Abstract
Operative correction of tetralogy of Fallot (TOF) frequently results in pulmonary insufficiency and chronic volume overload that have been linked to increased risk of adverse outcome. No consensus recommendations for timing of pulmonary valve replacement (PVR) exist. We examined the pattern of PVR in the US from 2004 to 2012. We used The Pediatric Health Information Systems Database to perform an observational study of children and adults, age ≥10 years with a diagnosis of TOF undergoing PVR at 35 centers in the United States between 2004 and 2012, to assess the rate of PVR and the age at which is performed. Mixed effects multivariable regression was used to account for patient-level covariates and center-level covariance. Additional analyses assessed for trends in cost, hospital length of stay (LOS), intensive care unit LOS, and in-hospital mortality over the study period. In total, 799 subjects at 35 centers underwent PVR over the study period. The number of PVR performed per year increased significantly over the study period. There was significant between-center heterogeneity in age at PVR (p<0.001). Age at PVR, intensive care unit LOS, hospital LOS, and cost did not change over the study period. In conclusion, PVR in TOF is being performed more frequently, without an accompanying change in the age at PVR or other measurable outcomes. There is significant variability in the age at which PVR is performed between centers across the United States. This highlights the need for additional research guiding the optimal timing of PVR.
Keywords: outcomes research, healthcare economics, administrative data, PHIS
Introduction
The purpose of this study was to analyze trends in the rate of pulmonary valve replacement (PVR) in subjects with tetralogy of Fallot (TOF) at centers in the US and the average age at PVR. Given the number of publications addressing timing of PVR in TOF during the study period, we hypothesized that the rate of PVR in subjects with TOF would increase with time, and that this would be accompanied by decreasing age at PVR. We also sought to determine if changes in practice were accompanied by trends in perioperative outcomes.
Methods
The Pediatric Health Information Systems (PHIS) database is an administrative database that contains data from inpatient, emergency department, ambulatory surgery, and observation encounters from 43 not-for-profit, tertiary care pediatric hospitals in the United States, affiliated with the Children’s Hospital Association (CHA) (Overland Park, KS)1. Data quality and reliability are assured through a joint effort between CHA and participating hospitals. The PHIS data warehouse is managed by Truven Health Analytics (Ann Arbor, MI). Participating hospitals provide discharge/encounter data including demographics, diagnoses, and procedures. Forty-two of these hospitals submit resource utilization data (e.g. pharmacy products, radiologic studies, and laboratory studies) to PHIS. Data are de-identified at the time of submission and are subjected to reliability and validity checks before inclusion. A data-use agreement was signed between study investigators and CHA. The institutional review board of The Children’s Hospital of Philadelphia reviewed the project and determined that it did not represent human subjects research in accordance with the Common Rule (45 CFR 46.102(f)).
We included children and adults ≥10 years of age with the diagnosis of TOF (International Classification of Disease, ninth revision code (ICD-9): 745.2) who underwent either operative (ICD-9: 35.25, 35.26) or trans-catheter (ICD-9: 35.07) PVR at any of the 43 PHIS centers between 1/1/2004 and 12/31/2012. The lower limit for age was chosen to reduce error in cohort identification, specifically the inclusion of subjects receiving other operations incorrectly coded as PVR, such as placement or replacement of right ventricle to pulmonary artery conduit. Centers were excluded if they did fewer than 50 cardiac surgical procedures per year, fewer than 5 PVR procedures (all ages) over the study period, or if they did not report cardiac surgical procedures in at least 66% (6/9 years) of years during the study period. This was intended to restrict analysis to centers with stable reporting practices and operative volumes.
Data were extracted from the PHIS database by direct query and included subject age, sex, race, insurance payor (private, public insurance, other), and presence of genetic syndrome (ICD-9) codes: 758.1, 758.2, 758.0, 758.31, 758.32, 758.33, 758.9, 758.6, 758.83, and 758.89)1. In-hospital death, length of stay (LOS), ICU LOS, and adjusted cost were also extracted. Center total cardiac surgical volume was calculated by admissions with ICD-9 codes 35.00 through 35.99 and 37.5, 37.51, 37.52, and 39.0 and 39.212.
Hospital charges are sent directly from billing records of each institution to PHIS. Cost is calculated by multiplying charge data by center-specific department level cost to charge ratios (RCC), allowing comparison across centers in whom charging practices varies. In this study, costs were standardized to 2012 United States Dollars (2012US$) using consumer price index for medical care, complied by the Bureau of Labor Statistics (data.bls.gov/cgi-bin/dsrv).
Descriptive statistics were expressed as mean ± standard deviation, median (range and inter-quartile range (IQR)), and percentages and counts as appropriate. We could not measure the total number of subjects with TOF during the study period and, therefore, could not calculate the incidence of PVR. The rate of PVR was assessed in three ways: 1) the total number of PVR performed across included centers per year, 2) the average number of PVR operations per center per year (analyzed both with and without adjustment for center volume), and 3) the proportion of each center’s total cardiac surgical volume accounted for by PVR. Linear regression was used to assess for trends in each of these rates per year over the study period. The latter two measures of PVR rate were chosen to differentiate between changes in the rate of PVR and changes in cardiac surgical volume at centers over time. Though not the true incidence of PVR, the rates generated provide information about the trend in the number of PVR being performed. The association between date of PVR and age at PVR in years was assessed using mixed effects models, based on generalized linear models using normal/Gaussian distribution and canonical identity link. Fixed effects were included for pre-specified covariates (listed above). All covariates were included, without use of bi-variable screening. To account for covariance within centers, a random intercept was added, and to account for covariance in the association between age of repair (outcome) and year of repair (exposure) by center, a random slope was added. Post-hoc, additional models were tested to assess for non-linearity in the association between year of operation and age at operation, including tests for changes in slope: 1) at the mid-point of the study period and 2) at the introduction of trans-catheter pulmonary valve replacement in the database (2011), as well as the addition of a quadratic term to assess for a non-linear change in age with time. Goodness of fit was compared using likelihood ratio tests.
Association between year of PVR and secondary outcomes was performed using generalized linear models with fixed effects and random effects. Risk of in-hospital death was modeled using binomial distribution. Length of stay, ICU duration, and RCC-adjusted cost were modeled using log gamma distribution. For each model, the canonical link for the listed distribution was used. In addition to previously included covariates, age at PVR (centered at 16 years) was added to models as a covariate. Because the number of in-hospital deaths was small, multi-variable models could not be employed to assess risk factors for in-hospital death. For other outcomes, standardized values for outcomes of interest were determined using conditional standardization, holding fixed effects covariates equal to the referent group. Heterogeneity in age of repair by center was assessed using likelihood ratio test of random intercept term. Heterogeneity in the response in age of PVR over time by center was assessed using the likelihood ratio test for random slope term.
Sensitivity analyses were performed for the effect of various age inclusion/exclusion criteria on the results of the study. First, the lower limit on age was varied over a range of ages from 6 years to 18 years, with primary analysis performed for each. This was performed to determine whether different age criteria were biasing study results in regards to temporal changes in age at PVR. A second sensitivity analysis for the upper limit of age was also performed restricting subject age <60 to <25 in five year increments. This was performed to insure that high-age outliers were not exerting excessive influence on study results. Third, subjects who underwent trans-catheter PVR were excluded to insure that this subgroup of subjects was not exerting excessive influence on study results.
Introduction of trans-catheter valve replacement was identified as a factor that might potentially have influence on study results. Several steps were taken to characterize these effects and account for them. First, the characteristics of subjects undergoing trans-catheter and operative PVR were summarized. Comparisons between the trans-catheter PVR (TC-PVR) and operative PVR (S-PVR) were made using either two-tailed Fisher’s exact or Wilcoxon rank sum tests as appropriate. Second, TC-PVR (compared to S-PVR) was included as a covariate in each multi-variable model to condition for its effect. Third, sensitivity analyses of the primary model were performed to determine the influence of TC-PVR’s introduction on the age at PVR, specifically 1) including the year of the introduction of TC-PVR in the PHIS database (2011) as a potential hinge point in age of PVR, and 2) restricting analyses to SC-PVR subjects.
All analysis was performed using Stata MP v13 (Statacorp, College Station TX).
Results
Between 2004 and 2012, a total of 799 subjects with TOF underwent PVR at 35 centers across the United States contributing data to the PHIS database (Table 1). Eight centers (10 subjects) did not meet inclusion criteria and were excluded. Median age at PVR was 17 years (range: 10–64). The majority of the population was male (57%) and white (75%). A small minority (10%) carried a diagnosis of a genetic syndrome. Median hospital LOS was 4 days (range: 1–126) with a median duration of ICU stay 2 days (range 1–41 days), median cost of hospitalization of 2012US$44,029 (range: 4,399 to 866,720), and in-hospital mortality of 0.9% (7 subjects).
Table 1.
Characteristics of the study population
| Number | 799 at 35 centers |
| Age at pulmonary valve replacement (years) | 17 (range: 10–64 IQR: 13–22) |
| Female Gender | 347 (43%) |
| Race | |
| White | 601 (75%) |
| Black | 78 (10%) |
| Asian | 27 (3%) |
| Other | 93 (12%) |
| Known genetic syndrome | 79 (10%) |
| Transcatheter pulmonary valve | 30 (4%) |
| Payor | |
| Private insurance | 467 (58%) |
| Medicaid | 213 (27%) |
| Other | 199 (16%) |
| Mortality | 7 (0.9%) |
| Cost (2012US$)* | 44,029 (range: 4,399 – 866,720, IQR: 35,294 – 57,514) |
| Hospital length of stay (days) | 4 (range: 1–126, IQR: 3–5) |
| ICU stay (days) † | 2 (range: 1–41, IQR: 2–4) |
n= 739 subjects
n= 656 subjects
Abbreviations: ICU: Intensive care unit, IQR inter-quartile range, 2012US$ United States dollars adjusted for inflation to year 2012
Characteristics of subjects who underwent SC-PVR and TC-PVR are summarized in Table 2. Subjects undergoing TC-PVR were older (p=0.046) and more likely to be black (p=0.04). Mortality was not significantly different (p=1.0). Duration of ICU stay was not significantly different between TC-PVR and S-PVR (p=0.21) but was available in a lower percentage of subjects following TC-PVR than S-PVR (27% vs. 84%, p<0.001). Cost was not significantly different, but hospital LOS was less following TC-PVR than S-PVR (median 1 range: 1–14 IQR: 1–2, p<0.0001).
Table 2.
Characteristics of operative and trans-catheter pulmonary valve replacement subgroups
| Operative valve replacement | Trans-catheter valve replacement | p | |
|---|---|---|---|
| Number | 769 at 35 centers | 30 at 18 centers | |
| Age at pulmonary valve replacement (years) | 17 (range: 10–61 IQR: 13–22) | 20.5 (range: 10–64, IQR: 15–28) | 0.046 |
| Female Gender | 333 (43%) | 15 (50%) | 0.46 |
| Race | |||
| White | 574 (76%) | 19 (63%) | 0.04 |
| Black | 71 (9%) | 7 (23%) | |
| Asian | 25 (3%) | 2 (7%) | |
| Other | 91 (12%) | 2 (7%) | |
| Known genetic syndrome | 76 (10%) | 3 (10%) | 1.0 |
| Payer | |||
| Private insurance | 450 (59%) | 17 (57%) | 0.34 |
| Medicaid | 207 (27%) | 6 (20%) | |
| Other | 112 (15%) | 7 (23%) | |
| Mortality | 7 (0.9%) | 0 | 1.0 |
| Cost (2012US$)* | 43,877 (range: 4,399–866,720, IQR: 35,294 – 57,045) | 47,609 (range: 10,532–89,771, IQR: 35,310–72,024) | 0.45 |
| Hospital length of stay (days) | 4 (range: 1–126, IQR: 3–5) | 1 (range: 1–14, IQR: 1–2) | <0.0001 |
| ICU stay (days) † | 2 (range: 1–41, IQR: 2–4) | 2 (range: 1–4, IQR: 1.5–3) | 0.21 |
n=713 for SVR and n=26 for TVR
n=648 for SVR and n=8 for TVR
Abbreviations: ICU: Intensive care unit, IQR inter-quartile range, 2012US$ United States dollars adjusted for inflation to year 2012
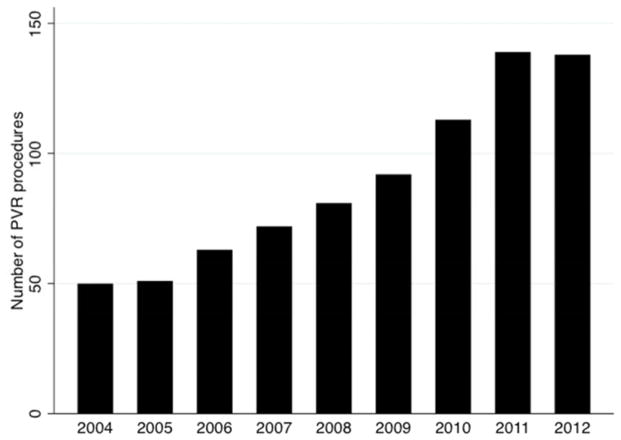
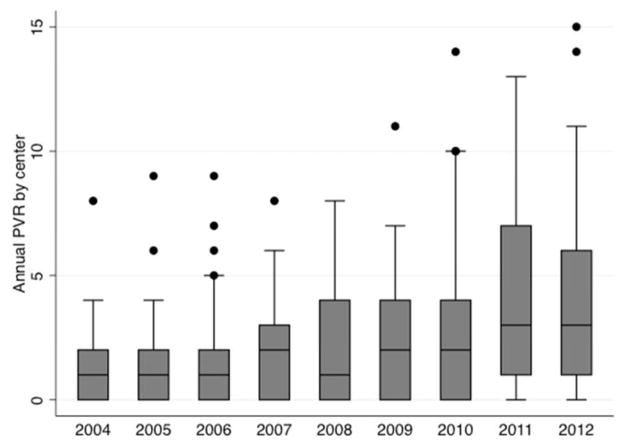
The annual rate of PVR increased over the study period. The total rate of PVR across 35 centers increased significantly (beta: 12.3 more PVR each year 95% CI: 9.8 to 14.7 p<0.001, Figure 1). Forty PVR were performed in 2004, which more than tripled by 2012 when 138 PVR were performed. The mean rate of PVR by center also increased significantly (beta=0.3 PVR per year, 95% CI: 0.2 to 0.5 p<0.001 Figure 2), with an annual rate of 1.1 PVR per year in 2004 increased to 3.9 PVR per year in 2012. When adjusted for center total surgical volume, the PVR per center continued to increase without a change in the rate (beta=0.3 PVR per year, 95% CI: 0.19 to 0.40, p<0.001). The proportion of PVR within each center’s annual cardiac surgical volume also increased significantly (beta: 0.7 PVR cases per 1000 cardiac surgical cases per center per year, 95% CI: 0.4–0.9, p<0.001).
Figure 1. Total number of pulmonary valve interventions (2004–2012) across PHIS centers.
This bar plot demonstrates the total number of PVR across all 35 centers meeting inclusion criteria between 2004 and 2012.
Figure 2. Number of pulmonary valve interventions per center (2004–2012) across PHIS centers.
This box and whiskers plot demonstrates changes across in number of PVR procedures performed at each of the 35 centers in PHIS database reported PVR. Horizontal line marks the median number of procedures. Upper and lower limits of the box 25th and 75th percentiles of the range. Whiskers are drawn to the adjacent value under the limit of 1.5 times the inter-quartile range. Values outside this limit are marked with filled circles.
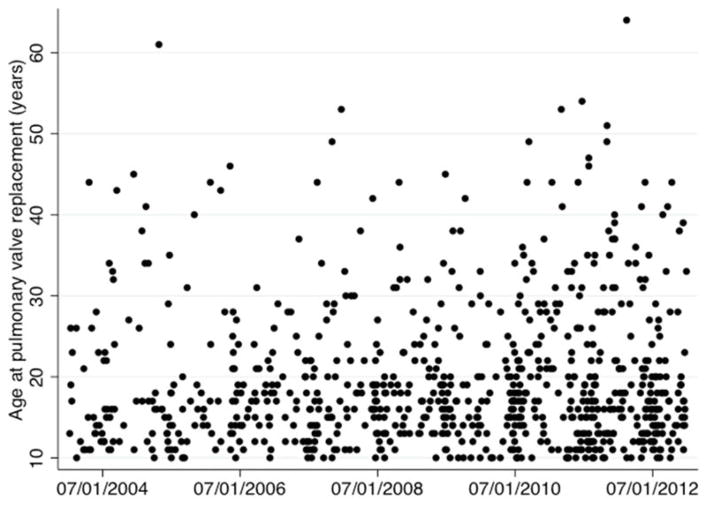
The multivariable mixed effects model assessing the association between date of PVR and age at PVR is summarized in Table 3. The adjusted expected age of PVR is 16.0 years (95% CI: 13.5 to 18.5). There was no association between date of PVR and age of subjects undergoing PVR (beta: 0.09 95% CI: −0.1 to 0.3 p=0.5) (Figure 3). Several covariates demonstrated a significant association with age at PVR. Black and Asian race ((beta: −2.9 p=0.003 and beta: −2.1, p=0.04, respectively) were both associated with earlier PVR relative to subjects with white race, as was presence of genetic syndrome (beta: −1.8, p=0.03). Male sex was associated with older age at PVR (beta=1.8, p=0.003).
Table 3.
Results of multivariate mixed effects regression model of age at PVR vs. date of PVR
| beta | 95% CI | p | |
|---|---|---|---|
| Date of PVR (per year from 2004) | 0.08 | −0.3 to 0.4 | 0.65 |
| Race (compared to white) | |||
| Black | −2.9 | −4.7 to −1.0 | 0.002 |
| Asian | −2.1 | −4.2 to −0.1 | 0.04 |
| Other | −1.0 | −2.7 to 0.6 | 0.23 |
| Male sex (compared to female) | 1.8 | 0.6 to 3.0 | 0.003 |
| Known genetic syndrome | −1.8 | −3.4 to −0.14 | 0.03 |
| Payer (compared to private insurance) | |||
| Medicaid | 0.4 | −1.4 to 2.2 | 0.66 |
| Other | −1.1 | −2.8 to 0.6 | 0.20 |
| Trans-catheter pulmonary valve replacement | 3.9 | −0.7 to 8.5 | 0.10 |
Abbreviations: CI confidence interval, IQR Inter-quartile range
Standardized expected age of PVR for 2004 is 16.0 years (95% CI: 13.5–18.5).
Figure 3. Age at PVR over the study period.
This scatter plot depicts the date of PVR on the x-axis and individual subject ages at PVR on the y-axis. Multivariate mixed effects regression model demonstrated that there was not a significant relationship between year and age at PVR (p=0.65).
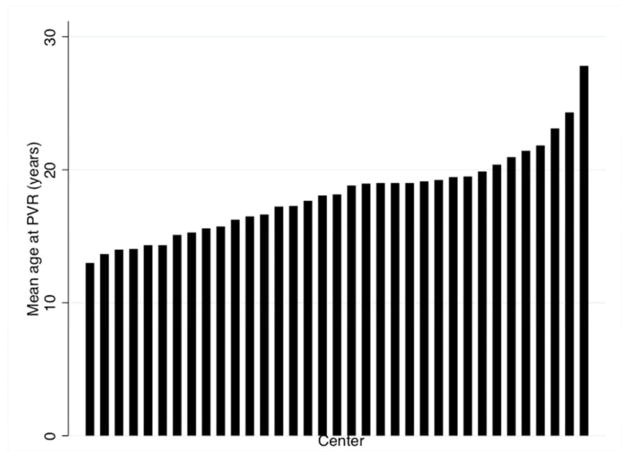
Age at PVR was significantly heterogeneous between centers (p<0.001) (Figure 4), with mean age by center ranging from 13 years to 27.8 years. The association between age at PVR and date of PVR did not differ significantly between centers (p=0.86).
Figure 4. Mean age at PVR (2004-2012) by center.
This bar graph depicts the mean age at PVR across the 35 PHIS centers meeting inclusion criteria for this study. Test for heterogeneity in age between centers demonstrated significant heterogeneity in age at PVR by center mixed effects model (p<0.001).
Three sensitivity analyses were performed, as described in the methods section. The first assessed for non-linear associations between date of PVR and age at PVR, with no improvement in model fit (data not shown). The second assessed the effect of different exclusion criteria based on age by varying them, with no change in the magnitude or direction of main effects or covariates (data not shown). The third excluded subjects who underwent trans-catheter PVR, with no change in the magnitude or direction of main effects and covariates (data not shown).
Standardized hospital LOS was 5.4 days (95% CI: 4.1 to 7.1 days) (Table 4). It was not related to date of operation (coefficient: 0.98, 95% CI: 0.95 to 1.0, p=0.06) or age of operation (coefficient: 1.0, 95% CI: 1.0 to 1.0 p=0.41). Presence of genetic syndrome (coefficient: 1.30, 95% CI: 1.09 to 1.60, p=0.01) and Medicaid (vs. private insurance, coefficient: 1.29, 95% CI: 1.09 to 1.53, p=0.003) were associated with longer hospital LOS. TC-PVR was associated with reduced hospital LOS (coefficient: 0.39, 95% CI: 0.26 to 0.61, p<0.001). Duration of intensive care was available in 81% (656/799) of subjects from all 36 centers. Standardized ICU duration was 2.8 days (95% CI: 2.0 to 3.9 days). Duration of ICU stay did not vary across the study period (95% CI 95% CI: 1.0 to 1.07, p=0.08) (Table 5). Genetic syndrome (coefficient: 1.26, 95% CI: 1.01 to 1.56, p=0.04), and older age (coefficient: 1.01 per year greater than 16, 95% CI: 1.00 to 1.01, p=0.03) and Medicaid (vs. private insurance coefficient: 1.17, 95% CI: 1.01 to 1.36, p=0.03) were independently associated with increased ICU stay.
Table 4.
Results of multivariate mixed effects regression model of hospital length of stay vs. date of PVR
| Relative LOS | 95% CI | P | |
|---|---|---|---|
| Date of PVR (per year relative to 2004) | 0.98 | 0.95 to 1.00 | 0.06 |
| Age (years) centered on 16 years | 1.00 | 1.00 to 1.00 | 0.41 |
| Race (compared to white) | |||
| Black | 1.35 | 0.95 to 1.93 | 0.10 |
| Asian | 1.07 | 0.80 to 1.45 | 0.64 |
| Other | 1.10 | 0.98 to 1.23 | 0.12 |
| Male sex (compared to female) | 0.94 | 0.86 to 1.04 | 0.23 |
| Known genetic syndrome | 1.30 | 1.06 to 1.60 | 0.01 |
| Payer (compared to private insurance) | |||
| Medicaid | 1.29 | 1.09 to 1.53 | 0.003 |
| Other | 1.03 | 0.88 to 1.21 | 0.71 |
| Trans-catheter pulmonary valve replacement | 0.39 | 0.26 to 0.61 | <0.001 |
Abbreviations: CI confidence interval, IQR Inter-quartile range
Standardized length of stay is 5.4 days (95% CI: 4.1 to 7.1 days). Relative LOS is the relative increase in the in hospital length of stay associated with the variable of interest.
Table 5.
Results of multivariate mixed effects regression model of ICU length of stay vs. date of PVR
| Relative ICU LOS | 95% CI | P | |
|---|---|---|---|
| Date of PVR (per year relative to 2004) | 1.03 | 1.0 to 1.07 | 0.08 |
| Age (years) centered on 16 years | 1.01 | 1.00 to 1.01 | 0.03 |
| Race (compared to white) | |||
| Black | 1.18 | 0.96 to 1.44 | 0.10 |
| Asian | 1.22 | 0.89 to 1.67 | 0.21 |
| Other | 1.01 | 0.84 to 1.22 | 0.89 |
| Male sex (compared to female) | 0.93 | 0.82 to 1.05 | 0.23 |
| Known genetic syndrome | 1.26 | 1.01 to 1.56 | 0.04 |
| Payer (compared to private insurance) | |||
| Medicaid | 1.17 | 1.01 to 1.36 | 0.03 |
| Other | 1.01 | 0.83 to 1.24 | 0.90 |
| Trans-catheter pulmonary valve replacement | 0.92 | 0.55 to 1.56 | 0.77 |
Abbreviations: CI confidence interval, IQR Inter-quartile range
Standardized expected ICU length of stay is 2.8 days (95% CI: 2.0 to 3.9 days). Relative ICU LOS is the relative increase in ICU duration associated with the variable of interest
Cost of hospitalization was available in 92% (739/799) of subjects from all centers). Standardized cost of hospitalization for PVR was 2012US$45,659 (95% CI: 39,647 to 52,582). Cost of hospitalization (standardized to 2012 United States dollars) increased by 2% per year of the study (coefficient: 1.02, 95% CI: 1.00 to 1.04, p=0.04) (Table 6). Older age (coefficient: 1.005 for every year older than 16, 95% CI: 1.001 to 1.008, p=0.01), genetic syndrome (coefficient: 1.24, 95% CI: 1.08 to 1.43, p=0.003), and Medicaid (coefficient: 1.14, 95% CI: 1.03 to 1.27, p=0.01) were associated with increased hospital cost.
Table 6.
Results of multivariate mixed effects regression model of inflation-adjusted total cost vs. date of PVR
| Relative cost | 95% CI | p | |
|---|---|---|---|
| Date of PVR (per year) | 1.02 | 1.0 to 1.04 | 0.04 |
| Age (years) centered on 16 years | 1.005 | 1.001 to 1.008 | 0.01 |
| Race (compared to white) | |||
| Black | 1.18 | 0.99 to 1.40 | 0.06 |
| Asian | 1.09 | 0.86 to 1.38 | 0.48 |
| Other | 1.04 | 0.86 to 1.38 | 0.48 |
| Male sex (compared to female) | 0.97 | 0.90 to 1.03 | 0.28 |
| Known genetic syndrome | 1.24 | 1.08 to 1.43 | 0.003 |
| Payer (compared to private insurance) | |||
| Medicaid | 1.14 | 1.03 to 1.27 | 0.01 |
| Other | 1.00 | 0.87 to 1.14 | 0.84 |
| Trans-catheter pulmonary valve replacement | 0.98 | 0.80 to 1.19 | 0.81 |
Abbreviations: CI confidence interval, IQR Inter-quartile range
Standardized expected cost is 2012US$45,659 (95% CI: 39,647 to 52,582). Relative cost is the relative increase in cost of hospitalization associated with the variable of interest.
Discussion
In this observational study from 35 children’s hospitals in the United States, the annual number of PVR in children and adults with TOF increased significantly over 9 years. While the age of subjects undergoing PVR did not change significantly, there was also significant heterogeneity between centers in the age at which patients had PVR. Older age was associated with increased ICU duration and cost. Presence of a genetic syndrome was associated with younger age at PVR, longer hospital LOS, longer ICU LOS, and increased cost. TC-PVR was associated with reduced hospital LOS, but had no significant reduction in cost compared with operative PVR.
PVR results in improved symptoms3,4, shorter QRS duration5–9, smaller RV dimensions4,6,8–14, and improvement in RV systolic function3,4,10,11,15 and LV systolic function4,14. However, these have not been accompanied by improvement in performance on exercise testing10,16,17, functional status17, risk of arrhythmia, sudden cardiac death, and all-cause mortality10,18. A number of studies have attempted to identify thresholds on electrocardiogram19 and non-invasive imaging20 that identify asymptomatic patients who are at risk for irreversible RV dysfunction, arrhythmia, or sudden cardiac death to guide optimal timing of PVR. Cardiology and surgical societies have not, to our knowledge, published guidelines with criteria for PVR in asymptomatic patients with TOF, but some authors have advocated pulmonary valve replacement with increasingly aggressive criteria21–23. The current study demonstrates an increase in PVR across the centers in the PHIS database, whether expressed in terms of the number of operations per center, the mean number of PVR adjusted for total number of cardiac operations, and as the proportion of cardiac operations represented by PVR. However, no association was demonstrated between date of PVR and age at PVR.
The current study demonstrates significant heterogeneity in practice patterns amongst pediatric hospitals in the United States. This finding is corroborated by a recent meta-analysis, in which the median age of patients in the 48 included studies ranged from 8 to 30 years11. Variability in practice reflects lack of consensus in patterns of PVR across children’s hospitals in the United States and uncertainty regarding optimal timing for PVR in TOF, highlighting the importance of determining these factors in children and adults with TOF.
We were also interested in determining if the increased rate of PVR was accompanied by in changes in mortality, ICU LOS, hospital LOS, and cost. The number of in-hospital deaths was too small to allow for meaningful analysis, but was consistent with pooled risk from meta-analyses11,24. No significant changes were seen in hospital or ICU LOS. Cost increased by 2% per year in excess of inflation, the reasons for which are unclear.
The study design is limited in its ability to make inferences about the relationship between subject-level factors and outcomes, and caution should be exercised when interpreting these associations. However, older age was associated with increased ICU duration and cost. Presence of a genetic syndrome was associated with younger age at PVR, longer hospital LOS, longer ICU LOS, and increased cost. The observed increased LOS and resource utilization is consistent with previous studies of perioperative outcomes in infants and younger children with 22q11.2 microdeletion25,26. TC-PVR was associated with reduced hospital LOS, but was not associated with significant reduction in cost compared with S-PVR. The only contemporaneous case series of TC- and S-PVR27 does not compare cost or LOS, and previous studies comparing the cost of TC- and S- PVR have had equivocal results28,29.
TC-PVR first was reported in the PHIS database in 2011. The number of reported cases is small, but we sought to determine the effect whether they affected observed outcomes. There was no detectable difference in the risk of in-hospital mortality or cost. However, LOS was reduced (both in comparison of recorded numbers and in multivariable models). ICU stay was not different between TC-PVR and S-PVR, but the number of subjects with TC-PVR with missing ICU data was large (88%). Not all subjects who undergo TC-VR under ICU admission during recovery, and it is not possible in the database to determine the proportion of “missing” ICU days are truly missing and those who were not admitted to an ICU. Sensitivity analyses demonstrated that at this point, there was no significant change in the age of PVR after the introduction of PVR, nor did excluding TC-PVR subjects alter the observed results.
The current study has several limitations. Administrative databases do not contain all of the covariates that potentially affects timing of PVR. In addition, the PHIS database is restricted to pediatric hospitals. In a previous study utilizing the National Inpatient Sample, investigators reported that a minority (37%) of operations for patients age>18 with congenital heart disease, including those with TOF, occurred in children’s hospitals30. This potentially limits generalizability of the findings to the practice patterns of pediatric institutions and may fail to capture trends that would be seen by including general hospitals. The prevalent population with TOF cannot be measured with the current database, and we acknowledge that demographic changes over the nine-year study period could influence rates of PVR.
Acknowledgments
The investigators thank Zeinab Mohammad for assistance in data extraction and A. Russell Localio PhD fors consultation during statistical analysis.
Dr. O’Byrne receives support from the NIH [T32 HL007915] and Entelligence Young Investigator grant. Dr. Mercer Rosa receives support from the NIH [NIH 3U01HL098153-03S1]. Dr. Kawut is supported by the NIH [K24 HL103844].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pasquali SK, Hall M, Li JS, Peterson ED, Jaggers J, Lodge AJ, Marino BS, Goodman DM, Shah SS. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the Pediatric Health Information Systems database. Circulation. 2010;122:2123–2130. doi: 10.1161/CIRCULATIONAHA.110.948737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasquali SK, Hall M, Slonim AD, Jenkins KJ, Marino BS, Cohen MS, Shah SS. Off-label use of cardiovascular medications in children hospitalized with congenital and acquired heart disease. Circ Cardiovasc Qual Outcomes. 2008;1:74–83. doi: 10.1161/CIRCOUTCOMES.108.787176. [DOI] [PubMed] [Google Scholar]
- 3.Discigil B, Dearani JA, Puga FJ, Schaff HV, Hagler DJ, Warnes CA, Danielson GK. Late pulmonary valve replacement after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 2001;121:344–351. doi: 10.1067/mtc.2001.111209. [DOI] [PubMed] [Google Scholar]
- 4.Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G, Mist B, Walker F, van Doorn C, Bonhoeffer P, Taylor AM. Biventricular Response After Pulmonary Valve Replacement for Right Ventricular Outflow Tract Dysfunction: Is Age a Predictor of Outcome? Circulation. 2008;118:S182–S190. doi: 10.1161/CIRCULATIONAHA.107.756825. [DOI] [PubMed] [Google Scholar]
- 5.Lim C, Lee JY, Kim W-H, Kim S-C, Song J-Y, Kim S-J, Choh J-H, Kim CW. Early replacement of pulmonary valve after repair of tetralogy: is it really beneficial? Eur J Cardiothorac Surg. 2004;25:728–734. doi: 10.1016/j.ejcts.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 6.van Huysduynen BH. Reduction of QRS duration after pulmonary valve replacement in adult Fallot patients is related to reduction of right ventricular volume. Eur Heart J. 2005;26:928–932. doi: 10.1093/eurheartj/ehi140. [DOI] [PubMed] [Google Scholar]
- 7.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk APJ, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, Mulder BJM. Preoperative Thresholds for Pulmonary Valve Replacement in Patients With Corrected Tetralogy of Fallot Using Cardiovascular Magnetic Resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 8.Buechel ERV. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J. 2005;26:2721–2727. doi: 10.1093/eurheartj/ehi581. [DOI] [PubMed] [Google Scholar]
- 9.Doughan AR, McConnell ME, Lyle TA, Book WM. Effects of pulmonary valve replacement on QRS duration and right ventricular cavity size late after repair of right ventricular outflow tract obstruction. Am J Cardiol. 2005;95:1511–1514. doi: 10.1016/j.amjcard.2005.01.100. [DOI] [PubMed] [Google Scholar]
- 10.Gengsakul A, Harris L, Bradley TJ, Webb GD, Williams WG, Siu SC, Merchant N, McCrindle BW. The impact of pulmonary valve replacement after tetralogy of Fallot repair: a matched comparison. Eur J Cardiothorac Surg. 2007;32:462–468. doi: 10.1016/j.ejcts.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Ferraz Cavalcanti PE, Sá MPBO, Santos CA, Esmeraldo IM, de Escobar RR, de Menezes AM, de Azevedo OM, de Vasconcelos Silva FP, de Lins RFA, de Lima RC. Pulmonary valve replacement after operative repair of tetralogy of Fallot: meta-analysis and meta-regression of 3,118 patients from 48 studies. J Am Coll Cardiol. 2013;62:2227–2243. doi: 10.1016/j.jacc.2013.04.107. [DOI] [PubMed] [Google Scholar]
- 12.Graham TP, Bernard Y, Arbogast P, Thapa S, Cetta F, Child J, Chugh R, Davidson W, Hurwitz R, Kay J, Sanders S, Schaufelberger M. Outcome of pulmonary valve replacements in adults after tetralogy repair: a multi-institutional study. Congenit Heart Dis. 2008;3:162–167. doi: 10.1111/j.1747-0803.2008.00189.x. [DOI] [PubMed] [Google Scholar]
- 13.van Straten A, Vliegen HW, Hazekamp MG, Bax JJ, Schoof PH, Ottenkamp J, van der Wall EE, de Roos A. Right Ventricular Function after Pulmonary Valve Replacement in Patients with Tetralogy of Fallot. Radiology. 2004;233:824–829. doi: 10.1148/radiol.2333030804. [DOI] [PubMed] [Google Scholar]
- 14.Chalard A, Sanchez I, Gouton M, Henaine R, Salami FA, Ninet J, Douek PC, Di Filippo S, Boussel L. Effect of Pulmonary Valve Replacement on Left Ventricular Function in Patients With Tetralogy of Fallot. AmJ Cardiol. 2012;110:1828–1835. doi: 10.1016/j.amjcard.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 15.Henkens IR, van Straten A, Schalij MJ, Hazekamp MG, de Roos A, van der Wall EE, Vliegen HW. Predicting outcome of pulmonary valve replacement in adult tetralogy of Fallot patients. Ann Thorac Surg. 2007;83:907–911. doi: 10.1016/j.athoracsur.2006.09.090. [DOI] [PubMed] [Google Scholar]
- 16.Eyskens B, Reybrouck T, Bogaert J, Dymarkowsky S, Daenen W, Dumoulin M, Gewillig M. Homograft insertion for pulmonary regurgitation after repair of tetralogy of Fallot improves cardiorespiratory exercise performance. Am J Cardiol. 2000;85:221–225. doi: 10.1016/s0002-9149(99)00640-2. [DOI] [PubMed] [Google Scholar]
- 17.Geva T, Gauvreau K, Powell AJ, Cecchin F, Rhodes J, Geva J, del Nido P. Randomized Trial of Pulmonary Valve Replacement With and Without Right Ventricular Remodeling Surgery. Circulation. 2010;122:S201–S208. doi: 10.1161/CIRCULATIONAHA.110.951178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrild DM, Berul CI, Cecchin F, Geva T, Gauvreau K, Pigula F, Walsh EP. Pulmonary valve replacement in tetralogy of Fallot: impact on survival and ventricular tachycardia. Circulation. 2009;119:445–451. doi: 10.1161/CIRCULATIONAHA.108.775221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, Webb GD, Redington AN. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 20.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 21.Tweddell JS, Simpson P, Li S-H, Dunham-Ingle J, Bartz PJ, Earing MG, Pelech AN. Timing and Technique of Pulmonary Valve Replacement in the Patient With Tetralogy of Fallot. Sem Thorac Cardiovasc Surg: Pediatric Cardiac Surgery Annual. 2012;15:27–33. doi: 10.1053/j.pcsu.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Cheung MMH, Konstantinov IE, Redington AN. Late Complications of Repair of Tetralogy of Fallot and Indications for Pulmonary Valve Replacement. Sem Thorac Cardiovasc Surg. 2005;17:155–159. doi: 10.1053/j.semtcvs.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Geva T. Indications for pulmonary valve replacement in repaired tetralogy of Fallot: the quest continues. Circulation. 2013;128:1855–1857. doi: 10.1161/CIRCULATIONAHA.113.005878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheung EW-Y, Wong WH-S, Cheung Y-F. Meta-analysis of pulmonary valve replacement after operative repair of tetralogy of fallot. AmJ Cardiol. 2010;106:552–557. doi: 10.1016/j.amjcard.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 25.Mercer-Rosa L, Pinto N, Yang W, Tanel R, Goldmuntz E. 22q11. 2 Deletion syndrome is associated with perioperative outcome in tetralogy of Fallot. J Thorac Cardiovasc Surg. 2013;146:868–873. doi: 10.1016/j.jtcvs.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Byrne ML, Yang W, Mercer-Rosa L, Parnell AS, Oster ME, Levenbrown Y, Tanel R, Goldmuntz E. 22q11.2 Deletion syndrome is associated with increased perioperative events and more complicated postoperative course in infants undergoing infant operative correction of truncus arteriosus communis or interrupted aortic arch. J Thorac Cardiovasc Surg. 2014:1–9. doi: 10.1016/j.jtcvs.2014.02.011. e-published ahead of print. http://dx.doi.org/10.1016/j.jtcvs.2014.02.011. [DOI] [PMC free article] [PubMed]
- 27.Frigiola A, Tsang V, Nordmeyer J, Lurz P, van Doorn C, Taylor AM, Bonhoeffer P, de Leval M. Current approaches to pulmonary regurgitation. European Journal of Cardio-Thoracic Surgery. 2008;34:576–581. doi: 10.1016/j.ejcts.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 28.Gatlin SW, Kim DW, Mahle WT. Cost Analysis of Percutaneous Pulmonary Valve Replacement. Am J Cardiol. 2011;108:572–574. doi: 10.1016/j.amjcard.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 29.Vergales JE, Wanchek T, Novicoff W, Kron IL, Lim DS. Cost-analysis of percutaneous pulmonary valve implantation compared to surgical pulmonary valve replacement. Cathet Cardiovasc Intervent. 2013;82:1147–1153. doi: 10.1002/ccd.25128. [DOI] [PubMed] [Google Scholar]
- 30.Karamlou T, Diggs BS, Ungerleider RM, Welke KF. Adults or big kids: what is the ideal clinical environment for management of grown-up patients with congenital heart disease? Ann Thorac Surg. 2010;90:573–579. doi: 10.1016/j.athoracsur.2010.02.078. [DOI] [PubMed] [Google Scholar]