Abstract
Background
Humans are exposed to persistent organic pollutants (POPs) through various routes, including consumption of contaminated food and accidental ingestion of settled dust.
Objectives
We aimed to identify key routes of exposure to organochlorine pesticides, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs) in California women of reproductive age.
Methods
Blood was collected from 48 mothers participating in the California Childhood Leukemia Study from 2006–2007 and analyzed for POPs using gas chromatography-mass spectrometry. Multivariable linear regression models of natural-log transformed serum concentrations were used to identify determinants of exposure from available questionnaire information on dietary habits, reproductive history, and demographic characteristics, as well as vacuum cleaner dust-POP levels.
Results
After adjusting for blood lipid levels, age, body mass index, cumulative lactation, and sampling date, serum concentrations of multiple major PCBs were positively associated with fish consumption, but not dust-PCB levels. After adjusting for blood lipid levels, Hispanic ethnicity, country of origin, and household annual income, serum concentrations of multiple major PBDEs were positively associated with dust-PBDE levels.
Conclusions
Our findings suggest that the relative contribution of specific exposure routes to total POP intake varies by chemical class, with dust being a relatively important source of PBDEs and diet being a relatively important source of PCBs.
Keywords: Environmental monitoring, house dust, organochlorine pesticides, polybrominated diphenyl ethers, polychlorinated biphenyls
1. INTRODUCTION
Persistent organic pollutants (POPs) are stable and widespread in the environment, they accumulate in fatty tissue of biota, and they are toxic to humans and wildlife. Three important classes of POPs are organochlorine (OC) pesticides, polychlorinated biphenyls (PCBs), and polybrominated diphenyl ethers (PBDEs). Chemicals from each of these classes were produced globally in large volumes, but the time period of peak production varied by chemical. For example, DDT [i.e., p-p’-1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane)] has been used extensively to control insects on agricultural crops and to control insect-borne diseases, with perhaps as much as four million metric tons produced worldwide since 1945; however, DDT use peaked circa 1970 and all uses of DDT in the U.S. have been banned since 1973 (Li and Macdonald 2005). Likewise, as components of electrical, heat transfer, and hydraulic equipment, PCBs had a similar production history to DDT, with over 1 million metric tons produced worldwide since 1929 and peak production in 1970, followed shortly thereafter by a ban of U.S. production and distribution (Breivik, et al. 2004). At the same time that DDT and PCBs were being phased out, PBDEs began to be used as chemical flame retardants to treat plastics and textiles in consumer products, and an estimated global market demand of 67,000 metric tons in 1999 suggests that peak PBDE production was on the same order of magnitude as peak PCB production (Breivik, et al. 2004). In the U.S., the manufacture and import of the commercial mixtures, Penta-BDE (composed primarily of BDEs 47, 99, 100, 153, and 154) and Octa-BDE (composed primarily of BDEs 153, 183, 196, 197, and 207) were phased out, as of January 1, 2005, and the Deca-BDE mixture (composed primarily of BDE-209) was subsequently phased out, as of December 31, 2013 (U.S. EPA 2014).
Adults are exposed to POPs through various routes, including consumption of contaminated food, accidental ingestion of settled dust, dermal contact with settled dust, and inhalation of contaminated air. The relative contribution of each exposure route to total POP intake may vary by chemical class and by chemicals within the same class. For example, because consumer goods that have been treated with PBDEs can still be found readily in U.S. homes (Stapleton, et al. 2012b), PBDE levels in settled dust remain high [e.g., median concentrations > 1 ppm for BDEs 47, 99, and 209 in 2010 (Whitehead, et al. 2013)]. Accordingly, it has been suggested that dust ingestion is a major route of exposure to PBDEs for U.S. adults (Lorber 2008) and positive relationships have been observed between levels of PBDEs in dust and levels of PBDEs in matched samples of serum (Johnson, et al. 2010; Watkins, et al. 2012) and milk (Wu, et al. 2007) in U.S. adults. However, due to small sample sizes [i.e., N = 12 (Johnson, et al. 2010; Wu, et al. 2007) and 31 (Watkins, et al. 2012)], these previous studies had limited capacity to use multivariable regression to adjust for other factors, such as diet, that may influence serum PBDE concentrations. As such, the true role of dust ingestion in adults’ exposure to PBDEs remains a topic of interest.
In contrast, dust ingestion is hypothesized to be a relatively minor contributor to total PCB intake for U.S. adults compared to inhalation or diet (Harrad, et al. 2009). All PCB-containing consumer products in the U.S. are at least 35 years old and their presence in U.S. homes is increasingly rare; as a result, PCB levels in settled dust are relatively low compared to PBDE levels [e.g., median concentrations < 5 ppb for PCBs 138 and 153 in 2010 (Whitehead, et al. 2014)]. However, one study of 20 Wisconsin households did observe a relationship between dust-PCB concentrations and serum-PCB concentrations, after adjusting for fish consumption (Knobeloch, et al. 2012). To our knowledge, no other previous studies have evaluated the relationship between PCB levels in matched dust and biological samples in U.S. adults.
Given that women exposed to POPs may experience various adverse reproductive effects, including decreased birth weight (Govarts, et al. 2012; Harley, et al. 2011), increased time to pregnancy (Chevrier, et al. 2013; Harley, et al. 2010), increased risk of spontaneous abortion (Korrick, et al. 2001), and increased risk of leukemia (Bailey, et al. 2014) and other cancers (Infante-Rivard and Weichenthal 2007; Vinson, et al. 2011) in their offspring, we sought to identify key routes of exposure to OC pesticides, PCBs, and PBDEs in California mothers of reproductive age participating in the California Childhood Leukemia Study.
2. MATERIAL AND METHODS
2.1. Study population
The California Childhood Leukemia Study (CCLS) is a case–control study of childhood leukemia conducted in the San Francisco Bay area and California Central Valley that seeks to identify genetic and environmental risk factors for childhood leukemia. Cases 0–14 years of age were ascertained from pediatric clinical centers; controls, matched to cases on date of birth, sex, Hispanic ethnicity, and mother’s race were selected from the California birth registry. We have employed several strategies to characterize the children’s exposure to chemicals, including the measurement of POPs in serum collected from the children’s mothers and in dust samples collected from the children’s homes. Mothers of case and control participants who were initially interviewed from 2005–2007 were eligible for blood collection if the participating child was 0–7 years old. Households of case and control participants who were enrolled in the study from December 1999 through November 2007 were eligible for dust collection if the participating child was 0–7 years old and living in the same home occupied at the time of diagnosis (or a similar reference date for controls). Among 112 mothers who provided a blood sample and had a vacuum-bag-dust sample analyzed for PBDEs, we selected a group of 50 mothers for POP analysis. Because we previously observed that Hispanic families, families with annual income below $75,000, and families residing in the Sacramento Valley and Sierra Mountains had higher PBDE-dust concentrations compared to other CCLS families (Whitehead, et al. 2013), we prioritized samples from these groups for POP analysis. Of the 50 blood samples analyzed for POPs, two did not pass quality control and were excluded from further analyses. Figure S1 (see Supplementary Materials) shows a complete flow diagram of the 48 mothers comprising the study population.
2.2 POPs analysis in serum
Blood samples were collected from the mothers at their homes from January 2006 through October 2007 by a physician’s assistant. Blood samples were collected in red-top vacutainer tubes without anticoagulant, shipped or hand delivered on ice packs for receipt at the laboratory no more than one day after collection, centrifuged, and the separated serum was stored at −20°C or colder prior to analysis.
The serum sample preparation protocol (see, Supplementary Materials Figure S2 and Table S1) was adapted from Sjodin et al. (Sjodin, et al. 2004b). After thawing, 1-mL serum samples were diluted to 2 mL with HPLC-grade water, spiked with internal standards (Tetrachloro-m-xylene, PCB-14, PCB-65, PCB-165, and BDE-139), denatured with formic acid, and extracted via an automated sample extraction system (RapidTrace, Biotage; Uppsala Sweden) that used a 540-mg Oasis HLB solid phase extraction cartridge (Waters Corp.; Milford, MA) and 12 mL of 1:1 hexane:methylene chloride for elution. After extraction, samples were concentrated to 1 mL using an automated nitrogen evaporation system (TurboVap LV, Biotage; Uppsala, Sweden). Subsequently, concentrated extracts were cleaned using manually packed acid-silica chromatography columns, followed by a further concentration to 80 µL, and a solvent exchange into isooctane. Finally, 13C12-labeled PCB-209 was added as an injection standard.
Samples (1-µL injections) were analyzed for 17 PCBs (PCBs 28, 66, 74, 99, 101, 105, 118, 138, 153, 156, 167, 170, 180, 183, 187, 194, 203), 5 PBDEs (BDEs 47, 99, 100, 153, 154, major components of the Penta-BDE commercial mixture), and 7 OC pesticides or metabolites thereof, including β-hexachlorocyclohexane (β-HCH, the major metabolite of the insecticide HCH), o,p'-DDT (a minor component of the insecticide DDT), p,p'-DDT (the major component of DDT), p,p'-Dichlorodiphenyldichloroethylene (p,p'-DDE, the major metabolite of DDT), hexachlorobenzene (HCB, a fungicide), trans-nonachlor (a minor component of the insecticide chlordane), and oxychlordane (the major metabolite of chlordane) using gas chromatography (7890 GC, Agilent Technologies; Sunnyvale, CA)-triple quadrupole mass spectrometry (7000B Series, Agilent Technologies; Sunnyvale, CA). Chromatographic conditions included pulsed splitless injection at 250°C, helium carrier gas at 1mL/min, and a 30-m DB-5ms column with 0.25-mm diameter and 0.25-µm film thickness (Agilent Technologies; Sunnyvale, CA). The GC temperature program was initiated at 90°C, held for 1 min, ramped at 50°C/min to 150°C, held for 1 min, ramped at 8°C/min to 225°C, held for 6.5 min, ramped at 14°C/min to 310°C, and finally held for 6 min. The mass spectrometer was operated in electron impact ionization mode using multiple ion detection, source temperature of 275°C, ionization energy of 70 eV, and mass resolution of 1.2 amu (see, Supplementary Materials Table S2).
Serum samples were analyzed in batches of 20 including 14 field samples, two bovine serum (HyClone bovine serum, Thermo Fisher Scientific, Inc; Waltham, MA) blanks as negative laboratory controls, two bovine serum blanks spiked with each target analyte as positive laboratory controls, and two standard reference materials (National Institute of Standards and Technology SRM 1958; Gaithersburg, MD). Results from 8 SRM samples (see, Supplementary Materials Table S3) were suitably accurate (e.g., average percent errors of −1%, −9%, and 22% for p,p’-DDE, PCB-153, and BDE-47, respectively) and precise (e.g., coefficients of variation of 6%, 3%, and 7%, respectively). The method detection limit (MDL) for each analyte was defined as three times the standard deviation of the masses in six blanks (see, Supplementary Materials Table S4). In regression analyses, values below the MDL were replaced by MDL/√2. POP concentrations were normalized by the percent recovery of PCB-165, an internal standard, on a sample-to-sample basis. Two samples had PCB-165 recovery below 50% and were not used in statistical analyses. For the remaining 48 samples, average PCB-165 recovery was 93% (±16%). Total cholesterol and triglyceride levels were measured by the Boston Children’s Hospital and used in Phillips’ formula to calculate total lipid content [i.e., TL = 2.27×TC + TG + 0.673 (Phillips, et al. 1989)].
2.3. POPs analysis in dust
From June 2005 through October 2007, dust samples were collected from the 48 participants’ vacuum cleaners, which had been used for day-to-day household cleaning prior to the sampling visit. There was no standardized dust sampling protocol and past locations of vacuum cleaner use were not known. Dust samples were stored away from heat (≤ 4°C) and light before chemical analysis.
The laboratory protocol used to analyze dust samples for PCBs (Whitehead, et al. 2014) and PBDEs (Whitehead, et al. 2013) has been described elsewhere. Briefly, dust particles smaller than 150 µm were obtained with a 100-mesh sieve and 0.2-g portions were spiked with 13C12-labeled internal standards, extracted via accelerated solvent extraction, purified by silica-gel column chromatography and gel permeation chromatography, concentrated to 250 µL, solvent exchanged into tetradecane, and spiked with 13C12-labeled injection standards. Samples were analyzed for p,p'-DDT, p,p'-DDE, 15 PCBs (PCBs 28, 52, 101, 105, 114, 118, 138, 153, 156, 157, 167, 180, 189, 194, and 209) and 22 PBDEs (BDEs 28, 32, 47, 66, 71, 99, 100, 153, 154, 155, 179, 183, 190, 196, 197, 201, 202, 203, 206, 207, 208, and 209) by isotope-dilution/gas chromatography-high resolution mass spectrometry.
2.4. Questionnaire information
In this analysis, we used information from three questionnaires administered on separate occasions. On average, 10 months (range: 2 to 40 months) after leukemia diagnosis (or a corresponding reference date for controls), participating mothers completed a structured in-home interview designed to ascertain information about a wide variety of topics that are potentially relevant to childhood leukemia etiology. Relevant information collected at the primary interview included the mother’s age, educational attainment, place of birth, Hispanic ethnicity, height, weight prior to the index pregnancy, and number of births prior to index pregnancy, as well as the length of time that the index child was breastfed, and the household annual income. We estimated cumulative lactation as the product of parity*breastfeeding duration for the index child. At the same interview, mothers completed a modified version of the Block food frequency questionnaire describing their eating habits during the year before the index pregnancy; this questionnaire has been previously validated (Block, et al. 1990). From this information we estimated the daily consumption of fish, fat, and meat, as previously described (Kwan, et al. 2009).
During the dust sampling visit, participating mothers completed a second interview designed to ascertain information relevant to chemical exposures in the home. Relevant information collected at the second interview included the construction date of the residence. At the same time, a global positioning device was used to determine the latitude and longitude for each residence and each residence was classified as belonging to one of six geographic regions. We linked each location to the corresponding U.S. Census block and identified each residence as urban, suburban, or rural based on the Census Bureau's delineations. Finally, during a subsequent telephone interview, participating mothers answered questions designed to identify possible PBDE sources in the home. Relevant information collected during the third interview included the presence of upholstered furniture with crumbling or exposed foam.
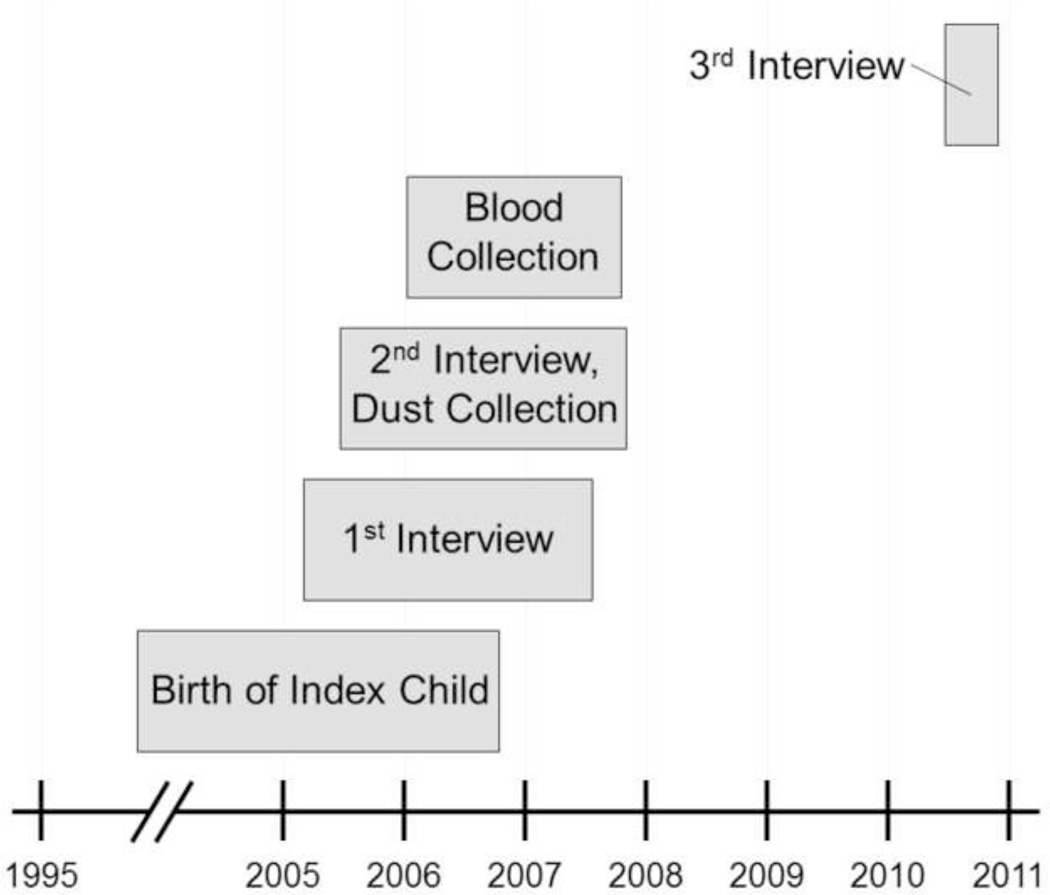
Blood collection and the three interviews were each conducted at separate times (see Figure 1). The average interval between the date of primary interview and the date of blood collection was 7 months (range: <1 to 23 months). The average interval between the date of blood collection and the date of dust collection was 5 months (range: <1 to 18 months) and blood collection preceded dust collection in 56% of the homes. The average interval between the date of blood collection and the date of the third interview was 3.8 years (range: 2.8 to 4.5 years). As indicated above, some of the questionnaire information used in this analysis, including dietary habits, was based on mothers’ recall of their index pregnancy and the average interval between the index child’s date of birth and the date of blood collection was 6 years (range: <1 to 11 years).
Figure 1.
Timing of sample collections and interviews for 48 mothers participating in the California Childhood Leukemia Study.
Because some participants did not complete all parts of the questionnaires, missing responses were replaced by the population medians from non-missing households in regression analyses (e.g., breastfeeding duration was missing for three respondents and was replaced by the population median of 24 weeks; see, Supplementary Materials Table S5).
2.5. Statistical analysis
Bivariate relationships between explanatory factors and lipid-normalized POP concentrations were evaluated using non-parametric Spearman rank correlation coefficients (for continuous and ordinal factors) and Kruskal-Wallis tests (for categorical and dichotomous factors). For each POP, we also evaluated a multivariable linear regression model of natural-log transformed wet-weight serum concentrations (continuous, ln[ng/mL serum]), which included the following explanatory factors: total serum lipids (continuous, mg/mL), age at blood collection (continuous, years), ethnicity (Hispanic or not), country of origin (U.S. or elsewhere), pre-pregnancy body mass index (BMI, continuous, kg/m2), daily fish consumption (continuous, g/day), cumulative lactation (continuous, years), blood sampling date (continuous, day), household annual income (ordinal, <$15k, $15–29k, $30–44k, $45–59k, $60–74k, ≥$75k), and the natural-log transformed dry-weight concentration of the same POP in dust (continuous, ln[ng/g]). Other variables were evaluated in bivariate analyses, but not included in multivariable models, because they were not related to serum-POP concentrations (residence location, residence construction date, urban density, fat consumption), they were co-linear with other explanatory factors (education, presence of upholstered furniture with crumbling or exposed foam in the residence), or they were heavily influenced by one observation (meat consumption). Whereas bivariate relationships were evaluated using lipid-normalized POP concentrations, the multivariable regression analysis evaluated wet-weight serum concentrations as outcome variables with total serum lipids used as an additional explanatory factor, due to the potential for underlying correlation between serum lipids and other explanatory factors (e.g., age, ethnicity, BMI) (Wolff, et al. 2005).
3. RESULTS
Table 1 shows summary statistics for lipid-adjusted concentrations of OC pesticides, PCBs, and PBDEs in serum samples from 48 mothers. Among the 29 chemicals analyzed, p,p’-DDE was found at the highest levels, with a median concentration of 180 ng/g lipid. In comparison, we observed lower median concentrations for the two components of the parent insecticide (4.1 and 5.3 ng/g lipid for p,p’-DDT and o,p’-DDT, respectively). Median concentrations of trans-nonachlor, oxychlordane, HCB, and β- HCH were lower than DDE and on the same order of magnitude as the DDT isomers (medians of 8.8, 5.0, 7.8, and 5.9 ng/g lipid, respectively). Each of 17 PCB congeners was detected in at least 60% of the serum samples, with median concentrations of at least 1 ng/g lipid. Of the PCBs analyzed, PCBs 118, 138, 153, 170, and 180 had the highest median concentrations (i.e., 4.0, 6.4, 9.7, 4.4, and 9.3 ng/g lipid, respectively) and each of these congeners was detected in every sample. Of the 5 PBDEs analyzed, BDE-47 was found at the highest levels, with a median concentration of 35 ng/g lipid. Median concentrations of BDEs 99, 100, and 153 were comparable to the median concentrations of the most prevalent PCBs (i.e., 10, 10, and 8.9 ng/g lipid, respectively) and each of these congeners was detected in at least 96% of samples. Additional results and discussion will focus on the major chemicals in each class (i.e., p,p’-DDE; trans-nonachlor; PCBs 118, 138, 153, 170, and 180; and BDEs 47, 99, 100, and 153).
Table 1.
Lipid-adjusted concentrations (ng/g lipid) of organochlorine pesticides, polychlorinated biphenyls, and polybrominated diphenyl ethers in serum samples collected from 48 mothers participating in the California Childhood Leukemia Study (2006–2007).
| Chemical | Detection Frequency |
Method Detection Limit (MDL) |
Percentiles of Serum Concentrations |
Geometric Mean |
|||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 90th | ||||
| Organochlorine pesticides | |||||||
| β-BHC | 69% | 1.6 | <MDL | 5.9 | 10 | 29 | 5.7 |
| o,p'-DDT | 69% | 1.9 | <MDL | 5.3 | 7.1 | 8.2 | 3.7 |
| p,p'-DDT | 88% | 1.6 | 3.3 | 4.1 | 6.3 | 9.0 | 4.3 |
| p,p'-DDE | 100% | 4.3 | 120 | 180 | 290 | 420 | 190 |
| HCB | 100% | 1.6 | 6.8 | 7.8 | 8.5 | 10 | 7.7 |
| trans-Nonachlor | 100% | 1.7 | 6.7 | 8.8 | 11 | 15 | 8.8 |
| Oxychlordane | 98% | 1.6 | 3.9 | 5.0 | 5.8 | 7.1 | 4.6 |
| Polychlorinated biphenyls (PCBs) | |||||||
| PCB-28 | 100% | 1.6 | 3.1 | 3.6 | 4.3 | 6.8 | 4.1 |
| PCB-66 | 85% | 1.6 | 1.7 | 2.3 | 2.9 | 3.4 | 2.2 |
| PCB-74 | 98% | 1.6 | 2.4 | 3.2 | 3.8 | 4.2 | 3.0 |
| PCB-99 | 90% | 1.6 | 2.2 | 3.0 | 3.5 | 4.3 | 2.7 |
| PCB-101 | 65% | 1.6 | <MDL | 1.8 | 2.3 | 2.9 | 1.7 |
| PCB-105 | 73% | 1.6 | <MDL | 2.0 | 2.5 | 2.7 | 1.9 |
| PCB-118 | 100% | 1.7 | 3.2 | 4.0 | 5.4 | 6.7 | 4.0 |
| PCB-138 | 100% | 1.6 | 4.8 | 6.4 | 9.4 | 14 | 6.8 |
| PCB-153 | 100% | 1.6 | 6.5 | 9.7 | 17 | 28 | 11 |
| PCB-156 | 94% | 1.6 | 1.9 | 2.5 | 3.4 | 4.1 | 2.6 |
| PCB-167 | 60% | 1.6 | <MDL | 1.6 | 2.1 | 2.3 | 1.6 |
| PCB-170 | 100% | 1.6 | 3.2 | 4.4 | 6.6 | 9.9 | 4.6 |
| PCB-180 | 100% | 1.6 | 6.1 | 9.3 | 15 | 25 | 10 |
| PCB-183 | 81% | 1.8 | 1.6 | 2.4 | 2.8 | 3.3 | 2.2 |
| PCB-187 | 100% | 1.6 | 2.5 | 3.4 | 4.6 | 7.2 | 3.5 |
| PCB-194 | 100% | 1.6 | 2.3 | 3.2 | 4.2 | 5.4 | 3.1 |
| PCB-203 | 100% | 1.6 | 2.2 | 3.1 | 4.0 | 5.3 | 3.0 |
| Polybrominated diphenyl ethers (PBDEs) | |||||||
| BDE-47 | 98% | 12 | 23 | 35 | 68 | 110 | 43 |
| BDE-99 | 100% | 2.9 | 6.5 | 10 | 13 | 25 | 11 |
| BDE-100 | 100% | 2.4 | 8.0 | 10 | 15 | 22 | 12 |
| BDE-153 | 96% | 2.4 | 5.0 | 8.9 | 16 | 46 | 10 |
| BDE-154 | 48% | 2.4 | <MDL | <MDL | 3.6 | 5.7 | 2.7 |
Table 2 shows Spearman correlation coefficients between lipid-adjusted POP serum concentrations and vacuum cleaner-dust POP concentrations. Positive correlation between dust and serum levels was observed for BDEs 47 and 99 (rs of 0.45 and 0.39, respectively). No significant correlation between dust and serum levels was observed for the major PCBs or p,p’-DDE. Dust-serum relationships were not evaluated for trans-nonachlor or PCB-170, because dust measurements were not available for these POPs.
Table 2.
Spearman correlation coefficients between lipid-adjusted serum concentrations (ng/g lipid) and vacuum cleaner-dust concentrations (ng/g dust) of selected persistent organic pollutants for 48 mothers participating in the California Childhood Leukemia Study.
| Chemical | rs | p-value |
|---|---|---|
| Organochlorine pesticide | ||
| p,p'-DDE | −0.21 | 0.15 |
| Polychlorinated biphenyls | ||
| PCB-118 | −0.07 | 0.62 |
| PCB-138 | −0.05 | 0.72 |
| PCB-153 | 0.01 | 0.95 |
| PCB-180 | 0.04 | 0.81 |
| Polybrominated diphenyl ethers | ||
| BDE-47 | 0.45 | 0.001 |
| BDE-99 | 0.39 | 0.006 |
| BDE-100 | 0.17 | 0.25 |
| BDE-153 | 0.10 | 0.50 |
Note: Dust-serum relationships were not evaluated for trans-nonachlor or PCB-170, because dust measurements were not available for these POPs.
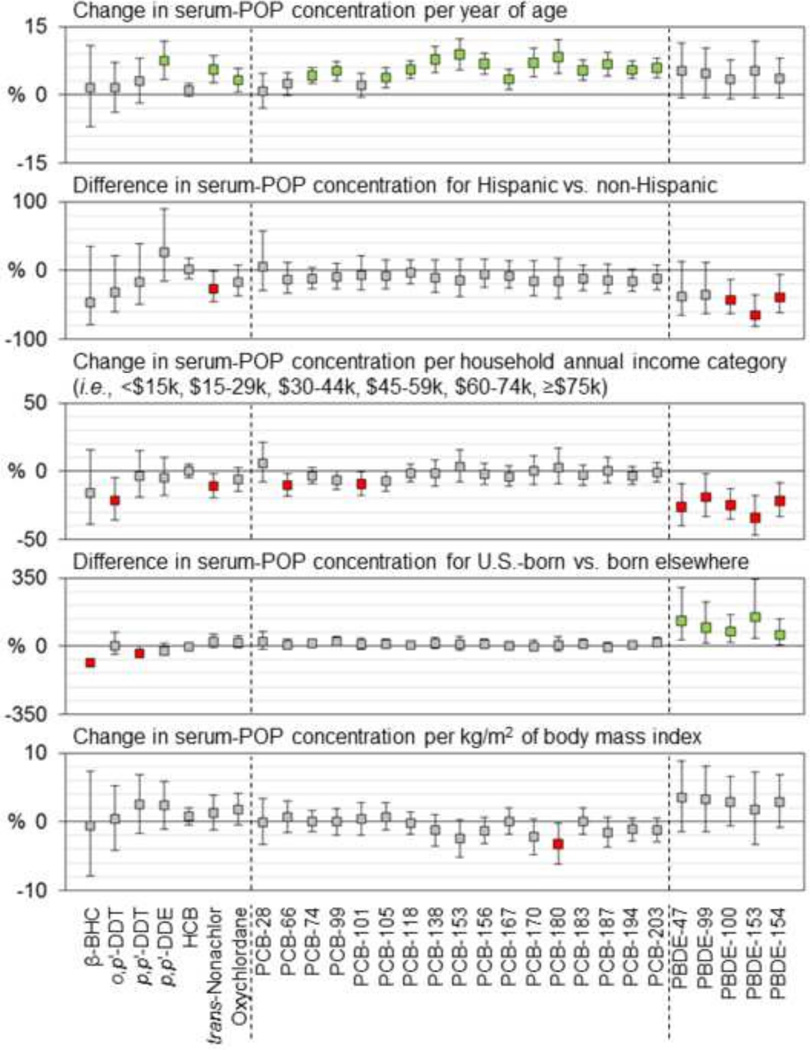
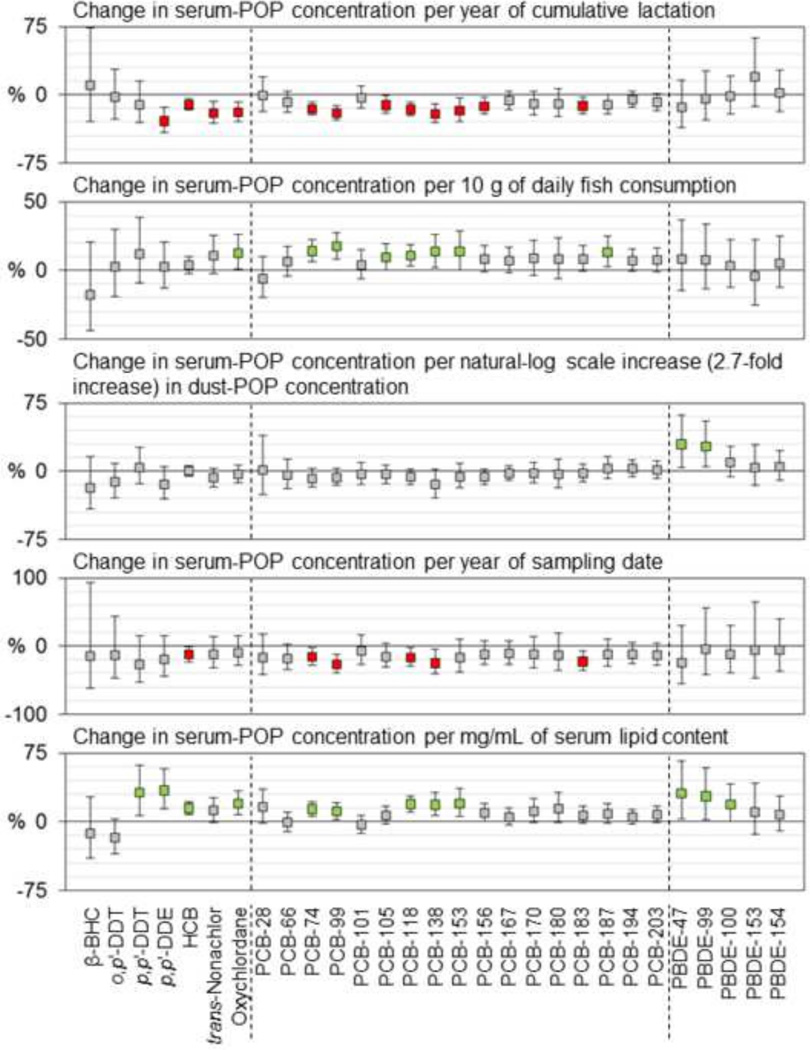
Table 3 shows bivariate relationships between lipid-adjusted POP concentrations and various explanatory factors. Figure 2 shows the percent change in POP serum concentrations associated with a unit increase in each explanatory factor included in the multivariable models. Age at blood collection was positively correlated with serum concentrations of p,p’-DDE and each of five major PCBs in bivariate analyses (Table 3). As shown in Figure 2, in multivariable models, a yearly increment of age was associated with a 6–9% increase in serum concentrations of p,p’-DDE, each of five major PCBs, and trans-nonachlor (shown as green boxes). In bivariate analyses, concentrations of PCBs 170 and 180 differed by race/ethnicity, with lower median concentrations in sera from Hispanic women than in sera from non-Hispanic White and Asian women (Table 3); however, in multivariable analyses, these differences did not reach statistical significance, whereas concentrations of trans-nonachlor and BDEs 100 and 153 were significantly lower in sera from Hispanic women than in sera from non-Hispanic women (Figure 2). Household annual income was positively correlated with serum concentrations of PCBs 138, 153, 170, and 180 and negatively correlated with concentrations of BDEs 47, 99, and 100 in bivariate analyses (Table 3); however only the income-PBDE relationships remained significant in multivariable models (Figure 2). Median concentrations of the four major PBDEs were higher in sera from women born in the U.S. than in sera from foreign-born women; while the differences were not statistically significant in bivariate analyses (Table 3), they were significant in multivariable models (Figure 2). Pre-pregnancy BMI was negatively correlated with serum concentrations of PCBs 153 (p=0.09), 170, and 180 (Table 3), and these relationships were marginally significant in multivariable models (i.e., p<0.1; Figure 2). Concentrations of major POPs were not correlated with cumulative lactation in bivariate analyses (Table 3); however, in multivariable models, p,p’-DDE, trans-nonachlor, and PCBs 118, 138, and 153 were inversely associated with cumulative lactation (Figure 2). As a group, women who consumed more than 10 g of fish per day (N=15) had higher median concentrations of each of five major PCBs and trans-nonachlor than women who consumed less than 5 g or 5–10 g of fish per day, daily fish consumption was positively correlated with serum concentrations of PCB-170 in bivariate analyses (Table 3), and fish consumption was associated with serum concentrations of PCBs 118, 138, and 153 in multivariable models (Figure 2). Women who had upholstered furniture with crumbling or exposed foam in their homes (N=7) had higher serum levels of BDEs 47 and 99 than women without such furniture (Table 3); however, this variable was not included in multivariable analyses that contained dust concentrations of PBDEs, as dust and foam were considered to represent the same route of exposure to PBDEs. Logged dust concentrations of BDEs 47 and 99 were positively associated with serum-PBDE concentrations in multivariable models (Figure 2). Concentrations of major POPs did not differ by region (Table 3). Sampling date was negatively correlated with serum concentrations of each of five major PCBs in bivariate analyses (Table 3), and these relationships remained significant in multivariable models of PCBs 118 and 138 (Figure 2). Serum lipid content was positively associated with p,p’-DDE, PCBs 118, 138, and 153, and BDEs 47, 99, and 100 in multivariable models (Figure 2).
Table 3.
Bivariate relationships between explanatory factors and lipid-adjusted concentrations (ng/g lipid) of selected persistent organic pollutants in serum samples collected from 48 mothers participating in the California Childhood Leukemia Study (2006–2007).
| Explanatory Factor | OCPs | PCBs | PBDEs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N |
p,p' DDE |
trans- Nona- chlor |
PCB- 118 |
PCB- 138 |
PCB- 153 |
PCB- 170 |
PCB- 180 |
BDE- 47 |
BDE- 99 |
BDE- 100 |
BDE- 153 |
|
| Age | + | + | + | + | + | + | ||||||
| <34.0 | 12 | 140 | 7.4 | 3.2 | 5.3 | 6.9 | 3.2 | 5.9 | 29 | 7.4 | 8.3 | 5.4 |
| 34.0–37.4 | 12 | 150 | 10 | 3.4 | 5.8 | 8.0 | 3.7 | 8.2 | 43 | 11 | 12 | 8.7 |
| 37.5–40.9 | 12 | 260 | 10 | 5.3 | 6.4 | 9.7 | 4.5 | 9.7 | 43 | 10 | 12 | 9.8 |
| >40.9 | 12 | 250 | 9.2 | 5.4 | 9.6 | 19 | 7.0 | 17 | 28 | 9.2 | 10 | 11 |
| Race/ethnicity |  |
 |
||||||||||
| Hispanic | 15 | 220 | 7.5 | 3.7 | 5.5 | 6.9 | 3.4 | 7.0 | 37 | 12 | 10 | 7.6 |
| Non-Hispanic, White | 27 | 180 | 9.9 | 4.7 | 7.3 | 13 | 5.0 | 12 | 31 | 8.9 | 10 | 11 |
| Non-Hispanic, Asian | 5 | 200 | 5.7 | 3.3 | 4.9 | 9.5 | 4.4 | 10 | 21 | 6.1 | 8.1 | 11 |
| Non-Hispanic, Native | 1 | 260 | 9.0 | 3.9 | 6.4 | 8.0 | 2.5 | 5.9 | 540 | 88 | 110 | 130 |
| Household annual income | + | + | + | + | − | − | − | |||||
| <$45,000 | 11 | 260 | 10 | 3.7 | 5.4 | 6.9 | 3.1 | 5.9 | 37 | 12 | 12 | 9.7 |
| $45,000-$74,999 | 13 | 150 | 9.2 | 3.9 | 5.9 | 9.6 | 4.5 | 9.2 | 69 | 13 | 14 | 8.3 |
| ≥$75,000 | 24 | 190 | 8.1 | 4.5 | 7.8 | 13 | 5.0 | 11 | 28 | 7.5 | 9.6 | 8.1 |
| Country of origin | ||||||||||||
| U.S. | 35 | 160 | 9.9 | 4.0 | 6.4 | 9.7 | 4.4 | 9.5 | 50 | 11 | 11 | 9.4 |
| Other | 13 | 260 | 8.0 | 4.4 | 6.2 | 9.6 | 4.4 | 8.5 | 31 | 7.0 | 9.5 | 6.0 |
| Body mass index | − | − | + | |||||||||
| Underweight | 2 | 200 | 9.0 | 4.6 | 9.2 | 16 | 6.2 | 15 | 44 | 11 | 11 | 15 |
| Normal | 28 | 170 | 9.1 | 4.2 | 7.1 | 13 | 5.0 | 12 | 29 | 7.7 | 10 | 8.3 |
| Overweight | 13 | 260 | 8.3 | 4.4 | 6.0 | 8.6 | 4.0 | 8.4 | 58 | 13 | 13 | 9.9 |
| Obese | 4 | 180 | 11 | 3.7 | 5.6 | 6.4 | 3.4 | 6.0 | 52 | 11 | 13 | 5.8 |
| Cumulative lactation | ||||||||||||
| <6 months | 19 | 220 | 8.7 | 4.1 | 6.6 | 9.9 | 4.0 | 8.5 | 36 | 11 | 10 | 6.0 |
| 6–12 months | 13 | 200 | 9.9 | 5.3 | 5.9 | 9.6 | 4.6 | 10 | 50 | 11 | 12 | 11 |
| >1 year | 16 | 140 | 7.7 | 3.4 | 6.1 | 9.5 | 4.5 | 10 | 29 | 7.8 | 10 | 9.8 |
| Fish consumption | + | |||||||||||
| <5 g/day | 19 | 220 | 7.8 | 3.7 | 6.0 | 9.3 | 4.0 | 9.5 | 51 | 11 | 12 | 8.5 |
| 5–10 g/day | 14 | 150 | 7.8 | 4.0 | 5.5 | 8.2 | 3.4 | 7.5 | 29 | 10 | 9.9 | 7.0 |
| >10 g/day | 15 | 190 | 10 | 4.4 | 7.3 | 13 | 5.0 | 12 | 36 | 8.5 | 12 | 9.9 |
| Crumbling/exposed foam |  |
 |
||||||||||
| Yes | 7 | 230 | 7.6 | 4.7 | 8.1 | 13 | 6.4 | 14 | 61 | 12 | 12 | 11 |
| No | 38 | 180 | 8.5 | 4.0 | 6.3 | 9.6 | 4.1 | 9.0 | 30 | 7.8 | 10 | 8.4 |
| Region | ||||||||||||
| California Central Coast | 4 | 180 | 8.2 | 3.8 | 5.8 | 7.8 | 3.8 | 6.8 | 43 | 10 | 10 | 5.2 |
| Metro San Francisco | 17 | 220 | 7.4 | 4.4 | 6.5 | 9.7 | 4.0 | 8.5 | 37 | 11 | 9.8 | 6.6 |
| North San Francisco | 5 | 140 | 11 | 5.4 | 7.1 | 13 | 6.8 | 15 | 25 | 5.2 | 13 | 11 |
| Sacramento Valley | 9 | 130 | 7.6 | 3.9 | 6.4 | 9.9 | 4.2 | 9.5 | 28 | 7.2 | 10 | 8.5 |
| San Joaquin Valley | 7 | 160 | 10 | 3.7 | 5.4 | 6.4 | 3.3 | 5.5 | 57 | 13 | 17 | 9.4 |
| Sierra Mountains | 5 | 230 | 9.5 | 4.7 | 8.3 | 12 | 5.1 | 11 | 50 | 8.5 | 10 | 13 |
| Sampling year | − | − | − | − | − | |||||||
| 2006 | 32 | 180 | 9.3 | 4.4 | 6.8 | 10 | 4.8 | 9.9 | 50 | 11 | 11 | 9.6 |
| 2007 | 16 | 190 | 8.4 | 3.3 | 4.5 | 6.5 | 3.3 | 6.6 | 27 | 7.7 | 9.8 | 7.0 |
OCPs=Organochlorine pesticides, PCBs=Polychlorinated biphenyls, PBDEs=Polybrominated diphenyl ethers
+ Indicates positive Spearman rank correlation coefficient with p<0.05
− Indicates negative Spearman rank correlation coefficient with p<0.05
 Indicates that serum concentrations differ by category using Kruskal-Wallis test with p<0.05
Indicates that serum concentrations differ by category using Kruskal-Wallis test with p<0.05
Figure 2.
Percent change (95% confidence interval) in wet-weight serum concentrations (ng/mL serum) of persistent organic pollutants associated with a unit increase in each explanatory factor included in the multivariable models. Statistically significant (p<0.05) regression coefficients are indicated with green (increase) and red (decrease) fill.
4. DISCUSSION
While numerous studies have tried to identify factors that influence serum concentrations of POPs, our analysis is one of the few to combine serum concentrations, dust concentrations, and questionnaire information on dietary habits, reproductive history, and demographic characteristics in multivariable regression. Our novel graphical approach to presenting multivariable regression results (Figure 2) affords a useful opportunity to compare and contrast factors that influence serum concentrations of different classes of POPs.
We confirmed established relationships between serum concentrations of the legacy POPs, PCBs and OC pesticides, and various factors. Serum lipid content and age were both positively related to serum concentrations of PCBs and OC pesticides, as has been noted in previous studies (Agudo, et al. 2009; Bachelet, et al. 2011; Garabrant, et al. 2009; Glynn, et al. 2007; Hardell, et al. 2010; Ibarluzea, et al. 2011; Laden, et al. 1999; McGraw and Waller 2009a; Moysich, et al. 2002; Schade and Heinzow 1998; Weiss, et al. 2006; Wolff, et al. 2005). In multivariable models, cumulative lactation time was associated with lower levels of several major legacy POPs, consistent with the hypothesis that mothers excrete POPs during lactation (Hardell, et al. 2010; Ibarluzea, et al. 2011; Laden, et al. 1999; Moysich, et al. 2002; Schade and Heinzow 1998; Wolff, et al. 2005). In multivariable models, we found that fish consumption was associated with serum-PCB concentrations. Taken together with the observation that serum-PCB concentrations were highest in the group of mothers that consumed the most fish, our regression results suggest that fish consumption may be an important source of exposure to PCBs for mothers participating in the CCLS. Fish consumption is known to be an important source of exposure to PCBs in many other populations as well (Agudo, et al. 2009; Bachelet, et al. 2011; Garabrant, et al. 2009; Glynn, et al. 2007; Ibarluzea, et al. 2011; Knobeloch, et al. 2012; McGraw and Waller 2009a; Moysich, et al. 2002; Schade and Heinzow 1998; Uemura, et al. 2010; Weiss, et al. 2006). Serum concentrations of p,p’-DDE were not associated with fish consumption, and the observed effect of country of origin on p,p’-DDT suggests that early-life exposures may have been more influential sources of DDT than recent dietary habits. We did not find any relationships between levels of PCBs or OC pesticides in dust and serum, which suggests that dust ingestion is not a major source of exposure to these legacy POPs for mothers participating in the CCLS.
PCB concentrations in the sera of U.S. adults have decreased since their ban in the 1970s (Sjodin, et al. 2004a) and sampling date has been inversely associated with PCB concentrations in contemporary biospecimens from several cross-sectional studies that span short time intervals (Agudo, et al. 2009; Glynn, et al. 2007; Hardell, et al. 2010; Schade and Heinzow 1998). Likewise, in our cross-sectional analysis, serum concentrations of PCBs 118 and 138 decreased with sampling date during the study period (2006–2007). Moreover, when compared to other studies of PCBs in sera from California adults, our results are consistent with a decreasing trend over time, for example our median PCB-153 concentration (9.7 ng/g lipid) is lower than that for samples collected in the 1960s (73 ng/g lipid) and 1990s (41 ng/g lipid) (Petreas, et al. 2003) and higher than that for samples collected from 2008–2009 (3.8 ng/g lipid) and 2011–2012 (3.1 ng/g lipid) (Zota, et al. 2013). Wolff et al. (Wolff, et al. 2005) postulated that in populations where POP uptake is ongoing, the dilution effect of high adiposity results in an inverse relationship between BMI and POP-serum concentrations; whereas, in populations where current POP uptake is negligible, POPs trapped in adipose tissues are released more slowly in normal weight women than in overweight women, resulting in a positive relationship between BMI and POP-serum concentrations. Along with similar findings from other studies conducted in the 1990s and 2000s (Agudo, et al. 2009; Bachelet, et al. 2011; Glynn, et al. 2007; Ibarluzea, et al. 2011), our observation of an inverse relationship between BMI and PCB-180 in serum suggests that mothers participating in the CCLS were still being exposed to environmental sources of PCBs, presumably via diet.
In contrast to legacy POPs, longitudinal studies suggest that levels of BDEs 47, 99, and 153 in blood from U.S. adults increased from the 1970s to the early 2000s (Sjodin, et al. 2004a) and plateaued in the mid-2000s (Turyk, et al. 2010) corresponding with the Penta-BDE phase out. In the context of other studies of PBDEs in sera from California adults, our results suggest a similar time trend, for example our median BDE-47 concentration for samples collected from 2006–2007 (35 ng/g lipid) is similar to that for samples collected in 2003–2004 (36 ng/g lipid) (Zota, et al. 2008) and 2008–2009 (42 ng/g lipid) (Zota, et al. 2013) and higher than that for samples collected earlier [<10, 10, and 15 ng/g lipid, for the 1960s (Petreas, et al. 2003), 1997–1999 (Petreas, et al. 2003), and 1999–2000 (Castorina, et al. 2011), respectively] or more recently [21.8 ng/g lipid for 2011–2012 (Zota, et al. 2013)]. Thus, our blood collection dates correspond to the period of peak exposure to Penta-BDE in California.
Given that PCBs, OC pesticides, and PBDEs have different production histories and different sources in the environment, adults may be exposed to these chemical classes via different routes. In our multivariable analysis, we observed positive associations between dust concentrations and serum concentrations of BDEs 47 and 99, suggesting that dust is an important source of exposure to PBDEs for mothers participating in the CCLS. In other adult populations, similar positive relationships have been observed between dust-PBDE concentrations and concentrations of PBDEs in serum (Johnson, et al. 2010; Watkins, et al. 2012), cord blood (Frederiksen, et al. 2010), breast milk (Bjorklund, et al. 2012; Wu, et al. 2007), placental tissue (Vorkamp, et al. 2011), and hair (Tang, et al. 2013). When relationships between dust and biological levels of PBDEs are evaluated on a congener-specific basis, a null relationship for BDE-153 is consistently noted (Bjorklund, et al. 2012; Frederiksen, et al. 2010; Johnson, et al. 2010; Tang, et al. 2013; Vorkamp, et al. 2011). Likewise, in our analysis, we observed positive associations between dust concentrations and serum concentrations of BDEs 47 and 99, but not BDE-153. We suggest that the serum concentrations of BDE-153 are indicative of cumulative PBDE exposure, whereas serum concentrations of BDEs 47 and 99 are more heavily influenced by current PBDE environmental exposures, due to the relatively long biological half-life of BDE-153 compared to BDEs 47 and 99 (Trudel, et al. 2011).
Concentrations of BDEs 47, 99, and 153 in dust and serum, are higher in North America than the rest of the world (Frederiksen, et al. 2009), especially in the State of California, due to a history of unique flammability standards (Zota, et al. 2008). Consistent with these geographic trends, California women born in the U.S. tend to have higher serum concentrations of BDEs 47, 99, and 153 than California women born outside of the U.S. (Castorina, et al. 2011). Likewise, in our analysis, birth in the U.S. was associated with higher levels of each major PBDE.
In the U.S., lower income (Anderson, et al. 2008; Bradman, et al. 2012; Fraser, et al. 2009; Herbstman, et al. 2007; Zota, et al. 2008) and educational attainment (Herbstman, et al. 2007; Rose, et al. 2010; Stapleton, et al. 2012a; Windham, et al. 2010; Zota, et al. 2008) are generally associated with higher body burdens of BDE-47. Differences in BDE-47 body burdens by socioeconomic status have been attributed to potential differences in building materials and furniture quality (Zota, et al. 2008). Indeed, we previously found elevated concentrations of BDEs 28, 47, 99, and 153 in dust from homes that contained upholstered furniture with crumbling or exposed foam and from low-income homes (Whitehead, et al. 2013). However, neither dust-PBDE concentrations (Figure 2) nor furniture quality (data not shown) explained the income-based disparity in serum concentrations of BDEs 47, 99, 100, and 153 in our multivariable regression analysis.
Studies of NHANES data have suggested that Mexican Americans have higher serum concentrations of BDE-47 than non-Hispanic Whites (Fraser, et al. 2009; Sjodin, et al. 2008), however this relationship is not evident after adjusting for income, geographic location, and country of origin (Zota, et al. 2008). Using multivariable models that adjusted for income, we previously reported higher dust concentrations of BDEs 28, 47, and 99 in samples collected from Hispanic households participating in the CCLS compared to non-Hispanic households (Whitehead, et al. 2013). Investigations of other U.S. populations have reported higher serum concentrations of lower-brominated PBDEs in African American children compared to White children, after adjustment for the educational attainment of the child’s caretaker (Horton, et al. 2013; Windham, et al. 2010). In our multivariable analysis that adjusted for income, we found lower serum-PBDE concentrations in Hispanic mothers compared to non-Hispanic mothers. Differences in serum-PBDE concentrations by race/ethnicity may be due to differences between groups in the magnitude of exposure to PBDEs or differences in xenobiotic metabolism by ethnicity (McGraw and Waller 2009b).
Although adults may be exposed to PCBs and OC pesticides via different routes and sources than PBDEs, these chemical classes also share some physicochemical similarities. For example, like PCBs, PBDEs are lipophilic and associations between PBDE concentrations and lipid content in plasma have been reported (Spliethoff, et al. 2008). Likewise, in our analysis, we observed associations between PBDE concentrations and lipid content in serum. It follows that PBDEs would tend to accumulate in blood with age; however, the relationship between serum concentrations of PBDEs and age is also influenced by changes in the magnitude of exposure to PBDEs during different life stages. For example, due to their tendency to make hand-to-mouth contact and their proximity to the floor, young children are exposed to high levels of PBDEs via the accidental ingestion of dust and, indeed, cross-sectional (Thomsen, et al. 2002; Toms, et al. 2009) and longitudinal (Eskenazi, et al. 2011) studies suggest that serum concentrations of PBDEs peak prior to age 5. Using 2,062 serum samples collected as part of the National Health and Nutrition Examination Survey (NHANES), Sjodin et al. (Sjodin, et al. 2008) demonstrated a U-shaped relationship between age and concentrations of BDEs 47 and 153, with the highest levels found in 12–19 year-olds, the youngest age group. In adult populations, most studies have reported no relationship between serum concentrations of PBDEs and age; however, some investigators have observed positive relationships (Anderson, et al. 2008; Turyk, et al. 2010; Uemura, et al. 2010; Weiss, et al. 2006). In our analysis, there was a non-significant positive relationship between serum-PBDE concentrations and age.
The CCLS was not designed to evaluate determinants of serum POP levels in participating mothers. The information used to describe explanatory factors in this analysis was collected because it was potentially relevant to childhood leukemia. Consequently, the blood, dust, and questionnaire information was collected on separate occasions, and this approach to data collection may have weakened observed relationships between explanatory factors and serum POP levels. For example, we assessed the presence of upholstered furniture with crumbling or exposed foam, on average, four years after blood was collected. Moreover, information about parity, breastfeeding, body mass index, and dietary habits was based on a mother’s recall of her index pregnancy and was not specific to the time of blood collection. Additionally, potentially useful information regarding hand washing, nail biting and finger licking, and changes in body mass index was not available for this analysis, nor did we have a complete inventory of household items that were potential POP sources. Finally, we did not measure POP concentrations directly in food items, but rather inferred the contribution of diet to total POP exposure using a food frequency questionnaire, an approach which may have limited our ability to recognize the true role of the dietary route of exposure to POPs.
Despite these limitations, we were able to identify two distinct sources of exposure to POPs for mothers participating in the CCLS. After adjusting for lipid content, age, BMI, cumulative lactation, and sampling date, serum-PCB concentrations were associated with fish consumption. In contrast, serum concentrations of BDE-47 were associated with dust concentrations, country of origin, income, and Hispanic ethnicity. Our findings are consistent with the hypothesis that the relative contribution of specific exposure routes to total POP intake varies by chemical, with dust being a relatively important source of PBDEs and diet being a relatively important source of PCBs. It is possible that moderating fatty fish consumption or dust ingestion may reduce POP exposures.
Supplementary Material
Highlights.
Blood from 48 California mothers (2006–2007) analyzed for POPs
Serum-PCBs were positively associated with fish consumption
Serum-PBDEs were positively associated with dust-PBDEs
Effects of age, ethnicity, income, birthplace, BMI, and lactation differed by POP
ACKNOWLEDGEMENTS
We thank the families for their participation. We also thank the clinical investigators at the following collaborating hospitals for help in recruiting patients: University of California Davis Medical Center (Dr. Jonathan Ducore), University of California San Francisco (Drs. Mignon Loh and Katherine Matthay), Children's Hospital of Central California (Dr. Vonda Crouse), Lucile Packard Children's Hospital (Dr. Gary Dahl), Children's Hospital Oakland (Dr. James Feusner), Kaiser Permanente Oakland (Drs. Daniel Kronish and Stacy Month), Kaiser Permanente Roseville (Drs. Kent Jolly and Vincent Kiley), Kaiser Permanente Santa Clara (Drs. Carolyn Russo, Denah Taggart, and Alan Wong), and Kaiser Permanente San Francisco (Dr. Kenneth Leung). We acknowledge the late Dr. Patricia Buffler, the founding Principal Investigator of the California Childhood Leukemia Study, and her leadership of the study for nearly 20 years. Finally, we acknowledge Praphopphat Adhatamsoontra, Warren Li, and Lea Pearlman, as well as the rest of the study staff for their effort and dedication.
FUNDING SOURCES
This work was supported in part by the National Institute of Environmental Health Sciences (NIEHS, grant numbers R01ES009137, R01ES015899, P42ES0470518, and P01ES018172); by the Intramural Research Program of the National Cancer Institute (NCI), National Institute of Health (subcontracts 7590-S-04, 7590-S-01); by the NCI (contract N02-CP-11015); and by the Environmental Protection Agency (EPA, grant number RD83451101). Funding sources were not involved with the study design; collection, analysis, and interpretation of data; writing of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
HUMAN SUBJECTS
The human subjects protocol of the California Childhood Leukemia Study has been reviewed and approved by the Institutional Review Board at the University of California, Berkeley. We obtained written informed consent from participating mothers prior to conducting the study.
Contributor Information
Todd P. Whitehead, Email: ToddPWhitehead@Berkeley.edu.
Sabrina Crispo Smith, Email: Sabrina.CrispoSmith@DTSC.CA.gov.
June-Soo Park, Email: June-Soo.Park@DTSC.CA.gov.
Myrto X. Petreas, Email: Myrto.Petreas@DTSC.CA.gov.
Stephen M. Rappaport, Email: SRappaport@Berkeley.edu.
Catherine Metayer, Email: CMetayer@Berkeley.edu.
REFERENCES
- Agudo A, Goni F, Etxeandia A, Vives A, Millan E, Lopez R, Amiano P, Ardanaz E, Barricarte A, Chirlaque MD, Dorronsoro M, Jakszyn P, Larranaga N, Martinez C, Navarro C, Rodriguez L, Sanchez MJ, Tormo MJ, Gonzalez CA. Polychlorinated biphenyls in Spanish adults: determinants of serum concentrations. Environ. Res. 2009;109:620–628. doi: 10.1016/j.envres.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Anderson HA, Imm P, Knobeloch L, Turyk M, Mathew J, Buelow C, Persky V. Polybrominated diphenyl ethers (PBDE) in serum: findings from a US cohort of consumers of sport-caught fish. Chemosphere. 2008;73:187–194. doi: 10.1016/j.chemosphere.2008.05.052. [DOI] [PubMed] [Google Scholar]
- Bachelet D, Truong T, Verner MA, Arveux P, Kerbrat P, Charlier C, Guihenneuc-Jouyaux C, Guenel P. Determinants of serum concentrations of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and polychlorinated biphenyls among French women in the CECILE study. Environ. Res. 2011;111:861–870. doi: 10.1016/j.envres.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Bailey HD, Fritschi L, Infante-Rivard C, Glass DC, Miligi L, Dockerty JD, Lightfoot T, Clavel J, Roman E, Spector LG, Kaatsch P, Metayer C, Magnani C, Milne E, Polychronopoulou S, Simpson J, Rudant J, Sidi V, Rondelli R, Orsi L, Kang AY, Petridou E, Schuz J. Parental occupational pesticide exposure and the risk of childhood leukemia in the offspring: Findings from the childhood leukemia international consortium. Int. J. Cancer. 2014 doi: 10.1002/ijc.28854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund JA, Sellstrom U, de Wit CA, Aune M, Lignell S, Darnerud PO. Comparisons of polybrominated diphenyl ether and hexabromocyclododecane concentrations in dust collected with two sampling methods and matched breast milk samples. Indoor Air. 2012;22:279–288. doi: 10.1111/j.1600-0668.2011.00765.x. [DOI] [PubMed] [Google Scholar]
- Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology. 1990;1:58–64. doi: 10.1097/00001648-199001000-00013. [DOI] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Sjodin A, Fenster L, Jones RS, Harley KG, Chevrier J, Holland NT, Eskenazi B. Factors associated with serum polybrominated diphenyl ether (PBDE) levels among school-age children in the CHAMACOS cohort. Environ. Sci. Technol. 2012;46:7373–7381. doi: 10.1021/es3003487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik K, Alcock R, Li YF, Bailey RE, Fiedler H, Pacyna JM. Primary sources of selected POPs: regional and global scale emission inventories. Environ. Pollut. 2004;128:3–16. doi: 10.1016/j.envpol.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Castorina R, Bradman A, Sjodin A, Fenster L, Jones RS, Harley KG, Eisen EA, Eskenazi B. Determinants of serum polybrominated diphenyl ether (PBDE) levels among pregnant women in the CHAMACOS cohort. Environ. Sci. Technol. 2011;45:6553–6560. doi: 10.1021/es104295m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, Cordier S. Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology. 2013;24:251–260. doi: 10.1097/EDE.0b013e31827f53ec. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Fenster L, Castorina R, Marks AR, Sjodin A, Rosas LG, Holland N, Guerra AG, Lopez-Carillo L, Bradman A. A comparison of PBDE serum concentrations in Mexican and Mexican-American children living in California. Environ. Health Perspect. 2011;119:1442–1448. doi: 10.1289/ehp.1002874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, McClean MD. Diet contributes significantly to the body burden of PBDEs in the general U.S. population. Environ. Health Perspect. 2009;117:1520–1525. doi: 10.1289/ehp.0900817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Thomsen C, Froshaug M, Vorkamp K, Thomsen M, Becher G, Knudsen LE. Polybrominated diphenyl ethers in paired samples of maternal and umbilical cord blood plasma and associations with house dust in a Danish cohort. Int. J. Hyg. Environ. Health. 2010 doi: 10.1016/j.ijheh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE. Human internal and external exposure to PBDEs--a review of levels and sources. Int. J. Hyg. Environ. Health. 2009;212:109–134. doi: 10.1016/j.ijheh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Garabrant DH, Franzblau A, Lepkowski J, Gillespie BW, Adriaens P, Demond A, Hedgeman E, Knutson K, Zwica L, Olson K, Towey T, Chen Q, Hong B, Chang CW, Lee SY, Ward B, Ladronka K, Luksemburg W, Maier M. The University of Michigan Dioxin Exposure Study: predictors of human serum dioxin concentrations in Midland and Saginaw, Michigan. Environ. Health Perspect. 2009;117:818–824. doi: 10.1289/ehp.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A, Aune M, Darnerud PO, Cnattingius S, Bjerselius R, Becker W, Lignell S. Determinants of serum concentrations of organochlorine compounds in Swedish pregnant women: a cross-sectional study. Environ. Health. 2007;6:2. doi: 10.1186/1476-069X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govarts E, Nieuwenhuijsen M, Schoeters G, Ballester F, Bloemen K, de Boer M, Chevrier C, Eggesbo M, Guxens M, Kramer U, Legler J, Martinez D, Palkovicova L, Patelarou E, Ranft U, Rautio A, Petersen MS, Slama R, Stigum H, Toft G, Trnovec T, Vandentorren S, Weihe P, Kuperus NW, Wilhelm M, Wittsiepe J, Bonde JP OBELIX ENRIECO. Birth weight and prenatal exposure to polychlorinated biphenyls (PCBs) and dichlorodiphenyldichloroethylene (DDE): a meta-analysis within 12 European Birth Cohorts. Environ. Health Perspect. 2012;120:162–170. doi: 10.1289/ehp.1103767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardell E, Carlberg M, Nordstrom M, van Bavel B. Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci. Total Environ. 2010;408:4412–4419. doi: 10.1016/j.scitotenv.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Harley KG, Chevrier J, Schall RA, Sjodin A, Bradman A, Eskenazi B. Association of prenatal exposure to polybrominated diphenyl ethers and infant birth weight. Am. J. Epidemiol. 2011;174:885–892. doi: 10.1093/aje/kwr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women's serum and fecundability. Environ. Health Perspect. 2010;118:699–704. doi: 10.1289/ehp.0901450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrad S, Ibarra C, Robson M, Melymuk L, Zhang X, Diamond M, Douwes J. Polychlorinated biphenyls in domestic dust from Canada, New Zealand, United Kingdom and United States: implications for human exposure. Chemosphere. 2009;76:232–238. doi: 10.1016/j.chemosphere.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Apelberg BJ, Witter FR, Patterson DG, Halden RU, Jones RS, Park A, Zhang Y, Heidler J, Needham LL, Goldman LR. Determinants of prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) in an urban population. Environ. Health Perspect. 2007;115:1794–1800. doi: 10.1289/ehp.10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton MK, Bousleiman S, Jones R, Sjodin A, Liu X, Whyatt R, Wapner R, Factor-Litvak P. Predictors of serum concentrations of polybrominated flame retardants among healthy pregnant women in an urban environment: a cross-sectional study. Environ. Health. 2013;12 doi: 10.1186/1476-069X-12-23. 23–069X-12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarluzea J, Alvarez-Pedrerol M, Guxens M, Marina LS, Basterrechea M, Lertxundi A, Etxeandia A, Goni F, Vioque J, Ballester F, Sunyer J INMA Project. Sociodemographic, reproductive and dietary predictors of organochlorine compounds levels in pregnant women in Spain. Chemosphere. 2011;82:114–120. doi: 10.1016/j.chemosphere.2010.09.051. [DOI] [PubMed] [Google Scholar]
- Infante-Rivard C, Weichenthal S. Pesticides and childhood cancer: an update of Zahm and Ward's 1998 review. J. Toxicol. Environ. Health B Crit. Rev. 2007;10:81–99. doi: 10.1080/10937400601034589. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Stapleton HM, Sjodin A, Meeker JD. Relationships between Polybrominated Diphenyl Ether Concentrations in House Dust and Serum. Environ. Sci. Technol. 2010 doi: 10.1021/es100697q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobeloch L, Turyk M, Imm P, Anderson H. Polychlorinated biphenyls in vacuum dust and blood of residents in 20 Wisconsin households. Chemosphere. 2012;86:735–740. doi: 10.1016/j.chemosphere.2011.10.048. [DOI] [PubMed] [Google Scholar]
- Korrick SA, Chen C, Damokosh AI, Ni J, Liu X, Cho SI, Altshul L, Ryan L, Xu X. Association of DDT with spontaneous abortion: a case-control study. Ann. Epidemiol. 2001;11:491–496. doi: 10.1016/s1047-2797(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Kwan ML, Jensen CD, Block G, Hudes ML, Chu LW, Buffler PA. Maternal diet and risk of childhood acute lymphoblastic leukemia. Public Health Rep. 2009;124:503–514. doi: 10.1177/003335490912400407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laden F, Neas LM, Spiegelman D, Hankinson SE, Willett WC, Ireland K, Wolff MS, Hunter DJ. Predictors of plasma concentrations of DDE and PCBs in a group of U.S. women. Environ. Health Perspect. 1999;107:75–81. doi: 10.1289/ehp.9910775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Macdonald RW. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: a review. Sci. Total Environ. 2005;342:87–106. doi: 10.1016/j.scitotenv.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- McGraw JE, Waller DP. Fish ingestion and congener specific polychlorinated biphenyl and p,p'-dichlorodiphenyldichloroethylene serum concentrations in a great lakes cohort of pregnant African American women. Environ. Int. 2009a;35:557–565. doi: 10.1016/j.envint.2008.10.003. [DOI] [PubMed] [Google Scholar]
- McGraw JE, Waller DP. The role of african american ethnicity and metabolism in sentinel polychlorinated biphenyl congener serum levels. Environ. Toxicol. Pharmacol. 2009b;27:54–61. doi: 10.1016/j.etap.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moysich KB, Ambrosone CB, Mendola P, Kostyniak PJ, Greizerstein HB, Vena JE, Menezes RJ, Swede H, Shields PG, Freudenheim JL. Exposures associated with serum organochlorine levels among postmenopausal women from western New York State. Am. J. Ind. Med. 2002;41:102–110. doi: 10.1002/ajim.10043. [DOI] [PubMed] [Google Scholar]
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, Zhao G, Bhatia R, Charles MJ. High body burdens of 2,2',4,4'-tetrabromodiphenyl ether (BDE-47) in California women. Environ. Health Perspect. 2003;111:1175–1179. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips DL, Pirkle JL, Burse VW, Bernert JT, Henderson LO, Jr, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch. Environ. Contam. Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Rose M, Bennett DH, Bergman A, Fangstrom B, Pessah IN, Hertz-Picciotto I. PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ. Sci. Technol. 2010;44:2648–2653. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade G, Heinzow B. Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in northern Germany: current extent of contamination, time trend from 1986 to 1997 and factors that influence the levels of contamination. Sci. Total Environ. 1998;215:31–39. doi: 10.1016/s0048-9697(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Focant JF, Lapeza C, Wang RY, McGahee EE, Zhang Y, 3rd, Turner WE, Slazyk B, Needham LL, Patterson DG., Jr Retrospective time-trend study of polybrominated diphenyl ether and polybrominated and polychlorinated biphenyl levels in human serum from the United States. Environ. Health Perspect. 2004a;112:654–658. doi: 10.1289/ehp.112-1241957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjodin A, Jones RS, Lapeza CR, Focant JF, McGahee EE, 3rd, Patterson DG., Jr Semiautomated high-throughput extraction and cleanup method for the measurement of polybrominated diphenyl ethers, polybrominated biphenyls, and polychlorinated biphenyls in human serum. Anal. Chem. 2004b;76:1921–1927. doi: 10.1021/ac030381+. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, Patterson DG., Jr Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol. 2008;42:1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Spliethoff HM, Bloom MS, Vena J, Sorce J, Aldous KM, Eadon G. Exploratory assessment of sportfish consumption and polybrominated diphenyl ether exposure in New York State anglers. Environ. Res. 2008;108:340–347. doi: 10.1016/j.envres.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Sjodin A, Webster TF. Serum PBDEs in a North Carolina toddler cohort: associations with handwipes, house dust, and socioeconomic variables. Environ. Health Perspect. 2012a;120:1049–1054. doi: 10.1289/ehp.1104802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and High Volume Use Flame Retardants in US Couches Reflective of the 2005 PentaBDE Phase Out. Environ. Sci. Technol. 2012b;46:13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Lei B, Xu G, Ma J, Lei JQ, Jin SQ, Hu GY, Wu MH. Polybrominated diphenyl ethers in human hair from the college environment: comparison with indoor dust. Bull. Environ. Contam. Toxicol. 2013;91:377–381. doi: 10.1007/s00128-013-1056-x. [DOI] [PubMed] [Google Scholar]
- Thomsen C, Lundanes E, Becher G. Brominated flame retardants in archived serum samples from Norway: a study on temporal trends and the role of age. Environ. Sci. Technol. 2002;36:1414–1418. doi: 10.1021/es0102282. [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF. Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults. Environ. Health Perspect. 2009;117:1461–1465. doi: 10.1289/ehp.0900596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel D, Scheringer M, von Goetz N, Hungerbuhler K. Total consumer exposure to polybrominated diphenyl ethers in North America and Europe. Environ. Sci. Technol. 2011;45:2391–2397. doi: 10.1021/es1035046. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Anderson HA, Steenport D, Buelow C, Imm P, Knobeloch L. Longitudinal biomonitoring for polybrominated diphenyl ethers (PBDEs) in residents of the Great Lakes basin. Chemosphere. 2010;81:517–522. doi: 10.1016/j.chemosphere.2010.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA. Technical Fact Sheet – Polybrominated Diphenyl Ethers (PBDEs) and Polybrominated Biphenyls (PBBs) EPA 505 -F-14–006. 2014 [Google Scholar]
- Uemura H, Arisawa K, Hiyoshi M, Dakeshita S, Kitayama A, Takami H, Sawachika F, Yamaguchi M, Sasai S. Congener-specific body burden levels and possible determinants of polybrominated diphenyl ethers in the general Japanese population. Chemosphere. 2010;79:706–712. doi: 10.1016/j.chemosphere.2010.02.050. [DOI] [PubMed] [Google Scholar]
- Vinson F, Merhi M, Baldi I, Raynal H, Gamet-Payrastre L. Exposure to pesticides and risk of childhood cancer: a meta-analysis of recent epidemiological studies. Occup. Environ. Med. 2011;68:694–702. doi: 10.1136/oemed-2011-100082. [DOI] [PubMed] [Google Scholar]
- Vorkamp K, Thomsen M, Frederiksen M, Pedersen M, Knudsen LE. Polybrominated diphenyl ethers (PBDEs) in the indoor environment and associations with prenatal exposure. Environ. Int. 2011;37:1–10. doi: 10.1016/j.envint.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, McClean MD, Fraser AJ, Weinberg J, Stapleton HM, Sjodin A, Webster TF. Impact of dust from multiple microenvironments and diet on PentaBDE body burden. Environ. Sci. Technol. 2012;46:1192–1200. doi: 10.1021/es203314e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J, Wallin E, Axmon A, Jonsson BA, Akesson H, Janak K, Hagmar L, Bergman A. Hydroxy-PCBs, PBDEs, and HBCDDs in serum from an elderly population of Swedish fishermen's wives and associations with bone density. Environ. Sci. Technol. 2006;40:6282–6289. doi: 10.1021/es0610941. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Dhaliwal J, Petreas MX, Buffler PA, Rappaport SM. Polychlorinated Biphenyls in Residential Dust: Sources of Variability. Environmental Science and Technology. 2014;48:157–164. doi: 10.1021/es403863m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Brown FR, Metayer C, Park JS, Does M, Petreas MX, Buffler PA, Rappaport SM. Polybrominated diphenyl ethers in residential dust: Sources of variability. Environ. Int. 2013;57–58:11–24. doi: 10.1016/j.envint.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Pinney SM, Sjodin A, Lum R, Jones RS, Needham LL, Biro FM, Hiatt RA, Kushi LH. Body burdens of brominated flame retardants and other persistent organo-halogenated compounds and their descriptors in US girls. Environ. Res. 2010;110:251–257. doi: 10.1016/j.envres.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Britton JA, Teitelbaum SL, Eng S, Deych E, Ireland K, Liu Z, Neugut AI, Santella RM, Gammon MD. Improving organochlorine biomarker models for cancer research. Cancer Epidemiol. Biomarkers Prev. 2005;14:2224–2236. doi: 10.1158/1055-9965.EPI-05-0173. [DOI] [PubMed] [Google Scholar]
- Wu N, Herrmann T, Paepke O, Tickner J, Hale R, Harvey LE, La Guardia M, McClean MD, Webster TF. Human exposure to PBDEs: associations of PBDE body burdens with food consumption and house dust concentrations. Environ. Sci. Technol. 2007;41:1584–1589. doi: 10.1021/es0620282. [DOI] [PubMed] [Google Scholar]
- Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 2013;47:11776–11784. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: unintended consequences of furniture flammability standards? Environ. Sci. Technol. 2008;42:8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.