Abstract
Importance
Previous studies have reported that histopathologically amelanotic melanoma is associated with poorer survival than pigmented melanoma; however, small numbers of amelanotic melanomas, selected populations, lack of centralized pathology review, or no adjustment for stage limit interpretation or generalization of results from prior studies.
Objective
To compare melanoma-specific survival between patients with histopathologically amelanotic and those with pigmented melanoma in a large international population-based study.
Design
Survival analysis with median follow-up of 7.6 years.
Setting
The Genes, Environment, and Melanoma study enrolled incident cases of melanoma diagnosed in 1998-2003 from international population-based cancer registries.
Participants
A total of 2,995 patients with 3,486 invasive primary melanomas centrally scored for histologic pigmentation.
Main Outcomes and Measurements
Clinicopathologic predictors and melanoma-specific survival of histologically amelanotic and pigmented melanoma were compared using generalized estimating equations and Cox regression models, respectively.
Results
Eight percent of melanomas (275 of 3,467) were histopathologically amelanotic. Female sex, nodular and unclassified or other histologic subtypes, increased Breslow thickness, presence of mitoses, severe solar elastosis, and lack of a co-existing nevus were independently associated with amelanotic melanoma (each P < .05). Amelanotic melanoma was generally of a higher American Joint Committee on Cancer (AJCC) tumor stage at diagnosis (P for trend <.001) than pigmented melanoma. Hazard of death from melanoma was higher for amelanotic than pigmented melanoma [hazard ratio (HR), 2.0; 95% confidence interval (CI), 1.4-3.0; P< .001], adjusted for age, sex anatomic site, and study design variables; but survival did not differ once AJCC tumor stage was also taken into account, (HR, 0.8; 95% CI, 0.5-1.2; P = .36).
Conclusions and Relevance
At the population level, survival after diagnosis of amelanotic melanoma is poorer than after pigmented melanoma because of its more advanced stage at diagnosis. It is probable that amelanotic melanomas present at more advanced tumor stages because they are difficult to diagnose. The association of amelanotic melanoma with presence of mitoses independently of Breslow thickness and other clinicopathologic characteristics suggests that amelanotic melanomas might also grow faster than pigmented melanomas. New strategies for early diagnosis and investigation of the biology of amelanotic melanoma are warranted.
Keywords: amelanotic melanoma, epidemiology, pathology, skin cancer, pigmentation, neoplasm staging
Introduction
The American Cancer Society has estimated that 76,100 new invasive melanomas will be diagnosed and 9,710 people will die from melanoma in 2014 in the United States.1 Despite newly available targeted and immunomodulatory agents,2-5 systemic therapies rarely lead to cures. Thus, early detection of primary melanomas followed by surgical excision remains critical for good outcomes. While recognition of all melanomas can be difficult, visual lack of brown or black color in amelanotic melanoma at the time of clinical diagnosis removes a defining characteristic for melanoma identification. Several studies indicate that amelanotic melanoma is associated with adverse survival because the tumors are more advanced at diagnosis; however, the majority of studies included only small numbers of amelanotic melanoma cases entered at single study sites and used a clinical, not pathologic, definition of amelanotic melanoma or lacked centralized pathology review.
Amelanotic melanoma has been defined differently between studies, either clinically as melanomas devoid of pigment on visual inspection before biopsy6-8 or lacking melanin pigment in melanoma cells on routine hematoxylin & eosin (H&E) stained sections.9-11 Approximately 2-20% of melanomas have been classified as amelanotic.6-11 Amelanotic melanomas can be found among all histologic subtypes, including superficial spreading (SSM), nodular (NM), lentigo maligna (LMM), and acral lentiginous (ALM) melanoma.6,12
Of all investigations using either a clinical or histopathologic definition, we are aware of only three studies that included more than 100 amelanotic cases.11,13,14 Moreau et al.14 using Surveillance, Epidemiology, and End Results (SEER) registry data, found that amelanotic melanoma was more advanced at diagnosis and more lethal than pigmented melanoma. However, misclassification of histolopathogically amelanotic and pigmented melanomas could occur in SEER data. SEER extracts the term ‘amelanotic’ from pathology reports and codes it under an ICD-O-3 morphology code, but the SEER registry allows documentation of only one morphology code.14,15 Thus, melanomas coded under a different morphology code (such as SSM, NM, LMM, or ALM) would not be coded as amelanotic. In addition, pathologists may not report ‘amelanotic’ on pathology reports, leading to missing data.
The two other studies with more than 100 amelanotic cases conducted centralized pathology review of the melanomas.11,13 In a Norwegian population-based study, Larsen et al.13 found that overall survival of patients was significantly worse for amelanotic than pigmented melanoma but these results were not adjusted for other melanoma characteristics.In a Danish hospital-based study, Sondergaard et al.11 found that amelanotic melanoma patients did not have poorer melanoma-specific survival once other clinicopathological characteristics including Breslow thickness were taken into account; however, generalization of the findings from a single hospital study would be limited.
We examined histopathologically diagnosed amelanotic and pigmented melanomas in the international population-based Genes, Environment and Melanoma (GEM) study of patients with single primary melanomas (SPMs) and multiple primary melanomas (MPMs).16-19 Expert pathologists reviewed and scored the histologic features, including pigmentation, of 3,486 invasive primary melanomas diagnosed in 1998-2003 from 2,995 GEM study patients from Australia, the United States, Italy and Canada. We used a precise definition of histologic pigmentation; melanomas were recorded as histopathologically amelanotic if on light microscopic examination of H&E-stained sections no melanin granules were seen in the cytoplasm of the tumor cells. Our goals were to more fully characterize the relationship of histopathologic pigmentation to clinicopathologic features and current American Joint Committee on Cancer (AJCC) tumor stage20 and to compare melanoma-specific and overall survival between patients with histopathologically amelanotic and pigmented melanoma.
Methods
Population
The GEM study population included incident primary cutaneous melanomas notified to population-based cancer registries in Australia, Canada, Italy and the United States.16,17,21-23 Patients with SPM were diagnosed in 2000 and those with MPM were diagnosed with a second or higher order invasive or in situ melanoma in 1998–2003. We included incident melanomas (SPMs and index MPMs), and, for patients with MPM, also ascertained the previous (usually the first) melanoma (previous MPM) in local cancer registry records.In situ melanomas were eligible as index MPMs when the patient had a previous invasive melanoma. The institutional review board at the coordinating center (Memorial Sloan-Kettering Cancer Center) and each participating institution approved the study protocol. Physician approval was sought before contacting eligible participants. All study participants provided written informed consent, including for obtaining diagnostic slides of their melanoma(s) for centralized review.
In GEM, there were 3,578 participants with a total of 4,784 primary cutaneous melanomas. This analysis excluded in situ melanomas (n = 302) because the aims were to determine the association of histopathologic pigmentation with clinical and pathologic features of and survival from invasive melanomas. The analyses reported here included only primary invasive melanomas for which the diagnostic slides were available for review and centrally scored for histopathologic pigmentation, a total of 3,486 (78% of 4,482) primary invasive melanomas from 2,955 (82% of 3,578) GEM participants. They comprised 2,007 index SPMs (85% of 2,372), 716 index MPMs (79% of 904) and 763 previous MPMs (63% of 1,206). The 716 index MPMs and 763 previous MPMs occurred in 948 MPM patients (79% of 1,206), among whom 185 had pathology reviewed for only the index MPM, 232 for only the previous MPM and 531 for both.
Centralized Pathology Review
Patient age, sex, and melanoma body site were extracted from pathology reports and confirmed during patient interview; histologic subtype and Breslow thickness were also extracted from pathology reports. Centralized review of the melanoma H&E-stained slides recorded histologic subtype, Breslow thickness, pigmentation, mitoses, ulceration, tumor infiltrating lymphocytes, adjacent solar elastosis, and co-existing nevus. Melanomas were classified according to previously reported criteria.24,25 Mitoses were defined as present or absent.26
Melanomas were recorded as histopathologically amelanotic if on light microscopic examination of H&E-stained sections no melanin granules were seen in the cytoplasm of the tumor cells. In a test set of 19 sections scored for melanin pigmentation by the three dermatopathologists who reviewed the GEM melanomas, the kappa statistic for agreement between the pathologists was 0.48, which indicates moderate agreement.
From one study center (North Carolina), we extracted pre-biopsy impression of lesional (‘clinical’) pigmentation from the pathology reports. ‘Clinical’ pigmentation was recorded on the pathology reports for only 23% (64 of 274) of the melanomas. Melanomas described as ‘tan, brown, blue, grey, black, or hyperpigmented’ were grouped as ‘clinically pigmented’, while melanomas noted as ‘pink, red, white or amelanotic’ were grouped as ‘clinically amelanotic’. Ninety-five percent (57 of 60) of ‘clinically pigmented’ melanomas were also histopathologically pigmented (as determined by centralized pathology review); while 80% (4 of 5) of ‘clinically amelanotic’ melanomas were histopathologically amelanotic (P < .0001; Fisher exact test). As ‘clinical’ pigmentation was often missing on the pathology reports but was associated with histopathologic pigment, we chose histopathologic pigmentation from centralized pathology review for all analyses. The North Carolina cancer registry ascertained these population-based cases, which were originally diagnosed by multiple providers across North Carolina. We did not have access to provider chart notes to abstract pre-biopsy melanoma color descriptions.
All data items were available for the T classification describing the state of the primary tumor in the AJCC TNM (tumor, regional nodes, distant metastasis) melanoma staging system; data on regional nodes and distant metastases were not available.
Melanoma treatment information was not available; however, the follow-up period at all study centers ended before US FDA, Health Canada, European Union and Australian Therapeutic Goods Administration approvals of CTLA-4, BRAF, and MEK inhibitors for treatment of metastatic melanoma.
Information about deaths from melanoma or other causes was obtained for all participants from National Death Indexes, cancer registries, and municipal records.Patient follow-up for vital status finished at the end of 2007 in most centers and at the end of 2008 in British Columbia and Italy. See eMethods for additional follow-up information.
Statistical Analysis
The association of clinical and pathologic characteristics with amelanotic and pigmented melanoma was examined including all single and multiple (both index and previous) primary melanomas. We used marginal logistic regressions with an independent correlation structure implemented in generalized estimating equations (GEE) to account for the clustering of melanomas for MPM patients. All models included study center and lesion status (SPM, index MPM, previous MPM), the design variables. To identify factors that independently distinguished amelanotic from pigmented melanoma, a multivariable model was developed that included all clinicopathologic features and study design variables [study center and lesion status (SPM, index MPM, or previous MPM)]. Statistical significance was evaluated based on Wald tests. We also report results from similar models examining the association of amelanotic versus pigmented melanoma with AJCC tumor stage. Linear trend was tested using the Wald statistic when AJCC tumor stage was treated as a single ordinal variable.
Previous analyses of GEM data required the inclusion of an age by sex interaction term because of the higher population incidence of melanoma in younger women than men but lower incidence in women than men at older ages.17 In this report, we tested for the presence of such an interaction by adding an age by sex interaction term to the model. We also examined the relation of pigmentation to Breslow thickness and age categories stratified by sex using Pearson chi-squared tests.
Survival by amelanotic and pigmented melanoma was examined in all patients, including all SPM and MPM patients. For MPM patients with review data for both the index and a previous MPM, we used the pathology characteristics of the tumor with the greatest Breslow thickness in the analysis. When thickness was the same for both melanomas, we used the characteristics of the index MPM; and, if one MPM had thickness missing (n = 30), the pathology characteristics of the other were used. Since the parent study involved population-based ascertainment of incident SPM and MPM, survival time was accumulated from the diagnosis date of the index lesion, whether SPM or MPM. The endpoint was date of death due to melanoma or the end of complete follow-up (censored patients). For melanoma-specific survival, patients were censored at the time of death from any cause other than melanoma.
Survival curves by lesion pigmentation were constructed using the Kaplan-Meier method and compared using a log-rank test. Hazard ratios (HR) and 95% confidence intervals (95% CI) for melanoma-specific survival by pigmentation were estimated in Cox regression models. The time-scale used in the Cox regression models was follow-up time, adjusting for baseline age as a covariate.For 96 patients enrolled as SPM patients who experienced a subsequent melanoma during the period of participant recruitment, a time-dependent covariate for MPM status was included in the model at the date of diagnosis of a second melanoma. An initial Cox model was adjusted for age, sex, site, and study center and whether SPM or MPM and another model also included AJCC tumor stage. A separate fully adjusted Cox model for overall survival by amelanotic compared to pigmented melanoma is also presented.
Tests based on Schoenfeld residuals and graphical methods using Kaplan-Meier curves in STATA/IC 12.1 (StataCorp LP, College Station, TX) showed no evidence that proportional hazards assumptions were violated for pigmentation. All significance tests were two-sided. SAS (SAS Institute, Cary, NC) version 9.3 was used for all analyses except for Kaplan-Meier curves, which were implemented in in STATA/IC 12.1 (StataCorp LP, College Station, TX).
Results
Clinicopathologic Characteristics
Overall 275 (8%) of the melanomas were amelanotic (Table 1). We examined associations of the clinicopathologic characteristics with amelanotic melanoma for 3,207 SPMs and MPMs with complete data for all variables of interest (Table 2). Notably, the median thickness of amelanotic melanomas (1.60 mm) was much greater than pigmented melanomas (0.68 mm). We observed some differences between the sexes depending on age and Breslow thickness. More of the melanomas in men age >70 years were amelanotic (10%) compared to men 50-69 years (7%) or men <50 years (4%) (P = .007), while in women 8-10% of melanomas were amelanotic in each age group (P = .55) (eTable 1). Amelanotic melanoma was associated (P < .001) with Breslow thickness >2.00 mm in each sex, but a higher percentage of men had thicker amelanotic melanomas (48% >2mm) than women (35% >2mm) (P=.05).
Table 1. Characteristics of 3,486 Primary Invasive Cutaneous Melanomas Centrally Reviewed for Histopathologic Pigmentation from 2,995 Patientsa.
| No. (%) of Melanomas | ||||
|---|---|---|---|---|
| All SPM and MPMs | SPMs | Index MPMs | Previous MPMs | |
| Characteristic | (n=3,486) | (n=2,007) | (n = 716) | (n =763) |
| Sex | ||||
| Male | 2,029 (58) | 1,020 (52) | 496 (69) | 513 (67) |
| Female | 1,457 (42) | 987 (48) | 220 (31) | 250 (33) |
| Age at diagnosis, y | ||||
| Median (IQR) | 60 (47-71) | 54 (44-68) | 67 (56-75) | 64 (53-72) |
| Histopathologically amelanoticb | ||||
| No | 3,211 (92) | 1,823 (91) | 670 (94) | 718 (94) |
| Yes | 275 (8) | 184 (9) | 46 (6) | 45 (6) |
Abbreviations: IQR, interquartile range; SD, standard deviation; SPM, single primary melanoma; MPM, multiple primary melanoma.
Patients were included who had histologic pigment scored in their melanoma.
Melanomas were recorded as amelanotic if on light microscopic examination of H&E-stained sections no melanin granules were seen in the cytoplasm of the tumor cells on centralized slide review by expert dermatopathologists.
Table 2. Relationship Between Histopathological Pigmentation and Clinicopathologic Features for 3,207 Primary Invasive Melanomas From 2,761 Patientsa.
| No. (%) of Melanomas | Amelanotic vs. Pigmented Melanoma | |||||
|---|---|---|---|---|---|---|
| Pigmented | Amelanotic | Adjusted for Design Variables Onlyb | Fully Adjustedc | |||
| Characteristic | (n=2,962) | (n=245) | OR (95% CI) | P Valued | OR (95% CI) | P Valued |
| Sex | ||||||
| Male | 1728 (58) | 134 (55) | 1 [Reference] | .53 | 1 [Reference] | .04 |
| Female | 1234 (42) | 111 (45) | 1.1 (0.8-1.4) | 1.4 (1.0-2.0) | ||
| Age at diagnosis, yr | ||||||
| Median (IQR) | 60 (47-71) | 62 (51-76) | ||||
| <50 | 851 (29) | 57 (23) | 1 [Reference] | <.001 | 1 [Reference] | |
| 50-69 | 1272 (43) | 99 (40) | 1.4 (1.0-2.0) | 1.1 (0.7-1.6) | .75 | |
| >70 | 839 (28) | 89 (36) | 2.0 (1.4-2.9) | 1.2 (0.8-1.8) | ||
| Anatomic site | ||||||
| Trunk | 1353 (46) | 80 (33) | 1 [Reference] | <.001 | 1 [Reference] | |
| Head, neck | 480 (16) | 63 (26) | 2.2 (1.5-3.2) | 1.1 (0.7-1.7) | .67 | |
| Upper extremities | 523 (18) | 51 (21) | 1.6 (1.2-2.4) | 1.0 (0.7-1.6) | ||
| Lower extremities | 606 (20) | 51 (21) | 1.6 (1.1-2.4) | 1.3 (0.8-2.0) | ||
| Melanoma subtype | ||||||
| Superficial spreading | 2149 (73) | 92 (38) | 1 [Reference] | <.001 | 1 [Reference] | |
| Nodular | 222 (7) | 62 (25) | 8.4 (5.8-12.2) | 2.7 (1.7-4.2) | <.001 | |
| Lentigo maligna | 421 (14) | 27 (11) | 1.6 (1.0-2.5) | 1.1 (0.7-1.9) | ||
| Unclassified/othere | 170 (6) | 64 (26) | 8.1 (5.6-11.8) | 3.5 (2.3-5.4) | ||
| Breslow thickness, mm | ||||||
| Median (IQR) | 0.68 (0.45-1.20) | 1.60 (0.80-3.50) | ||||
| 0.01-1.00 | 2,078 (70) | 81 (33) | 1 [Reference] | <.001 | 1 [Reference] | |
| 1.01-2.00 | 548 (19) | 61 (25) | 3.1 (2.1-4.5) | 1.6 (1.1-2.6) | <.001 | |
| 2.01-4.00 | 256 (9) | 50 (20) | 6.4 (4.3-9.6) | 2.4 (1.4-4.0) | ||
| >4.00 | 80 (3) | 53 (22) | 21.9 (13.9-34.4) | 6.9 (3.8-12.3) | ||
| Mitoses | ||||||
| Absent | 1,860 (63) | 71 (29) | 1 [Reference] | <.001 | 1 [Reference] | .003 |
| Present | 1,102 (37) | 174 (71) | 4.5 (3.3-6.1) | 1.9 (1.3-2.9) | ||
| Ulceration | ||||||
| Absent | 2,762 (93) | 191 (78) | 1 [Reference] | <.001 | 1 [Reference] | .70 |
| Present | 200 (7) | 54 (22) | 4.3 (3.1-6.2) | 1.1 (0.7-1.7) | ||
| TIL grade | ||||||
| Absent | 589 (20) | 67 (27) | 1 [Reference] | .003 | 1 [Reference] | |
| Nonbrisk | 1,890 (64) | 161 (66) | 0.7 (0.5-1.0) | 1.0 (0.7-1.4) | .95 | |
| Brisk | 483 (16) | 17 (7) | 0.4 (0.2-0.7) | 0.9 (0.5-1.7) | ||
| Solar elastosis | ||||||
| Absent | 913 (31) | 49 (20) | 1 [Reference] | <.001 | 1 [Reference] | |
| Mild to moderate | 1534 (52) | 117 (48) | 1.2 (0.9-1.8) | 1.4 (0.9-2.1) | .04 | |
| Severe | 515 (17) | 79 (32) | 2.4 (1.7-3.5) | 2.0 (1.1-3.3) | ||
| Co-existing nevus | ||||||
| Absent | 2072 (70) | 216 (88) | 1 [Reference] | <.001 | 1 [Reference] | .03 |
| Present | 890 (30) | 29 (12) | 0.4 (0.3-0.6) | 0.6 (0.4-1.0) | ||
Abbreviations: OR, odds ratio; CI, confidence interval; TIL, tumor-infiltrating lymphocytes.
Invasive single melanomas and multiple melanomas (index and previous) were included. Melanomas with one or more data points missing for Breslow thickness (n = 10), ulceration (n = 159), mitoses (n = 147), tumor-infiltrating lymphocytes (n = 165), solar elastosis (n = 98), and co-existing nevus (n = 11) were excluded. We used marginal logistic regression with an independent correlation structure implemented in generalized estimating equations to account for the clustering of melanomas for patients with multiple primary melanoma (MPM).
Adjusted for study center and lesion status (single primary melanoma, index MPM, or previous MPM).
Included all variables in the table and adjusted for study center and lesion status (SPM, index MPM, or previous MPM).
P values were calculated in in the generalized estimating equation models.
Other includes acral lentiginous, spindle cell, nevoid, and Spitzoid melanomas.
Age and each of the clinicopathologic characteristics, but not sex, were associated (P < .05) with amelanotic melanoma when adjusted only for the design variables – study center and lesion status (SPM, index MPM, or previous MPM) (Table 2). When all variables were included in one fully adjusted model, the variables independently associated (P< .05) with amelanotic melanoma were female sex, nodular and unclassified or other histologic subtypes, increased Breslow thickness, presence of mitoses, severe solar elastosis, and lack of a co-existing nevus.
The odds ratio (OR) for amelanotic melanoma in women relative to men was significantly increased in the fully adjusted model (OR, 1.4; 95% CI, 1.0-2.0) but not in the model adjusted only for study design variables (OR, 1.1; 95% CI, 0.8-1.4), an effect accounted for by the inclusion of thickness in the fully adjusted model. Ulceration and tumor-infiltrating lymphocytes each became non-significant in the fully adjusted model due to addition of thickness to the model. The age by sex interaction term was not significant when added to the fully adjusted model (P for interaction=.22) and no OR in the model changed by >10%; thus, we did not include the interaction term in the models.
We examined in more detail the association of amelanotic melanoma with individual AJCC tumor stage adjusted for factors known to be associated with survival (sex, age, and anatomic site) along with the design variables (Table 3). Melanomas with higher AJCC tumor stage were more likely to be amelanotic, with Ors between 2.9 and 11.1 for tumor stages between T1b and T3b and ORs of 24.6 for T4a and 29.1 for T4b relative to T1a (P for trend <.001).
Table 3. Relationship Between Histopathologic Pigmentation and AJCC Tumor Stage for 3,325 Primary Invasive Melanomas From 2,845 Patientsa.
| No. (%) of Melanomas | ||||
|---|---|---|---|---|
| Pigmented | Amelanotic | Amelanotic vs. Pigmented Melanoma | ||
| AJCC Tumor Stageb | (n=3,058) | (n=267) | Adjusted OR (95% CI)c | Ptrendd |
| T1a | 1,720 (97) | 54 (3) | 1 [Reference] | |
| T1b | 412 (92) | 36 (8) | 2.9 (1.8-4.6) | |
| T2a | 506 (91) | 53 (9) | 3.5 (2.3-5.4) | |
| T2b | 62 (79) | 16 (21) | 11.1 (5.8-21.2) | <.001 |
| T3a | 168 (82) | 38 (18) | 9.0 (5.6-14.5) | |
| T3b | 103 (89) | 13 (11) | 4.6 (2.3-9.2) | |
| T4a | 51 (64) | 29 (36) | 24.6 (13.6-44.4) | |
| T4b | 36 (56) | 28 (44) | 29.1 (15.5-54.9) | |
Abbreviations: AJCC, American Joint Committee on Cancer; OR, odds ratio; CI, confidence interval.
Invasive single melanomas and multiple melanomas (index and previous) with missing data for stage (n = 161) were excluded. We used marginal logistic regression with an independent correlation structure implemented in generalized estimating equations to account for the clustering of melanomas for multiple primary melanoma (MPM) patients.
T1a, Breslow thickness ≤1.0 mm and absence of ulceration or mitoses; T1b, Breslow thickness ≤ 1.0 mm and presence of ulceration or mitoses; T2a, Breslow thickness 1.01-2.0 mm without ulceration; T2b, Breslow thickness 1.01-2.0 mm with ulceration; T3a, Breslow thickness 2.01-4.0 mm without ulceration; T3b, Breslow thickness 2.01-4.0 mm with ulceration, T4a, Breslow thickness > 4.0 mm without ulceration; T4b, Breslow thickness > 4.0 mm with ulceration.
Adjusted for age (<50, 50-69, >70), sex, anatomic site (trunk, head/neck, upper extremities, lower extremities), study center, and lesion status (single primary melanoma, index MPM, or previous MPM).
Ptrend was calculated in the generalized estimating equation model including stage as an ordinal variable.
Melanoma-Specific Survival
There were 208 melanoma deaths in 2,736 GEM patients with complete AJCC tumor stage information; the median follow-up time was 7.6 years. Kaplan-Meier survival curves show 5-year melanoma-specific survival of 88% (95% CI, 84-92%) in amelanotic and 95% (95% CI, 94-96%) in pigmented melanoma (P<.001, log-rank test) (Figure 1). The HR for melanoma death was 2.0 (95% CI, 1.4-3.0; P < .001) for amelanotic relative to pigmented melanoma in a Cox regression model adjusted for age, sex, anatomic site, study center and whether SPM or MPM (Table 4). However, addition of AJCC tumor stage to the above model removed the association of amelanotic relative to pigmented melanoma with melanoma-specific survival (HR, 0.8; 95% CI, 0.5-1.2; P=.36).
Fig 1. Kaplan-Meier Survival Curves.
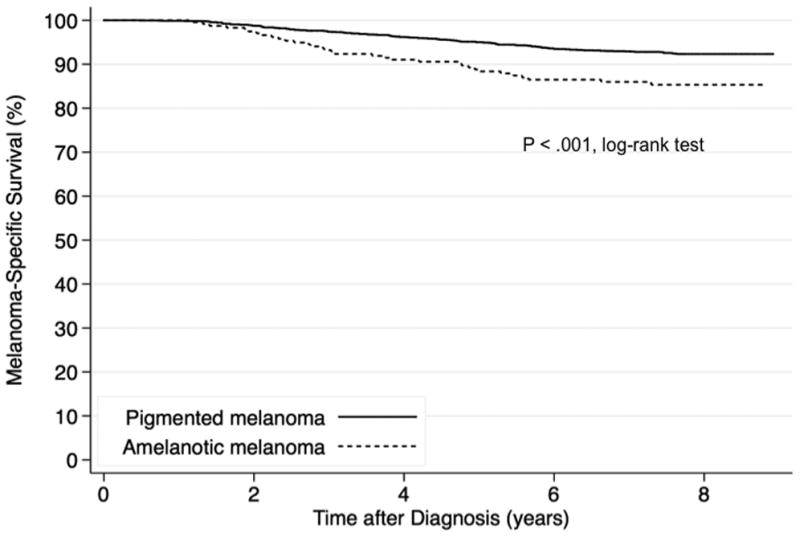
Kaplan-Meier melanoma-specific survival probabilities by histopathologically amelanotic and pigmented melanoma are shown for patients with melanoma (n = 2,736) with median follow-up of 7.6 years.
Table 4. Hazard Ratios for Melanoma-Specific Death According to Histopathologically Amelanotic and Pigmented Melanoma Among Patients with Primary Melanomas (n=2,736)a.
| No. (%) of Patients | ||||||
|---|---|---|---|---|---|---|
| Censored | Death as a Result of Melanoma | Partially Adjustedb | Fully Adjustedc | |||
| Characteristic | (n=2,528) | (n=208) | HR (95% CI) | P Value | HR (95% CI) | P Value |
| Histopathologically amelanotic | ||||||
| No | 2,322 (93) | 175 (7) | 1 [Reference] | <.001 | 1 [Reference] | .36 |
| Yes | 206 (86) | 33 (14) | 2.0 (1.4-3.0) | 0.8 (0.5-1.2) | ||
| Age, y | ||||||
| Increase in 10-y increments | – | – | 1.2 (1.0-1.3) | <.001 | 1.2 (1.0-1.3) | .005 |
| Sex | ||||||
| Male | 1389 (90) | 150 (10) | 1 [Reference] | .004 | 1 [Reference] | .26 |
| Female | 1139 (95) | 58 (5) | 0.6 (0.4-0.9) | 0.8 (0.6-1.2) | ||
| Anatomic site | ||||||
| Trunk | 1,133 (93) | 89 (7) | 1 [Reference] | .003 | 1 [Reference] | .04 |
| Head, neck | 380 (86) | 61 (14) | 1.7 (1.2-2.3) | 1.4 (1.0-2.0) | ||
| Upper extremities | 471 (94) | 29 (6) | 0.9 (0.6-1.3) | 0.7 (0.5-1.1) | ||
| Lower extremities | 544 (95) | 29 (5) | 0.8 (0.5-1.3) | 0.7 (0.5-1.1) | ||
| AJCC tumor staged | ||||||
| T1a | 1,290 (98) | 21 (2) | – | 1 [Reference] | <.001 | |
| T1b | 373 (97) | 13 (3) | – | 2.2 (1.1-4.3) | ||
| T2a | 466 (90) | 52 (10) | – | 6.3 (3.8-10.5) | ||
| T2b | 57 (80) | 14 (20) | – | 14.1 (7.1-28.1) | ||
| T3a | 165 (82) | 37 (18) | – | 11.1 (6.4-19.0) | ||
| T3b | 78 (73) | 29 (27) | – | 18.5 (10.4-32.8) | ||
| T4a | 58 (75) | 19 (25) | – | 16.0 (8.5-30.3) | ||
| T4b | 41 (64) | 23 (36) | – | 26.9 (14.4-50.1) | ||
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; CI, confidence interval.
Of the 2,995 participants in this study, multiple primary melanomas patients (MPMs) who were missing histolopathologic pigmentation (n = 141) or AJCC tumor stage (n = 32) for their selected (usually thicker) melanoma and single primary melanoma patients (SPMs) missing AJCC tumor stage (n = 86) were excluded. Patients who entered the study as SPMs who developed a subsequent melanoma were treated as time-dependent.
This Cox model was adjusted for age, sex, anatomic site, study center and whether SPM or MPM.
This Cox model also included study center and whether SPM or MPM.
T1a, Breslow thickness ≤1.0 mm and absence of ulceration or mitoses; T1b, Breslow thickness ≤ 1.0 mm and presence of ulceration or mitoses; T2a, Breslow thickness 1.01-2.0 mm without ulceration; T2b, Breslow thickness 1.01-2.0 mm with ulceration; T3a, Breslow thickness 2.01-4.0 mm without ulceration; T3b, Breslow thickness 2.01-4.0 mm with ulceration, T4a, Breslow thickness > 4.0 mm without ulceration; T4b, Breslow thickness > 4.0 mm with ulceration.
In a reanalysis of SPMs alone, death from melanoma remained higher for amelanotic than pigmented melanoma (HR, 2.4; 95% CI, 1.5-3.8); P < .001) adjusted for age, sex anatomic site, and study design variables; but survival did not differ once AJCC tumor stage was also taken into account (HR 0.8; 95% CI, 0.5-1.2; P = .26) (not shown in tables).
Overall Survival
Hazard of death from all causes was higher for histopathologically amelanotic than pigmented melanoma (HR, 1.6; 95% CI, 1.2-2.1), adjusted for age, sex anatomic site, and study design variables (P = .001; not shown in tables); however, survival did not differ once AJCC tumor stage was also taken into account, (HR, 0.9, 95% CI, 0.7-1.2; P = .44) (eTable 2).
Discussion
Female sex, nodular and unclassified or other subtypes, increased Breslow thickness, presence of mitoses, severe solar elastosis, and lack of a co-existing nevus were independently associated with histopathologically amelanotic melanoma in the international population-based GEM study. Amelanotic melanomas were more frequent among melanomas with higher AJCC stage at diagnosis. Melanoma-specific fatality was higher for amelanotic compared to pigmented melanoma but not once AJCC tumor stage was taken into account.
The 8% frequency of histopathologic amelanotic melanoma in our study is within the range of the 2-20% of melanomas previously reported as histopathologically amelanotic.7,9-11,13,14,14 Other studies have found amelanotic melanoma to be associated with older age6,14 head/neck14 site and sun-damaged skin,27 as we did. However, solar elastosis, which we have shown is associated with cumulative site-specific ambient erythemal UV exposure,28 was a stronger predictor of amelanotic melanoma in GEM than age or site. Like us, others have reported that NMs6,13,29 were more likely to be amelanotic. We contribute to the literature that unclassified and histological types other than NM, SSM or LMM were also independently associated with amelanotic melanoma. Further, we found lack of a co-existing nevus to be independently associated with amelanotic melanoma also, apparently, for the first time.
Other studies have variably reported predilection of amelanotic melanoma for females,6,7 males14,30 or neither sex.31 In unadjusted analyses, we observed that younger (< 50 years) females were more likely than males of a similar age to have amelanotic than pigmented melanoma but there was little difference at older (>50 years) ages. Men generally had thicker melanomas. Being female increased the likelihood that a melanoma was an amelanotic melanoma but only after taking into account Breslow thickness and age. We speculate that women on the whole might self-refer for suspicious lesions that prove to be amelanotic melanomas more often than men do.
As we found, amelanotic melanoma has been reported as positively associated with increased Breslow thickness10,14,29 and presence of mitoses in other studies.13 Notably, Liu et al. reported rapid rate of melanoma growth, which they found correlated moderately to strongly with mitotic rate, to be associated with histopathologically amelanotic melanoma.32 We report that the association of mitoses with amelanotic melanoma is independent of Breslow thickness and other clinicopathologic characteristics. Because mitoses are generally considered as a marker for tumor growth,33 our finding suggests that amelanotic melanomas grow faster than pigmented melanomas.
Previous studies variably found ulceration to be more frequent in14,34 or not associated with6 amelanotic melanoma. In GEM, ulceration and higher tumor-infiltrating lymphocyte (TIL) grade were each positively associated with amelanotic melanoma in GEM patients but not after accounting for thickness. No study that we are aware of has examined TIL grade in relationship to histopathologic pigmentation of melanoma; however, amelanotic melanomas have been reported to frequently retain pigment cell differentiation antigens.30,35,36 We speculate that this may render histopathologically amelanotic and pigmented melanomas similarly antigenic and lead to comparable lymphocytic infiltrates.
Like GEM, one other large study that included greater than 100 amelanotic melanomas and conducted centralized pathology review found no difference in melanoma-specific survival for histopathologically amelanotic versus pigmented melanoma after accounting for their generally more advanced tumor stage.11 It is probable that amelanotic melanomas tend to present at a more advanced tumor stage because they are more difficult to diagnose. A high rate of clinical misdiagnosis for amelanotic melanoma has been reported.6,27,37 Lack of melanin granules in histopathologically amelanotic melanoma could affect use of the color criterion in the ABCDE algorithm38 and impact early recognition. One group attempted to measure diagnostic delay using a clinical, not pathologic, definition of amelanotic melanoma. Betti et al.39 reported a greater delay in amelanotic melanoma diagnosis due principally to physician diagnostic delay but the same group29 subsequently reported no significant diagnostic delay. Additional studies could clarify the issues surrounding delays in diagnosis of amelanotic melanoma.
We are not aware of another international study comparing survival from amelanotic and pigmented melanoma, nor has another population-based study examined survival by pigmentation of centrally reviewed melanomas while taking into account tumor stage. Other advantages of our study include its large size, collection of information about cause of death, objective histopathologic definition of tumor pigmentation, use of current AJCC tumor staging, and long observational period ending before recent US Food and Drug Administration approvals of new agents,2-5 which could differentially alter the natural course of disease. As BRAF-mutant melanoma has been reported to be associated with histologic pigmentation,40 future survival studies of amelanotic melanoma in relationship to survival could be confounded by treatments targeting the BRAF-MEK pathway.2,4,5
A study weakness is that histopathologically amelanotic and pigmented melanoma may be misclassified for some cases; the interobserver agreement for scoring of histopathologic pigmentation was moderate (kappa = 0.48). Others have also noted difficulty grading pigmentation,13 although we are not aware of any prior interobserver studies for histopathologic pigmentation. Another limitation is that we were unable to access pre-biopsy color descriptions of the melanomas from provider charts.
In conclusion, the poorer melanoma-specific and overall survival for histopathologically amelanotic compared to pigmented melanoma in GEM was entirely due to higher tumor stage at diagnosis of amelanotic melanomas. It is very likely that amelanotic tumors presented at a higher AJCC tumor stage due to delayed diagnosis. The positive association of mitoses with amelanotic melanoma independent of tumor thickness and other clinicopathologic characteristics suggests that amelanotic melanomas may grow more rapidly than pigmented melanomas. If this is the case, amelanotic melanomas may not only be more difficult to diagnose but the window of opportunity for early diagnosis might be smaller. Although amelanotic melanoma was associated with severe solar elastosis, it occurred on all body sites, supporting complete skin examinations for its detection. Studying a subset of cases, we found that ‘clinical’ pigmentation was associated with ‘histopathologic’ pigmentation. Future research is needed to identify the best methods for diagnosis of amelanotic melanoma. Studying the biology of amelanotic melanoma could shed light on the possibility that it has a faster growth rate than pigmented melanoma.
Supplementary Material
Acknowledgments
Funding/Support: National Cancer Institute (NCI) grants R01CA112243, R01CA112524, R01CA112243-05S1, R01CA112524-05S2, CA098438, U01CA83180, R33CA160138, and P30 CA014089; National Institute of Environmental Health Sciences (P30ES010126); University of Sydney Medical Foundation Program grant (Bruce Armstrong); Michael Smith Foundation for Health Research Infrastructure Award (Richard Gallagher).
Abbreviations
- AJCC
American Joint Committee on Cancer
- CI
confidence interval
- GEE
generalized estimating equation
- GEM
Genes, Environment, and Melanoma Study
- HR
hazard ratio
- MPM
multiple primary melanoma
- OR
odds ratio
- SD
standard deviation
- SPM
single primary melanoma
Footnotes
Author Contributions: Dr. Nancy E. Thomas had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thomas, Kricker, Busam, From, Armstrong, Anton-Culver, Gallagher, Zanetti, Rosso, Paine, Ollila, Berwick
Acquisition of data: Thomas, Kricker, Busam, From, Groben, Armstrong, Anton-Culver, Gruber, Marrett, Gallagher, Zanetti, Rosso, Dwyer, Venn, Hao, Orlow, Paine, Frank, Berwick
Analysis and interpretation of data: Thomas, Kricker, Waxweiler, Dillon, Busam, From, Armstrong, Anton-Culver, Gruber, Gallagher, Kanetsky, Orlow, Reiner, Luo, Begg, Berwick
Drafting of the manuscript: All authors
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Thomas, Armstrong, Anton-Culver, Gallagher, Berwick
Administrative, technical, and material support: Thomas, Groben, Armstrong, Gruber, Paine, Hao, Berwick
Study supervision: Thomas, Kricker, Busam, Armstrong, Anton-Culver, Gruber, Gallagher, Rosso, Berwick
Conflict of Interest: Disclosures: None reported.
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
GEM Study Group: Coordinating Center, Memorial Sloan-Kettering Cancer Center, New York, NY: Marianne Berwick, M.P.H., Ph.D. (Principal Investigator (PI), currently at the University of New Mexico), Colin B. Begg, Ph.D. (co-PI), Irene Orlow, Ph.D. (co-Investigator), Klaus J. Busam, M.D. (Dermatopathologist), Anne S. Reiner, M.P.H. (Biostatistician), Pampa Roy, Ph.D. (Laboratory Technician), Ajay Sharma, M.S. (Laboratory Technician), Emily La Pilla (Laboratory Technician. University of New Mexico, Albuquerque: Marianne Berwick, M.P.H., Ph.D. (PI), Li Luo, Ph.D. (Biostatistician), Kirsten White, MSc (Laboratory Manager), Susan Paine, M.P.H. (Data Manager). Study centers included the following: The University of Sydney and The Cancer Council New South Wales, Sydney, Australia: Bruce K. Armstrong M.B.B.S.; D.Phil., (PI), Anne Kricker, Ph.D. (co-PI), Anne E. Cust, Ph.D. (co-Investigator); Menzies Research Institute Tasmania, University of Tasmania, Hobart, Australia: Alison Venn, Ph.D. (current PI), Terence Dwyer, M.D. (PI, currently at International Agency for Research on Cancer, Lyon, France), Paul Tucker, M.D. (Dermatopathologist); British Columbia Cancer Research Centre, Vancouver, Canada: Richard P. Gallagher, M.A. (PI), Donna Kan (Coordinator); Cancer Care Ontario, Toronto, Canada: Loraine D. Marrett, Ph.D. (PI), Elizabeth Theis, M.Sc. (co-Investigator), Lynn From, M.D. (Dermatopathologist); CPO, Center for Cancer Prevention, Torino, Italy: Roberto Zanetti, M.D (PI), Stefano Rosso, M.D. (co-PI); University of California, Irvine, CA: Hoda Anton-Culver, Ph.D. (PI), Argyrios Ziogas, Ph.D. (Statistician); University of Michigan, Ann Arbor, MI: University of Michigan, Ann Arbor: Stephen B. Gruber, M.D., M.P.H., Ph.D. (PI, currently at University of Southern California, Los Angeles, CA), Timothy Johnson, M.D. (Director of Melanoma Program), Shu-Chen Huang, M.S., M.B.A. (co-Investigator, joint at USC-University of Michigan); University of North Carolina, Chapel Hill, NC: Nancy E. Thomas, M.D., Ph.D. (PI), Robert C. Millikan, Ph.D. (previous PI, deceased), David W. Ollila, M.D. (co-Investigator), Kathleen Conway, Ph.D. (co-Investigator), Pamela A. Groben, M.D. (Dermatopathologist), Sharon N. Edmiston, B.A. (Research Analyst), Honglin Hao (Laboratory Specialist), Eloise Parrish, MSPH (Laboratory Specialist), Jill S. Frank, M.S. (Research Assistant); University of Pennsylvania, Philadelphia, PA: Timothy R. Rebbeck, Ph.D. (PI), Peter A. Kanetsky, M.P.H., Ph.D. (co-Investigator); UV data consultants: Julia Lee Taylor, Ph.D. and Sasha Madronich, Ph.D., National Centre for Atmospheric Research, Boulder, CO.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011 Jun 30;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012 Jul 28;380(9839):358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 5.Robert C, Flaherty KT, Hersey P, et al. METRIC phase III study: Efficacy of trametinib (T), a potent and selective MEK inhibitor (MEKi), in progression-free survival (PFS) and overall survival (OS) compared with chemotherapy (C) in patients (pts) with BRAFV600/k mutant advanced or metastatic melanoma (MM) J Clin Oncol. 2012;30(15) suppl. [Google Scholar]
- 6.McClain SE, Mayo KB, Shada AL, Smolkin ME, Patterson JW, Slingluff CL., Jr Amelanotic melanomas presenting as red skin lesions: a diagnostic challenge with potentially lethal consequences. Int J Dermatol. 2012 Apr;51(4):420–426. doi: 10.1111/j.1365-4632.2011.05066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huvos AG, Shah JP, Goldsmith HS. A clinicopathologic study of amelanotic melanoma. Surg Gynecol Obstet. 1972 Dec;135(6):917–920. [PubMed] [Google Scholar]
- 8.Ariel IM. Amelanotic melanomas: an analysis of 77 patients. Curr Surg. 1981 May-Jun;38(3):151–155. [PubMed] [Google Scholar]
- 9.Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer. 1996 Aug 1;78(3):427–432. doi: 10.1002/(SICI)1097-0142(19960801)78:3<427::AID-CNCR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 10.Balch CM, Murad TM, Soong SJ, Ingalls AL, Halpern NB, Maddox WA. A multifactorial analysis of melanoma: prognostic histopathological features comparing Clark's and Breslow's staging methods. Ann Surg. 1978 Dec;188(6):732–742. doi: 10.1097/00000658-197812000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sondergaard K, Schou G. Survival with primary cutaneous malignant melanoma, evaluated from 2012 cases. A multivariate regression analysis. Virchows Arch A Pathol Anat Histopathol. 1985;406(2):179–195. doi: 10.1007/BF00737084. [DOI] [PubMed] [Google Scholar]
- 12.Massi D, Pinzani P, Simi L, et al. BRAF and KIT somatic mutations are present in amelanotic melanoma. Melanoma Res. 2013 Aug 9; doi: 10.1097/CMR.0b013e32836477d4. Epub ahead of prinnt. [DOI] [PubMed] [Google Scholar]
- 13.Larsen TE, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 2. The relation of cell type, pigmentation, atypia and mitotic count to histological type and prognosis. Acta Pathol Microbiol Scand A. 1978 Nov;86A(6):513–522. doi: 10.1111/j.1699-0463.1978.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 14.Moreau JF, Weissfeld JL, Ferris LK. Characteristics and survival of patients with invasive amelanotic melanoma in the USA. Melanoma Res. 2013 Jul 23; doi: 10.1097/CMR.0b013e32836410fe. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.SEER Training: Morphology & Grade. http://training.seer.cancer.gov/melanoma/abstract-code-stage/morphology.html.
- 16.Begg CB, Hummer AJ, Mujumdar U, et al. A design for cancer case-control studies using only incident cases: experience with the GEM study of melanoma. Int J Epidemiol. 2006 Jun;35(3):756–764. doi: 10.1093/ije/dyl044. [DOI] [PubMed] [Google Scholar]
- 17.Millikan RC, Hummer A, Begg C, et al. Polymorphisms in nucleotide excision repair genes and risk of multiple primary melanoma: the Genes Environment and Melanoma Study. Carcinogenesis. 2006 Mar;27(3):610–618. doi: 10.1093/carcin/bgi252. [DOI] [PubMed] [Google Scholar]
- 18.Thomas NE, Busam KJ, From L, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013 Nov 20;31(33):4252–4259. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kricker A, Armstrong BK, Goumas C, et al. Survival for Patients With Single and Multiple Primary Melanomas: The Genes, Environment, and Melanoma Study. JAMA Dermatol. Aug;149(8):921–927. doi: 10.1001/jamadermatol.2013.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009 Dec 20;27(36):6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begg CB, Hummer A, Mujumdar U, et al. Familial aggregation of melanoma risks in a large population-based sample of melanoma cases. Cancer Causes Control. 2004 Nov;15(9):957–965. doi: 10.1007/s10522-004-2474-2. [DOI] [PubMed] [Google Scholar]
- 22.Orlow I, Begg CB, Cotignola J, et al. CDKN2A germline mutations in individuals with cutaneous malignant melanoma. J Invest Dermatol. 2007 May;127(5):1234–1243. doi: 10.1038/sj.jid.5700689. [DOI] [PubMed] [Google Scholar]
- 23.Murali R, Goumas C, Kricker A, et al. Clinicopathologic features of incident and subsequent tumors in patients with multiple primary cutaneous melanomas. Ann Surg Oncol. 2012 Mar;19(3):1024–1033. doi: 10.1245/s10434-011-2058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969 Mar;29(3):705–727. [PubMed] [Google Scholar]
- 25.McGovern VJ, Mihm MC, Jr, Bailly C, et al. The classification of malignant melanoma and its histologic reporting. Cancer. 1973 Dec;32(6):1446–1457. doi: 10.1002/1097-0142(197312)32:6<1446::aid-cncr2820320623>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 26.Piris A, Mihm MC, Jr, Duncan LM. AJCC melanoma staging update: impact on dermatopathology practice and patient management. J Cutan Pathol. 2011 May;38(5):394–400. doi: 10.1111/j.1600-0560.2011.01699.x. [DOI] [PubMed] [Google Scholar]
- 27.Adler MJ, White CR., Jr Amelanotic malignant melanoma. Semin Cutan Med Surg. 1997 Jun;16(2):122–130. doi: 10.1016/s1085-5629(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 28.Thomas NE, Kricker A, From L, et al. Associations of cumulative sun exposure and phenotypic characteristics with histologic solar elastosis. Cancer Epidemiol Biomarkers Prev. 2010 Nov;19(11):2932–2941. doi: 10.1158/1055-9965.EPI-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gualandri L, Betti R, Crosti C. Clinical features of 36 cases of amelanotic melanomas and considerations about the relationship between histologic subtypes and diagnostic delay. J Eur Acad Dermatol Venereol. 2009 Mar;23(3):283–287. doi: 10.1111/j.1468-3083.2008.03041.x. [DOI] [PubMed] [Google Scholar]
- 30.Cheung WL, Patel RR, Leonard A, Firoz B, Meehan SA. Amelanotic melanoma: a detailed morphologic analysis with clinicopathologic correlation of 75 cases. J Cutan Pathol. 2012 Jan;39(1):33–39. doi: 10.1111/j.1600-0560.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AE, Cochran AJ, Morton DL. Melanoma from unknown primary site and amelanotic melanoma. Semin Oncol. 1982 Dec;9(4):442–447. [PubMed] [Google Scholar]
- 32.Liu W, Dowling JP, Murray WK, et al. Rate of growth in melanomas: characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006 Dec;142(12):1551–1558. doi: 10.1001/archderm.142.12.1551. [DOI] [PubMed] [Google Scholar]
- 33.Chung KT, Nilson EH, Case MJ, Marr AG, Hungate RE. Estimation of growth rate from the mitotic index. Applied microbiology. 1973 May;25(5):778–780. doi: 10.1128/am.25.5.778-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000 May;42(5 Pt 1):731–734. doi: 10.1067/mjd.2000.103981. [DOI] [PubMed] [Google Scholar]
- 35.Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008 May;35(5):433–444. doi: 10.1111/j.1600-0560.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 36.Pouryazdanparast P, Brenner A, Haghighat Z, Guitart J, Rademaker A, Gerami P. The role of 8q24 copy number gains and c-MYC expression in amelanotic cutaneous melanoma. Mod Pathol. 2012 Sep;25(9):1221–1226. doi: 10.1038/modpathol.2012.75. [DOI] [PubMed] [Google Scholar]
- 37.Andersen WK, Silvers DN. ‘Melanoma? It can't be melanoma!’ A subset of melanomas that defies clinical recognition. JAMA. 1991 Dec 25;266(24):3463–3465. [PubMed] [Google Scholar]
- 38.Rigel DS, Friedman RJ, Kopf AW, Polsky D. ABCDE--an evolving concept in the early detection of melanoma. Arch Dermatol. 2005 Aug;141(8):1032–1034. doi: 10.1001/archderm.141.8.1032. [DOI] [PubMed] [Google Scholar]
- 39.Betti R, Vergani R, Tolomio E, Santambrogio R, Crosti C. Factors of delay in the diagnosis of melanoma. Eur J Dermatol. 2003 Mar-Apr;13(2):183–188. [PubMed] [Google Scholar]
- 40.Viros A, Fridlyand J, Bauer J, et al. Improving melanoma classification by integrating genetic and morphologic features. PLoS Med. 2008 Jun 3;5(6):e120. doi: 10.1371/journal.pmed.0050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


