Abstract
Pluripotent stem cells have become powerful tools for both research and regenerative medicine. To date, however, only mouse and rat embryonic stem cells (ESCs)/induced pluripotent stem cells (iPSCs) have the ability to contribute to the formation of germline-competent chimeras. These stem cells are thus considered as “naïve” pluripotent stem cells. Several signaling pathways have been identified to play a critical role in the induction and maintenance of this naïve pluripotent state. Understanding how these pathways induce and maintain naïve pluripotency will likely lead to the generation of germline-competent naïve ESCs/iPSCs from humans and animals phylogenetically close to humans.
Introduction
Pluripotent stem cells can be established from pre-implantation embryos, e.g., embryonic stem cells (ESCs), post-implantation epiblasts, e.g., epiblast stem cells (EpiSCs), and somatic cells through reprogramming, e.g., induced pluripotent stem cells (iPSCs) [1–5]. These pluripotent stem cells can be maintained in culture indefinitely while retaining the ability to differentiate into all cell lineages of the three germ layers. To date, although ESC-like cells from many species have been reported, only ESCs and iPSCs derived from mice and rats possess the unique “naïve” pluripotent state, characterized by the expression of pluripotency markers (e.g., Rex1 and Nr0b1), two active X chromosomes in the female cell, and the ability to generate germline-competent chimeric offspring [1,2,6,7]. In contrast, mouse EpiSCs represent a “primed” pluripotent state, characterized by the low-level expression of the naïve pluripotency genes, a silent X chromosome in the female cell and inability to colonize embryos after being injected into blastocysts [3,4] (Table 1). Interestingly, current available human ESCs/iPSCs are more similar to mouse EpiSCs than to mouse ESCs/iPSCs in their self-renewal requirements, morphology and gene expression patterns [8]. Mouse EpiSCs have been successfully reprogrammed to the naïve pluripotent state through genetic manipulation [9•–12•]. More recently, several studies reported that naïve pluripotent human stem cells that resemble mouse ESCs could also be generated from already established human ESCs or human fibroblasts through reprogramming and/or extrinsic stimulation [13–17••]. Current available data suggest that naïve pluripotent stem cells from different species may share a common mechanism that governs their self-renewal. In this review, we will discuss the conservative signaling pathways involved in inducing and maintaining naïve pluripotency.
Table 1.
Comparison of naïve and primed pluripotent states
| Naïve | Primed | References | |
|---|---|---|---|
| Origin | ICM of blastocyst | Post-implantation epiblast | [1–4,6,7] |
| Colony Morphology | Dome | Flat | [1–4,6,7] |
| Single-cell Mortality | Low | High | [1–4,6,7] |
| XX Chromosomes | Dual Activation | Single Activation | [1–4] |
| GermlineTransmission | High | Low | [1–4,6,7] |
| Culture Conditions | LIF+BMP4, 2i | Activin+bFGF, CHIR+IWR1 | [3,4,18–20•] |
Self-renewal-promoting pathways in naïve ESCs and iPSCs
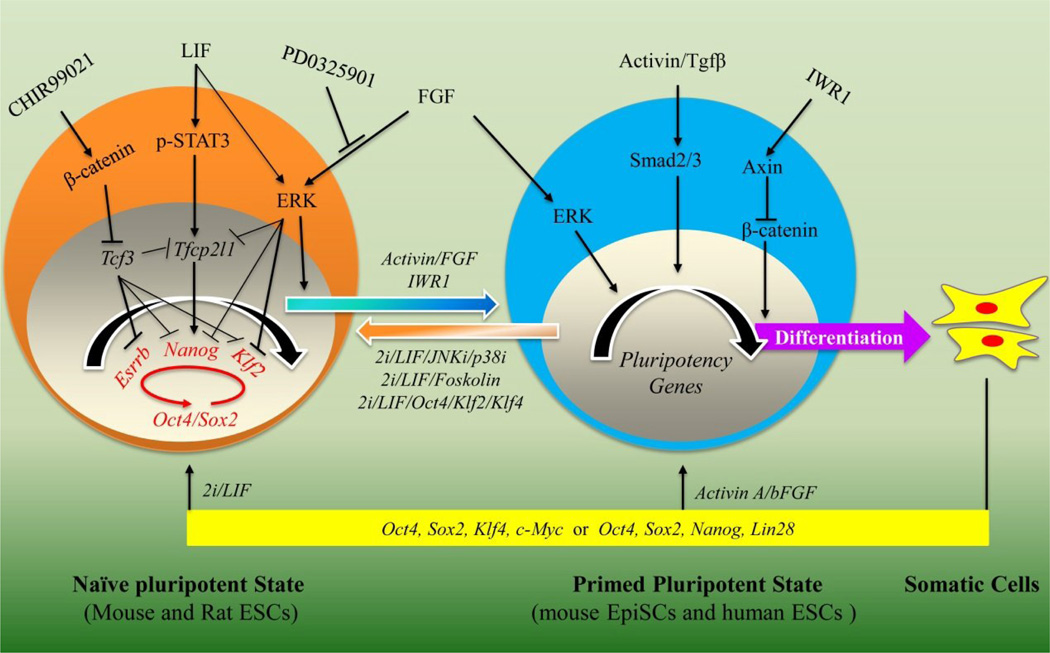
Mouse ESCs were first established in 1981 by culturing them on top of a layer of mitotically inactivated fibroblasts (known as feeders) in serum-containing medium [1,2]. The essential cytokine secreted by feeders is leukaemia inhibitory factor (LIF) and the serum can be replaced by bone morphogenetic protein 4 (BMP4) [18]. LIF and BMP4 are dispensable for mouse ESC self-renewal if two small-molecule inhibitors, CHIR99021 and PD0325901 (known as “2i”), are used [19••]. CHIR99021 and PD0325901 inhibit glycogen synthase kinase 3 (GSK3) and mitogen activated kinase kinase (MEK), respectively. LIF/2i has been used to generate germline-competent ESCs and iPSCs from mice and rats [6,7,19••]; and recently, from humans, albeit, with additional cytokines/small molecule inhibitors required [13–17••]. Therefore, it is proposed that the LIF/2i-mediated signaling pathways are likely conserved among naïve pluripotent stem cells derived from different species (Figure 1) In mouse ESCs, Tfcp2l1, Esrrb and Klf2 are the three key downstream targets of LIF/STAT3 and CHIR99021/β-catenin signaling pathways and PD0325901-mediated inhibition of FGF/ERK signaling pathway, respectively, and overexpression of Tfcp2l1, Esrrb and Klf2 can largely replace the effect of LIF, CHIR99021 and PD0325901 in the maintenance of naïve pluripotent state [11•,12•,21,22]. Notably, Tfcp2l1 and Nanog are at the intersection of self-renewal pathways mediated by LIF, CHIR99021 and PD0325901 [12•]. Primed pluripotent state mouse EpiSCs and human ESCs, however, depend on FGF/ERK and Activin A/Smad signaling pathways for self-renewal [3,4]. Recently, it has been shown that the combination of two small molecules, CHIR99021 and IWR1, can also promote mouse EpiSC and human ESC self-renewal through retention of stabilized β-catenin in the cytoplasm [20•]. The primed pluripotent state and somatic cells can both be reprogrammed into the naïve ESC-like state by overexpression of ectopic genes and addition of small molecules [5,9•].
Figure 1.
Signaling pathways involved in the maintenance of naïve and primed pluripotent states.
LIF/STAT3 pathway: an essential cue for naïve pluripotent state induction
The binding of LIF to LIF/gp130 receptors results in activation of JAK1 and, the latter phosphorylates STAT3 at a single site tyrosine 705. Phosphorylated STAT3 then translocates into the nucleus and induces target gene transcription [23]. Activation of LIF/STAT3 signaling not only promotes, but is also required for the establishment of naïve pluripotency during somatic cell reprogramming [24•], as overexpression of a constitutively active STAT3 mutant (STAT3C) significantly increases the total number of Oct4-GFP-positive colonies in Yamanaka factors-infected mouse embryonic fibroblasts, whereas inhibition of the LIF/STAT3 signaling using JAK1 inhibitor abolishes iPSC generation [24•]. Furthermore, activated STAT3 can promote partially reprogrammed cells (pre-iPSCs) to become fully reprogrammed iPSCs [25•,26•]. This is in line with the finding that hyperactivation of STAT3 is sufficient to convert mouse EpiSCs into naïve pluripotent states, even in the presence of FGF and Activin A [25•,26•], a condition that instructs and maintains the primed pluripotent state of EpiSCs [3,4]. The reprogramming effect of STAT3 in mouse EpiSCs can be amplified by Nanog, which drives elevation of phosphorylated STAT3 partly through repressing Socs3 expression, a negative regulator of STAT3 signaling [27,28]. Moreover, many STAT3-regulated genes are co-regulated by Nanog [29], therefore, Nanog and phosphorylated STAT3 could synergistically induce activation of many naïve pluripotency genes, such as Klf4 (A STAT3 target and can reprogram the primed pluripotent state EpiSCs into the naïve pluripotent state iPSCs when overexpressed [9•]), resulting in rapid and efficient EpiSC reprograming [27]. However, Klf4 is not the only factor responsible for STAT3-induced reprogramming, since LIF is also required [9•].
Recently, more STAT3 targets have been identified, such as c-Myc, Pim1, Pim3, Pramel7, Rhox5, Gbx2 and Tfcp2l1 [10–12•]. Forced expression of each of these STAT3 targets can partially recapitulate the self-renewal-promoting effect of LIF. However, knockdown of Tfcp2l1, but not other STAT3 targets, impairs STAT3-mediated ESC self-renewal and EpiSC reprogramming [11•,12•]. Tfcp2l1 provides a major connection between extrinsic LIF and intrinsic core pluripotency factors, such as Oct4, Nanog, and Sox2 [11•,12•]. Notably, Tfcp2l1 is highly expressed in the inner cell mass of human blastocysts, but becomes significantly downregulated during derivation of human ESCs [30], similar to its expression pattern during the transition from mouse ESCs to EpiSCs [12•], suggesting that it may play a role in establishing and maintaining the naïve pluripotent state.
Canonical Wnt/β-catenin pathway: an accelerator of naïve pluripotent state induction
In addition to the LIF/STAT3 pathway, the canonical Wnt/β-catenin signaling pathway is also beneficial for the establishment of germline-competent pluripotent stem cells [6,7,19••]. β-catenin is the key mediator of the Wnt/β-catenin pathway. Activation of Wnt signaling by Wnt3a or GSK3 inhibitor leads to the stabilization of β-catenin. Stabilized β-catenin enters the nucleus, where it regulates gene transcription through interaction with the Tcf/Lef1 family of transcription factors [31]. Activation of Wnt/β-catenin signaling in mouse ESCs significantly enhances the ability of ESCs to reprogram somatic cells into a naïve pluripotent state during ESC-somatic cell fusion [32]. Activation of Wnt/β-catenin signaling can also dramatically increase iPSC generation efficiency from Oct4/Sox2/Klf4-infected somatic cells. This reprogramming-promoting effect is greatly repressed by inhibition of Wnt/β-catenin signaling [33••]. CHIR99021 is sufficient to convert Oct4/Klf4-transduced mouse embryonic fibroblasts into chimaera-forming iPSCs if combined with BIX01294, a histone methyltransferase G9a inhibitor [34]. In another approach, Oct4/Klf4-transduced neural stem cells treated with CHIR99021 could attain naïve pluripotent state in the presence of PD0325901 and LIF [35]. Furthermore, CHIR99021 is also one of the pivotal factors in inducing fully-competent iPSCs from mouse EpiSCs [9•], human ESCs [13–17••], mouse fibroblasts transfected with Oct4 only [34], and chemically treated non-transgenic mouse neural stem cells [37]. Wnt/β-catenin signaling-mediated mouse ESC self-renewal has been attributed to the stabilization of the intracellular β-catenin protein [38,39] and the repression of Tcf3 by stabilized β-catenin [39]. Tcf3 localizes to the promoters of many pluripotency genes, including Nanog, Nr0b1, Tfcp2l1 and Esrrb [21], and acts as a transcriptional repressor. Among these genes, Esrrb is required for mediating ESC self-renewal downstream of GSK3 inhibition. Depletion of Esrrb results in loss of expression of pluripotency genes in response to CHIR99021, while its overexpression replicates the effect of GSK3 inhibitor or Tcf3 deletion [21]. Thus either Esrrb overexpression or Tcf3 deletion can enhance the efficiency of neural precursor cell (NPC) reprogramming to iPSCs [40,41]. Notably, there are two phases of Wnt signaling in reprogramming somatic cells [42•]. In the early stage, Tcf/Lef1 family members Tcf1 and Lef1 inhibit while Tcf3 and Tcf4 promote reprogramming. In the late stage, however, deletion of Tcf3/Tcf4 enhances iPSC generation in a Tcf1- or Lef1-dependent manner [42•]. Accordingly, manipulating the levels of Tcf3, from slight early overexpression to late depletion, could enable efficient reprogramming in the absence of ectopic Sox2 [42•]. The fact that modulation of Wnt signaling activity enables reprogramming of somatic cells to iPSCs in the absence of Sox2 and c-Myc suggests that Wnt signaling pathway is likely important in achieving chemical-defined reprograming in combination with other transient cues that can replace the remaining Yamanaka factors.
FGF signaling pathway: diverse functions in naïve pluripotent state induction
FGF signaling pathway is an evolutionarily conserved pathway that plays an important role in regulating cell fates during development [43]. FGFs bind to FGF receptors (FGFR) and activate multiple signaling cascades, including MEK, JAK/STAT, PI3K and phosphoinositide phospholipase C (PLCγ) pathways [43]. FGF signaling is required for the generation and maintenance of human ESCs and iPSC [8,44–46]. Interestingly, FGF signaling also promotes mouse iPSC generation in the early phase but functions adversely in the late phase of mouse iPSC induction [47•]. In mouse ESCs, however, FGF-mediated activation of MEK signaling drives lineage commitment [48••]. Inhibition of FGF/MEK signaling enables the derivation of germline-competent ESCs from different strains of mice and rats [6,7], and might also facilitate reprogramming to the naïve pluripotent state. Consistently, the medium containing FGF/MEK inhibitor allows the conversion of human ESCs to the naïve pluripotent state through the overexpression of Oct4, Klf4, and Klf2 [13]. A chemical-defined medium containing LIF/2i, JNK inhibitor SP600126, p38 inhibitor SB203580, Tgfβ and bFGF has also been used to derive naïve human ESCs from already established human ESCs, the ICM of the human blastocyst and Yamanaka factors-infected somatic cells [16••]. Notably, the expression profile exhibited by these naïve human ESCs does not appear to be fundamentally different from conventional primed human ESCs for key naïve pluripotency marker genes such as Prdm14, Tbx3 and Klf4. Additionally, the methylation profile of primed and supposedly naïve human pluripotent state also appears to be relatively similar in contrast to mouse primed and naïve pluripotent state in which a more dramatic difference in the methylation profile is observed [16••]. However, these naïve human ESCs are highly similar to mouse ESCs in their growth properties, gene expression profile, X chromosome activation status and signaling pathway dependence [16••]. Importantly, these naïve human ESCs can contribute to the formation of cross-species chimaeras when microinjected into mouse morula-stage embryos [16••]. LIF/2i, when combined with other factors, such as Yamanaka factors/Lrh1/Rarg [14], HDAC inhibitors (HDACi) plus bFGF [17••], Rock inhibitor plus bFGF [49] or BMP4 inhibitor [15], can also induce and maintain naïve human ESCs/iPSCs. Intriguingly, bFGF is included in some of the culture conditions [16••,17••,49], indicating that FGF signaling might have a positive effect on self-renewal of both naïve and primed human pluripotent stem cells, although the underlying mechanism is largely unknown [17••].
How FGF/MEK inhibitor maintains and induces naïve pluripotency is not well understood. A recent study reports that FGF signaling inhibition in ESCs drives rapid genome-wide demethylation via negative regulation of the de novo methyltransferase genes Dnmt-3a, -3b and -3l, a pattern remarkably resembling the epigenomes of ICM cells in the blastocyst [50]. A further study shows that Prdm14 is involved in the FGF/MEK inhibitor-mediated suppression of Dnmt-3 gene expression. Prdm14 is highly expressed in 2i-treated ESCs. It binds to the promoter region of Dnmt-3b gene and the knockout of the Prdm14 gene leads to significant upregulation of Dnmt-3b expression and increased methylation in ESCs cultured in 2i conditions [50]. However, depletion of Prdm14 has no obvious effect on 2i-mediated ESC self-renewal, therefore, Prdm14 is unlikely to be the primary mediator of the FGF/MEK inhibitor [50,51]. Forced expression of each of the pluripotency factors Tfcp2l1, Nanog and Klf2 can substitute for FGF/MEK inhibitor in promoting ESC self-renewal. However, neither Tfcp2l1 nor Nanog is essential for the maintenance of the naïve pluripotency state by 2i [12•,22,52]. Interestingly, Klf2-null mouse ESCs are not viable under 2i, suggesting that Klf2 has an essential role in 2i-mediated ESC self-renewal [22]. Activation of FGF/MEK signaling in mouse ESCs leads to the phosphodependent degradation of Klf2 protein, and therefore it has been suggested that MEK inhibitor PD0325901 maintains naïve pluripotent state partially through prevention of Klf2 protein phosphodegradation [22]. Recently, Tee et al found that loss of FGF signaling results in alterations in chromatin accessibility and profoundly impacts the expression of developmental genes [53]. It will be interesting to further explore the precise molecular mechanisms of the FGF signaling pathway in inducing and maintaining naïve pluripotency.
Conclusion
LIF/2i-mediated activation of LIF/STAT3 and Wnt/β-catenin signaling along with inhibition of FGF/MEK are necessary and sufficient for inducing and maintaining naïve pluripotency in mouse and rat ESCs/iPSCs. Manipulation of other signaling pathways through additional factors might be required for generation of naïve pluripotent stem cells from species other than mice and rats. Understanding how naïve pluripotency is induced and maintained will facilitate the application of pluripotent stem cell-based therapies.
Acknowledgement
This work is supported, in part, by 211 Project of Anhui University (10117700027, 32030081). The research in Ying lab is supported by Chen Yong Foundation of the Zhongmei Group, National Institutes of Health (R01 OD010926), California Institute for Regenerative Medicine (CIRM) New Faculty Award II (RN2-00938), and CIRM Scientific Excellence through Exploration and Development (SEED) Grant (RS1-00327).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 3.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191–195. doi: 10.1038/nature05950. [DOI] [PubMed] [Google Scholar]
- 4.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196–199. doi: 10.1038/nature05972. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Buehr M, Meek S, Blair K, Yang J, Ure J, Silva J, McLay R, Hall J, Ying QL, Smith A. Capture of authentic embryonic stem cells from rat blastocysts. Cell. 2008;135:1287–1298. doi: 10.1016/j.cell.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Li P, Tong C, Mehrian-Shai R, Jia L, Wu N, Yan Y, Maxson RE, Schulze EN, Song H, Hsieh CL, et al. Germline competent embryonic stem cells derived from rat blastocysts. Cell. 2008;135:1299–1310. doi: 10.1016/j.cell.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9. Guo G, Yang J, Nichols J, Hall JS, Eyres I, Mansfield W, Smith A. Klf4 reverts developmentally programmed restriction of ground state pluripotency. Development. 2009;136:1063–1069. doi: 10.1242/dev.030957. This paper demonstrates for the first time that EpiSCs can be reprogrammed to naïve pluripotency by transfection with a single factor, Klf4.
- 10.Tai CI, Ying QL. Gbx2, a LIF/Stat3 target, promotes reprogramming to and retention of the pluripotent ground state. J Cell Sci. 2013;126:1093–1098. doi: 10.1242/jcs.118273. [DOI] [PubMed] [Google Scholar]
- 11.Martello G, Bertone P, Smith A. Identification of the missing pluripotency mediator downstream of leukaemia inhibitory factor. EMBO J. 2013;32:2561–2574. doi: 10.1038/emboj.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ye S, Li P, Tong C, Ying QL. Embryonic stem cell self-renewal pathways converge on the transcription factor Tfcp2l1. EMBO J. 2013;32:2548–2560. doi: 10.1038/emboj.2013.175. These papers [Refs 11,12] show that Tfcp2l1 is the major target of the LIF/STAT3 signaling pathway. Forced expression of Tfcp2l1 can mimic the effect of LIF/STAT3 signaling in promoting mouse ESC self-renewal and converting EpiSCs into naïve pluripotency.
- 13.Hanna J, Cheng AW, Saha K, Kim J, Lengner CJ, Soldner F, Cassady JP, Muffat J, Carey BW, Jaenisch R. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Yang J, Liu H, Lu D, Chen X, Zenonos Z, Campos LS, Rad R, Guo G, Zhang S, et al. Rapid and efficient reprogramming of somatic cells to induced pluripotent stem cells by retinoic acid receptor gamma and liver receptor homolog 1. Proc Natl Acad Sci U S A. 2011;108:18283–18288. doi: 10.1073/pnas.1100893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan YS, Goke J, Ng JH, Lu X, Gonzales KA, Tan CP, Tng WQ, Hong ZZ, Lim YS, Ng HH. Induction of a human pluripotent state with distinct regulatory circuitry that resembles preimplantation epiblast. Cell Stem Cell. 2013;13:663–675. doi: 10.1016/j.stem.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Gafni O, Weinberger L, Mansour AA, Manor YS, Chomsky E, Ben-Yosef D, Kalma Y, Viukov S, Maza I, Zviran A, et al. Derivation of novel human ground state naive pluripotent stem cells. Nature. 2013;504:282–286. doi: 10.1038/nature12745. [DOI] [PubMed] [Google Scholar]
- 17. Ware CB, Nelson AM, Mecham B, Hesson J, Zhou W, Jonlin EC, Jimenez-Caliani AJ, Deng X, Cavanaugh C, Cook S, et al. Derivation of naive human embryonic stem cells. Proc Natl Acad Sci U S A. 2014;111:4484–4489. doi: 10.1073/pnas.1319738111. In these papers [Refs 16,17], the authors demonstrate that LIF, 2i, and bFGF are all required for inducing naïve pluripotency from already established human ESCs and from human somatic cells through iPSC reprogramming.
- 18.Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- 19. Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. The authors demonstrate for the first time that mouse ESCs can be derived and maintained in a chemical-defined condition supplemented with small molecules that specifically inhibit GSK3 and MEK.
- 20. Kim H, Wu J, Ye S, Tai CI, Zhou X, Yan H, Li P, Pera M, Ying QL. Modulation of beta-catenin function maintains mouse epiblast stem cell and human embryonic stem cell self-renewal. Nat Commun. 2013;4:2403. doi: 10.1038/ncomms3403. The authors show that activation of Wnt/β-catenin exerts opposite roles in the maintenance of naïve and primed pluripotent states, promoting mouse ESCs self-renewal while inducing EpiSC differentiation.
- 21.Martello G, Sugimoto T, Diamanti E, Joshi A, Hannah R, Ohtsuka S, Gottgens B, Niwa H, Smith A. Esrrb is a pivotal target of the Gsk3/Tcf3 axis regulating embryonic stem cell self-renewal. Cell Stem Cell. 2012;11:491–504. doi: 10.1016/j.stem.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeo JC, Jiang J, Tan ZY, Yim GR, Ng JH, Goke J, Kraus P, Liang H, Gonzales KA, Chong HC, et al. Klf2 Is an Essential Factor that Sustains Ground State Pluripotency. Cell Stem Cell. 2014;14:864–872. doi: 10.1016/j.stem.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Niwa H, Burdon T, Chambers I, Smith A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3. Genes Dev. 1998;12:2048–2060. doi: 10.1101/gad.12.13.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang Y, Luo Y, Jiang Z, Ma Y, Lin CJ, Kim C, Carter MG, Amano T, Park J, Kish S, et al. Jak/Stat3 signaling promotes somatic cell reprogramming by epigenetic regulation. Stem Cells. 2012;30:2645–2656. doi: 10.1002/stem.1225. The authors show that activation of STAT3 during reprogramming enhances the efficiency of iPSC generation, whereas STAT3 inhibition abolishes the emergence of iPSCs. This study suggests that activated STAT3 not only promotes, but also is essential for the pluripotency establishment of mouse embryonic fibroblasts during reprogramming.
- 25.Yang J, van Oosten AL, Theunissen TW, Guo G, Silva JC, Smith A. Stat3 activation is limiting for reprogramming to ground state pluripotency. Cell Stem Cell. 2010;7:319–328. doi: 10.1016/j.stem.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Oosten AL, Costa Y, Smith A, Silva JC. JAK/STAT3 signalling is sufficient and dominant over antagonistic cues for the establishment of naïve pluripotency. Nat Commun. 2012;3:817. doi: 10.1038/ncomms1822. The results in these papers[Refs 25,26] suggest that activation of STAT3 is sufficient to reprogram EpiSCs to naïve ESCs.
- 27.Stuart HT, van Oosten AL, Radzisheuskaya A, Martello G, Miller A, Dietmann S, Nichols J, Silva JC. NANOG amplifies STAT3 activation and they synergistically induce the naive pluripotent program. Curr Biol. 2014;24:340–346. doi: 10.1016/j.cub.2013.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kershaw NJ, Murphy JM, Liau NP, Varghese LN, Laktyushin A, Whitlock EL, Lucet IS, Nicola NA, Babon JJ. SOCS3 binds specific receptor-JAK complexes to control cytokine signaling by direct kinase inhibition. Nat Struct Mol Biol. 2013;20:469–476. doi: 10.1038/nsmb.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourillot PY, Aksoy I, Schreiber V, Wianny F, Schulz H, Hummel O, Hubner N, Savatier P. Novel STAT3 target genes exert distinct roles in the inhibition of mesoderm and endoderm differentiation in cooperation with Nanog. Stem Cells. 2009;27:1760–1771. doi: 10.1002/stem.110. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary T, Heindryckx B, Lierman S, van Bruggen D, Goeman JJ, Vandewoestyne M, Deforce D, de Sousa Lopes SM, De Sutter P. Tracking the progression of the human inner cell mass during embryonic stem cell derivation. Nat Biotechnol. 2012;30:278–282. doi: 10.1038/nbt.2135. [DOI] [PubMed] [Google Scholar]
- 31.Valenta T, Hausmann G, Basler K. The many faces and functions of beta-catenin. EMBO J. 2012;31:2714–2736. doi: 10.1038/emboj.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lluis F, Pedone E, Pepe S, Cosma MP. Periodic activation of Wnt/beta-catenin signaling enhances somatic cell reprogramming mediated by cell fusion. Cell Stem Cell. 2008;3:493–507. doi: 10.1016/j.stem.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 33. Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. The authors demonstrate that activation of Wnt/β-catenin signaling by Wnt3a significantly increases the reprogramming efficiency in Yamanka factors-infected mouse fibroblasts, while inhibition of this pathway represses the production of iPSCs.
- 34.Li W, Zhou H, Abujarour R, Zhu S, Young Joo J, Lin T, Hao E, Scholer HR, Hayek A, Ding S. Generation of human-induced pluripotent stem cells in the absence of exogenous Sox2. Stem Cells. 2009;27:2992–3000. doi: 10.1002/stem.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Zhang Q, Yin X, Yang W, Du Y, Hou P, Ge J, Liu C, Zhang W, Zhang X, et al. Generation of iPSCs from mouse fibroblasts with a single gene, Oct4, and small molecules. Cell Res. 2011;21:196–204. doi: 10.1038/cr.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, et al. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013;341:651–654. doi: 10.1126/science.1239278. [DOI] [PubMed] [Google Scholar]
- 38.Ye S, Tan L, Yang R, Fang B, Qu S, Schulze EN, Song H, Ying Q, Li P. Pleiotropy of glycogen synthase kinase-3 inhibition by CHIR99021 promotes self-renewal of embryonic stem cells from refractory mouse strains. PLoS One. 2012;7:e35892. doi: 10.1371/journal.pone.0035892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wray J, Kalkan T, Gomez-Lopez S, Eckardt D, Cook A, Kemler R, Smith A. Inhibition of glycogen synthase kinase-3 alleviates Tcf3 repression of the pluripotency network and increases embryonic stem cell resistance to differentiation. Nat Cell Biol. 2011;13:838–845. doi: 10.1038/ncb2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Festuccia N, Osorno R, Halbritter F, Karwacki-Neisius V, Navarro P, Colby D, Wong F, Yates A, Tomlinson SR, Chambers I. Esrrb is a direct Nanog target gene that can substitute for Nanog function in pluripotent cells. Cell Stem Cell. 2012;11:477–490. doi: 10.1016/j.stem.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lluis F, Ombrato L, Pedone E, Pepe S, Merrill BJ, Cosma MP. T-cell factor 3 (Tcf3) deletion increases somatic cell reprogramming by inducing epigenome modifications. Proc Natl Acad Sci USA. 2011;108:11912–11917. doi: 10.1073/pnas.1017402108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho R, Papp B, Hoffman JA, Merrill BJ, Plath K. Stage-specific regulation of reprogramming to induced pluripotent stem cells by Wnt signaling and T cell factor proteins. Cell Rep. 2013;3:2113–2126. doi: 10.1016/j.celrep.2013.05.015. The authors show that the four Tcf/Lef1 transcription factors play distinct roles during reprogramming. Modulation of the function of Tcf3 could replace the role of Sox2 in iPSC generation.
- 43.Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–3360. doi: 10.1242/dev.050146. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 45.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 46.Eiselleova L, Matulka K, Kriz V, Kunova M, Schmidtova Z, Neradil J, Tichy B, Dvorakova D, Pospisilova S, Hampl A, et al. A complex role for FGF-2 in self-renewal, survival, and adhesion of human embryonic stem cells. Stem Cells. 2009;27:1847–1857. doi: 10.1002/stem.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jiao J, Dang Y, Yang Y, Gao R, Zhang Y, Kou Z, Sun XF, Gao S. Promoting reprogramming by FGF2 reveals that the extracellular matrix is a barrier for reprogramming fibroblasts to pluripotency. Stem Cells. 2013;31:729–740. doi: 10.1002/stem.1318. This study shows that addition of FGF2 during the early phase of reprogramming promotes mouse iPSC generation, partially by accelerating cell proliferation and facilitating mesenchymal–epithelial transition through downregulation of mesenchymal-specific gene expression.
- 48. Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. The authors demonstrate that Erk1/2 signaling is activated by autocrine fibroblast growth factor 4 (Fgf4) in mouse ESCs and that Fgf4 stimulation of Erk1/2 is an autoinductive stimulus for naïve ESCs to exit the self-renewal program.
- 49.Valamehr B, Robinson M, Abujarour R, Rezner B, Vranceanu F, Le T, Medcalf A, Lee TT, Fitch M, Robbins D, et al. Platform for Induction and Maintenance of Transgene-free hiPSCs Resembling Ground State Pluripotent Stem Cells. Stem Cell Reports. 2014;2:366–381. doi: 10.1016/j.stemcr.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ficz G, Hore TA, Santos F, Lee HJ, Dean W, Arand J, Krueger F, Oxley D, Paul YL, Walter J, et al. FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. Cell Stem Cell. 2013;13:351–359. doi: 10.1016/j.stem.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamaji M, Ueda J, Hayashi K, Ohta H, Yabuta Y, Kurimoto K, Nakato R, Yamada Y, Shirahige K, Saitou M. PRDM14 ensures naive pluripotency through dual regulation of signaling and epigenetic pathways in mouse embryonic stem cells. Cell Stem Cell. 2013;12:368–382. doi: 10.1016/j.stem.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 52.Silva J, Nichols J, Theunissen TW, Guo G, van Oosten AL, Barrandon O, Wray J, Yamanaka S, Chambers I, Smith A. Nanog is the gateway to the pluripotent ground state. Cell. 2009;138:722–737. doi: 10.1016/j.cell.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tee WW, Shen SS, Oksuz O, Narendra V, Reinberg D. Erk1/2 activity promotes chromatin features and RNAPII phosphorylation at developmental promoters in mouse ESCs. Cell. 2014;156:678–690. doi: 10.1016/j.cell.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]