Abstract
Leptin is an important pleiotropic hormone involved in the regulation of nutrient intake and energy expenditure, and is known to influence body weight in infants and adults. High maternal levels of arsenic have been associated with reduced infant birth weight, but the mechanism of action is not yet understood. This study aimed to investigate the association between in utero arsenic exposure and infant cord blood leptin concentrations within 156 mother-infant pairs from the New Hampshire Birth Cohort Study (NHBCS) who were exposed to low to moderate levels of arsenic through well water and diet. In utero arsenic exposure was obtained from maternal second trimester urinary arsenic concentration, and plasma leptin levels were assessed through immunoassay. Results indicate that urinary arsenic species concentrations were predictive of infant cord blood leptin levels following adjustment for creatinine, infant birth weight for gestational age percentile, infant sex, maternal pregnancy-related weight gain, and maternal education level amongst 149 white mother-infant pairs in multivariate linear regression models. A doubling or 100% increase in total urinary arsenic concentration (iAs + MMA + DMA) was associated with a 10.3% (95% CI: 0.8–20.7%) increase in cord blood leptin levels. A 100% increase in either monomethylarsonic acid (MMA) or dimethylarsinic acid (DMA) was also associated with an 8.3% (95% CI: −1.0–18.6%) and 10.3% (95% CI: 1.2–20.2%) increase in cord blood leptin levels, respectively. The association between inorganic arsenic (iAs) and cord blood leptin was of similar magnitude and direction as other arsenic species (a 100% increase in iAs was associated with a 6.5% (95% CI: −3.4–17.5%) increase in cord blood leptin levels), albeit not significant. These results suggest in utero exposure to low levels of arsenic influences cord blood leptin concentration and presents a potential mechanism by which arsenic may impact early childhood growth.
Keywords: arsenic, cord blood, leptin, pregnancy, prenatal exposure
1 Introduction
New research suggests an association between arsenic exposure and leptin (Ahmed et al., 2011; Fei et al., 2013). Leptin is an adipocyte hormone encoded by the LEP gene in humans (Li, 2011) that is well known for its role in regulating satiety, energy expenditure, and fat mass in both adults and infants (Friedman, 2002). Prenatal exposure to arsenic during pregnancy has also been associated with decreased fetal size at birth – specifically in the lower ranges of arsenic exposure (Rahman et al., 2009). In a Bangladeshi cohort of mother-infant pairs, arsenic exposure during pregnancy was found to be positively associated with leptin expression in the placenta as detected by immunohistochemistry, when using measures of maternal urinary arsenic collected at 30 weeks of gestation as the exposure (Ahmed et al., 2011). To our knowledge, no studies have investigated the association between maternal urinary arsenic and leptin expression in infant cord blood.
The placenta (Linnemann et al., 2004) and fetal adipose tissues (Lepercq et al., 2001) produce the majority of leptin that circulates in the fetus; in addition, some leptin is actively transported across the placenta from the mother (Linnemann et al., 2004). Cord blood leptin levels have been shown to closely reflect intravenous leptin levels in infants within the first 6 hours of life, indicating that cord blood concentrations are similar to circulating leptin levels within the infant at birth (Harigaya et al., 1997). Interestingly, female infants have been shown to have higher cord blood leptin levels at birth when compared to male infants (Alexe et al., 2006). Cord blood leptin levels are positively correlated with birth weight as well as birth weight for gestational age (Fonseca et al., 2004; Forhead and Fowden, 2009; Geary et al., 1999; Karakosta et al., 2013; Mantzoros et al., 2009; Mullis and Tonella, 2008; Schubring et al., 1997) and explain ~21% of the variation in birth weight between infants (Karakosta et al., 2011). Fetal leptin levels seem to reflect fetal adiposity rather than affect fetal weight gain as infants with congenital leptin deficiency (Montague et al., 1997; Strobel et al., 1998) or leptin receptor mutations (Clement et al., 1998) are born with normal birth weights.
Leptin plays an important role in the regulation of weight gain during infancy and early childhood growth (Fonseca et al., 2004; Mantzoros et al., 2009; Montague et al., 1997; Ong et al., 1999; Strobel et al., 1998). Infants with leptin deficiency or non-functional leptin receptors have rapid weight gain in early life (Clement et al., 1998; Montague et al., 1997; Strobel et al., 1998). Amongst healthy and preterm infants (Fonseca et al., 2004) without monogenic forms of obesity, English and American studies have associated higher cord blood leptin with less weight gain or catch-up growth up to 6 months (Mantzoros et al., 2009) and 2 years of age (Ong et al., 1999), and lower adiposity at 3 years of age (Mantzoros et al., 2009). This work suggests that slight imbalances to infant leptin levels due to gestational exposures may influence future weight phenotypes.
Arsenic (As) is a metalloid element that is ubiquitous in the environment (Jomova et al., 2011; Watanabe and Hirano, 2012), and has well-documented toxicity in humans (Chen et al., 2007; Watanabe and Hirano, 2012). The most common routes of non-occupational arsenic exposure is through food and drinking water (Jomova et al., 2011; Watanabe and Hirano, 2012). In the state of New Hampshire, unregulated private wells are commonly used as the home water supply, and an estimated one in ten New Hampshire homes rely on a well with water arsenic concentrations exceeding the Environmental Protection Agency’s recommended maximum of 10 μg/L (Environmental Protection Agency, 2001; Jomova et al., 2011; Karagas et al., 2002; Karagas et al., 1998). Upon ingestion, inorganic arsenic (iAs) is sequentially converted to monomethylated (MMA) and dimethylated (DMA) arsenic species, and all three species are primarily excreted through urine (Jomova et al., 2011; Watanabe and Hirano, 2012).
Arsenic is a contaminant of particular concern for pregnant women, as it is known to freely transport from mother to fetus through the placenta, with in utero arsenic exposure concentrations approximating those experienced by the mother throughout pregnancy (Concha et al., 1998; Vahter, 2009). Furthermore, even low levels of in utero exposure to arsenic may result in decreased fetal birth size (Rahman et al., 2009). The previously observed associations between leptin and infant weight, and prenatal arsenic exposure and infant birth weight, support the investigation of a relationship between arsenic exposure and infant leptin levels, as arsenic may influence infant weight through leptin.
This study investigates whether exposure to arsenic in utero (as measured through maternal excretion of arsenic species in urine) can predict infant cord blood leptin levels in a population exposed to relatively low levels of arsenic through diet and well water.
2 Materials & Methods
2.1 Study population
The study population consisted of 156 mother-infant pairs who were participants in the ongoing New Hampshire Birth Cohort Study (NHBCS), and were enrolled between January 2009 and June 2009. The mother-infant pairs chosen for this study represent a convenience sample of the first 156 mother infant pairs from the NHBCS with sufficient maternal and cord blood plasma for leptin analysis, as well as sufficient maternal urine samples for arsenic analysis. The NHBCS has been described in detail elsewhere (Farzan et al., 2013; Fei et al., 2013; Gilbert-Diamond et al., 2011; Koestler et al., 2013). Briefly, mothers were enrolled at ~24–28 gestational weeks at study clinics in New Hampshire, USA, beginning January, 2009. Mothers included in the cohort were literate in English, mentally competent, between 18–45 years old, and reported using a private, unregulated well for their home drinking water since their last menstrual period. Infants included in the cohort were singleton, live pregnancies. Self-reported sociodemographic (age, race/ethnicity, marital status, level of education), lifestyle (including tobacco and alcohol use, previous pregnancies, complications, birth outcomes), and clinical data were derived from pre- and post-delivery questionnaires and a medical records review. Participants provided informed consent in accordance with the policies set up by the Institutional Review Board (IRB) and Dartmouth College.
2.2 Sample collection
Infant cord blood samples were collected at the time of delivery by trained obstetrical staff. Cord blood samples were centrifuged and separated into components which were then aliquoted, and the plasma component was stored at −80 °C in liquid nitrogen until plasma leptin concentrations were assayed.
Urine samples were requested from study participants during a routine second trimester prenatal visit. Maternal spot urine samples were collected from study participants at the time of enrollment (between ~24–28 weeks of gestation). Samples were collected in pre-labelled, acid washed bottles containing 30 μL of 10 nM diammonium diethyldithiocarbamate to stabilize arsenic species, and were subsequently frozen within 24 hours of sample collection at −80 °C until analysis. Analysis occurred at the University of Arizona Hazard Identification Core.
2.3 Leptin concentration analysis
Infant cord blood leptin concentration levels (pg/mL) were measured using the standard manufacturer’s protocol for the MILLIPLEX MAP® Human Adipokine Magnetic Bead Panel 2 (Millipore, Billerica, Massachusetts) by DartLab, Geisel School of Medicine at Dartmouth College. Standards and spikes were measured in triplicate, undiluted cord blood samples were measured once, and blank values were subtracted from all readings. The mean intra-plate and inter-plate assay quality controls were 8.27% and 13.51%, respectively. In total, n = 156 infant cord blood samples were assayed for leptin protein levels. All leptin concentrations were within the detection range of the assay (0.192 to 600 ng/mL) and fell within the quality control/assurance acceptability limits of 70–130%.
2.4 Urinary arsenic concentration analysis
The urinary concentrations (μg/L) of arsenite (AsIII), arsenate (AsV), monomethylarsonic acid (MMAV), dimethylarsinic acid (DMAV), and arsenobetaine were measured using a high performance liquid chromatography (HPLC) ICP-MS system at the University of Arizona Hazard Identification Core, and has been described in detail elsewhere (Farzan et al., 2013; Fei et al., 2013; Gilbert-Diamond et al., 2011; Koestler et al., 2013). Detection limits ranged from 0.10 to 0.15 μg/L for individual arsenic species (no samples were below the detection limit). Urinary creatinine levels (mg/dL) were also measured to control for urinary dilution (Barr et al., 2005; Gamble and Liu, 2005; Nermell et al., 2008) using Cayman’s creatinine assay kit and protocol. Inorganic arsenic (iAs) was calculated as the sum of AsIII and AsV. Total As was calculated as the sum of iAs, MMAV and DMAV. Arsenobetaine was excluded from the total, because it is believed to be nontoxic.
2.5 Statistical analysis
All statistical analyses were performed in R version 3.0.1. We investigated individual predictors of arsenic and infant leptin with univariable linear regression models. Median maternal urinary arsenic concentrations were compared using the Mann-Whitney-Wilcoxon test and Kruskal-Wallis test for binary and categorical independent variables, respectively.
Linear regression models were then used to regress natural log-transformed infant cord blood leptin levels (outcome) on natural log-transformed maternal urinary arsenic species concentrations (predictor). The loge-transformation was employed to normalize the distributions. Models were adjusted for infant birth weight for gestational age percentile (Fenton and Kim, 2013) due to its known association with infant leptin and potential association with arsenic. Models also included urinary creatinine concentration to control for urinary dilution (Barr et al., 2005; Gamble and Liu, 2005; Nermell et al., 2008). Additional potential confounders were evaluated to see if they altered the relationship between urinary arsenic and cord blood leptin, and were retained in the final adjusted models if they resulted in a ±10% change in the coefficient estimate for total urinary arsenic concentration. The potential confounders selected a priori for evaluation were: infant sex and race, as well as maternal pre-pregnancy BMI, pregnancy-related weight gain, age at delivery, race, parity, education level, smoking status during pregnancy, and alcohol consumption during pregnancy. The final adjusted models controlled for continuous infant birth weight for gestational age percentile, continuous creatinine concentration, infant sex, continuous maternal pregnancy-related weight gain, and categorical maternal education level (any high school, any college or technical school, college graduate, or any postgraduate). Non-white mother-infant pairs were excluded from the analysis due to their small numbers (n=7 mother-infant pairs), resulting in the analysis of 149 exclusively white mother-infant pairs. We performed additional analyses stratified by sex, as differential leptin concentration by sex has been reported in some studies (Alexe et al., 2006; Harigaya et al., 1997; Schubring et al., 1997).
We calculated the increase in cord blood leptin according to tertile of maternal total urinary arsenic amongst the white mother-infant pairs using the adjusted regression coefficient estimates and the following covariate values: creatinine = 71.43 mg/dL (median), birth weight for gestational age percentile = 0.55 (mean), female infant sex, maternal pregnancy-related weight gain = 30.7 lbs (mean), and college graduate for maternal education (most common).
Infant race was used to impute maternal race when missing (n = 16) and maternal race was used to impute infant race when missing (n = 2). Missing creatinine was imputed using the median value, 71.43 mg/dL (n = 10). A missing stratum method (Jones, 1996) was used to deal with missing maternal education level (n = 17), alcohol consumption during pregnancy (n = 42), and smoking status during pregnancy (n = 17).
In a sensitivity analysis, mothers with gestational diabetes (n = 8) during the current pregnancy were excluded. In another sensitivity analysis, participants with maternal urinary total arsenic concentrations or infant cord blood leptin levels ≥ 3 standard deviations for each biological measure (n = 5) were excluded. Further covariate adjustment for maternal pre-pregnancy BMI was performed. Finally, to investigate the sensitivity of results to adjustment by creatinine in comparison to using creatinine-adjusted arsenic concentrations (where the concentration of arsenic is divided by the concentration of creatinine prior to loge-transformation), we created a model that used creatinine-adjusted arsenic concentration as the predictor.
3 Results
3.1 Descriptive statistics
The study participants consisted of 156 women and their infants enrolled in the NHBCS (Table 1). There were 78 female and 78 male infants. Approximately 85% of infants were of average birth weight for gestational age based on national standards and growth curves for each infant sex (Fenton and Kim, 2013). The mean gestational age was 39.6 weeks (standard deviation = 1.4 weeks), and gestational age ranged from 35.1 to 44.8 weeks. Mothers’ age ranged from 19.9 to 40.0 years at delivery with a mean age of 31.5 years. Mothers were primarily of healthy BMI (18.5–25 kg/m2, 53%), white (96%), married (84%), and college educated (38%).
Table 1.
Measures of maternal total urinary arsenic and infant cord blood leptin by maternal and infant characteristics (n = 156 mother-infant pairs from the New Hampshire Birth Cohort Study).
| n (%) | Median Total urinary As (μg/L) | P-value1 | Median Cord blood Leptin (ng/mL) | P-value1 | |
|---|---|---|---|---|---|
| All samples | 156 (100) | 4.30 (IQR: 2.15–6.75) | 27.31 (IQR: 17.81–38.33) | ||
| Maternal characteristics | |||||
| Age (years) | 0.272 | 0.500 | |||
| 18–24.9 | 10 (6.4) | 5.33 | 27.60 | ||
| 25–29.9 | 43 (27.6) | 3.58 | 24.11 | ||
| 30–34.9 | 69 (44.2) | 4.04 | 30.54 | ||
| 35–45 | 34 (21.8) | 5.40 | 27.63 | ||
| BMI classification | 0.947 | 0.718 | |||
| Underweight | 5 (3.2) | 2.23 | 36.67 | ||
| Normal | 82 (52.6) | 4.33 | 25.38 | ||
| Overweight | 38 (24.4) | 3.84 | 26.31 | ||
| Obese | 26 (16.7) | 5.08 | 28.97 | ||
| Missing | 5 (3.2) | 4.42 | 82.42 | ||
| Gestational weight gain (lbs) | 0.139 | 0.236 | |||
| <15 | 16 (10.3) | 4.22 | 27.65 | ||
| 15–25 | 21 (13.5) | 4.49 | 24.17 | ||
| 25–35 | 63 (40.4) | 5.30 | 22.83 | ||
| >35 | 56 (35.9) | 3.70 | 30.41 | ||
| Race or ethnicity | 0.032 | 0.331 | |||
| White | 150 (96.2) | 4.19 | 27.21 | ||
| Non-white | 6 (3.8) | 8.25 | 31.71 | ||
| Parity | 0.804 | 0.405 | |||
| None | 51 (32.7) | 3.80 | 27.44 | ||
| 1 child | 64 (41.0) | 4.42 | 24.10 | ||
| 2 children | 21 (13.5) | 4.68 | 31.90 | ||
| ≥ 3 children | 20 (12.8) | 3.46 | 31.86 | ||
| Education level | 0.645 | 0.552 | |||
| Any high school or graduate | 14 (9.0) | 3.57 | 27.52 | ||
| Junior college graduate or some college or technical school | 37 (23.7) | 4.68 | 29.49 | ||
| College graduate | 60 (38.5) | 4.28 | 29.74 | ||
| Any post-graduate schooling | 28 (17.9) | 4.45 | 21.75 | ||
| Missing | 17 (10.9) | 4.49 | 21.52 | ||
| Relationship status | 0.960 | 0.960 | |||
| Married | 131 (84.0) | 4.26 | 27.18 | ||
| Not married | 8 (5.1) | 4.54 | 28.76 | ||
| Missing | 17 (10.9) | 4.49 | 21.52 | ||
| Delivery method | 0.556 | 0.349 | |||
| Normal, spontaneous vaginal | 95 (60.9) | 4.16 | 27.86 | ||
| Vaginal birth after c-section | 2 (1.3) | 3.86 | 12.69 | ||
| C-section | 53 (34.0) | 4.40 | 26.44 | ||
| Induced vaginal delivery | 3 (1.9) | 10.0 | 29.47 | ||
| Missing | 3 (1.9) | 9.96 | 24.11 | ||
| Smoking status prior to pregnancy | 0.072 | 0.855 | |||
| Never | 120 (76.9) | 3.98 | 26.31 | ||
| Sometimes | 10 (6.4) | 6.95 | 30.59 | ||
| Everyday | 9 (5.8) | 5.55 | 29.49 | ||
| Missing | 17 (10.9) | 4.49 | 21.52 | ||
| Smoked during pregnancy | 0.388 | 0.948 | |||
| Yes | 8 (5.1) | 5.53 | 27.18 | ||
| No | 131 (84.0) | 4.21 | 29.85 | ||
| Missing | 17 (10.9) | 4.49 | 21.52 | ||
| Alcohol consumption during pregnancy | 0.438 | 0.490 | |||
| Yes | 19 (12.2) | 5.86 | 27.86 | ||
| No | 95 (60.9) | 4.21 | 21.38 | ||
| Missing | 42 (26.9) | 4.39 | 28.42 | ||
| Infant characteristics | |||||
| Cord blood leptin levels (ng/mL) | 0.716 | ||||
| 1st Quartile (2.813–17.81) | 39 (25) | 4.42 | 12.32 | ||
| 2nd Quartile (17.81–27.31) | 39 (25) | 4.37 | 21.38 | ||
| 3rd Quartile (27.31–38.33) | 39 (25) | 3.60 | 31.83 | ||
| 4th Quartile (38.33–416.4) | 39 (25) | 4.35 | 64.53 | ||
| Sex | 0.500 | <0.001 | |||
| Male | 78 (50) | 4.04 | 21.21 | ||
| Female | 78 (50) | 4.33 | 32.71 | ||
| Birth weight group (Fenton 2013) | 0.318 | <0.001 | |||
| Small for gestational age | 5 (3.2) | 3.54 | 21.03 | ||
| Average for gestational age | 132 (84.6) | 4.24 | 25.38 | ||
| Large for gestational age | 18 (11.5) | 5.27 | 38.90 | ||
| Missing | 1 (0.6) | 9.96 | 10.93 | ||
| Gestational age (weeks) | 0.772 | 0.164 | |||
| 35–39.9 | 92 (59.0) | 4.36 | 25.79 | ||
| 40–45 | 63 (40.4) | 4.04 | 29.16 | ||
| Missing | 1 (0.6) | 9.96 | 10.93 | ||
| Race or ethnicity | 0.048 | 0.598 | |||
| White | 154 (98.7) | 4.28 | 27.31 | ||
| Non-white | 2 (1.3) | 13.8 | 47.54 | ||
P-value from Mann-Whitney-Wilcoxon test and Kruskal-Wallis test for binary and categorical variables, respectively.
The mean gestational age at enrollment and maternal urine collection was 24.7 weeks (standard deviation = 2.3 weeks). For our study participants, concentration of total arsenic in well water samples ranged from 0.024 to 58.01 μg/L with a median of 1.18 μg/L. Maternal urinary total arsenic concentrations had a median (interquartile range, IQR) of 4.3 μg/L (2.15–6.75 μg/L) (Table 1), and maternal urinary iAs concentrations had a median of 0.28 μg/L (0.18–0.55 μg/L). Median maternal urinary MMA and DMA concentrations were 0.34 μg/L (0.18–0.53 μg/L) and 3.59 μg/L (1.72–5.67 μg/L), respectively. Maternal urinary total arsenic concentration was positively associated with non-white maternal and infant race (P = 0.03 and 0.05, respectively). Maternal urinary arsenic was not associated with any other maternal or infant characteristics in this sample.
Median leptin levels were significantly higher amongst female infants (32.7 ng/mL) when compared to male infants (21.2 ng/mL, P < 0.001) (Table 1). Leptin levels were positively correlated with infant birth weight for gestational age percentile (Pearson’s r=0.31, P < 0.001). Infant cord blood leptin was not associated with any other maternal or infant characteristics in this sample.
3.2 Association between arsenic and leptin
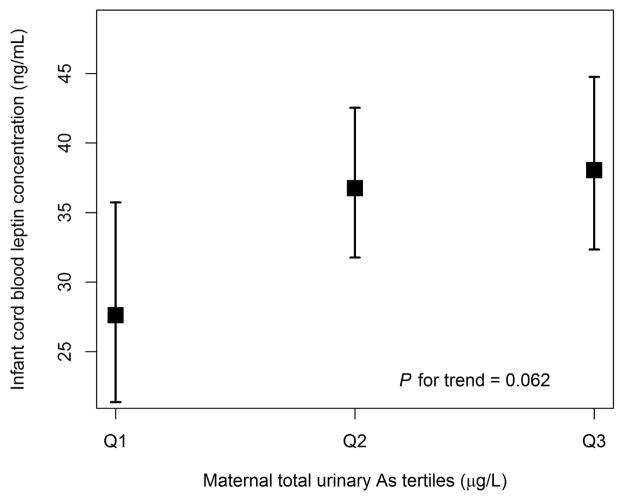
Maternal urinary concentrations of iAs, MMA, DMA, and total arsenic showed a positive association with infant cord blood leptin concentrations when unadjusted for potential confounders amongst 149 white mother-infant pairs, and were also positively associated with infant cord blood leptin concentrations following covariate adjustment (Table 2). After adjustment for potential confounders, each 100% increase or doubling in iAs was associated with a 6.5% (95% CI: −3.4–17.5%) increase in infant cord blood leptin; each 100% increase in MMA was associated with an 8.3% (95% CI: −1.0–18.6%) increase in infant cord blood leptin; each 100% increase in DMA was associated with a 10.3% (95% CI: 1.2–20.2%) increase in infant cord blood leptin; and each 100% increase in total urinary arsenic was associated with a 10.3% (95% CI: 0.8–20.7%) increase in infant cord blood leptin. There was a 37.7% increase in cord blood leptin concentration when comparing the 3rd tertile of maternal total urinary arsenic to the 1st tertile (P = 0.06) in adjusted models (Figure 1), and similar increases in cord blood leptin for tertiles of iAs, MMA and DMA were observed (Supplemental Figures S1–S3).
Table 2.
Unadjusted and adjusted estimates (95% CI) for the association between natural log-transformed maternal urinary arsenic concentration and natural log-transformed cord blood leptin concentration (n=149*).
| Predictor | iAs | MMA | DMA | Total As |
|---|---|---|---|---|
| Ln[Arsenic species (μg/L)]1 | 0.02 (−0.12, 0.17) | 0.01 (−0.12, 0.14) | 0.04 (−0.08, 0.16) | 0.04 (−0.09, 0.16) |
| Ln[Arsenic species (μg/L)]2 | 0.09 (−0.05, 0.23) | 0.12 (−0.01, 0.25) | 0.14 (0.02, 0.26) | 0.14 (0.01, 0.27) |
| Creatinine (mg/dL) | 0.00 (−0.01,0.00) | 0.00 (−0.01, 0.00) | 0.00 (−0.01, 0.00) | 0.00 (−0.01, 0.00) |
| Infant birth weight %3 | 1.05 (0.64, 1.47) | 1.08 (0.66, 1.50) | 1.10 (0.68, 1.51) | 1.09 (0.68, 1.51) |
| Infant is Male | −0.38 (−0.60, −0.16) | −0.37 (−0.59, −0.15) | −0.37 (−0.58, −0.24) | −0.37 (−0.58, −0.15) |
| Maternal weight gain (lbs) | 0.00 (0.00, 0.01) | 0.01 (0.00, 0.01) | 0.00 (0.00, 0.01) | 0.01 (0.00, 0.01) |
| Maternal Education Level | ||||
| any high school | Reference | Reference | Reference | Reference |
| any college | −0.15 (−0.56, 0.26) | −0.17 (−0.58, 0.24) | −0.17 (−0.58, 0.24) | −0.17 (−0.58, 0.24) |
| college graduate | −0.10 (−0.50, 0.29) | −0.13 (−0.53, 0.26) | −0.14 (−0.53, 0.25) | −0.14 (−0.53, 0.25) |
| any postgraduate | −0.29 (−0.73, 0.15) | −0.31 (−0.59, 0.36) | −0.34 (−0.58, 0.35) | −0.34 (−0.77, 0.35) |
Models unadjusted.
Models adjusted for all other variables in the table.
Infant birth weight for gestational age percentile from Fenton & Kim, 2013 (n=2 missing).
Urinary arsenic metabolites were measured via HPLC. Total urinary arsenic (Total As) is the sum of inorganic arsenic (iAs), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA); arsenobetaine was not included in this total.
Includes white mother-infant pairs only
Figure 1.
Increase in cord blood leptin according to tertile of maternal total urinary arsenic, adjusted as outlined in “Materials and Methods.” Values are regression estimates ± standard error. Median creatinine, mean birth weight for gestational age percentile, female infant sex, mean maternal pregnancy-related weight gain, and college graduate as maternal education were used to estimate calculations.
Aside from arsenic, Fenton (2013) birth weight for gestational age percentile and female sex were positively associated with cord blood leptin concentration (Table 2). Maternal urinary creatinine was negatively associated with cord blood leptin concentration in adjusted models. Lower maternal educational level was positively associated with cord blood leptin concentration, but only weakly so. No evidence for an interaction between infant sex and any maternal urinary arsenic species was found (P for interaction with total As = 0.82, with MMA = 0.71, with DMA = 0.74, and with iAs = 0.97). Sex-stratified results showed a similar positive association between maternal urinary arsenic concentrations and infant cord blood leptin concentration following adjustment for potential confounders amongst white mother-infant pairs (Supplemental Tables S2 and S3). In this small study, we did not observe an overall association with infant birth weight and maternal urinary arsenic concentrations (Table 1, Supplemental Table S1).
Sensitivity analyses that excluded participants with a gestational diabetes diagnosis, and women with extreme or outlying values of maternal urinary total arsenic or infant cord blood leptin levels (≥ 3 standard deviations for each biological measure) resulted in similar association estimates. Further adjustment for maternal pre-pregnancy BMI did not substantially change the results. Prediction of leptin levels by creatinine-adjusted arsenic concentrations resulted in estimates of similar magnitude and statistical significance. No statistically significant departure from linearity was observed in the relationship between maternal arsenic and cord blood leptin concentration in generalized additive models (data not shown for sensitivity analyses).
4 Discussion
We found maternal arsenic exposure during pregnancy to be positively associated with infant cord blood leptin levels after adjusting for potential confounders. Our observation of a positive association between in utero arsenic exposure and cord blood leptin concentration complements results from a Bangladeshi study that examined in utero arsenic exposure and placental leptin levels (Ahmed et al., 2011). In a region of rural Bangladesh subject to large variations in well water arsenic, urine samples were collected from 130 pregnant women around 30 weeks of gestation, and placental samples were collected at delivery (Ahmed et al., 2011). Amongst the Bangladeshi mothers, maternal urinary total arsenic concentrations were ~18 times greater than the mean urinary arsenic measured in the mothers from the NHBCS, and each quartile increase in total urinary arsenic concentration was associated with a 0.31% (95% CI: 0.15, 0.47%) increase in placental leptin expression measured by immunostaining following adjustment for maternal age, SES, and tobacco chewing (Ahmed et al., 2011). The upregulation of infant leptin associated with in utero arsenic exposure appears to be consistent between Bangladeshi and New Hampshire infants, despite the vastly different social and geographical contexts of each study, and leptin assessment in different tissues.
Several studies suggest that cord blood leptin concentration is related to early life growth trajectories (Boeke et al., 2013; Fleisch et al., 2007; Mantzoros et al., 2009; Ong et al., 1999). Among 197 infants of the British Avon Longitudinal Study of Pregnancy and Childhood, cord blood leptin concentration was inversely related to weight gain from birth to 4 months of age, and accounted for 6.6% of the variance in weight gain for that time period after adjusting for birth weight (Ong et al., 1999). Among 588 American children participating in the Project Viva birth cohort, higher cord blood leptin concentration was associated with a negative change in weight-for-length z scores during the first 6 months of age (unadjusted Spearman’s = −0.33, P < 0.0001) (Mantzoros et al., 2009). While speculative, even small increases in cord blood leptin levels associated with in utero arsenic exposure may influence infant growth following birth – but this will need further exploration.
In human epidemiologic studies, limited evidence for decreased birth weight and birth size exists in the context of maternal arsenic consumption through drinking water (Bloom et al., 2014). A Flemish study found the odds of having an SGA infant increase 1.65 (95% CI: 1.15–2.37) times with each interquartile range increase in cord blood arsenic concentrations (Govarts et al., 2012), and it was suggested that arsenic may influence fetal growth. Prenatal exposure to arsenic during pregnancy has been associated with decreased fetal birth size specifically in the lower ranges of exposure (Rahman et al., 2009). In a Bangladeshi cohort, birth weight decreased by 1.68 g (standard error = 0.62 g) for each 1 μg/L increase in maternal urinary arsenic in the exposure range of 0–100 μg/L of urinary arsenic (Rahman et al., 2009). Another study conducted in Bangladesh found maternal hair arsenic collected during a prenatal visit (a measure of historical arsenic exposure) to be associated with decreased infant birth weight (Huyck et al., 2007). We did not find evidence of an association between arsenic and birth weight in our study; however, our study may have been underpowered to examine this outcome.
Animal studies have also investigated the effects of arsenic exposure on birth outcomes and growth following birth. Pups of pregnant mice that consumed drinking water containing 100 μg/L arsenic from gestational day 8 to birth had lower birth weights compared to mice born of mothers who did not consume arsenic through drinking water – with male infant mice being more affected than females (Ramsey et al., 2013). The pups exposed to arsenic in utero experienced catch-up growth and eventually matched the weight of the unexposed offspring (Ramsey et al., 2013). Another mouse study found that pups exposed to water contaminated with arsenic at 10 μg/L postnatally had growth deficits compared to unexposed offspring (Kozul-Horvath et al., 2012). Therefore, arsenic may be an early life toxicant that impacts growth both prenatally and postnatally.
Infants with lower birth weight often experience more rapid weight gain during early infancy up to 2 years of age: a phenomenon termed “catch-up growth”, which may protect against mortality and hospital admissions (Hack et al., 1984; Ong et al., 2000; Victora and Barros, 2001). The lower leptin levels associated with low birth weight may facilitate this catch up growth through increased appetite signalling (Blundell et al., 2001). If arsenic is related to both lower birth weight (Rahman et al., 2009) and higher leptin (independent of birth weight), then infants exposed to in utero arsenic may not undergo the expected and beneficial catch-up growth due to suppressed hunger cravings (Blundell et al., 2001). A study of 2372 infants in Bangladesh found that maternal urinary arsenic at 30 weeks gestation was related to decreased attained weight during the first 2 years of life (Saha et al., 2012), though the associations were largely attenuated after adjustment for infant age, maternal BMI, and socioeconomic status. The association between in utero arsenic exposure and early growth has still not been studied in American populations.
This study is the first known, to our knowledge, to evaluate the association between arsenic exposure and cord blood leptin in a population with arsenic exposure levels that could be considered common in the United States population. Our study was strengthened by using sensitive methodologies to measure a biomarker of in utero exposure to both inorganic arsenic and its metabolites. A limitation of our study was the reliance on a single maternal second trimester urine sample to estimate in utero arsenic exposure. Studies suggest, however, that urinary arsenic is relatively stable over time (Kile et al., 2007; Navas-Acien et al., 2009) and we expect that uncaptured variation in in utero arsenic exposure would bias our results towards the null. Our analysis also relies on a single measurement of leptin concentration in cord blood. The observed association was statistically significant but small, and the public health implications or long-term health effects are unknown. Future studies, including ones based on the ongoing New Hampshire Birth Cohort Study, could examine whether in utero arsenic is related to leptin concentrations throughout early life and longitudinally over time. While we were unable to examine the underlying mechanism of the arsenic-leptin association, measuring the regulation of leptin pathway genes may provide insight in future studies. This study was also unable to examine whether arsenic-related differences in cord blood leptin concentrations were related to differences in early childhood growth.
5 Conclusion
In summary, maternal urinary arsenic concentrations were positively associated with infant cord blood leptin concentration in a New Hampshire population. Further research is needed to determine if arsenic-related differences in cord blood leptin concentration are associated with altered growth trajectories in early life.
Supplementary Material
HIGHLIGHTS.
We predicted infant cord blood leptin levels from in utero arsenic exposure.
Cord blood leptin increased with birth weight and was higher among female infants.
Low levels of arsenic exposure was unrelated to infant birth weight.
Increasing arsenic exposure was associated with increased cord blood leptin levels.
Suggests a mechanism by which arsenic may impact early childhood growth.
Acknowledgments
Funding sources: The work described in this manuscript was funded in part by grant P01ES022832 from the National Institute of Environmental Health at the NIH, and grant RD83544201 from the Environmental Protection Agency. Dr. Gilbert-Diamond is funded by grant NIHGMS P20GM104416 from the National Institutes of Health (NIH). This study was reviewed and approved by the Committee for the Protection of Human Subjects (CPHS) at Dartmouth College, Hanover, NH. All participants in the study provided informed consent in accordance with CPHS guidelines, the Institutional Review Board (IRB), and Dartmouth College.
The authors would like to thank the University of Arizona and the participants and study staff of the NHBCS, without whom this work would not be possible.
Abbreviations
- 95% CI
95% confidence interval
- AGA
average for gestational age
- As
arsenic
- BMI
body mass index
- DMA
dimethylarsinic acid
- iAs
inorganic arsenic
- IQR
interquartile range
- LEP
leptin gene
- LGA
large for gestational age
- MMA
monomethylarsonic acid
- NHBCS
New Hampshire Birth Cohort Study
- SGA
small for gestational age
Footnotes
This study was reviewed and approved by the Committee for the Protection of Human Subjects (CPHS) at Dartmouth College, Hanover, NH and all participants in the study provided informed consent in accordance with CPHS guidelines.
The authors have declared no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed S, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environmental health perspectives. 2011;119:258. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexe D-M, et al. Determinants of early life leptin levels and later life degenerative outcomes. Clinical medicine & research. 2006;4:326–335. doi: 10.3121/cmr.4.4.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, et al. Urinary creatinine concentrations in the US population: implications for urinary biologic monitoring measurements. Environmental health perspectives. 2005;113:192. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, et al. Maternal arsenic exposure and birth outcomes: A comprehensive review of the epidemiologic literature focused on drinking water. International journal of hygiene and environmental health. 2014 doi: 10.1016/j.ijheh.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell J, et al. Regulation of appetite: role of leptin in signalling systems for drive and satiety. International Journal of Obesity & Related Metabolic Disorders. 2001;25:S29–S34. doi: 10.1038/sj.ijo.0801693. [DOI] [PubMed] [Google Scholar]
- Boeke CE, et al. Differential associations of leptin with adiposity across early childhood. Obesity. 2013;21:1430–1437. doi: 10.1002/oby.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environmental health perspectives. 2007;115:1415–1420. doi: 10.1289/ehp.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Concha G, et al. Exposure to inorganic arsenic metabolites during early human development. Toxicological Sciences. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency . Monitoring Contaminants, 40 CFR Parts 9, 141 and 142. Federal Register. 2001;66:50961–50963. [Google Scholar]
- Farzan SF, et al. In utero arsenic exposure and infant infection in a United States cohort: A prospective study. Environmental Research. 2013;126:24–30. doi: 10.1016/j.envres.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei DL, et al. Association between In Utero arsenic exposure, placental gene expression, and infant birth weight: a US birth cohort study. Environmental Health. 2013;12:58. doi: 10.1186/1476-069X-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch AF, et al. Influence of serum leptin on weight and body fat growth in children at high risk for adult obesity. Journal of Clinical Endocrinology & Metabolism. 2007;92:948–954. doi: 10.1210/jc.2006-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca VM, et al. Early postnatal growth in preterm infants and cord blood leptin. Journal of perinatology. 2004;24:751–756. doi: 10.1038/sj.jp.7211188. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Fowden AL. The hungry fetus? Role of leptin as a nutritional signal before birth. The Journal of Physiology. 2009;587:1145–1152. doi: 10.1113/jphysiol.2008.167072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutrition reviews. 2002;60:S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- Gamble MV, Liu X. Urinary creatinine and arsenic metabolism. Environmental health perspectives. 2005;113:A442. doi: 10.1289/ehp.113-a442a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary M, et al. Leptin concentrations in maternal serum and cord blood: relationship to maternal anthropometry and fetal growth. BJOG: An International Journal of Obstetrics & Gynaecology. 1999;106:1054–1060. doi: 10.1111/j.1471-0528.1999.tb08113.x. [DOI] [PubMed] [Google Scholar]
- Gilbert-Diamond D, et al. Rice consumption contributes to arsenic exposure in US women. Proceedings of the National Academy of Sciences. 2011;108:20656–20660. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, et al. Exposure to Arsenic in Relation with Birth Outcome in Newborns of the 2nd Flemish Environment and Health Study (2007–2011) Epidemiology. 2012;23 [Google Scholar]
- Hack M, et al. Catch-up growth in very-low-birth-weight infants: clinical correlates. Archives of Pediatrics & Adolescent Medicine. 1984;138:370–375. doi: 10.1001/archpedi.1984.02140420036013. [DOI] [PubMed] [Google Scholar]
- Harigaya A, et al. Relationship between concentration of serum leptin and fetal growth. Journal of Clinical Endocrinology & Metabolism. 1997;82:3281–3284. doi: 10.1210/jcem.82.10.4321. [DOI] [PubMed] [Google Scholar]
- Huyck KL, et al. Maternal arsenic exposure associated with low birth weight in Bangladesh. Journal of Occupational and Environmental Medicine. 2007;49:1097–1104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- Jomova K, et al. Arsenic: toxicity, oxidative stress and human disease. Journal of Applied Toxicology. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- Jones MP. Indicator and stratification methods for missing explanatory variables in multiple linear regression. Journal of the American Statistical Association. 1996;91:222–230. [Google Scholar]
- Karagas MR, et al. Assessment of cancer risk and environmental levels of arsenic in New Hampshire. International journal of hygiene and environmental health. 2002;205:85–94. doi: 10.1078/1438-4639-00133. [DOI] [PubMed] [Google Scholar]
- Karagas MR, et al. Design of an epidemiologic study of drinking water arsenic exposure and skin and bladder cancer risk in a US population. Environmental health perspectives. 1998;106:1047–1050. doi: 10.1289/ehp.98106s41047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakosta P, et al. Leptin levels in cord blood and anthropometric measures at birth: a systematic review and meta-analysis. Paediatric and Perinatal Epidemiology. 2011;25:150–163. doi: 10.1111/j.1365-3016.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- Karakosta P, et al. Maternal Weight Status, Cord Blood Leptin and Fetal Growth: a Prospective Mother–Child Cohort Study (Rhea Study) Paediatric and Perinatal Epidemiology. 2013;27:461–471. doi: 10.1111/ppe.12074. [DOI] [PubMed] [Google Scholar]
- Kile ML, et al. Dietary arsenic exposure in Bangladesh. Environmental health perspectives. 2007;115:889–893. doi: 10.1289/ehp.9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler DC, et al. Differential DNA Methylation in Umbilical Cord Blood of Infants Exposed to Low Levels of Arsenic in Utero. Environmental health perspectives. 2013 doi: 10.1289/ehp.1205925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozul-Horvath CD, et al. Effects of low-dose drinking water arsenic on mouse fetal and postnatal growth and development. PloS one. 2012;7:e38249. doi: 10.1371/journal.pone.0038249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepercq J, et al. Prenatal leptin production: evidence that fetal adipose tissue produces leptin. Journal of Clinical Endocrinology & Metabolism. 2001;86:2409–2413. doi: 10.1210/jcem.86.6.7529. [DOI] [PubMed] [Google Scholar]
- Li M-D. Leptin and beyond: an odyssey to the central control of body weight. The Yale journal of biology and medicine. 2011;84:1–7. [PMC free article] [PubMed] [Google Scholar]
- Linnemann K, et al. 158 Materno-Fetal Transfer of 125-I-Leptin in The Dual in vitro Perfused Placenta Perfusion Model and Increased Leptin Mrna-Expression in Adipose Tissue of Pregnant Women. Pediatric Research. 2004;56:491–491. [Google Scholar]
- Mantzoros CS, et al. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics. 2009;123:682–689. doi: 10.1542/peds.2008-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague CT, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Mullis P-E, Tonella P. Regulation of fetal growth: Consequences and impact of being born small. Best Practice & Research Clinical Endocrinology & Metabolism. 2008;22:173–190. doi: 10.1016/j.beem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A, et al. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: the Strong Heart Study. Environmental health perspectives. 2009;117:1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermell B, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environmental research. 2008;106:212–218. doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ong KK, et al. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. British medical journal. 2000;320:967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong KK, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. Journal of Clinical Endocrinology & Metabolism. 1999;84:1145–1148. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]
- Rahman A, et al. Arsenic exposure during pregnancy and size at birth: a prospective cohort study in Bangladesh. American journal of epidemiology. 2009;169:304–312. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- Ramsey KA, et al. In utero exposure to low dose arsenic via drinking water impairs early life lung mechanics in mice. BMC Pharmacology and Toxicology. 2013;14:13. doi: 10.1186/2050-6511-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha KK, et al. Pre-and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural Bangladesh. Environmental health perspectives. 2012;120:1208–1214. doi: 10.1289/ehp.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubring C, et al. Levels of leptin in maternal serum, amniotic fluid, and arterial and venous cord blood: relation to neonatal and placental weight. Journal of Clinical Endocrinology & Metabolism. 1997;82:1480–1483. doi: 10.1210/jcem.82.5.3935. [DOI] [PubMed] [Google Scholar]
- Strobel A, et al. A leptin missense mutation associated with hypogonadism and morbid obesity. Nature genetics. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- Vahter M. Effects of arsenic on maternal and fetal health. Annual review of nutrition. 2009;29:381–399. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- Victora CG, Barros FC. Commentary: The catch-up dilemma—relevance of Leitch’s ‘low–high’pig to child growth in developing countries. International journal of epidemiology. 2001;30:217–220. doi: 10.1093/ije/30.2.217. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hirano S. Metabolism of arsenic and its toxicological relevance. Archives of toxicology. 2012;87:969–979. doi: 10.1007/s00204-012-0904-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.