Abstract
There is a sizeable literature suggesting that mercury (Hg) exposure affects cytokine levels in humans. In addition to their signaling role in the immune system, some cytokines are also integrally associated with sleep behavior. In this cross-sectional study of 9–11 year old children (N = 100), we measured total blood Hg in whole blood, serum levels of tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), and objectively measured sleep and activity using actigraphy. Increasing blood Hg was associated with significantly shorter sleep duration and lower levels of TNF-α. IL-6 was not associated with sleep or blood Hg. This study is the first to document an association between total blood Hg and sleep (albeit a small effect), and the first to consider the associations of total blood Hg with cytokines TNF-α and IL-6 in a pediatric sample. Further research using alternative designs (e.g., time-series) is necessary to determine if there is a causal pathway linking low-level Hg exposure to sleep restriction and reduced cytokines.
Keywords: Children, mercury, sleep, cytokines, IL-6, TNF-α
1. Introduction
Low-level mercury (Hg) exposure in children appears to increase systemic inflammatory markers (Gump et al. 2012). One explanation for this association could be underlying effects of Hg on inflammatory signaling cytokines. For example, in vitro studies of inorganic Hg (iHg) exposure show an increase in immune cell release of the proinflammatory cytokines interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6, and a decrease in the release of anti-inflammatory cytokines interleukin 1-receptor antagonist (IL-1Ra) and IL-10 (Gardner et al. 2009; Kempuraj et al. 2010). Similarly, a cross-sectional study demonstrated elevated pro-inflammatory cytokines (namely, IL-1β, TNF-α, and IFN-γ) in miners exposed to elemental and iHg relative to levels in miners without occupational Hg exposure (Gardner et al. 2010b). However, a different cross-sectional study of adults with Hg exposure via fish consumption along the Tapajos river system of Brazil found increases in some pro-inflammatory cytokines (IL-6, IFN- γ) but also an increase in an anti-inflammatory cytokine (IL-4; Nyland et al., 2011). Although this study presumed that the majority of total Hg in whole blood was methylmercury (MeHg) via fish consumption, Hg speciation in blood was not carried out so it is not possible to determine if associations with cytokines are a result of iHg levels, MeHg levels, or both. We are not aware of any studies that have considered potential associations between Hg exposure (either as total Hg, MeHg, or iHg) and cytokine levels in children.
We were particularly interested in potential associations of Hg exposure to sleep patterns in the present study because, although cytokines have pro-inflammatory functions, they also serve an important role in sleep regulation (Gamaldo et al. 2012). A number of studies have shown that alterations in cytokine activity affect sleep pattern (Raison et al. 2010; Ritter et al. 2013; Hayes et al. 2011) and other research suggests that sleep restriction produces decreases in TNF-α and IL-6 (Chennaoui et al. 2011; Vgontzas et al. 2004; Abedelmalek et al. 2013), suggesting bi-directional effects. Despite these documented links for Hg-cytokines and cytokines-sleep, we only know of one study investigating the potential effects of Hg on sleep (Moen et al. 2008). This cross-sectional study of dental assistants exposed to Hg via dental amalgams found significant increases in self-reported sleep disturbances. We are not aware of any other studies considering potential Hg associations with sleep and none using objective measures of sleep such as actigraphy.
In the present study, we focused on children partially because they are presumed to be more sensitive than adults to the effects of environmental toxicants (Faustman et al. 2000) and therefore effects might be evident in children in the absence of any observable effects in adults. With respect to the focus on low-level exposure, chronic low-level Hg exposure is widespread and yet understudied relative to research of higher exposures (e.g, occupational exposures, high fish consumption). Nonessential metals may have adverse effects at levels below the current thresholds for identifying “elevated” levels (Bellinger et al. 1992; Gump et al. 2011; Freire et al. 2010). If these effects exist, it will be important for us to understand the pathophysiological changes that may occur during chronic low-level environmental metal exposure.
The aim of the present study was to evaluate associations between blood Hg, objectively measured sleep and activity patterns, and blood cytokine levels (specifically, TNF-α and IL-6). Our data was drawn from a slightly larger study (N = 140) designed to study cardiovascular responses to acute stress in children (ages 9–11) as a function of blood lead (Pb) levels. This subsample contains 100 children, as they were the ones for which both two days of wrist actigraphy data and serum cytokine measurements were obtained. Wrist actigraphy has been validated and shown to be a reliable method for the objective measurement of sleep quantity and quality (Marino et al. 2013). Finally, we used mediational analyses in an effort to understand the potential pathway for associations between these variables.
2. Patients and Methods
2.1. Study Population
Participants (N = 100) were recruited as part of an ongoing study designed to address the effects of nonessential metals on cardiovascular responses to acute stress. Using a direct mailing list, we mailed invitations to homes in Oswego County, NY, containing a child within our target age group of 9–11 year olds. This recruitment method elicits participation from a sample that closely resembles an eligible population and is cost effective (Hinshaw et al. 2007). Further inclusion criteria included: 1) reporting no use on the day of testing of medication that might affect cardiovascular functioning (e.g., Ritalin), and 2) having no significant developmental disorders that might affect task performance (a component of our broader study). A blood draw for measuring nonessential toxic metal levels was followed within 2 weeks by a laboratory visit. Children were paid $100 for their participation.
2.4. Child’s Blood Hg and Lead (Pb) Levels
Whole blood specimens (2 mL) were collected into Royal Blue Top Vacutainer® (BD Franklin Lakes, NJ, USA) tubes that had been pre-certified by the analyzing laboratory for low-level measurements of total Hg and Pb (a potential confound, see below). Blood specimens were refrigerated pending shipment to the Trace Elements section of the Laboratory of Inorganic and Nuclear Chemistry at the New York State Department of Health’s Wadsworth Center, Albany, NY. The analysis for Pb and Hg in whole blood was carried out using a Perkin Elmer Sciex Model ELAN DRC Plus inductively coupled plasma-mass spectrometer (Shelton, CT, USA) equipped with a dynamic reaction cell (DRC-ICP-MS) (Palmer et al. 2006). For this study, the instrument was operated in the “standard” rather than “DRC” mode. The method detection limits (MDL) were 0.24 μg/L (Hg) and 0.34 μg/dL (Pb). Given the lower levels of blood Hg that were expected, the superior detection limits of ICP-MS were appropriate for this study and have been used in several biomonitoring studies (McKelvey et al. 2007). Four levels of internal quality control materials were analyzed before, during and after each run. The method has been validated against NIST SRM 966 (Toxic Metals in Bovine Blood), as well as a new standard reference material SRM 955c (Toxic Metals in Caprine Blood) that has been certified for Pb and Hg (Murphy et al. 2009). Laboratory values below the MDL were used in data analyses with the assumption that such values constitute the best available estimate of the true value and are preferable to assigning a zero or an arbitrary constant such as ½ the MDL (Fitzgerald et al. 2004; Stewart et al. 2008).
2.2 Objective Sleep
Objective sleep was measured with the Actigraph Model GT1M (Actigraph Inc, Pensacola, Florida). The device was attached to the child’s wrist following the blood draw on Friday mornings and children wore the device until the following Monday morning. The GT1M has the following characteristics: dimensions of L 38 × W 37 × H 18 (mm), weight of 27 g, a micro-electro-mechanical systems (MEMS) solid state sensor, dynamic range of 0.05–2.5 g, and frequency range of 0.25–2.5 Hz (Actigraph 2008). Data (movement counts) were collected in 1-min epochs and were downloaded onto a desktop computer. All data were hand-edited with additional information from a sleep log completed by the participant where they recorded time to bed and time up in the morning. These times were used as a guide to determine the general window within which to determine sleep onset and waking time. The actual times adopted were those times within these windows when there were 10 consecutive minutes with no activity (sleep time) or 10 consecutive minutes of some activity (wake time). A primary coder (blind to values on all other variables) recorded these values for each child and these values allowed us to then calculate the additional variables of sleep duration (wake time – sleep time), sleep quality (mean activity counts during sleep time), and daily activity (mean activity counts during day). A second independent coder (also blind to other variables) recorded sleep time and wake time for a subset of 12 children (3 days/nights of data for each, so, 36 data points) and these values were highly correlated with our primary coder (r = 0.96, p < .001). We selected only Friday and Saturday for inclusion in our current analyses, as data from these nights would afford the least constraint on sleep (i.e., they were not school nights) and were temporally closest to the Friday morning blood draw.
2.5. Cytokine Analysis of Serum
Fasting blood samples were collected in the morning using Vacutainers (BD Vacutainer, REF 367820) and serum was produced by centrifuging twice to remove any cellular debris. Samples were aliquoted to reduce freeze-thaws and stored in Protein LoBind microcentrifuge tubes (Brinkmann Instruments) at −80°C until analyzed. Ninety-six well microplates were washed using a Bio-Tek ELx50 plate washer (Winooski, VT), absorbances were read using a Bio-Tek Power Wave XS plate reader, and concentrations were determined from standard curves using KC Junior software (Bio-Tek). All standards and blanks were run in duplicate, and samples (N=99) and controls (pooled human serum) were run in triplicate. The absorbance average of the blank wells was subtracted from each sample, standard, and control. TNF-α and IL-6 concentrations of the samples and control were determined from the standards using a 4-parameter sigmoid curve. All steps were completed at room temperature.
Serum TNF-α was measured by an enzyme linked immunoassay (ELISA) kit (ALPCO) per the manufacturer’s instructions. Briefly, standards, samples, controls, and blanks were added to a 96-well microplate coated with monoclonal antibodies specific for human TNF-α. After incubating for two hours and washing four times, biotinylated anti-TNF-α antibodies were added, the plate was incubated for another hour, and then it was washed four times. Horseradish peroxidase-labeled streptavidin and tetramethylbenzidine were added, the plate was incubated in the dark for 30 minutes, the reaction was halted by a stop solution, and the absorbance was measured at 450 nm.
Serum IL-6 was measured using the Quantikine HS ELISA (R&D Systems) per the manufacturer’s instructions. Briefly, standards, samples, controls, and blank were added to a 96-well microplate coated with mouse monoclonal antibodies against human IL-6 and the plate was incubated for two hours while shaking. The plate was washed six times, polyclonal antibodies against IL-6 conjugated to alkaline phosphatase were added, and the plate was incubated for two hours while shaking. The plate was washed six times, substrate solution was added, and the plate was incubated for 1 hour before adding amplifier solution and incubating again for 30 minutes. A stop solution was added and the absorbances were measured at 490 nm using a correction wavelength of 690 nm.
2.6. Potential Confounds
Potential confounders were chosen based on the National Institutes of Health guidelines for the inclusion of covariates in multivariate models, which recommends a prior selection of a limited set of variables shown in prior literature to relate to the outcome (Ewout and Harrell 2009). This approach avoids over-fitting a model that occurs when “cherry picking” covariates from a larger set of potential confounds (Babyak 2004). The following covariates were included: gender, age, race, BMI percentile standing (age and gender adjusted using a SAS program developed by the Centers for Disease Control and Prevention (Centers for Disease Control and Prevention 2008), family history of cardiovascular disease (yes vs. no for parents or grandparents), and socioeconomic status (SES). SES was a single measure derived from normalizing (using z-scores) and averaging the parents’ education (using an 8 level item), occupation (using a 9 level occupational classification developed by Hollingshead (Hollingshead 1975), and income (using a 9-level item). We did not anticipate effects of Pb exposure on sleep (or cytokines), nevertheless, all analyses of Hg associations reported below were unchanged when this variable was also added. Attention deficit disorder (ADD) might affect actigraphy data; however, only two parents reported that their child had ADD and therefore we did not control for this variable. Finally, we previously reported (Gump et al., 2012, Environ Res, 112, 204–11) an association between fish consumption (coded as yes vs. no) and Hg. Although fish consumption is associated with blood Hg levels in the current cohort (see Gump 2012), fish consumption was not significantly related to sleep duration or cytokine levels.
2.7. Data Analyses
All statistical analyses were performed using SAS software (SAS, Cary, NC). Hg was log transformed because the distribution of blood Hg levels was not normally distributed and showed a significant positive skew (skewness = 6.77, standard error of skewness = 0.24, z = 28.04, p < 0.05). Log Hg was analyzed in regression models (using SAS PROC REG). In addition to this approach, we analyzed nontransformed Hg by creating quartiles that contained a roughly equal number of participants and corresponded to the following blood Hg levels: < 0.24 μg/L (1st quartile; N = 25), 0.25 – 0.45 μg/L (2nd quartile; N = 25), 0.46 – 0.79 μg/L (3rd quartile; N = 24), and 0.80 – 11.82 μg/L (4th quartile; N = 26). For the analysis of quartiles, SAS PROC GLM was used with a linear contrast to test the effects of increasing blood Hg levels. In all analytic models, the six covariates outlined above were entered first. Because sleep duration may be artificially constrained by when a child goes to bed and may be affected by their daily activity level, all models also included covariate control for these two variables (except when analyzing these variables as outcomes). TNF-α was normally distributed (skewness = −0.03, standard error of skewness = 0.24, z = −0.11, p > .10); however, IL-6 was not normally distributed (skewness = 3.95, standard error of skewness = 0.24, z = 16.29, p < .05) and this variable was therefore log transformed prior to analyses. Sample size varied slightly across analyses due to missing data (as outlined for each measure above).
Because cytokines are integrally related to sleep patterns (Gamaldo et al. 2012) and this relationship may be bi-directional, we tested sleep as a potential mediator of the associations between Hg and cytokines as well as cytokines as a potential mediator for an association between Hg and sleep. Since we measured two cytokines, this resulted in testing four different mediational models. Mediational modeling was performed using the online calculator for conducting a Sobel test (Preacher and Leonardelli 2001).
3. Results and Discussion
3.1. Characteristics of Participants
Table 1 presents characteristics of children in our sample. Our sample included 9, 10, and 11 year-olds (Ns = 47, 50, and 3, respectively), a roughly equal number of males and females (57 and 43, respectively), and was predominantly white (87%). The parents of these children had, on average, “some college” (scale score = 5.4), a family income between $45,000 and $65,000, and an occupational status between “Clerical and Sales” (Hollingshead score = 5) and “Technician or Semiprofessional” (Hollingshead score = 6). The mean BMI percentile for our sample was 75.1% and the mean BMI for our sample was 20.37 kg/m2 (SD = 4.07). This is higher than 2009–2010 NHANES data for 6–11 year old children (Ogden et al. 2012); males: M = 18.3 kg/m2 and females: M = 18.5 kg/m2). These differences are consistent with 2008–2010 school data showing that Oswego County, NY, had the 2nd highest percentage of overweight or obese middle and high school students (46.1%) when compared to all other counties in New York State (New York State Department of Health 2011). In our sample, 79.0% of the children had a family history of high cardiovascular disease (at least 1 parent and grandparent with known high cholesterol). Mean blood Hg was 0.77 μg/L and the median was 0.46 μg/L. With the exception of one participant at 11.82 μg/L, all participants had Hg levels below 3.27 μg/L. By log transforming this variable, the one outlier was reduced from 8.63 SDs above the mean to 2.88 SDs above the mean. In addition, all analyses were repeated with this participant excluded and results were attenuated only slightly and remained significant. The 90th percentile for Hg was 1.55 μg/L. The average for our study population is well below the Environmental Protection Agency’s established level of 5.8 μg/L for potential health risks (US Environmental Protection Agency 2011).
Table 1.
Sample Characteristics (N = 100)
| Variable | M(SD) | % |
|---|---|---|
| Age | 10.01 (0.62) | |
| Gender (% female) | 43.0 | |
| Race (% white) | 87.0 | |
| Childs’ BMI (percentile1) | 75.13 (23.19) | |
| Family History of CVD (% yes) | 79.0 | |
| Family Income2 | 7.83 (1.82) | |
| Parent’s Occupation (score) | 5.92 (1.71) | |
| Parent’s Education (score) | 5.40(1.11) | |
| Blood Hg (μg/L) | 0.77 (1.28) | |
| Blood Pb (μg/dL) | 1.00 (0.47) | |
| Average Sleep Duration (hrs) | 9.27 (0.95) | |
| TNF-α (pg/mL) | 17.88 (3.53) | |
| IL-6 (pg/mL) | 0.99 (0.97) |
Percentiles are derived from national age and gender adjusted BMI tables provided by the CDC.
On this income scale, a score of 8 corresponds to “45,000–65,000”. As outlined in the methods section, this income was subsequently adjusted for the number of people living in the household.
3.2. Increasing blood Hg is associated with reduced levels of TNF-α and IL-6
In apparent contrast to our prior research in this same cohort demonstrating positive associations between Hg and proteomic-derived measures of systemic inflammatory markers (Gump et al. 2012), we found a significant negative association between Hg and TNF-α (β = −0.20 p < 0.05; full model R2 = 0.15), but not IL-6 (β = −0.13 p > 0.10; full model R2 = 0.11), as shown in Table 2. Parallel analyses evaluating linear trends across Hg quartiles revealed significant negative associations for both TNF-α and IL-6 (F (1, 86) = 4.11, p < 0.05, and F (1, 86) = 4.82, p < 0.05, respectively). It is unclear why prior studies of Hg exposure and proinflammatory cytokine levels have generally observed a positive association (e.g., Gardner et al. 2009; Gardner et al. 2010b) while we observed a negative one in this pediatric population (N=100).
Table 2.
Association of blood Hg (analyzed as continuous variables and then analyzed separately as a trend across quartiles) with actigraphy measures and blood cytokine levels (N = 100).
| Logged Hg (β) | Hg Quartile | Linear Contrast | ||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |||
| Actigraphy | ||||||
| Time going to sleep (hrs:mins) | −0.17 | 10:16 pm | 10:37 pm | 10:16 pm | 10:18 pm | |
| Sleep Duration (hrs:mins) | −0.24* | 9:25 | 9:19 | 9:13 | 8:47 | * |
| Sleep Quality (avg counts/min) | −0.07 | 51.23 | 45.97 | 48.96 | 42.69 | |
| Daily Activity (avg counts/min) | −0.08 | 1837.86 | 1912.58 | 1670.34 | 1821.78 | |
| Cytokines | ||||||
| TNF-α (pg/mL) | −0.20* | 19.28 | 18.64 | 18.47 | 17.17 | * |
| IL-6 (log pg/mL) | −0.12 | 0.99 | 0.96 | 0.88 | 0.84 | * |
p < 0.05
Note. Each measure from actigraphy data was analyzes in a separate model. All analyses include covariate control for gender, age, race, SES, BMI, percentile, and family history of CVD. Analyses also included covariate control for time going to sleep and daily activity as these variables relate to sleep duration, the focus of our mediational models. Also, IL-6 levels were analyzed in log-transformed form but here after reported following conversion back to original units.
3.3. Elevated Hg associated with reduced sleep duration
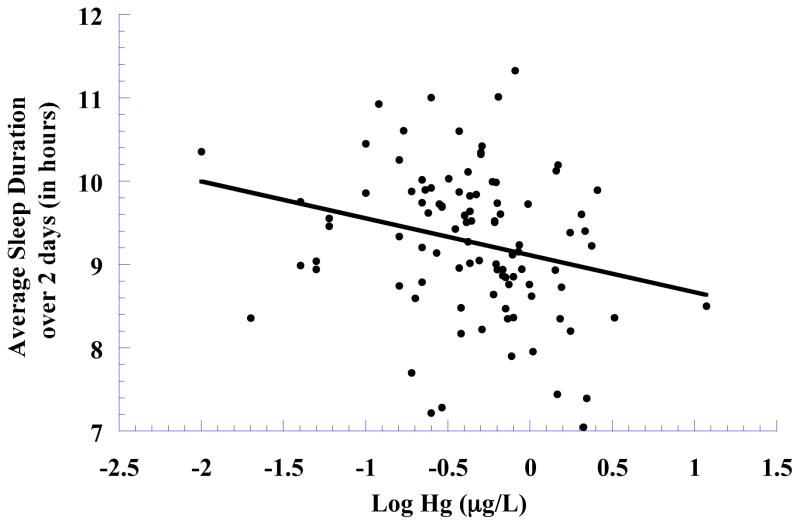
In regression models predicting objectively measured sleep and activity (also shown in Table 2), we found a significant negative association between Hg and sleep duration (β = −0.24, p < 0.05; full model R2 = 0.16), but no significant association with sleep quality (β = −0.13, p > 0.10; full model R2 = 0.11), time going to sleep (β = −0.13, p > 0.10; full model R2 = 0.11), or activity while awake (β = −0.13 p > 0.10; full model R2 = 0.11). Based on the regression model for sleep duration (and as illustrated in Figure 1), for each 1 μg/L increase in Hg, children were getting 9 minutes less sleep. Parallel analyses evaluating linear trends across Hg quartiles also revealed a significant negative association for sleep duration (F (1, 86) = 4.02, p < 0.05) but not sleep quality (F (1, 86) = 1.19, p > 0.10), time going to sleep (F1, 88) = 0.07, p > 0.10), or activity while awake (F (1, 90) = 0.48, p > 0.10).
Figure 1.
Sleep duration (adjusted for covariates) in relation to log blood Hg, with an ordinary least squares line added.
3.4. Associations between sleep duration, Hg, and TNF-α
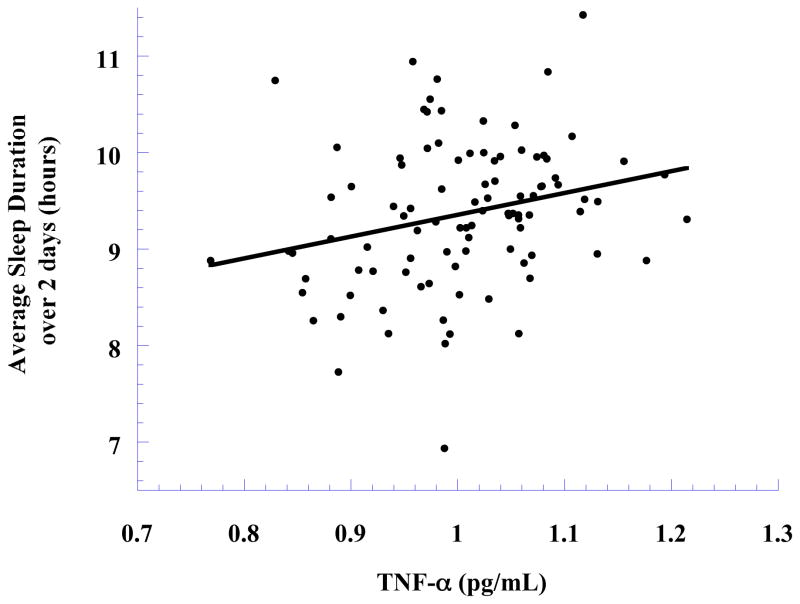
After controlling for covariates, sleep duration was significantly associated with TNF-α (β = 0.38, p < 0.001, see Figure 2). Based on all the significant associations outlined above, we conducted a Sobel test for mediation to test whether the association between Hg and TNF-α can be explained by differences in sleep duration or if, alternatively, the association between Hg and sleep duration can be explained by differences in TNF-α. The results of these analyses did not enable us to establish the appropriate ordering of variables in this pathway. The Hg-sleep association was changed slightly by the addition of TNF-α to the model (β = −0.24, p < 0.01 = in original model, β = −0.18, p = 0.05, when adding TNF-alpha) and the Sobel test for that mediational model was marginally significant (z = 1.71, p = .08). Similarly, the Hg-TNF-α association was reduced slightly by the addition of sleep duration to the model (β = 0.36, p < 0.0001, in original model, β = 0.32, p < 0.001, when adding TNF-alpha) and the Sobel test for that mediational model was significant (z = 1.99, p < .05). Therefore, it appears that two models are possible: Hg → Sleep → TNF-α or Hg → TNF-α → Sleep. It should be noted, mediational models do not allow us to establish the directionality of pathways (MacKinnon et al. 2000). Since there is no empirical evidence or logical rationale to suggest that either cytokines or sleep duration affect blood Hg levels, the bi-directional nature of cytokine-sleep associations suggests at least two additional models that are possible and therefore need to be explored in the future: Hg → Sleep ← TNF-α or Hg → TNF-α ← Sleep.
Figure 2.
TNF-α (adjusted for covariates) in relation to sleep duration, with an ordinary least squares line added.
3.5. Possible limitations
First, it always remains possible that significant associations occur by chance (i.e., a Type I error is made). We have tried to limit the number of analyses conducted to avoid this error; however, a replication of our findings would help alleviate this concern. Second, the cross-sectional design has known weaknesses with respect to establishing causality. For example, although improbable, it remains possible that sleep duration and/or cytokine levels affect the toxicokinetics of this nonessential environmental metal and thereby alter blood Hg levels. In general, the associations we observed provide the basis for generating hypotheses to be tested in subsequent research. Third, we measured total Hg in our samples and therefore cannot address the relationship between the various forms of Hg (elemental, inorganic, organometallic) and sleep duration and cytokine levels. While most of the Hg present in human originates from fish in the food supply in the form of methylmercury (MeHg; (Ravichandran 200), we cannot exclude the possible contributions of other Hg species such as elemental Hg exposure through dental amalgams (Hansen et al. 2004). Moreover, there is some evidence that the pattern of associations with cytokines varies as a function of the type of Hg exposure considered (Gardner et al. 2010a). Fourth, the association between Hg and sleep duration in the present study is relatively small (9 minutes sleep lost for every 1 μg/L of Hg increase). However, literature suggests that even relatively small changes in sleep duration in children (< 1 hour) can impair neurocognitive functioning (Molfese et al. 2013), increase risk of overweight/obesity (Chaput et al. 2006), and increase emotional lability and impulsivity (Gruber et al. 2012), to name just a few of the potential consequences. Fifth, our sample size is small in a population that has a relatively high SES and is almost exclusively Anglo American. As such, replication of our findings with a larger more diverse sample is necessary. Finally, it is not logical for Hg-induced sleep reduction to continue indefinitely – at some point it will plateau. In our current study of low-level Hg exposure, we were unable to determine the level at which the association plateaus. Of importance to future research, research with high Hg exposure cohorts may be unable to detect an association between Hg and sleep duration as the effect would have already plateaued at some floor for sleep duration.
Further research using alternative designs (e.g., time-series) is necessary to determine if there is a causal pathway linking low-level Hg exposure to sleep restriction and reduced TNF-α. If low-level Hg exposure affects sleep and cytokines, this could have important consequences to children’s health and behaviors. Importantly, the associations demonstrated here were significant at Hg levels below the potential health risk level of 5.8 μg/L that has been established by the Environmental Protection Agency (2011). However, in order to determine if low-level Hg exposure presents any risk with respect to sleep or TNF-α, further study is necessary to adequately rule out Type I error or confounding.
4. Conclusions
This is the first study to suggest that increasing blood Hg is associated with less sleep duration and reduced TNF-α (but not IL-6) in children. Although we controlled for a number of potential confounds, it remains possible that an unknown (uncontrolled) variable has created spurious associations between blood Hg, sleep duration, and TNF-α.
Highlights.
Total blood mercury, sleep, and cytokines (TNF-α, IL-6) were measured in children.
Higher mercury levels were associated with less sleep and lower levels of TNF-α.
Mediational models considered pathways that might explain these associations.
Acknowledgments
We are grateful for the assistance of the Oswego Hospital Laboratory and Dr. Robert Morgan (Oswego Family Physicians) for their help with blood specimen collection. The Human Research Committee of SUNY Oswego approved this study. Written informed assent and consent was obtained from all participants and their parents, respectively. This work was supported by National Institutes of Health grants ES15619, ES15619-1S1, and ES023252 (to B.B.G., K.B., and JAM).
Abbreviations
- CDC
Centers for Disease Control and Prevention
- CVD
cardiovascular diseases
- Hg
mercury
- MDL
method detection limits
- Pb
lead
- SES
socioeconomic status
Footnotes
The authors have no conflict of interests associated with this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abedelmalek S, Souissi N, Chtourou H, Denguezli M, Aouichaoui C, Ajina M, Aloui A, Dogui M, Haddouk S, Tabka Z. Effects of partial sleep deprivation on proinflammatory cytokines, growth hormone, and steroid hormone concentrations during repeated brief sprint interval exercise. Chronobiol Int. 2013;30:502–509. doi: 10.3109/07420528.2012.742102. [DOI] [PubMed] [Google Scholar]
- Actigraph. GT1M specifications. 2008. [Google Scholar]
- Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med. 2004;66:411–421. doi: 10.1097/01.psy.0000127692.23278.a9. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM, Needleman HL. Low-level exposure, intelligence and academic achievement: A long-term follow-up study. Pediatrics. 1992;90:855–861. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [December 20, 2008];A SAS Program for the CDC Growth Charts. 2008 < http://www.cdc.gov/nccdphp/dnpa/growthcharts/resources/sas.htm>.
- Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the ‘Quebec en Forme’ Project. Int J Obes (Lond) 2006;30:1080–1085. doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- Chennaoui M, Sauvet F, Drogou C, Van Beers P, Langrume C, Guillard M, Gourby B, Bourrilhon C, Florence G, Gomez-Merino D. Effect of one night of sleep loss on changes in tumor necrosis factor alpha (TNF-alpha) levels in healthy men. Cytokine. 2011;56:318–324. doi: 10.1016/j.cyto.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Ewout WF, Harrell E., Jr . Statistical models for prognostication. In: Max MBJ, editor. Lynn (Hrsg.): Symptom research: Methods and Opportunities. Bethesda: 2009. [Google Scholar]
- Faustman EM, Silbernagel SM, Fenske RA, Burbacher TM, Ponce RA. Mechanisms underlying children’s susceptibility to environmental toxicants. Environ Health Perspect. 2000;108(Suppl 1):13–21. doi: 10.1289/ehp.00108s113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Hwang SA, Langguth K, Cayo M, Yang BZ, Bush B, Worswick P, Lauzon T. Fish consumption and other environmental exposures and their associations with serum PCB concentrations among Mohawk women at Akwesasne. Environ Res. 2004;94:160–170. doi: 10.1016/s0013-9351(03)00133-6. [DOI] [PubMed] [Google Scholar]
- Freire C, Ramos R, Lopez-Espinosa MJ, Díez S, Vioque J, Ballester F, Fernández MF. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environmental research. 2010;110:96–104. doi: 10.1016/j.envres.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Gamaldo CE, Shaikh AK, McArthur JC. The sleep-immunity relationship. Neurol Clin. 2012;30:1313–1343. doi: 10.1016/j.ncl.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silbergeld EK. Differential immunotoxic effects of inorganic and organic mercury species in vitro. Toxicol Lett. 2010a;198:182–190. doi: 10.1016/j.toxlet.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Evans SL, Wang SB, Doyle KM, Crainiceanu CM, Silbergeld EK. Mercury induces an unopposed inflammatory response in human peripheral blood mononuclear cells in vitro. Environ Health Perspect. 2009;117:1932–1938. doi: 10.1289/ehp.0900855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RM, Nyland JF, Silva IA, Ventura AM, de Souza JM, Silbergeld EK. Mercury exposure, serum antinuclear/antinucleolar antibodies, and serum cytokine levels in mining populations in Amazonian Brazil: a cross-sectional study. Environ Res. 2010b;110:345–354. doi: 10.1016/j.envres.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber R, Cassoff J, Frenette S, Wiebe S, Carrier J. Impact of sleep extension and restriction on children’s emotional lability and impulsivity. Pediatrics. 2012;130:e1155–61. doi: 10.1542/peds.2012-0564. [DOI] [PubMed] [Google Scholar]
- Gump BB, MacKenzie JA, Bendinskas K, Morgan R, Dumas AK, Palmer CD, Parsons PJ. Low-level Pb and cardiovascular responses to acute stress in children: The role of cardiac autonomic regulation. Neurotoxicology and Teratology. 2011;33:212–219. doi: 10.1016/j.ntt.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gump BB, MacKenzie JA, Dumas AK, Palmer CD, Parsons PJ, Segu ZM, Mechref YS, Bendinskas KG. Fish consumption, low-level mercury, lipids, and inflammatory markers in children. Environ Res. 2012;112:204–211. doi: 10.1016/j.envres.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen G, Victor R, Engeldinger E, Schweitzer C. Evaluation of the mercury exposure of dental amalgam patients patients by the Mercury Triple Test. Occup Environ Med. 2004;61:535–540. doi: 10.1136/oem.2003.009555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AL, Xu F, Babineau D, Patel SR. Sleep duration and circulating adipokine levels. Sleep. 2011;34:147–152. doi: 10.1093/sleep/34.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw LB, Jackson SA, Chen MY. Direct mailing was a successful recruitment strategy for a lung-cancer screening trial. J Clin Epidemiol. 2007;60:853–857. doi: 10.1016/j.jclinepi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four factor index of social status. New Haven, CT: 1975. [Google Scholar]
- Kempuraj D, Asadi S, Zhang B, Manola A, Hogan J, Peterson E, Theoharides TC. Mercury induces inflammatory mediator release from human mast cells. J Neuroinflammation. 2010;7:20. doi: 10.1186/1742-2094-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention Science. 2000;1:173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, Dulin H, Berkman LF, Buxton OM. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36:1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKelvey W, Gwynn RC, Jeffery N, Kass D, Thorpe LE, Garg RK, Palmer CD, Parsons PJ. A biomonitoring study of lead, cadmium, and mercury in the blood of New York City adults. Environmental Health Perspectives. 2007;115:1435. doi: 10.1289/ehp.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen B, Hollund B, Riise T. Neurological symptoms among dental assistants: a cross-sectional study. J Occup Med Toxicol. 2008;3:10. doi: 10.1186/1745-6673-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molfese DL, Ivanenko A, Key AF, Roman A, Molfese VJ, O’Brien LM, Gozal D, Kota S, Hudac CM. A one-hour sleep restriction impacts brain processing in young children across tasks: evidence from event-related potentials. Dev Neuropsychol. 2013;38:317–336. doi: 10.1080/87565641.2013.799169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KE, Guthrie WF, Vetter TW, Turk GC, Palmer CD, Lewis ME, Geraghty CM, Parsons PJ. Comparison of clinical methods with isotope dilution inductively coupled plasma mass spectrometry for the new standard reference material 955c lead in caprine blood. Journal of Analytical Atomic Spectrometry. 2009;24:1170–1178. [Google Scholar]
- New York State Department of Health. [May 30 2014];Student Weight Status Category Reporting Survey Results: County-Level Report, 2008–2010. 2011 < http://www.health.ny.gov/prevention/obesity/statistics_and_impact/docs/2008-2010_student_weight_status_by_county_and_level.pdf>.
- Ogden Cynthia L, Carroll Margaret D, Kit, Flegal Brian K, Katherine M. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CD, Lewis ME, Jr, Geraghty C, Barbosa F, Jr, Parsons PJ. Determination of lead, cadmium and mercury in blood for assessment of environmental and occupational exposures: A comparison between inductively coupled plasma-mass spectrometry and atomic absorption spectrometry. Spectrochimica Acta B-Atomic Spectrometry. 2006;61:980–990. [Google Scholar]
- Preacher KJ, Leonardelli GJ. Calculation for the Sobel test: An interactive calculation tool for mediation tests [Computer software] 2001 [Google Scholar]
- Raison CL, Rye DB, Woolwine BJ, Vogt GJ, Bautista BM, Spivey JR, Miller AH. Chronic interferon-alpha administration disrupts sleep continuity and depth in patients with hepatitis C: association with fatigue, motor slowing, and increased evening cortisol. Biol Psychiatry. 2010;68:942–949. doi: 10.1016/j.biopsych.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran M. Interactions between mercury and dissolved organic matter - a review. Chemosphere. 2004;55:319–331. doi: 10.1016/j.chemosphere.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Ritter PS, Kretschmer K, Pfennig A, Soltmann B. Disturbed sleep in bipolar disorder is related to an elevation of IL-6 in peripheral monocytes. Med Hypotheses. 2013 doi: 10.1016/j.mehy.2013.09.025. [DOI] [PubMed] [Google Scholar]
- Stewart PW, Lonky E, Reihman J, Pagano J, Gump BB, Darvill T. The relationship between prenatal PCB exposure and intelligence (IQ) in 9-year-old children. Environ Health Perspect. 2008;116:1416–1422. doi: 10.1289/ehp.11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Environmental Protection Agency. Methylmercury (MeHg) (CASRN 22967-92-6) Integrated Risk Information System; 2011. [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89:2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]