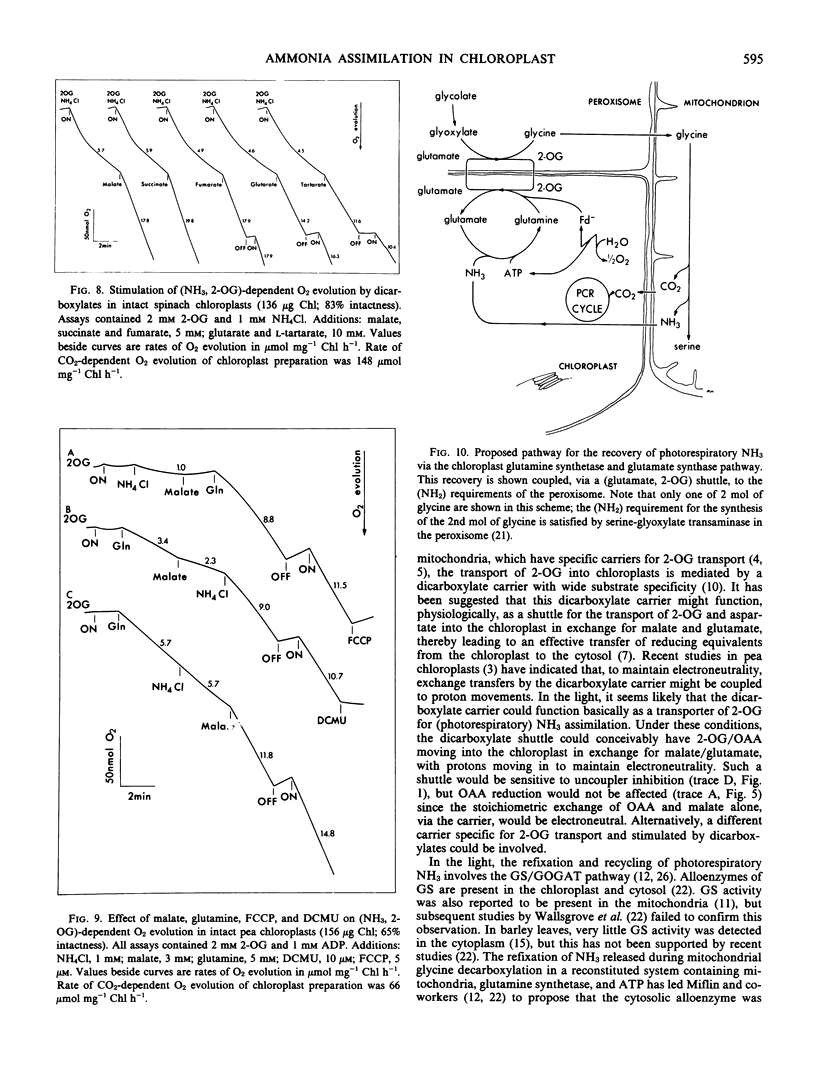
Intact chloroplasts isolated from spinach (Spinacia oleracea L.) leaves showed a light-dependent O2 evolution (5.5 ± 0.75 micromoles per milligram chlorophyll per hour) when supplied with ammonia and 2-oxoglutarate. This (ammonia, 2-oxoglutarate)-dependent O2 evolution was stimulated 2- to 4-fold by the dicarboxylates, malate, succinate, fumarate, glutarate, and l-tartarate. Evolution of O2 in the presence of malate was dependent on the presence of both 2-oxoglutarate and NH4Cl; malate with only either 2-oxoglutarate and NH4Cl alone did not support O2 evolution. Furthermore, in the presence of malate, the amount of O2 evolved was solely dependent on the amount of NH4Cl or 2-oxoglutarate added and malate did not affect the ratio of O2 evolved to NH4Cl or 2-oxoglutarate consumed. Studies with inhibitors (2-(3,4-dichlorophenyl)-1,1-dimethyl urea, methionine sulfoximine, and azaserine) indicated that the above activity was directly linked to glutamine synthetase and glutamate synthase activity in the chloroplast and was not caused by the metabolism of malate. The Vmax/2 of (ammonia, 2-oxoglutarate)-dependent O2 evolution was reached at 32 micromolar NH4Cl and 6 millimolar (approximately) 2-oxoglutarate in the absence of malate, and at 22 micromolar NH4Cl and 73 micromolar 2-oxoglutarate when malate (3 millimolar) was present.
Intact chloroplasts isolated from pea (Pisum sativum) leaves also showed a stimulation of (ammonia, 2-oxoglutarate)-dependent O2 evolution by malate. However glutamine was required for this activity even though glutamine with only either NH4Cl or 2-oxoglutarate did not respond to malate stimulation.
The measured rates of (ammonia, 2-oxoglutarate)-dependent O2 evolution in isolated spinach chloroplasts in the presence of malate were about 19.5 ± 4.5 micromoles O2 evolved per milligram chlorophyll per hour. This is adequate to sustain photorespiratory NH3 recycling and the refixation of NH3 arising from NO3 under ambient conditions in the light. The role of the chloroplast in photorespiratory NH3 recycling and the nature of the associated transport of 2-oxoglutarate into the chloroplast is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. W., Done J. A polarographic study of glutamate synthase activity in isolated chloroplasts. Plant Physiol. 1977 Sep;60(3):354–359. doi: 10.1104/pp.60.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Done J. Polarographic study of ammonia assimilation by isolated chloroplasts. Plant Physiol. 1977 Oct;60(4):504–508. doi: 10.1104/pp.60.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- Heber U., Santarius K. A. Direct and indirect transfer of ATP and ADP across the chloroplast envelope. Z Naturforsch B. 1970 Jul;25(7):718–728. doi: 10.1515/znb-1970-0714. [DOI] [PubMed] [Google Scholar]
- Heber U. Stoichiometry of reduction and phosphorylation during illumination of intact chloroplasts. Biochim Biophys Acta. 1973 Apr 27;305(1):140–152. doi: 10.1016/0005-2728(73)90239-9. [DOI] [PubMed] [Google Scholar]
- Jackson C., Dench J. E., Morris P., Lui S. C., Hall D. O., Moore A. L. Photorespiratory nitrogen cycling: evidence for a mitochondrial glutamine synthetase [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1122–1124. doi: 10.1042/bst0071122. [DOI] [PubMed] [Google Scholar]
- Lea P. J., Miflin B. J. Alternative route for nitrogen assimilation in higher plants. Nature. 1974 Oct 18;251(5476):614–616. doi: 10.1038/251614a0. [DOI] [PubMed] [Google Scholar]
- Mann A. F., Fentem P. A., Stewart G. R. Identification of two forms of glutamine synthetase in barley (Hordeum vulgare). Biochem Biophys Res Commun. 1979 May 28;88(2):515–521. doi: 10.1016/0006-291x(79)92078-3. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A., Stocking C. R. Kinetics and Energetics of Light-driven Chloroplast Glutamine Synthesis. Plant Physiol. 1975 Jan;55(1):59–63. doi: 10.1104/pp.55.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibe R., Beck E. On the Mechanism of Activation by Light of the NADP-dependent Malate Dehydrogenase in Spinach Chloroplasts. Plant Physiol. 1979 Nov;64(5):744–748. doi: 10.1104/pp.64.5.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate S. S., Meister A. Regulation of rat liver glutamine synthetase: activation by alpha-ketoglutarate and inhibition by glycine, alanine, and carbamyl phosphate. Proc Natl Acad Sci U S A. 1971 Apr;68(4):781–785. doi: 10.1073/pnas.68.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]


