Abstract
Axon regeneration in the mature central nervous system is limited by extrinsic inhibitory signals and a postnatal decline in neurons' intrinsic growth capacity. Neuronal levels of the second messenger cAMP are important in regulating both intrinsic growth capacity and neurons' responses to extrinsic factors. Approaches which increase intracellular cAMP in neurons enhance neurite outgrowth and facilitate regeneration after injury. Thus, understanding the factors which affect cAMP in neurons is of potential therapeutic importance. Recently, soluble adenylyl cyclase (sAC, ADCY10), the ubiquitous, non-transmembrane adenylyl cyclase, was found to play a key role in neuronal survival and axon growth. sAC is activated by bicarbonate and cations and may translate physiologic signals from metabolism and electrical activity into a neuron's decision to survive or regenerate. Here we critically review the literature surrounding sAC and cAMP signaling in neurons to further elucidate the potential role of sAC signaling in neurite outgrowth and regeneration.
Introduction
Unlike immature neurons that demonstrate a robust capacity to regenerate, adult mammalian central nervous system (CNS) neurons fail to regenerate in response to injury [1,2]. Failed adult regeneration can be attributed to both intrinsic and extrinsic factors. In the adult CNS, injury results in the release or deposition of inhibitory molecules within myelin [3–6] and the glial scar [7–9]. A relative insufficiency of neurotrophic signaling also contributes [10]. Additionally, neurons decrease in their intrinsic growth capacity as a function of maturity, as reflected by a postnatal decline in ability for rapid neurite extension [11]. While simply blocking the expression or presentation of glial-assocaited inhibitory factors can promote axonal regeneration after injury [6,8], the relative ability of immature neurons to overcome inhibitory cues [12–14] suggest that therapeutic targeting of the factors that influence the intrinsic growth state of the neuron may be effective in regenerative medicine.
Levels of the second messenger adenosine 3′-5′-cyclic monophosphate (cAMP) correlate with intrinsic neurite outgrowth capacity of neurons [15]. The effect of cAMP on neurite outgrowth was first demonstrated in chick dorsal root ganglia cells, in which cAMP treatment increased both length and number of axons [16]. Since then, the role of cAMP in the growth and guidance of axons has been extensively studied. As neurons mature, intracellular cAMP levels decline along with the ability of axons to regenerate after injury [15,17]. In mature neurons, increasing cAMP reverses this effect, and attenuates inhibitory signaling derived from molecules in CNS myelin and the glial scar [18]. Understanding the mechanisms underlying cAMP production may be vital for the development of therapeutic strategies to address regenerative failure in CNS injury. In this review we will discuss soluble adenylyl cyclase (sAC), a member of the adenylyl cyclase (AC) family that has recently been recognized as a potent regulator of neurite outgrowth and neuronal survival [19].
cAMP in neurite outgrowth
The induction of neurite outgrowth by cAMP can be divided into 2 phases, the first acting at the growth cone/axonal tip and the second at the cell body and nucleus, inducing transcriptional changes that sustain neurite outgrowth [20,21]. Growth cones are a specialized form of lamellipodia responsible for guiding and exerting tension on the trailing axon [22]. At the growth cone, cAMP-mediated activation of protein kinase A (PKA) causes inactivation of the small G-protein Rho, which results in potentiation of neurite outgrowth [14]. While growth cone dynamics are important for proper axonal guidance and motility, their activities are largely independent from neurite assembly [23]. The ability of cAMP to influence growth cone directionality was first observed in experiments utilizing extracellular, cell-permeable, cAMP gradients, which induced turning and directional attraction of growth cones [24]. When a gradient of cell-permeable cAMP was replaced with a bath application, modulation of responses to other signaling molecules (see below) was observed [25]. In some cases, axons were even found to exhibit opposite reactions to guidance cues when cAMP was present versus absent, indicating a potent role of cAMP signaling in the intrinsic regulation of neuronal growth responses [26].
What are the cues being modulated by cAMP and how does cAMP exert these effects? Paracrine factors are critical for the proper guidance and development of neurons. One family of structurally related molecules referred to as neurotrophins have been widely studied and are involved in a variety of neuronal functions [27]. The first discovered neurotrophin was nerve growth factor (NGF) [28]. While NGF was the first discovered, and arguably most heavily studied neurotrophin, it may not be the best surrogate from which to infer a role for cAMP in neurotrophin signaling. Only a select few neuronal subtypes respond to NGF, indicating that the neurotrophic effects for this specific molecule may be unique and/or highly specialized [29]. Other well-characterized neurotrophins include brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin-4/5 (NT-4/5) [27,30]. Like NGF, other neurotrophins induce survival and neurite growth in various, often specific neuronal subtypes. All neurotrophins bind to a sub-family of receptor tyrosine kinases (RTKs) known as tropomyosin receptor kinase (Trk) receptors [31], in addition to binding with a lower affinity to the tumor necrosis alpha-receptor (TNFR) family member p75 [32]. While all neurotrophins bind p75, they differentially bind to for individual Trk receptors. TrkA is specific for NGF, TrkB binds both BDNF and NT4/5, and TrkC binds NT3 [33]. Activation of Trk receptors is similarly sufficient to promote survival and neurite growth [34,35].
BDNF activation of TrkB, as well as the trafficking of this receptor, is cAMP-dependent [36]. In contrast, NGF and NT-3-mediated phosphorylation of TrkA and TrkC, respectively, do not require cAMP. This is intriguing as NGF and NT-3 have considerably weaker capacity to promote survival or regeneration in retinal ganglion cells [37], while both neurotrophin ligands of TrkB (BDNF and NT-4/5) demonstrate benefit in both regards [38]. Additionally, ‘priming’ neurons via pre-treatment with BDNF overcomes inhibitory cues in CNS myelin in a cAMP-dependent manner [39,40]. The ability of TrkB ligands to modify regenerative capacity is consistent with the theory that cAMP is important in mediating growth and regenerative capacity of neurons.
Other signaling ligands similarly promote neurite growth, albeit through different signaling pathways. For example, pituitary adenylyl cyclase-activating peptide (PACAP) demonstrates similar neurite outgrowth promotion as NGF in PC12 cells [41] and also promotes neurite outgrowth and survival in primary neurons [42–44]. PACAP exerts these effects via activation of transmembrane ACs (tmACs) which associate with the PACAP receptor, PAC1 [41]. PACAP and NGF both activate the small GTP-binding protein Rap1, which leads to sustained activation of ERK mitogen-activated protein kinase, which is required for neurite outgrowth [41,45–48]. NGF activation of Rap1 has been suggested to be dependent on protein kinase A (PKA) activation, which requires cAMP [45,46], albeit whether NGF-dependent neurite extension is cAMP-dependent is controversial [49]. However, synergistic effects of cAMP and NGF on signaling and neurite outgrowth have been observed [50], and NGF-dependent neurite outgrowth is not supported when NGF is replaced by cAMP [51], which confirms that distinct signaling pathways exist for NGF and cAMP.
The role of cAMP in the nucleus-mediated promotion of neurite outgrowth has been most closely tied to phosphorylation of cAMP response element binding protein (CREB) [52,53]. Increased intracellular cAMP leads to phosphorylation of CREB, which is critical for the transcriptional programs needed for neuritogenesis [54]. Constitutively activated CREB results in robust neurite outgrowth and attenuated responsiveness to CNS molecules which inhibit regeneration [53]. Interestingly, these effects mirror the response of neurons pre-treated with exogenous BDNF and/or cAMP [18,53].
Besides neurotrophin stimulation, neuronal activity is beneficial for both neuronal survival and proper guidance and innervation during development [19,55,56]. In the eye, spontaneous retinal activity is required for proper development, and this spontaneous activity results in increased intracellular calcium and cAMP [57]. Such calcium transients are known to affect in vivo axon extension and pathfinding [58]. Damaged mature neurons have both decreased spontaneous electrical activity and decreased cAMP and calcium influx. The correlation between spontaneous activity of immature neurons and calcium/cAMP levels further supports the importance of cAMP signaling as a therapeutic target for CNS regeneration.
How is cAMP produced?
Adenylyl cyclases (ACs) are the family of enzymes that synthesize cAMP. There are 9 mammalian transmembrane ACs (tmACs) that are activated by G-protein coupled receptors (GPCRs). While all tmACs are activated by Gsα, these tmACs are differentially regulated by Gβγ, protein kinase phosphorylation and calcium. In addition to signaling in response to extracellular stimuli at the plasma membrane, tmACs also can continue cAMP production even after clearance from the plasma membrane associated with uptake and trafficking of membrane-derived endosomes [59,60]. In contrast, sAC (ACDY10) is unique among the ACs in lacking transmembrane domains, insensitivity to G-proteins, and bicarbonate-mediated activation, in addition to being activated by calcium. While sAC is most readily detectable in testis where it has a role in sperm maturation [61], bicarbonate-stimulated cAMP production has been observed in most other tissues and is thought to be associated with intracellular organelles like the mitochondrion and the nucleus [62]. Due to ubiquitous carbonic anhydrases, intracellular pH affects bicarbonate levels that in turn directly modulate sAC activity (Fig. 1) [63]. As mentioned above, sAC is also activated by divalent cations such as calcium [64], via an independent mechanism that can be synergistic with bicarbonate [65].
Figure 1. sAC associates with mitochondria and responds to decreasing pH via bicarbonate activation.
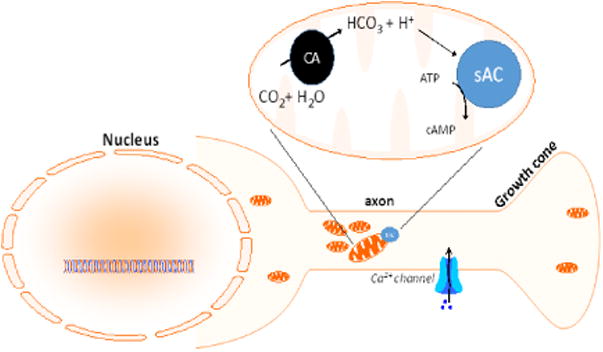
Increases in carbon dioxide (CO2) resulting from metabolic energy production results in decreased pH due to the ubiquitous presence of carbonic anhydrase (CA) which converts CO2 into bicarbonate (HCO3), which in turn activates sAC.
cAMP and tmAC function have classically been studied using forskolin, a potent pharmacologic activator of all tmACs except AC9 [66,67]. sAC is also insensitive to direct stimulation by forskolin [68]. Elucidating the individual functions of different ACs has been hindered by a lack of useful antibodies for the different ACs, as well as a lack of specific inhibitors [54,69,70]. KH7 is an inhibitor that shows selectivity for sAC and has been used in conjunction with RNA interference (RNAi) to demonstrate sAC-specific functions [71]. Recently, knock-out mice for individual ACs, including sAC, have become available that should allow assignment of specific cyclase functions.
Soluble adenylyl cyclase
The first reported observation of a “soluble” AC with no transmembrane association was in 1975 [72]. Over 2 decades later the purification and cloning of sAC was reported [73]. The newly discovered sAC cDNA encoded a 187-kDa protein containing 2 catalytic domains (C1 and C2) that demonstrated similarity to AC domains from different cyanobacterial species, suggesting that the sAC catalytic domains evolved as a fusion of 2 distinct bacterial cyclases [73]. The cyanobacterial ACs also demonstrate bicarbonate sensitivity, again supporting an evolutionarily conserved bicarbonate-mediated activity [61,73]. This confirmation of a mammalian, bicarbonate-sensitive AC provided a vital insight into the mechanisms underlying bicarbonate-induced spermatozoa competence and other tmAC-independent, cAMP-mediated cellular events [74–77].
Human sAC is the product of the ADCY10 gene, which is >100 kb and is comprised of >33 exons [64]. While there is a single mammalian sAC gene, there are a number of different sAC mRNAs that have been identified by PCR from various cell types. These isoforms are consistent with ADCY10 mRNA alternative RNA splicing and promoter usage [78,79]. However, validation of the existence of these various sAC isoforms has been difficult due to an inability to reliably detect sAC protein by western blotting in tissues other than testes. The original purification of sAC from testis in both rat and human also revealed an abundant, functional 48 kDa protein [80,81]. This smaller sAC may be a cleavage product of the larger 187-kDa protein, although an alternatively-spliced mRNA has been cloned that would encode this shorter isoform [73,78]. This N-terminal, catalytically-active fragment of full-length sAC contains the 2 catalytic domains [82]. C-terminal to the C2 domain in full-length testes sAC are a putative auto-inhibitory region [83] and canonical p-loop and leucine zipper motifs [73]. While the p-loop of the C-terminal domain of sAC is dispensable for catalytic activity, this region of sAC which is not present in tmACs may allow sAC to function uniquely as a cellular ATP sensor [65,73,83]. In general, the function of the long C-terminal tail of full-length remains unknown, but could function to target sAC via protein-protein interactions to specific intracellular sites of action.
sAC function has been studied in vivo using knock-out mice. The first mouse model used to study sAC function was a deletion in the 2nd-4th exons encoding the C1 catalytic domain, resulting in infertility due to dysfunctional sperm and dyslipidemia [79,83,84]. In these animals, a residual sAC mRNA was detected that encodes a protein containing the C2 domain and the remaining 140-kDa C-terminal region of full-length sAC, although whether this C1-deleted protein has a function remains unclear. Results obtained with the original C1-deleted mouse do show that the C1 domain is necessary for some sAC physiologic functions, at least in testis, and are consistent with the observation from tmACs that both C1 and C2 domains contribute to the active site required for AC activity [54], but whether a C1 and C2 domain are required within the same protein for sAC activity is still an open question. The sAC C1 and C2 domains are homologous to bacterial ACs, and within bacteria these domains function in response to bicarbonate as individual catalytic units [61], so sAC C2-only isoforms could also be catalytically active on their own. It is also possible that C2-only sAC could either self-associate or associate with catalytic domains of tmACs to reconstitute sAC activity in specific cell types. Studies conducted in Sf9 insect cells seem to contradict the theory that C1- or C2-only sAC variants can have catalytic function on their own, showing that only recombinant derivatives of human sAC containing both C1 and C2 were capable of adenylyl cyclase activity [64]. Whether sAC variants lacking catalytic domains can restore physiologic cellular function with or without catalytic activity remains an important question, for example as recently studied in bronchial epithelial cells from C2-null transgenic animals [85].
sAC is found in almost every mammalian tissue [62,64,86–88], including neurons [89]. Within the cell, sAC localization and activity are found in mitochondria, nuclei, and cytoplasmic compartments including in axons, dendrites and growth cones (Fig. 2) [19,88–94]. However, despite ubiquitous sAC-related activity throughout various tissues and organelles, there have been only 3 reported phenotypes for the C1-deleted sAC transgenic mouse: infertility and dyslipidemia [95], increased intraocular pressure [96], and altered insulin response to glucose stimulation [97]. As there are 10 ACs in all with multiple ACs expressed in every cell, deletion of a single cyclase may only demonstrate a limited phenotype in certain cell conditions. Given sAC's activation by bicarbonate, many sAC functions may only be manifest in mice stressed pathologically as in ischemia, for example.
Figure 2. sAC is found in various neuronal compartments.
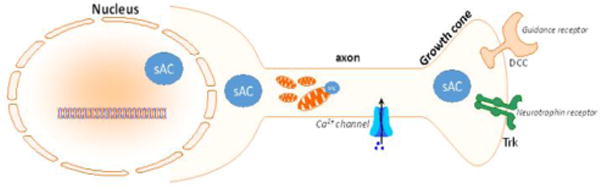
sAC localization and activity has been demonstrated in various neuronal compartments including the nucleus, axon, growth cone and cell body.
sAC in neurite outgrowth
What is the role of sAC in neurite outgrowth? sAC signaling associated neurite outgrowth appears to be regulated by neurotrophins. tmAC-catalyzed cAMP production appears to operate via distinct signaling pathways in synergy with NGF-dependent signaling, as demonstrated in PACAP signaling studies [50]. Although controversial, NGF relies on Rap1 activation for sustained Erk signaling, and sAC has been shown to be necessary for NGF-mediated activation of Rap1 [98]. A role for sAC in the signaling by TrkB ligands, BDNF and NT-4/5, is also supported: for example, trafficking and phosphorylation of TrkB has been shown to be cAMP-dependent [36,99], and the same intrinsic growth states associated with BDNF attraction by growth cones [100] has been shown to increase neuronal survival and regeneration [101].
Using pharmacological inhibitors and siRNA against sAC, our lab has recently demonstrated that sAC is necessary for retinal ganglion cell (RGC) survival and axon growth [19]. These data also demonstrated that other calcium-responsive tmACs, AC1 and AC8, were dispensable for the survival and neurite outgrowth of RGCs in vitro, whereas reduction in sAC expression by siRNA or function by pharmacologic inhibition significantly reduced RGC survival and neurite growth [19]. Evidence in support of a role for tmACs in neuronal survival and neurite outgrowth is mainly based on the use of the tmAC activator, forskolin. However, forskolin is a strong tmAC agonist that could result in non-physiological levels of cAMP that overwhelm normal intracellular cAMP compartmentation. As a result, it is possible that forskolin stimulation may not be representative of normal tmAC function in neurons. Taken together, these data suggest that sAC is a critical AC controlling cAMP-mediated intrinsic survival and neurite outgrowth properties of neurons, at least in RGCs.
Additionally, a novel role for sAC has been recently documented in the priming effect of BDNF on primary neurons which overcomes inhibition by CNS myelin [102]. In that paper it was shown that the benefit of BDNF pre-treatment was lost when sAC function was disrupted via pharmacologic inhibition or genetic silencing. In addition to this important role in BDNF-mediated neuronal ‘priming’, the authors also demonstrated that sAC overexpression in the optic nerve enhanced regeneration after optic nerve crush [102]. It was previously appreciated that cAMP promotes regeneration, so the degree to which this effect is specific to sAC or any other source of cAMP remains to be studied in future work on sAC function in neuronal regeneration. Nevertheless these data support the potential therapeutic utility of sAC activation in promoting neurite growth in the CNS such as after spinal cord injury.
Besides neurotrophin-stimulated neurite outgrowth, sAC has also been implicated in netrin-1 mediated growth cone guidance (Fig. 3). Netrin-1 is released at the optic nerve head and attracts RGC axons out of the eye into the optic nerve [15]. As RGCs age, their responsiveness to netrin-1 falls in association with a decline in cAMP. Interventions which increase cAMP restore netrin-1 responsiveness in a manner similar to the enhanced survival and neurite outgrowth of cAMP-rich immature neurons [15]. In cultured dorsal root ganglion neurons, attenuation of sAC function via pharmacologic inhibition or siRNA caused a loss of netrin-1 responsiveness, while sAC overexpression increased axonal extension and growth cone elaboration [103]. Interestingly, no gross defect in axonal path-finding during development was observed in the sAC C1 knock-out mouse [104]. These findings could suggest that redundant or complementary mechanisms exist within neurons for sAC function in vivo. However, defects in netrin-mediated guidance may not have been detectable in the sAC C1 knock-out mouse studies [104], as sAC's potential role may have yielded effects that could be either too subtle for the assays employed, or confined to specific or untested neuronal subpopulations. As such, it is difficult to conclude a complete absence of disrupted axonal path-finding solely from the assays utilized in this work [104]. Additionally, it may also be possible that C1-deficient sAC isoforms are readily expressed and may somehow compensate for the loss of the C1 domain [73].
Figure 3. sAC has been linked to various neurotrophic signaling events in the growth cone.
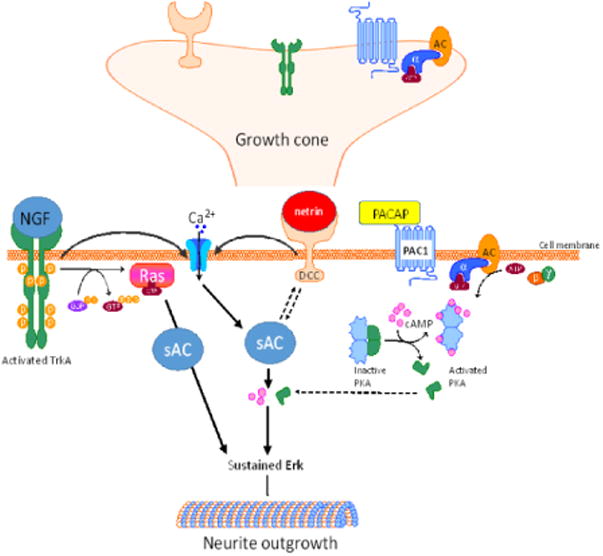
cAMP production, and subsequent protein kinase A (PKA) activation, by tmACs such as that stimulated by PACAP, has been shown to be distinct from other neurotrophic signaling pathways. However, sAC-mediated production of cAMP has been shown to be important for the neurite outgrowth signaling via nerve growth factor (NGF). sAC also mediates neurite outgrowth and growth cone guidance mediated by netrin, and sAC function also increases the neuronal response to netrin binding to the deleted in colorectal cancer (DCC) receptor. Interestingly, both netrin and NGF cause localized influx of calcium, which also activates sAC.
Besides being activated by bicarbonate, sAC is directly activated by calcium independently of calmodulin [88,105]. Like cAMP, calcium-dependent pathways play key roles in neuronal development and function. Netrin-1 signaling also relies on calcium influx from intra- and extracellular sources for function [106], and NGF induces intracellular calcium elevations [107]. As mentioned above, calcium transients secondary to spontaneous electrical activity in developing neurons affect in vivo axon extension and pathfinding by growth cones [58]. Damaged mature neurons may not exhibit the same spontaneous electrical activity, and this could explain a decrease in cAMP observed after injury, although acutely there may be other situations when there is an increase in calcium influx after injury [108]. That there was no decrease in survival and axonal extension in RGCs from the double knockout of the calcium-sensitive tmACs, AC1 and AC8, supports the hypothesis that sAC is a primary contributor to the activity- and calcium-dependent elevation of cAMP and promotion of of axon growth (Fig. 4) [19]. Finally, sAC has also been shown to play a role in the nucleus-associated promotion of neurite outgrowth, the transcriptional regulation needed for sustained axonal extension, as sAC enhances CREB phosphorylation (Fig. 5) [90].
Figure 4. Electrical activity leads to activation of sAC.
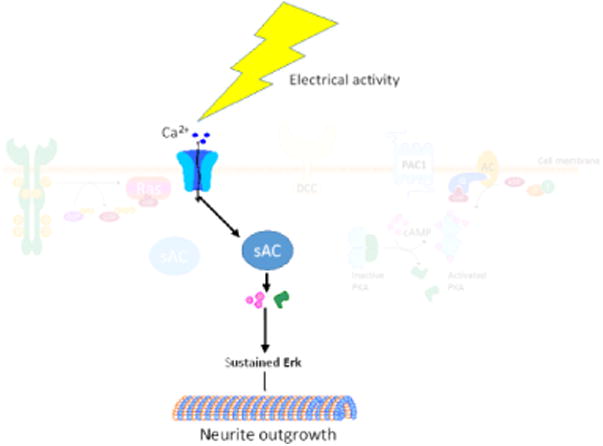
Electrical activity in the axon leads to influx, and intracellular release, of calcium which then directly activates sAC. This leads to increases in intracellular cAMP, which activates PKA and promotes neurite outgrowth.
Figure 5. sAC function leads to transcriptional changes in the nucleus.
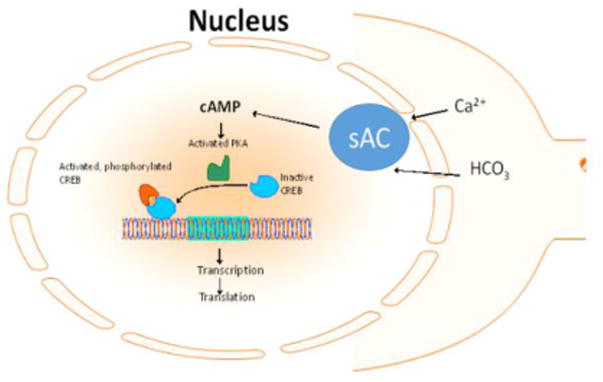
Nuclear-associated sAC responds to intracellular cues such as calcium and bicarbonate and leads to activation of CREB, which leads to transcriptional changes that support sustained neurite outgrowth.
Summary
Existing data show that sAC serves important functions in neuronal survival and neurite extension. However, mechanistically little is known regarding concerning sAC activity in these processes. While sAC is thought to be associated with organelles such as the nucleus and the mitochondrion, it is unknown whether these are relevant sites of sAC action in neurons during development or injury. Future studies evaluating the specific effects of sAC localization in different cellular compartments may lend to a better understanding of the role of sAC in these processes. In addition, how sAC is localized within cells is unknown and whether sAC is associated with scaffold proteins such as A-kinase anchoring proteins conferring sAC compartmentation requires further investigation. cAMP effectors include PKA, Epac and cyclic nucleotide gated ion channels. With little exception, which of these targets mediate sAC signals also has not been studied. sAC is unique among the ACs in being regulated by bicarbonate and calcium and, as such, is poised to play critical roles in neuronal disease and regeneration.
The inability of several studies to recapitulate effects in vivo of genetic or pharmacologic disruption of sAC function in vitro, at least in using currently available transgenic animals, is an obstacle to the study of sAC moving forward. As the currently available transgenic models only delete portions of the full-length protein, and only target the C1 or C2 domains individually, it is difficult to draw firm conclusions. There is a clear need for more comprehensive knock-out and perhaps knock-in models to better elucidate in vivo roles for this enzyme. Similarly, although the discovery of sAC-specific pharmacologic inhibitors has been invaluable, and may also prove potentially therapeutically useful, the lack of potent and specific pharmacological activators of sAC could greatly aid in assessing the therapeutic potential for targeting sAC. Investigations into the mechanisms underlying sAC function are justified by the functional data provided by several laboratories including our own and should reveal novel strategies for manipulating sAC activity in beneficial therapies.
Acknowledgments
The authors gratefully acknowledge funding support from the National Eye Institute (R01-022129 and P30-022589) and an unrestricted grant from Research to Prevent Blindness, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, et al. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 3.Ng WP, Cartel N, Roder J, Roach A, Lozano A. Human central nervous system myelin inhibits neurite outgrowth. Brain Res. 1996;720:17–24. doi: 10.1016/0006-8993(96)00062-5. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 6.Chaudhry N, Filbin MT. Myelin-associated inhibitory signaling and strategies to overcome inhibition. J Cereb Blood Flow Metab. 2007;27:1096–1107. doi: 10.1038/sj.jcbfm.9600407. doi:9600407 [pii] 10.1038/sj.jcbfm.9600407. [DOI] [PubMed] [Google Scholar]
- 7.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. doi:nrn1956 [pii] 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17:120–127. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Hynds DL, Snow DM. Neurite outgrowth inhibition by chondroitin sulfate proteoglycan: stalling/stopping exceeds turning in human neuroblastoma growth cones. Exp Neurol. 1999;160:244–255. doi: 10.1006/exnr.1999.7212. [DOI] [PubMed] [Google Scholar]
- 10.Tuszynski MH, Gabriel K, Gage FH, Suhr S, Meyer S, Rosetti A. Nerve growth factor delivery by gene transfer induces differential outgrowth of sensory, motor, and noradrenergic neurites after adult spinal cord injury. Exp Neurol. 1996;137:157–73. doi: 10.1006/exnr.1996.0016. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg JL, Klassen MP, Hua Y, a Barres B. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–4. doi: 10.1126/science.1068428. [DOI] [PubMed] [Google Scholar]
- 12.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010 doi: 10.1038/nn.2603. doi:nn.2603 [pii] 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150:1264–73. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandtlow CE. Regeneration in the central nervous system. Exp Gerontol. 2003;38:79–86. doi: 10.1016/s0531-5565(02)00165-1. [DOI] [PubMed] [Google Scholar]
- 15.Shewan D, Dwivedy A, Anderson R, Holt CE. Age-related changes underlie switch in netrin-1 responsiveness as growth cones advance along visual pathway. Nat Neurosci. 2002;5:955–62. doi: 10.1038/nn919. [DOI] [PubMed] [Google Scholar]
- 16.Roisen FJ, Murphy RA, Pichichero ME, Braden WG. Cyclic adenosine monophosphate stimulation of axonal elongation. Science. 1972;175:73–4. doi: 10.1126/science.175.4017.73. [DOI] [PubMed] [Google Scholar]
- 17.Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J Neurosci. 2001;21:4731–9. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aglah C, Gordon T, Posse de Chaves EI. cAMP promotes neurite outgrowth and extension through protein kinase A but independently of Erk activation in cultured rat motoneurons. Neuropharmacology. 2008;55:8–17. doi: 10.1016/j.neuropharm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Corredor RG, Trakhtenberg EF, Pita-Thomas W, Jin X, Hu Y, Goldberg JL. Soluble adenylyl cyclase activity is necessary for retinal ganglion cell survival and axon growth. J Neurosci. 2012;32:7734–44. doi: 10.1523/JNEUROSCI.5288-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- 21.Smith DS, Skene JH. A transcription-dependent switch controls competence of adult neurons for distinct modes of axon growth. J Neurosci. 1997;17:646–58. doi: 10.1523/JNEUROSCI.17-02-00646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent EW, Gertler FB. Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron. 2003;40:209–27. doi: 10.1016/s0896-6273(03)00633-0. [DOI] [PubMed] [Google Scholar]
- 23.a Lundquist E. Rac proteins and the control of axon development. Curr Opin Neurobiol. 2003;13:384–90. doi: 10.1016/S0959-4388(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 24.Gundersen RW, Barrett JN. Characterization of the turning response of dorsal root neurites toward nerve growth factor. J Cell Biol. 1980;87:546–54. doi: 10.1083/jcb.87.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–63. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–8. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- 27.Barde YA. The nerve growth factor family. Prog Growth Factor Res. 1990;2:237–48. doi: 10.1016/0955-2235(90)90021-b. [DOI] [PubMed] [Google Scholar]
- 28.Levi-Montalcini R. The nerve growth factor: its mode of action on sensory and sympathetic nerve cells. Harvey Lect. 1966;60:217–59. [PubMed] [Google Scholar]
- 29.Hofer MM, Barde YA. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988;331:261–2. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- 30.Ibáñez CF. Emerging themes in structural biology of neurotrophic factors. Trends Neurosci. 1998;21:438–44. doi: 10.1016/s0166-2236(98)01266-1. [DOI] [PubMed] [Google Scholar]
- 31.Bibel M, Barde YA. Neurotrophins: key regulators of cell fate and cell shape in the vertebrate nervous system. Genes Dev. 2000;14:2919–37. doi: 10.1101/gad.841400. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita T, Tucker KL, Barde YA. Neurotrophin binding to the p75 receptor modulates Rho activity and axonal outgrowth. Neuron. 1999;24:585–593. doi: 10.1016/s0896-6273(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 33.Barbacid M. The Trk family of neurotrophin receptors. J Neurobiol. 1994;25:1386–403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Cho S, Goldberg JL. Neurotrophic effect of a novel TrkB agonist on retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:1747–54. doi: 10.1167/iovs.09-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldberg JL, Barres BA. The relationship between neuronal survival and regeneration. Annu Rev Neurosci. 2000;23:579–612. doi: 10.1146/annurev.neuro.23.1.579. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–72. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 37.Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GTA, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- 38.Peinado-Ramón P, Salvador M, Villegas-Pérez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:489–500. [PubMed] [Google Scholar]
- 39.Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 40.Gao Y, Nikulina E, Mellado W, Filbin MT. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J Neurosci. 2003;23:11770–7. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, et al. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–5. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez BJ, Basille M, Vaudry D, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide promotes cell survival and neurite outgrowth in rat cerebellar neuroblasts. Neuroscience. 1997;78:419–30. doi: 10.1016/s0306-4522(96)00617-3. [DOI] [PubMed] [Google Scholar]
- 43.Fukiage C, Nakajima T, Takayama Y, Minagawa Y, Shearer TR, Azuma M. PACAP induces neurite outgrowth in cultured trigeminal ganglion cells and recovery of corneal sensitivity after flap surgery in rabbits. Am J Ophthalmol. 2007;143:255–262. doi: 10.1016/j.ajo.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 44.Villalba M, Bockaert J, Journot L. Pituitary adenylate cyclase-activating polypeptide (PACAP-38) protects cerebellar granule neurons from apoptosis by activating the mitogen-activated protein kinase (MAP kinase) pathway. J Neurosci. 1997;17:83–90. doi: 10.1523/JNEUROSCI.17-01-00083.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 46.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–6. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 47.Heidemann SR, Joshi HC, Schechter A, Fletcher JR, Bothwell M. Synergistic effects of cyclic AMP and nerve growth factor on neurite outgrowth and microtubule stability of PC12 cells. J Cell Biol. 1985;100:916–27. doi: 10.1083/jcb.100.3.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–180. doi: 10.1038/35044547. [DOI] [PubMed] [Google Scholar]
- 49.a Frazier W, Ohlendorf CE, Boyd LF, Aloe L, Johnson EM, a Ferrendelli J, et al. Mechanism of action of nerve growth factor and cyclic AMP on neurite outgrowth in embryonic chick sensory ganglia: demonstration of independent pathways of stimulation. Proc Natl Acad Sci U S A. 1973;70:2448–52. doi: 10.1073/pnas.70.8.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaudry D, Stork PJS, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: making the right connections. Science. 2002;296:1648–9. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 51.Gunning PW, Landreth GE, a Bothwell M, Shooter EM. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J Cell Biol. 1981;89:240–5. doi: 10.1083/jcb.89.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cao Z, Gao Y, Bryson JB, Hou J, Chaudhry N, Siddiq M, et al. The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J Neurosci. 2006;26:5565–73. doi: 10.1523/JNEUROSCI.0815-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao Y, Deng K, Hou J, Bryson JB, Barco A, Nikulina E, et al. Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron. 2004;44:609–21. doi: 10.1016/j.neuron.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 54.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17:5, 22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–52. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- 56.Corredor RG, Goldberg JL. Electrical activity enhances neuronal survival and regeneration. J Neural Eng. 2009;6:055001. doi: 10.1088/1741-2560/6/5/055001. [DOI] [PubMed] [Google Scholar]
- 57.Dunn TA, Wang CT, Colicos MA, Zaccolo M, DiPilato LM, Zhang J, et al. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J Neurosci. 2006;26:12807–15. doi: 10.1523/JNEUROSCI.3238-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–5. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- 59.Calebiro D, Nikolaev VO, Gagliani MC, de Filippis T, Dees C, Tacchetti C, et al. Persistent cAMP-signals triggered by internalized G-protein-coupled receptors. PLoS Biol. 2009;7:e1000172. doi: 10.1371/journal.pbio.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrandon S, Feinstein TN, Castro M, Wang B, Bouley R, Potts JT, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat Chem Biol. 2009;5:734–42. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y. Soluble Adenylyl Cyclase as an Evolutionarily Conserved Bicarbonate Sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. 80. [DOI] [PubMed] [Google Scholar]
- 62.Mittag TW, Guo WB, Kobayashi K. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am J Physiol. 1993;264:F1060–4. doi: 10.1152/ajprenal.1993.264.6.F1060. [DOI] [PubMed] [Google Scholar]
- 63.Pastor-Soler N, Beaulieu V, Litvin TN, Da Silva N, Chen Y, Brown D, et al. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J Biol Chem. 2003;278:49523–9. doi: 10.1074/jbc.M309543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geng W, Wang Z, Zhang J, Reed BY, Pak CYC, Moe OW. Cloning and characterization of the human soluble adenylyl cyclase. Am J Physiol Cell Physiol. 2005;288:C1305–16. doi: 10.1152/ajpcell.00584.2004. [DOI] [PubMed] [Google Scholar]
- 65.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–6. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 66.Simonds WF. G protein regulation of adenylate cyclase. Trends Pharmacol Sci. 1999;20:66–73. doi: 10.1016/s0165-6147(99)01307-3. [DOI] [PubMed] [Google Scholar]
- 67.Sunahara RK, Dessauer CW, Gilman AG. Complexity and diversity of mammalian adenylyl cyclases. Annu Rev Pharmacol Toxicol. 1996;36:461–80. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- 68.Kamenetsky M, Middelhaufe S, Bank EM, Levin LR, Buck J, Steegborn C. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–39. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steegborn C, Litvin TN, Levin LR, Buck J, Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol. 2005;12:32–7. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha. GTPgammaS. Science. 1997;278:1907–16. doi: 10.1126/science.278.5345.1907. [DOI] [PubMed] [Google Scholar]
- 71.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, Kamenetsky M, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–59. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braun T, Dods RF. Development of a Mn-2+-sensitive, “soluble” adenylate cyclase in rat testis. Proc Natl Acad Sci U S A. 1975;72:1097–101. doi: 10.1073/pnas.72.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci U S A. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Visconti PE, Galantino-Homer H, Moore GD, Bailey JL, Ning X, Fornes M, et al. The molecular basis of sperm capacitation. J Androl. 19:242–8. n.d. [PubMed] [Google Scholar]
- 75.Garty NB, Salomon Y. Stimulation of partially purified adenylate cyclase from bull sperm by bicarbonate. FEBS Lett. 1987;218:148–52. doi: 10.1016/0014-5793(87)81036-0. [DOI] [PubMed] [Google Scholar]
- 76.Okamura N, Tajima Y, Onoe S, Sugita Y. Purification of bicarbonate-sensitive sperm adenylylcyclase by 4-acetamido-4′-isothiocyanostilbene-2,2′-disulfonic acid-affinity chromatography. J Biol Chem. 1991;266:17754–9. [PubMed] [Google Scholar]
- 77.Okamura N, Onoe S, Kawakura K, Tajima Y, Sugita Y. Effects of a membrane-bound trypsin-like proteinase and seminal proteinase inhibitors on the bicarbonate-sensitive adenylate cyclase in porcine sperm plasma membranes. Biochim Biophys Acta. 1990;1035:83–9. doi: 10.1016/0304-4165(90)90177-x. [DOI] [PubMed] [Google Scholar]
- 78.Jaiswal BS, Conti M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J Biol Chem. 2001;276:31698–708. doi: 10.1074/jbc.M011698200. [DOI] [PubMed] [Google Scholar]
- 79.Farrell J, Ramos L, Tresguerres M, Kamenetsky M, Levin LR, Buck J. Somatic “soluble” adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex “knockout” mice. PLoS One. 2008;3:e3251. doi: 10.1371/journal.pone.0003251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gordeladze JO, Hansson V. Purification and kinetic properties of the soluble Mn2+-dependent adenylyl cyclase of the rat testis. Mol Cell Endocrinol. 1981;23:125–36. doi: 10.1016/0303-7207(81)90064-2. [DOI] [PubMed] [Google Scholar]
- 81.Gordeladze JO, Andersen D, Hansson V. Physicochemical and kinetic properties of the Mn2+-dependent adenylyl cyclase of the human testis. J Clin Endocrinol Metab. 1981;53:465–71. doi: 10.1210/jcem-53-3-465. [DOI] [PubMed] [Google Scholar]
- 82.Wuttke MS, Buck J, Levin LR. Bicarbonate-regulated soluble adenylyl cyclase. JOP. 2001;2:154–8. [PubMed] [Google Scholar]
- 83.Chaloupka Ja, Bullock Sa, Iourgenko V, Levin LR, Buck J. Autoinhibitory regulation of soluble adenylyl cyclase. Mol Reprod Dev. 2006;73:361–368. doi: 10.1002/mrd.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, Jaiswal BS, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–62. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Baumlin N, Buck J, Levin LR, Fregien N, Salathe M. A soluble adenylyl cyclase form targets to axonemes and rescues beat regulation in sAC knockout mice. Am J Respir Cell Mol Biol. 2014:1–11. doi: 10.1165/rcmb.2013-0542OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sinclair ML, Wang XY, Mattia M, Conti M, Buck J, Wolgemuth DJ, et al. Specific expression of soluble adenylyl cyclase in male germ cells. Mol Reprod Dev. 2000;56:6–11. doi: 10.1002/(SICI)1098-2795(200005)56:1<6::AID-MRD2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 87.Halm ST, Zhang J, Halm DR. beta-Adrenergic activation of electrogenic K+ and Cl- secretion in guinea pig distal colonic epithelium proceeds via separate cAMP signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2010;299:G81–95. doi: 10.1152/ajpgi.00035.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han H, Stessin A, Roberts J, Hess K, Gautam N, Kamenetsky M, et al. Calcium-sensing soluble adenylyl cyclase mediates TNF signal transduction in human neutrophils. J Exp Med. 2005;202:353–61. doi: 10.1084/jem.20050778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen J, Martinez J, Milner TA, Buck J, Levin LR. Neuronal expression of soluble adenylyl cyclase in the mammalian brain. Brain Res. 2013;1518:1–8. doi: 10.1016/j.brainres.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, a Fischman D, et al. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–34. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Acin-Perez R, Salazar E, Brosel S, Yang H, a Schon E, Manfredi G. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol Med. 2009;1:392–406. doi: 10.1002/emmm.200900046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Geng W, Hill K, Zerwekh JE, Kohler T, Müller R, Moe OW. Inhibition of osteoclast formation and function by bicarbonate: role of soluble adenylyl cyclase. J Cell Physiol. 2009;220:332–40. doi: 10.1002/jcp.21767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Păunescu TG, Ljubojevic M, Russo LM, Winter C, McLaughlin MM, Wagner CA, et al. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol. 2010;298:F643–54. doi: 10.1152/ajprenal.00584.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tresguerres M, Parks SK, Salazar E, Levin LR, Goss GG, Buck J. Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc Natl Acad Sci U S A. 2010;107:442–7. doi: 10.1073/pnas.0911790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tresguerres M, Levin LR, Buck J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 2011;79:1277–88. doi: 10.1038/ki.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee YS, Tresguerres M, Hess K, Marmorstein LY, Levin LR, Buck J, et al. Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. J Biol Chem. 2011;286:41353–8. doi: 10.1074/jbc.M111.284679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, et al. CO2/HCO3(-)- and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J Biol Chem. 2013;288:33283–91. doi: 10.1074/jbc.M113.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006;281:17253–8. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meyer-Franke A, Wilkinson GA, Kruttgen A, Hu M, Munro E, Hanson MG, et al. Depolarization and cAMP elevation rapidly recruit TrkB to the plasma membrane of CNS neurons. Neuron. 1998;21:681–93. doi: 10.1016/s0896-6273(00)80586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–9. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- 101.Hanson MG, Shen S, Wiemelt AP, McMorris FA, Barres BA. Cyclic AMP elevation is sufficient to promote the survival of spinal motor neurons in vitro. J Neurosci. 1998;18:7361–71. doi: 10.1523/JNEUROSCI.18-18-07361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jennifer Martinez MTF, Stessin Alexander M, Campana Aline, Hou Jianwei, Nikulina Elena, Buck Jochen, Levin Lonny R. The role of soluble adenylyl cyclase in neuronal outgrowth. J Neurosci. 2014 doi: 10.1523/JNEUROSCI.1434-14.2014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu KY, Zippin JH, Huron DR, Kamenetsky M, Hengst U, Buck J, et al. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat Neurosci. 2006;9:1257–64. doi: 10.1038/nn1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore SW, Lai Wing Sun K, Xie F, a Barker P, Conti M, Kennedy TE. Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J Neurosci. 2008;28:3920–4. doi: 10.1523/JNEUROSCI.0547-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci U S A. 2003;100:10676–81. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Low VF, Fiorini Z, Fisher L, Jasoni CL. Netrin-1 stimulates developing GnRH neurons to extend neurites to the median eminence in a calcium- dependent manner. PLoS One. 2012;7:e46999. doi: 10.1371/journal.pone.0046999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang H, Takeda K, Lazarovici P, Katagiri Y, Yu ZX, Dickens G, et al. Nerve growth factor (NGF)-induced calcium influx and intracellular calcium mobilization in 3T3 cells expressing NGF receptors. J Biol Chem. 1999;274:26209–16. doi: 10.1074/jbc.274.37.26209. [DOI] [PubMed] [Google Scholar]
- 108.Knöferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, et al. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107:6064–9. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]


