Summary
Osteonecrosis of the femoral head (ONFH) is a common complication of sickle cell disease (SCD). To examine the association between microparticles and ONFH in SCD, we compared plasma microparticle levels in 20 patients with and without ONFH. Microparticles were quantified using nanoparticle tracking analysis and found to be 2.3-fold higher in patients with ONFH compared to patients without ONFH, and 2.5-fold higher than in healthy controls. These results suggest that microparticles may be a clinically useful biomarker of ONFH in SCD. Further investigations are needed to determine the functional relevance of microparticles in the pathogenesis of ONFH in SCD.
Keywords: Anaemia, sickle cell disease, coagulation, clinical haematology, haemoglobinopathies
Introduction
Osteonecrosis of the femoral head (ONFH) is a chronic, debilitating complication of sickle cell disease, affecting 50% of patients homozygous for the sickle cell mutation (HbSS) by 35 years of age(Mahadeo, et al 2011, Milner, et al 1991). Clinical symptoms peak during adolescence, resulting in significant physical impairment and chronic pain and, ultimately, total hip replacement at an early age. Patients with ONFH are frequently asymptomatic during the early stages, delaying the diagnosis until it has progressed to advanced stages(Ware, et al 1991). Aside from co-inherited alpha thalassemia trait and elevated haemoglobin level, few predictors of ONFH in SCD exist (Lemonne, et al 2013, Milner, et al 1991).
SCD is characterized by chronic haemolysis and recurrent ischaemia due to micro-vascular occlusion. Repeated intravascular sickling triggers a complex cycle of sustained endothelial activation, inflammation and thrombosis eventuating in progressive vascular damage(Hebbel 2004). The pathogenesis of ONFH in SCD, as in other disease populations, has been attributed to the ischaemic consequences of vascular occlusion in the already vulnerable microcirculation of the femoral head.
Microparticles (MP) are small (0.1–1.0 μm diameter), cell membrane-derived vesicles generated in response to cellular activation or injury. Circulating MP expose phosphatidylserine (PS), a phospholipid normally maintained on the inner leaflet of the plasma membrane, and release vaso-active mediators involved in coagulation activation. MP originating from platelets, erythrocytes, endothelial cells and monocytes have been detected in patients with SCD(Shet, et al 2003, Wun, et al 1998). Both total and tissue factor-positive MP are elevated at baseline and increase further during vaso-occlusive events, suggesting their involvement in the thrombotic manifestations of the disease (Kasar, et al 2013, Shet, et al 2003, van Beers, et al 2009).
Alterations in the number and characteristics of circulating MP have been implicated in the pathophysiology of ONFH due to causes other than SCD, however, the extent to which MP may contribute to the development of ONFH in SCD is not known(Kang, et al 2008). In this pilot study, we investigated the association between MP levels and ONFH in SCD.
Methods
Study Subjects
Twenty patients with sickle cell anaemia (HbSS or HbS/β0thal genotype) were recruited at their baseline status during a routine clinic visit. Case subjects were characterized by X-ray or magnetic resonance imaging-documented osteonecrosis of one or both hips. Control subjects were selected based on a negative history of hip pain and a normal hip examination. Both case subjects and control subjects underwent a standardized hip examination. Patients treated with hydroxycarbamide at a stable dose (> 3 months) were eligible. Exclusion criteria consisted of transfusion within the past 30 days, hospitalization for a vaso-occlusive pain episode, acute chest syndrome, fever or surgery within the past 30 days, or imaging studies documenting bony lesions of the femur or hip due to causes unrelated to SCD. Ten healthy race- and age-matched subjects were included as a reference group. The Institutional Review Board at Children’s Hospital & Research Center Oakland (CHRCO) approved the study. All subjects provided written informed consent.
Measurement of Circulating Microparticles
A standard protocol was followed to ensure uniform collection, processing, and storage of samples. Blood samples were drawn with a 21-gauge needle, avoiding prolonged use of a tourniquet. Whole blood was collected in vacuum tubes containing sodium citrate and centrifuged for 15 min at 1500 × g at room temperature to generate platelet-poor plasma. Aliquots of plasma were immediately frozen and stored at −80°C until use. For MP isolation, 300 μl of plasma was diluted in phosphate-buffered saline (PBS) and centrifuged at 10,000 × g for 1 h and the supernatant centrifuged at 100,000 × g for 2 h. The MP pellet was re-suspended in 1 ml of PBS. Appropriate dilutions were made to allow the Brownian motion of a sufficient number of particles to be followed for a sufficient amount of time to analyse the nanoparticle tracks on a NanoSight LM-10 nanoparticle tracking analyser (Nanosight, Amesbury, UK).
Clinical laboratory measures of haemolysis, inflammation and coagulation were also analysed. Complete blood count, reticulocyte count, total bilirubin, lactate dehydrogenase (LDH), high-sensitivity C-reactive protein (hs-CRP), and von Willebrand Factor antigen (VWF:Ag) level were performed using standard laboratory techniques in the clinical laboratory at CHRCO. Plasma D-dimer and tissue factor (TF) levels were measured by enzyme-linked immunosorbent assay using commercial kits (IMUCLONE® D-dimer kit, IMUBIND® tissue factor kit, Sekisui Diagnostics, Lexington, MA, USA) following the manufacturer’s instructions.
Statistical Analysis
Sample size was calculated assuming a 50% higher level of total MP in case subjects compared to control subjects, a two-tailed α-error of 0.05 and 80% power. Data were examined for measures of normality and parametric and non-parametric analyses were performed when appropriate. Descriptive statistics were computed for each group. Differences between the 3 groups (+ONFH, −ONFH and controls) were evaluated using analysis of variance (ANOVA) and Tukey adjustment for multiple comparisons. Bivariate associations were estimated using Pearson’s correlation coefficient (r). All analyses were performed using SAS version 9.3 (SAS Inc., 2013, Cary, NC). A significance level of 0.05 was used for all statistical tests.
Results & Discussion
The mean age of ONFH-positive cases (n=10) was 25.8 (range, 19–43) years and ONFH-negative controls (n=10) was 28.9 (range, 19–46) years. There were 6 males and 4 females in each group. Eight case subjects and 6 control subjects were receiving treatment with hydroxycarbamide. Eight ONFH-positive subjects had bilateral hip involvement. The reference group was comprised of 10 healthy African-American subjects with mean age 27.6 (range 18–40) years.
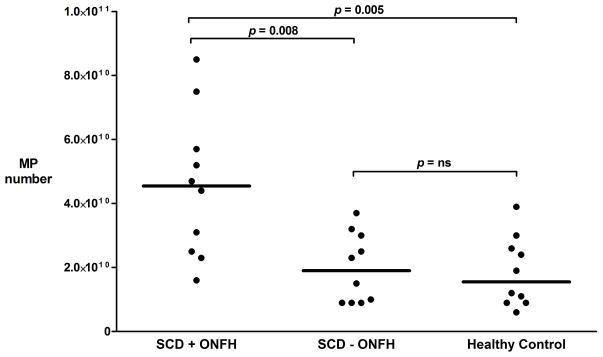
Total MP numbers were 2.3-fold greater in ONFH (+) patients than in ONFH(−) patients (p=0.008), and 2.5-fold greater than in reference subjects (controls; p=0.005) (Fig 1). Moderate to strong correlations were found between MP, reticulocyte, and D-dimer levels in the ONFH (+) group (Table I). Data comparing laboratory markers of haemolysis, coagulation and inflammation among the three groups are shown in Table I.
Figure 1.
Column scatter plot of MP levels obtained from SCD patients with ONFH (n = 10), SCD patients without ONFH (n = 10), and healthy controls (n =10). P-values represent non-parametric Kruskal-Wallis analysis. Horizontal lines indicate median values.
MP, microparticle; SCD, sickle cell disease; ONFH, osteonecrosis of the femoral head.
Table I.
Microparticle levels and laboratory markers of haemolysis, coagulation and inflammation.
| Mean Values (SD) | Normal Reference Range | P-value of least square means | Correlation, r (p-value) | |||||
|---|---|---|---|---|---|---|---|---|
| + ONFH | − ONFH | Control | + ONFH vs − ONFH | + ONFH vs Control | − ONFH vs Control | +ONFH | ||
| Microparticles 1010 particles/ml plasma | 4.55 (2.26) | 1.99 (1.08) | 1.85 (1.09) | N/A | 0.003 | 0.002 | ns | -- |
| WBC × 109/l | 10.54 (4.8) | 9.53 (5.29) | 7.25 (1.52) | 5–10 | ns | ns | ns | +0.25 (ns) |
| Hb g/l | 96 (13) | 94 (18) | 134 (13) | 135–173 | ns | <0.001 | <0.001 | +0.03 (ns) |
| Plt × 109/l | 340 (167) | 302 (100) | 260 (42) | 150–400 | ns | ns | ns | 0.00 (ns) |
| ARC 109/l | 258 (140) | 278 (140) | 61.4 (20) | 36–159 | ns | 0.0002 | 0.0006 | +0.80 (0.006) |
| Tbili umol/L | 36.1 (22.2) | 45 (28.2) | 10.3 (2.1) | 1.7–25.7 | ns | 0.03 | 0.003 | +0.43 (ns) |
| LDH u/l | 712 (159) | 862 (503) | 439 (52) | 313–618 | ns | ns | 0.01 | +0.10 (ns) |
| hsCRP mg/l | 9.26 (8.71) | 3.26 (2.83) | 2.45 (3.31) | 0.1–3.0 | ns | 0.002 | ns | +0.03 (ns) |
| VWF:Ag % | 143.4 (73.7) | 121.3 (46.0) | 83.6 (28.2) | 51–185 | ns | 0.04 | ns | −0.42 (ns) |
| Tissue Factor pg/ml | 169.7 (155.2) | 250.1 (150.7) | 161.3 (125.1) | N/A | ns | ns | ns | −0.28 (ns) |
| D-Dimer ng/ml | 1254.8 (926.1) | 1579.4 (1431.0) | 168.2 (131.1) | <400 | ns | 0.05 | 0.01 | +0.64 (0.05) |
ONFH, osteonecrosis of the femoral head; WBC, white blood cell count; Hb, haemoglobin; Plt, platelet count; ARC, absolute reticulocyte count; Tbili, total bilirubin; LDH, lactate dehydrogenase; hsCRP, highly-sensitive C-reactive protein; VWF:Ag, von Willebrand factor antigen; SD, standard deviation; N/A, not ns, not significant.
The results of this pilot study confirm previously reported associations of MP with ONFH in the general population and are the first to demonstrate a significant association with ONFH in SCD(Kang, et al 2008). Although functional activity was not assessed in this study, the significantly higher number of MP found in patients with ONFH compared to those without ONFH suggest their involvement in the pathogenesis of ONFH in SCD.
While speculative, possible mechanisms by which MP may contribute to the vascular pathophysiology of ONFH in SCD include pro-coagulant PS and TF expression (Kuypers, et al 2007). PS exposed on the surface of MP serves as a hydrolytic target for secretory phospholipase A2 (sPLA2)-mediated production of thromboxane A2 and induction of platelet aggregation(Fourcade, et al 1995, Neidlinger, et al 2006). Tissue factor is expressed on the surface of MP and may activate coagulation via its critical role as a binding site for factor VII (Shet, et al 2003).
This study confirmed the previously reported finding of a positive correlation between MP levels and the fibrinolytic marker, D-dimer, but was unable to replicate a correlation between haemoglobin, LDH and VWF:Ag (van Beers, et al 2009). This discrepancy may be explained by our analyses of total MP, rather than biologically distinct cellular subtypes. Our methods, employing nanoparticle-tracking analysis to detect and quantify MP, also differed from previous studies that used less sensitive methods. Indeed, the total MP number observed in our study population was four orders of magnitude greater than the numbers of MP previously reported in SCD patients using flow cytometry-based methods (Kasar, et al 2013, Shet, et al 2003, van Beers, et al 2009).
In conclusion, we have demonstrated increased levels of circulating MP in SCD patients with ONFH. Further studies are required to determine the predictive value and pathophysiological significance of this observation.
Acknowledgments
This publication was supported by NIH/NCRR UCSF-CTSI Grant Number UL1 RR024131.
Footnotes
Authorship Contributions
A.M. and C.H. wrote the manuscript, which was reviewed and agreed upon by all the authors. A.M., C.H., F.K., S.L, R.M.S. and W.W.S. collected, analysed and interpreted data. A.M., C.H. and G.G. performed statistical analysis.
Disclosure of Conflicts of Interest
The authors declare no competing financial interests.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- Fourcade O, Simon MF, Viode C, Rugani N, Leballe F, Ragab A, Fournie B, Sarda L, Chap H. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. 1995;80:919–927. doi: 10.1016/0092-8674(95)90295-3. [DOI] [PubMed] [Google Scholar]
- Hebbel RP. Special issue of Microcirculation: examination of the vascular pathobiology of sickle cell anemia. Foreword Microcirculation. 2004;11:99–100. [PubMed] [Google Scholar]
- Kang P, Shen B, Yang J, Pei F. Circulating platelet-derived microparticles and endothelium-derived microparticles may be a potential cause of microthrombosis in patients with osteonecrosis of the femoral head. Thrombosis Research. 2008;123:367–373. doi: 10.1016/j.thromres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Kasar M, Boga C, Yeral M, Asma S, Kozanoglu I, Ozdogu H. Clinical significance of circulating blood and endothelial cell microparticles in sickle-cell disease. Journal of Thrombosis & Thrombolysis. 2013;38:167–175. doi: 10.1007/s11239-013-1028-3. [DOI] [PubMed] [Google Scholar]
- Kuypers FA, Larkin SK, Emeis JJ, Allison AC. Interaction of an annexin V homodimer (Diannexin) with phosphatidylserine on cell surfaces and consequent antithrombotic activity. Journal of Thrombosis & Haemostasis. 2007;97:478–486. [PubMed] [Google Scholar]
- Lemonne N, Lamarre Y, Romana M, Mukisi-Mukaza M, Hardy-Dessources MD, Tarer V, Mougenel D, Waltz X, Tressieres B, Lalanne-Mistrih ML, Etienne-Julan M, Connes P. Does increased red blood cell deformability raise the risk for osteonecrosis in sickle cell anemia? Blood. 2013;121:3054–3056. doi: 10.1182/blood-2013-01-480277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadeo KM, Oyeku S, Taragin B, Rajpathak SN, Moody K, Santizo R, Driscoll MC. Increased prevalence of osteonecrosis of the femoral head in children and adolescents with sickle-cell disease. American Journal of Hematology. 2011;86:806–808. doi: 10.1002/ajh.22103. [DOI] [PubMed] [Google Scholar]
- Milner PF, Kraus AP, Sebes JI, Sleeper LA, Dukes KA, Embury SH, Bellevue R, Koshy M, Moohr JW, Smith J. Sickle cell disease as a cause of osteonecrosis of the femoral head. New England Journal of Medicine. 1991;325:1476–1481. doi: 10.1056/NEJM199111213252104. [DOI] [PubMed] [Google Scholar]
- Neidlinger NA, Larkin SK, Bhagat A, Victorino GP, Kuypers FA. Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction. Journal of Biological Chemistry. 2006;281:775–781. doi: 10.1074/jbc.M505790200. [DOI] [PubMed] [Google Scholar]
- Shet AS, Aras O, Gupta K, Hass MJ, Rausch DJ, Saba N, Koopmeiners L, Key NS, Hebbel RP. Sickle blood contains tissue factor-positive microparticles derived from endothelial cells and monocytes. Blood. 2003;102:2678–2683. doi: 10.1182/blood-2003-03-0693. [DOI] [PubMed] [Google Scholar]
- van Beers EJ, Schaap MC, Berckmans RJ, Nieuwland R, Sturk A, van Doormaal FF, Meijers JC, Biemond BJ. Circulating erythrocyte-derived microparticles are associated with coagulation activation in sickle cell disease. Haematologica. 2009;94:1513–1519. doi: 10.3324/haematol.2009.008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware HE, Brooks AP, Toye R, Berney SI. Sickle cell disease and silent avascular necrosis of the hip. Journal of Bone & Joint Surgery (British Volume) 1991;73:947–949. doi: 10.1302/0301-620X.73B6.1955442. [DOI] [PubMed] [Google Scholar]
- Wun T, Paglieroni T, Rangaswami A, Franklin PH, Welborn J, Cheung A, Tablin F. Platelet activation in patients with sickle cell disease. British Journal of Haematology. 1998;100:741–749. doi: 10.1046/j.1365-2141.1998.00627.x. [DOI] [PubMed] [Google Scholar]