Abstract
Hypercapnic acidosis activates Ca2+ channels and increases intracellular Ca2+ levels in neurons of the locus coeruleus (LC), a known chemosensitive region involved in respiratory control. We have also shown that large conductance Ca2+-activated K+ channels (BK), in conjunction with this pathway, limits the hypercapnic-induced increase in firing rate in LC neurons. Here, we present evidence that the Ca2+ current is activated by a HCO3−-sensitive pathway. The increase in HCO3− associated with hypercapnia activates HCO3−-sensitive adenylyl cyclase (sAC). This results in an increase in cAMP levels and activation of Ca2+ channels via cAMP-activated protein kinase A (PKA). We also show the presence of sAC in the cytoplasm of LC neurons, and that the cAMP analogue db-cAMP increases Ca2+i. Disrupting this pathway by decreasing HCO3− levels during acidification or inhibiting either sAC or PKA, but not transmembrane adenylyl cyclase (tmAC), can increase the magnitude of the firing rate response to hypercapnia in LC neurons from older neonates to the same extent as inhibition of BK channels.
Keywords: central control of breathing, L-type Ca2+ channel, panic disorder, KH7, development, transmembrane adenylyl cyclase
1. INTRODUCTION
Neurons that are sensitive to CO2/H+ exist in numerous brain regions and contribute to various functions and disorders including the control of breathing, learning and memory, depression and panic disorders (Wemmie et al., 2004; Coryell et al., 2009; Ziemann et al., 2009; Putnam, 2010). These CO2/H+-sensitive neurons are referred to as chemosensitive and the ability of a neuron to respond in this way is generally attributed to the presence of acid-sensitive ion channels on its surface membrane (Putnam et al., 2004; Putnam, 2010) We have focused on chemosensitive neurons within one brain stem area, the locus coeruleus (LC). There is considerable evidence that chemosensitive neurons from the LC play an important role in the hypercapnic ventilatory response (recently reviewed in Gargaglioni et al., 2010). Early studies showed that focal acidification of the LC alone resulted in increased ventilation (Coates et al., 1993), showing that the LC could drive increased breathing. Further, lesioning a large percentage of LC neurons resulted in a marked decrease in the hypercapnic ventilatory response to inspired CO2 (Li & Nattie, 2006; Biancardi et al., 2008). Finally, a high percentage of LC neurons have been shown to be chemosensitive (Elam et al., 1981; Pineda & Aghajanian, 1997; Filosa et al., 2002) and LC neurons in culture were shown to exhibit intrinsic chemosensitivity (Johnson et al., 2008). Taken together, these studies point to chemosensitive LC neurons as playing an important role in the control of breathing and in the ventilatory response to inspired hypercapnia.
The chemosensitive response to hypercapnia could be due to the sensing of changes of molecular CO2, to changes of intracellular and/or extracellular pH, or to changes of HCO3− in response to hypercapnia. Recently, evidence has been presented that CO2 itself could be directly sensed in leptomeniges and glial cells in the ventral medullary surface (Huckstepp et al., 2010). The mechanism probably involves CO2 directly modifying connexin hemichannels by forming a carbamate bridge between two residues that favors the open state in the hemichannel (Meigh et al., 2013). Numerous other studies have focused on the ability of changes of intracellular or extracellular pH during hypercapnia to alter the activity of ion channels (Putnam et al., 2004). Chemosensitive LC neurons have been shown to contain a variety of pH-sensitive channels, including inward rectifying K+ channels (Pineda & Aghajanian, 1997), transient A currents and delayed rectifying K+ currents (Li & Putnam, 2013), TASK channels (Bayliss et al., 2001) and TRP channels (Cui et al., 2011). Acidification alters these channels in such a way that LC neurons depolarize and increase their firing rate.
Our work has focused on the possible effects of Ca2+ channels on the chemosensitive response of LC neurons. We have previously reported that hypercapnia activates L-type Ca2+ channels in LC neurons (Filosa & Putnam, 2003; Imber et al., 2012; Imber & Putnam, 2012). In LC neurons from young neonates, this activation seems to contribute to the increased firing rate response induced by elevated CO2/H+ (Filosa & Putnam, 2003). However, in LC neurons from older neonates the activation of Ca2+ channels by hypercapnia stimulates KCa channels to produce a braking effect on the chemosensitive firing rate response (Imber et al., 2012). This effect of hypercapnia on Ca2+ channels is unexpected since acidification is commonly expected to inhibit Ca2+ channels (Tombaugh & Somjen, 1997; Shah et al., 2001). Recent findings provide evidence that elevated intracellular HCO3− is involved in the pathway by which hypercapnia activates the L-type Ca2+ current in LC neurons (Imber & Putnam, 2012). It was hypothesized that this activation might involve soluble adenylyl cyclase (sAC).
Little is known about a HCO3−-sensitive mechanism involved in the chemosensitive response of brainstem neurons, but a role for HCO3− in the chemosensitive response of peripheral chemoreceptors has been hypothesized (Summers et al., 2002). This study determined that the CO2/H+- activation of L-type Ca2+ channels in glomus cells was blocked by an inhibitor of protein kinase A (PKA) and occurred in association with an intracellular elevation of cAMP (Summers et al., 2002). These findings showed that hypercapnia induced elevation of L-type Ca2+ channels through activation of PKA. The authors speculated that hypercapnia could activate PKA through a HCO3−-dependent mechanism. However, a more recent study has shown that the mechanism is dependent on acidosis and does not involve a HCO3−-dependent mechanism (Nunes et al., 2013).
Soluble adenylyl cyclase (sAC) has been characterized as an intracellular HCO3−-dependent means of producing cAMP (Buck et al., 1999; Chen et al., 2000; Zippin et al., 2001; Li et al., 2011). Since intrinsic chemosensitivity requires that cells respond to CO2, and that the diffusion of increased CO2 across cell membranes results in elevated HCO3−, it follows that chemosensitive cells expressing sAC could increase their cAMP levels in response to hypercapnia. The presence of sAC in LC neurons has been shown in a preliminary report (Nunes et al., 2008), which raises the possibility that chemosensitive cells of the LC may utilize a sAC-dependent pathway for the activation of their L-type Ca2+ channels in response to hypercapnia.
In the current study, we hypothesize that a sAC-dependent pathway is responsible for the CO2/H+ activation of the L-type Ca2+ current in LC neurons. If so, the addition of dibutyryl-cAMP (db-cAMP) should mimic the hypercapnia-induced increase in Ca2+ current (Imber et al., 2012). Conversely, we expect that the nominal absence of CO2/HCO3− from the superfusion solution will decrease the sensitivity of this Ca2+ current to hypercapnia. In addition, we expect to find the presence of the HCO3−-dependent sAC enzyme in the cytoplasm of neonatal LC neurons using immunohistochemistry. We have shown that the activation of Ca2+ currents in LC neurons from rats older than ~P10 decreases the firing rate response to hypercapnia via the subsequent activation of large-conductance Ca2+-activated BK channels (Imber et al., 2012). If Ca2+ channels are activated by hypercapnia through a sAC-mediated mechanism, we further expect that the sAC inhibitors 2-hydroxyestradiol (2HE) or 2-(1H–benzo[d]imidazole-2-ylthio)-N’-(5-bromo-2-hydroxybenzylidene) propanehydrazide (KH7) (Schlicker et al., 2008; Li et al., 2011; Bitterman et al., 2013) or the PKA inhibitor H89 will increase the firing rate response to hypercapnia of LC neurons from older neonates to a similar extent as does the BK channel inhibitor paxilline (Imber et al., 2012) while the transmembrane adenylyl cyclase (tmAC) inhibitor 2’,5’-dideoxyadenosine (ddAdo) (Bitterman et al., 2013) will have no effect on the firing rate response to hypercapnia of LC neurons from older neonates. Our findings strongly support our hypothesis that a sAC-mediated pathway leads to the activation of L-type Ca2+ channels by hypercapnia in LC neurons.
A preliminary report of our findings has previously been published (Imber et al., 2012).
2. MATERIALS AND METHODS
2.1 Ethical approval
All procedures in which animals were involved were reviewed and approved by the Wright State University Institutional Animal Care and Use Committee and are in agreement with standards set out in the National Institutes of Health Guide for Care and Use of Laboratory Animals. Wright State University is accredited by AAALAC and is covered by NIH Assurance (no. A3632-01).
2.2 Slice preparation
Neonatal Sprague-Dawley rats postnatal (P) age P3-P16 of mixed sex were used in these studies. Depending on the age of the neonate, they were anesthetized using either 100% CO2 or hypothermia and then decapitated. The brainstem was removed and a vibratome (Pelco Vibratome 1000) was used to make coronal brain slices. Slicing was done in ice-cold (4–6°C) artificial cerebrospinal fluid (aCSF) solution. Slices of the pons (containing the LC) were maintained in aCSF equilibrated with 5% CO2/95% O2 at room temperature until used (1–4 hours after slicing). For all experiments, slices were continuously superfused at a rate of ~4 ml/min by gravity flow using solutions held at 35°C.
2.3 Solutions
All brain slices were immersed in aCSF solution unless indicated otherwise. This solution consisted of (in mM): 124 NaCl, 3 KCl, 1.3 MgSO4, 26 NaHCO3, 1.24 NaH2PO4, 10 glucose, and 2.4 CaCl2 and was equilibrated with 5% CO2/95% O2, pH ~7.45 (at 35°C). Hypercapnic solutions had the same composition but were equilibrated with 15% CO2/85% O2, pH ~7.0. This level of CO2 was chosen to maximize the activation of the cellular signaling pathway being studied (Ritucci et al., 2005; Hartzler et al., 2008). In nominally CO2/HCO3− free solutions, HEPES buffer isosmotically replaced the HCO3- in aCSF and the solution was equilibrated with 100% O2. The pH of the HEPES aCSF solution was adjusted to 7.45 and 7.0 (similar to the normal aCSF and hypercapnic solutions, respectively) using HCl and NaOH. The whole cell pipette filling solution consisted of (in mM): 130 K-gluconate, 0.4 EGTA, 1 MgCl2, 0.3 GTP, 2 ATP, and 10 HEPES, and was buffered to a pH of ~7.35 using KOH. For intracellular Ca2+ (Ca2+i) measurements, 250 µM of the Ca2+-sensitive fluorescent dye Fura-2 was also added to the pipette solution. The whole cell pipette filling solution for voltage clamp studies of the Ca2+ current consisted of (in mM): 130 CsCl, 10 EGTA, 1 MgCl2, 0.3 GTP, 2 ATP, 10 HEPES, and 10 tetraethylammonium (TEA), buffered to pH ~7.45 using CsOH. For immunohistochemistry studies of sAC, the phosphate buffered saline (PBS) solution contained (in mM): 137 NaCl, 2.7 KCl, 4.3 Na2HPO4, and 1.47 KH2PO4.
2.4 Measurement of intracellular Ca2+
We loaded LC neurons with the Ca2+-sensitive dye Fura-2 (250 µM) from the whole cell patch pipette. Dye-loaded neurons were alternately excited at 340 nm and 380 nm using a Sutter Lamda 10-2 filter wheel (light from a 75W xenon arc lamp). Emitted fluorescence (505 nm) was intensified by a GenIISys Image intensifier and captured by a CCD camera. Fluorescence images were acquired using a Gateway 2000 E-3100 computer and analyzed with MetaFluor 4.6r software. Images were acquired every 15 seconds (~2 seconds acquisition time). Photobleaching was reduced by blocking excitation light between acquisitions. We did not calibrate the Fura-2 fluorescence and instead used arbitrary fluorescence units to monitor increases or decreases in Rfl. For analysis, Rfl values were estimated by averaging at least 5 values before, during and after db-cAMP administration.
2.5 Electrophysiological recordings
All electrophysiological recordings used in this study were whole cell recordings. Whole cell pipettes were pulled to a tip resistance of ~5 MΩ using thin-walled borosilicate glass (outer diameter 1.5 mm, inner diameter 1.12 mm). We visualized LC neurons with an upright microscope (Nikon Eclipse 6600) using an x60 water-immersion objective. A visualized neuron was patched, forming a gigaohm seal. Membrane potential (Vm) was measured in both current and voltage clamp mode. An Axopatch 200B amplifier was used for the injection of either current or voltage. A slope/height window discriminator (FHC Model 700B, Bowdoinham, ME) was used to determine the integrated firing rate (FR). pCLAMP software version 10.0 was used to analyze Vm and FR. Recordings were started when Vm reached a stable resting value. A healthy neuron was defined as one with a stable resting Vm of −45 to −60 mV and a spontaneous firing rate of < 4 Hz. All electrophysiological responses were shown to be reversible by returning to baseline values when the solution was changed back to the initial aCSF. We were able to successfully patch LC neurons for longer than 45 minutes in current clamp without washout of the chemosensitive response (Filosa & Putnam, 2003). Most drugs/solutions exhibited their effects within less than 2 minutes. When more than one hypercapnic pulse was used in an experiment, we returned resting FR between pulses to within 0.5 Hz of the original FR by injecting current so that we could compare the chemosensitive responses to the two different pulses.
LC neurons were clamped at a holding potential of −70 mV in aCSF for voltage clamp experiments. TTX (1 µM) was added to block Na+ currents and 3 mM BaCl2, to maximize Ca2+ currents, replaced NaCl isosmotically. Depolarizations (600 ms duration) were applied in 10 mV steps from −60 mV to +50 mV and the resulting peak current determined. These measurements were made in either aCSF equilibrated with 5% CO2/95% O2 or in HEPES-buffered aCSF (nominal absence of CO2/HCO3−).
2.6 Immunohistochemistry
Brainstem slices (300 µm) from neonatal rats aged above P10 were fixed in freshly prepared 4% paraformaldehyde (4 g/100 ml) in PBS buffer, pH 7.4, for 72 hours. Fixed slices were washed three times for 15 minutes each in PBS. A blocking solution of 0.1M PBS + 0.3% Triton X-100 (0.3 ml/100 ml) + 10% donkey serum (10 ml/100 ml) (EMD Biosciences, Inc) was applied for 30 min. Slices were then rinsed three times (15 min per wash) in 0.1M PBS. Primary antibodies (rabbit derived anti-SAC-101-AP; FabGennix) were diluted in 0.1M PBS + 0.3% Triton X-100, at a dilution of 1:200. Fixed slices were incubated in primary antibody solution for 72 hours at 4°C. After incubation, slices were washed three times in PBS for 15 minutes for each wash. Donkey-derived secondary antibody (anti-rabbit cy3; Jackson ImmunoResearch Laboratories) was diluted 1:50 in 0.1M PBS + 0.3% Triton-X-100. Slices that had been incubated in primary antibody were incubated overnight at 4°C in the secondary antibody solution. The next day, samples were washed three times (15 min per wash) in PBS. Slices were mounted with Vectashield Mounting Medium (Vector Laboratories) and digital images (Z stack, 0.5 µm resolution) were obtained using an Olympus FV1000 Confocal Microscope (Olympus Corp.) and files were managed using Fluoview software (Olympus Corp.). Control slices were treated identically except that the second incubation was in 0.1M PBS + 0.3% Triton-X-100 with no primary antibody (SAC-101-AP).
2.7 Drugs
TTX, BSA, 2HE, db-cAMP, H89, ddAdo and Fura-2 were purchased from Sigma-Aldrich (St Louis, MO) while KH7 was a gift from Drs. Lonny Levin and Jochen Buck. 2HE (20 mM) was prepared as a stock solution in EtOH while TTX (1 mM), db-cAMP (10 mM), H89 (10 mM), and Fura-2 (10 mM) stocks were made in dH2O and KH7 (2.4 mM) and ddAdo (3 mM) stocks were made in DMSO. All drugs were diluted in aCSF to a final working concentration of 10 µm for 2HE, 1 µM for TTX, 250 µM for db-cAMP, 10 µM for H89, 30 µM for KH7 and 30 µM for ddAdo.
2.8 Data analysis and statistics
Absolute changes in firing rate (ΔFR) were quantified using the following equation: ΔFR = (((hypercapnic average firing rate - control average firing rate)/ (control average firing rate)) × 100%). Neurons that responded to hypercapnia with a 20% or greater increase in firing rate were considered chemosensitive. All values are expressed as mean ± SEM. Significant differences between two means were determined by student t-tests or paired t-tests. Differences were considered significant if P<0.05.
3. RESULTS
3.1 Effect of db-cAMP on Ca2+ currents and intracellular Ca2+
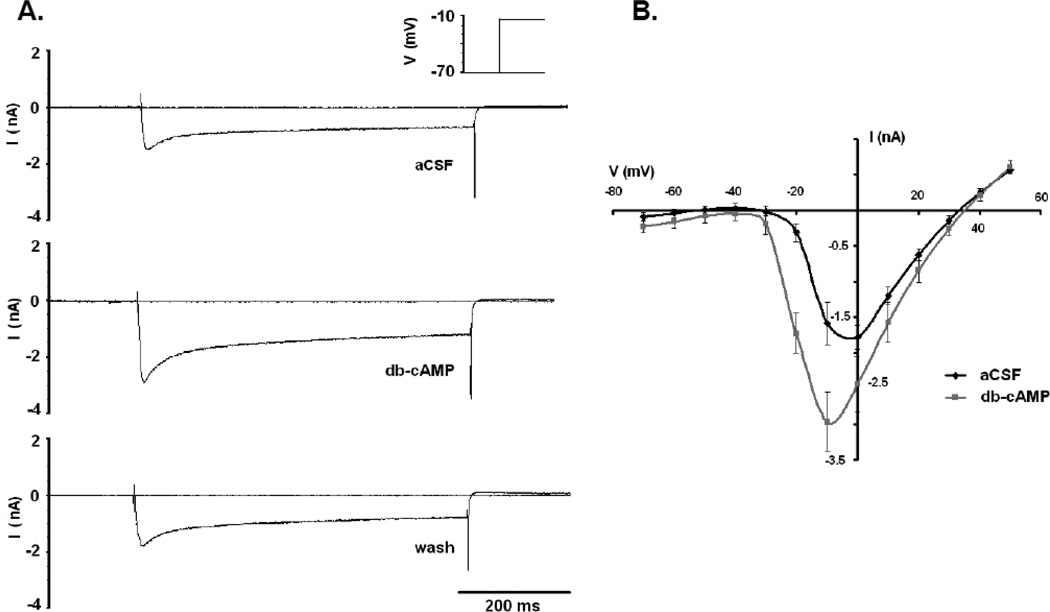
Whole cell voltage clamp of LC neurons in the presence of blockers of Na+ and K+ channels demonstrated slowly inactivating inward currents that activated at approximately −30 mV and reversed at around +45 mV, consistent with L-type Ca2+ currents previously reported for LC neurons (Imber et al., 2012) (Fig. 1). Since the peak Ca2+ current for LC neurons was observed after day P10 (Imber et al., 2012), only rats P10 and older were used for this study. Figure 1A shows a typical appearance for the peak Ca2+ current at −10 mV, including the long (>200 ms) inactivation time consistent with L-type Ca2+ channels (Hille, 2001; Imber et al., 2012). When 250 µM db-cAMP was added to the perfusion solution, a marked increase in the amplitude of the peak Ca2+ current was observed (Fig. 1A). The increase in current amplitude could be reversed when the slice was washed in aCSF for ~5 minutes (Fig. 1A). These data suggest that increased intracellular cAMP levels can increase the L-type Ca2+ current in LC neurons. Figure 1B shows the average Ca2+ IV plots for 5 neurons from 3 slices aged P10-P13. The addition of db-cAMP to the perfusion solution resulted in an apparent increase in current amplitude and voltage sensitivity, activation being shifted in the hyperpolarizing direction (Fig. 1B). The peak Ca2+ current (at −10 mV) was significantly higher (paired t-test) in the presence of db-cAMP (−2.79 ± 0.48 nA; n=5) than in the absence of db-cAMP (−1.50 ± 0.14 nA). These results mirror the activation of the Ca2+ current by CO2 noted previously, and are consistent with an activation of an L-type Ca2+ channel by phosphorylation (Sculptoreanu et al., 1993; Sculptoreanu et al., 1995; Hille, 2001; Dai et al., 2009; Imber et al., 2012).
Figure 1.
The effects of db-cAMP (250 µM) on the Ca2+ current in LC neurons from neonatal rats older than P10. (A) Peak voltage-sensitive currents activated by a step from −70 mV to −10 mV in the presence of Na+ and K+ blockade and 3 mM BaCl2. Top trace is in aCSF, while middle trace is in the presence of db-cAMP. Note the large difference in the amplitude of inward current in the presence of the cAMP analogue and the long inactivation time, typical of L-type currents. Bottom trace is after 5 minutes of wash in aCSF, and reverses the increase in current amplitude induced by the cAMP analogue. (B) Average IV plot of current recordings under the same conditions as in (A). Resulting IV plots are characteristic for high voltage activated Ca2+ channels. Black trace is control in aCSF, while gray trace is in db-cAMP. Traces represent the mean ± SEM for 5 neurons.
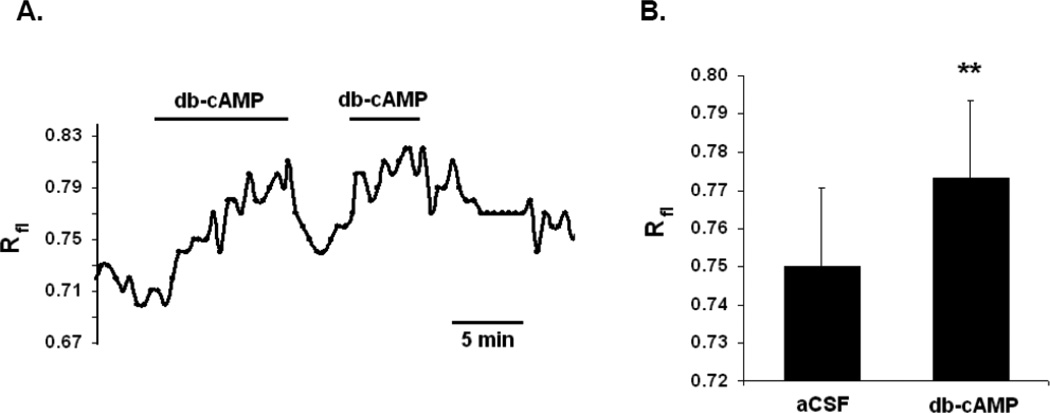
The activation of L-type Ca2+ currents by db-cAMP also increases resting intracellular Ca2+ levels in LC neurons, similar to the effects of 1) hypercapnia and 2) the voltage activation of nifedipine-sensitive Ca2+ currents previously reported (Imber et al., 2012; Imber & Putnam, 2012). Figure 2A shows the results from an LC neuron loaded with the Ca2+-sensitive dye Fura-2. When db-cAMP was added to the superfusate, an increase in intracellular Ca2+ levels was observed (Fig. 2A). This increase reversed when db-cAMP was washed from the slice and a second exposure to db-cAMP once again reversibly increased intracellular Ca2+ (Fig. 2A). Figure 2B shows the average significant (paired t-test) increase in Rfl values caused by the addition of db-cAMP in 3 neurons from 2 slices. In all cases, the membrane potential remained at rest or hyperpolarized slightly, and did not show an increase in firing rate (data not shown). The increase in intracellular Ca2+ levels by the addition of a cAMP analogue supports the enhanced activation of L-type Ca2+ channels by a cAMP-dependent pathway, similar to those reported previously (Dai et al., 2009).
Figure 2.
The effects of db-cAMP (250 µM) on Ca2+i levels in LC neurons from neonatal rats older than P10. (A) LC neuron loaded intracellularly with the Ca2+-sensitive dye Fura-2. Rfl is the ratio of fluorescence from excitation at 340nm/ 380nm. Exposure to the membrane permeable cAMP analogue db-cAMP causes a reversible and repeatable increase in intracellular Ca2+. (B) Average values for Rfl before (left bar) and after adding db-cAMP (right bar) to the superfusate (n=3). Increases in Rfl values were significant with a P<0.005 (paired t-test).
3.2 Dependence of Ca2+ currents on HCO3−
The nominal absence of CO2/HCO3− decreased both the current amplitude and voltage sensitivity of L-type Ca2+ channels in LC neurons. Replacing CO2/ HCO3−-buffered aCSF solution with HEPES solution (equilibrated with 100% O2) reduced the amplitude of the peak Ca2+ current, and was reversed by restoring CO2/ HCO3−-buffered aCSF (Fig 3A). In the nominal absence of CO2/ HCO3−, an IV plot (4 neurons from 3 slices) showed that the amplitude and voltage sensitivity of the Ca2+ current in LC neurons was decreased compared to CO2/HCO3−-buffered aCSF (Fig. 3B). The peak Ca2+ current at −10 mV was significantly (paired t-test) less in HEPES-buffered solutions (−1.58 ± 0.07 nA; n=4) than in aCSF solutions equilibrated with 5% CO2 and containing 24 mM HCO3− (−2.03 ± 0.09 nA). These data suggest a loss of activation of L-type Ca2+ channels in LC neurons in the nominal absence of intracellular HCO3−, consistent with our previous findings of a HCO3−-dependence of L-type Ca2+ channel activity in LC neurons (Imber & Putnam, 2012).
Figure 3.
The effects of HEPES-buffered aCSF on the Ca2+ current in LC neurons from neonatal rats older than P10. (A) Peak voltage-sensitive currents activated by a step from −70 mV to −10 mV. Top trace is in aCSF, while middle trace is in HEPES-buffered aCSF equilibrated with 100% O2. Note the decrease in the amplitude of inward current in the nominal absence of CO2/HCO3-. Bottom trace is after 5 minutes of wash in aCSF, and reverses the decrease in current amplitude. (B) Average IV plot of current recordings under the same conditions as in (A). Black trace is control in aCSF, while gray trace is in HEPES-buffered aCSF. Traces represent the mean ± SEM for 4 neurons.
3.3 The presence of sAC in LC neurons and its involvement in hypercapnic-activation of Ca2+ channels
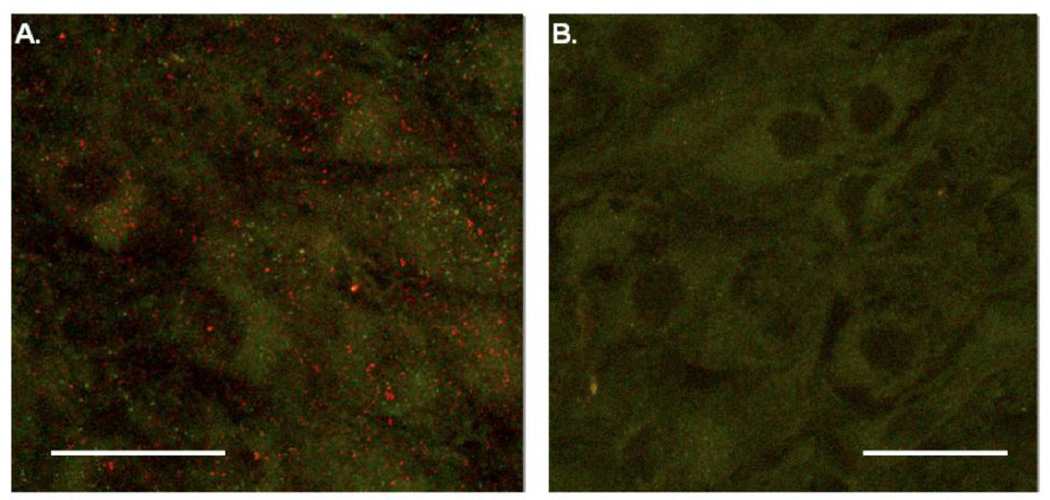
L-type Ca2+ channel activity could be HCO3−-dependent if LC neurons contain sAC. We performed immunohistochemistry experiments to look for the presence of sAC in LC neurons from neonatal rats. We observed positive staining for sAC (red puncta, Fig. 4A) in the cytoplasm of LC neurons from a P10 rat. The green is autofluorescence from the catecholamines in the largely catecholaminergic LC neurons, and readily identifies the cytoplasm of individual neurons (Fig. 4). Nonspecific staining was not seen in LC neurons (rat aged P12) in a control slice treated with secondary antibody only (Fig. 4B). These immunohistochemical findings indicate the presence of sAC in the cytoplasm of LC neurons from older neonatal rats.
Figure 4.
Immunohistochemistry studies of soluble adenylyl cyclase in LC neurons. (A) Immunohistochemical studies of LC neurons from a P10 rat incubated with an antibody for sAC and secondary antibody CY3 show the presence of the sAC enzyme in the cytoplasm (red puncta). Green fluorescence is autofluorescence from the catecholamines in the largely catecholaminergic LC neurons. (B) LC neurons from a P12 rat incubated with CY3 only. Notice the absence of red puncta. Scale bars represent 50 µm.
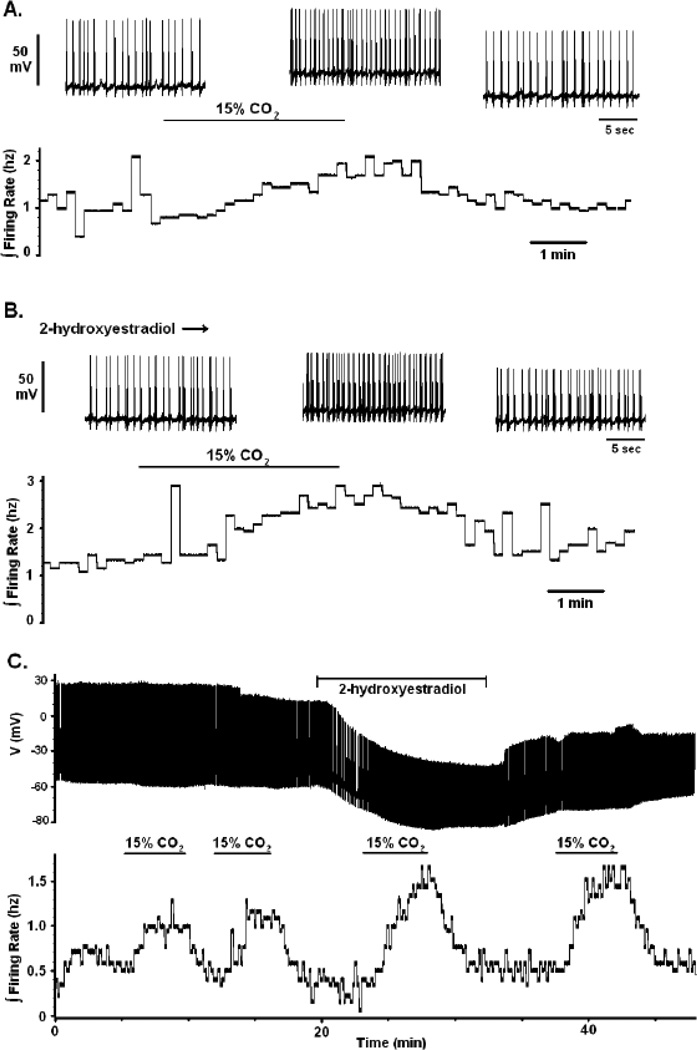
The possible role of sAC in a pathway involving HCO3− -activated L-type Ca2+ channels can be determined by studying the firing rate response of LC neurons to hypercapnia in the presence of an inhibitor of sAC, 2-hydroxyestradiol (2HE) (Schlicker et al., 2008; Li et al., 2011). The activation of an L-type Ca2+ current has previously been shown to significantly decrease the firing rate response of LC neurons to hypercapnia (a braking phenomenon) via the activation of BK channels in neonatal rats older than P10 (Imber et al., 2012). Since we hypothesize that the activation of sAC is the mechanism behind the hypercapnic activation of the Ca2+ current, it follows that inhibition of the sAC enzyme should increase the firing rate response of LC neurons to CO2 in a similar fashion, i.e. inhibition of the braking pathway should increase the firing rate response. Figure 5A shows a typical chemosensitive response of increased firing rate in response to hypercapnia (from 5 to 15% CO2) for an LC neuron from a P10 rat (Gargaglioni et al., 2010; Imber et al., 2012). When the same neuron was exposed to the sAC inhibitor 2HE (10 µM), the firing rate response to hypercapnia (5 to 15% CO2) was increased (ΔFR value of approximately 0.5 Hz in aCSF to 1.5 Hz in the presence of 2HE) (Fig. 5B). Another example of the effects of 2HE are shown in Fig. 5C, where repeated pulses of hypercapnia (going from 5 to 15% CO2) in an older (P14) neonate yield a similar increase in firing rate but the firing rate is markedly increased in the presence of 2HE. Note that the effects of 2HE do not rapidly wash off (Fig. 5C). Note also that the addition of 2HE during normocapnia (5% CO2) causes no change in the firing rate (Fig. 5C), suggesting that sAC has a very low activity under normal physiological conditions. The average effect of hypercapnia (going from 5% CO2 to 15% CO2) on the firing rate of LC neurons was an increase of firing rate of 0.80 ± 0.19 Hz (n=5). In these same neurons in the presence of 2HE, the increase in firing rate was significantly (paired t test) larger, 1.46 ± 0.35 Hz. These data show clearly that there is a significantly increased firing rate response to hypercapnia in the presence, compared to the absence, of 2HE, and support our hypothesis that sAC is involved in the activation of L-type Ca2+ channels in a HCO3−-dependent braking pathway that results in elevated intracellular Ca2+ and activation of BK channels.
Figure 5.
The effects of sAC inhibition on the magnitude of the chemosensitive response in LC neurons from neonatal rats older than P10. The inhibition of sAC enzyme by 2-hydroxyestradiol (2HE; 10 µM ) causes an increase in the chemosensitive response. (A) A typical chemosensitive response for whole cell current clamp experiments from neonatal rats older than P10 when CO2 is increased from 5 to 15%. The bottom trace represents the integrated firing rate (reported as Hz measured in 10 s bins). Note that 15% CO2 causes an increase in the integrated firing rate of LC neurons that is reversible upon return to 5% CO2. The top trace shows individual action potentials (voltage scale of 50 mV) at a faster time scale than the lower panel. The first set of action potentials is in the presence of 5% CO2, the middle set of action potentials is in the presence of 15% CO2 and the last set of action potentials is upon return to 5% CO2 at the end of the lower trace. (B) The same neuron as in (A) in the presence of 2HE. Notice the significant increase in the integrated firing rate response to hypercapnia (5 to 15% CO2) in the presence of 2HE shown in the lower trace. Just as in (A), the upper traces show individual action potentials at a faster time trace in the presence of 5% CO2 + 2HE (left trace), 15% CO2 +2HE (middle trace) and 5% CO2 + 2HE again (right trace) . (C) A complete current clamp record of a whole cell patch from a LC neuron from a P14 rat. In the lower trace is the integrated firing rate for this neuron in response to 15% CO2 in the presence and the absence of 2HE. Note that repeated bouts of hypercapnia (going from 5% to 15% CO2) result in a similar increase in integrated firing rate. The addition of 2HE does not increase the firing rate in control conditions (5% CO2) but leads to a substantially increased firing rate response to hypercapnia (15% CO2) that is not easily washed off upon removal of 2HE. The upper trace is a plot of action potentials vs. time for this neuron but at a slow time scale. This does not make individual action potentials clear but highlights the entire trace. Note the large apparent hyperpolarization in response to 2HE that reverses upon washing it off. This hyperpolarization is most likely due to a change in junction potential, caused by the addition of 2HE to the superfusate; however, the inhibition of sAC does not appear to reverse.
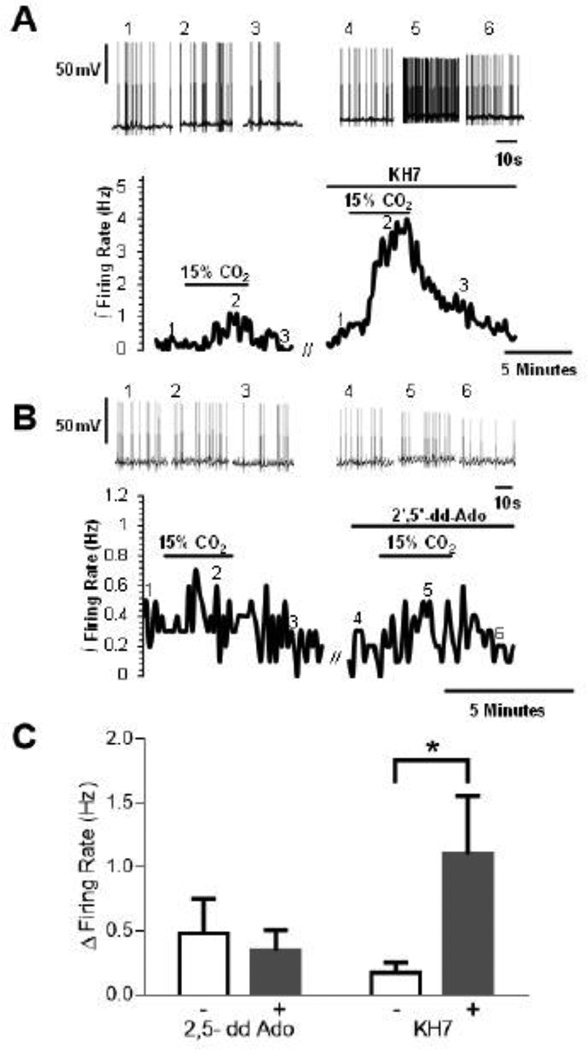
To further test for a role of sAC in activation of Ca2+ channels and restricting the firing rate response of LC neurons to hypercapnia, we used the more specific and potent sAC inhibitor KH7 (Bitterman et al., 2013). As with 2HE, inhibition of sAC by KH7 (30 µM) resulted in a significant increase in the firing rate response of LC neurons to hypercapnia in rats older than P10, with the firing rate response to hypercapnia being larger in the presence than in the absence of KH7 (Figs. 6A,C). To test the specificity of the involvement of sAC in this process, we examined the effect of a specific inhibitor of transmembrane adenylyl cyclases (tmAC), ddAdo (Bitterman et al., 2013), on the firing rate response to hypercapnia of LC neurons from rat pups older than P10. Unlike the large increase in firing rate seen with inhibitors of sAC, the inhibitor of tmAC, ddAdo (30 µM), did not affect the firing rate response of LC neurons to hypercapnia from rat pups older than P10 (Figs. 6B,C). The increase in firing rate in response to hypercapnia was not significantly different in the absence vs. the presence of ddAdo. These findings with inhibitors of sAC and tmAC suggest that the firing rate response of LC neurons to hypercapnia in rat pups older than P10 involves activation of sAC but not tmAC.
Figure 6.
The effects of sAC and tmAC inhibition on the magnitude of the chemosensitive response in LC neurons from neonatal rats older than P10. The inhibition of sAC enzyme by KH7 (30 µM ) causes an increase in the firing rate response induced by hypercapnia while the inhibition of tmAC by ddAdo (30 µM) has not effect on the firing rate rate response induced by hypercapnia. (A) A typical chemosensitive response for whole cell current clamp experiments from neonatal rats older than P10 when CO2 is increased from 5 to 15% and the effect of KH7 on that increased firing rate response. The bottom trace represents the integrated firing rate (reported as Hz measured in 10 s bins). Note that 15% CO2 causes a small increase in the integrated firing rate of LC neurons that is reversible upon return to 5% CO2. This increase is dramatically increased in response to 15% CO2 in the presence of the specific sAC inhibitor KH7. The top trace shows individual action potentials (voltage scale of 50 mV) at a faster time scale than the lower panel. The first three sets of action potentials depict the action potentials at the points indicated by the numbers on the lower trace (1, 2 and 3) for the control response. The second three sets of action potentials depict the action potentials at the points indicated by the numbers on the lower trace (4, 5 and 6) for the firing rate response to hypercapnia in the presence of KH7. (B) A similar set of traces to (A) but for the effects of the tmAC inhibitor 2’,5’-dd-Ado. In the lower trace, note that hypercapnia induces a small increase in firing rate that is similar in both the presence and in the absence of 2’,5’-dd-Ado. The upper traces show individual action potentials segments at the times indicated on the lower traces but at a faster time scale. (C) Bar graphs showing the mean ± SEM for the change in firing rate in going from 5% CO2 to 15% CO2 in the absence vs. the presence of 2,5-dd Ado (left two bars; n=5)) and in the absence vs. the presence of KH7 (right two bars; n=6). Note that 2’,5’-dd-Ado does not affect the firing rate response to hypercapnia in LC neurons but that KH7 results in a significantly higher (P<0.05) response of firing rate to hypercapnia.
3.4 The involvement of PKA in hypercapnic-activation of Ca2+ channels
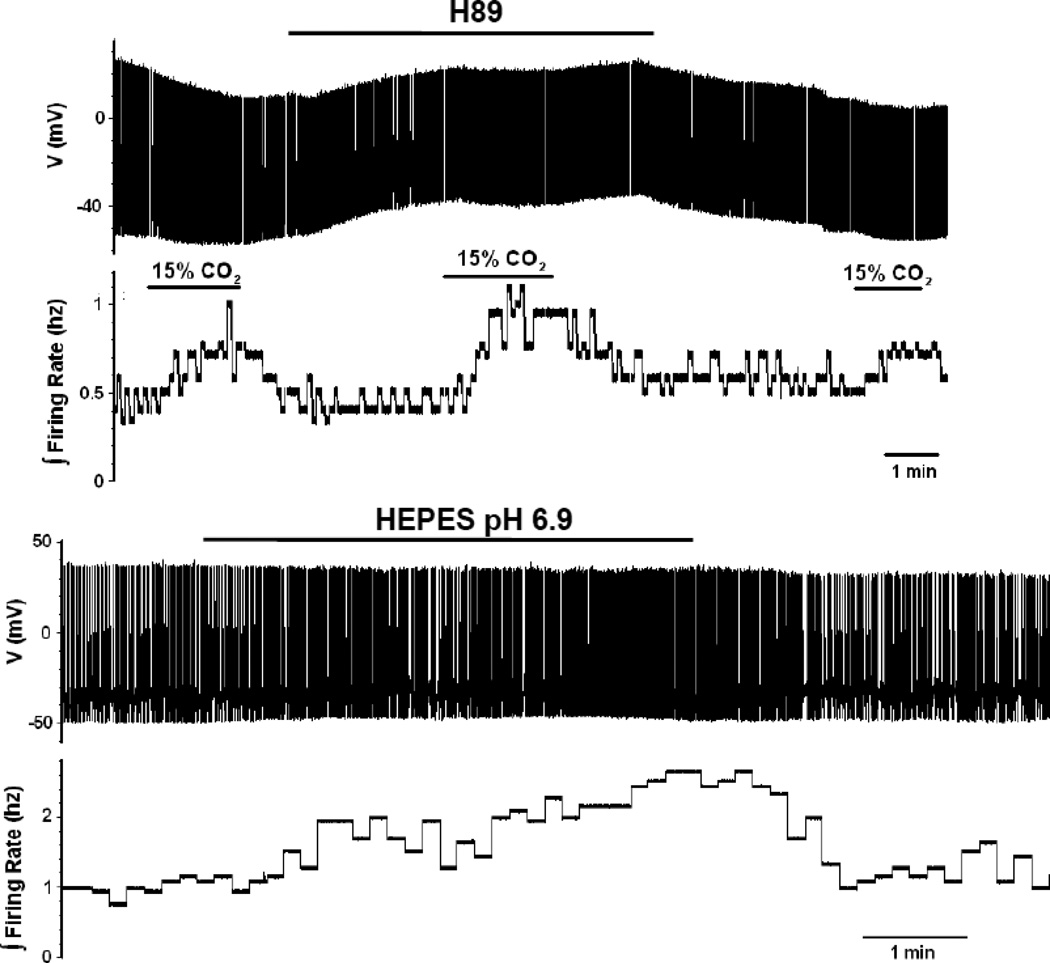
If sAC/cAMP is activating the Ca2+ currents in LC neurons via PKA phosphorylation, then the inhibition of PKA should also increase the firing rate response of LC neurons to hypercapnia. When the PKA inhibitor H89 (10 µM) was added to the superfusate during the whole cell patch of a LC neuron from a P12 rat, there was no increase in firing rate under control conditions (5% CO2) but there was an increase in the firing rate response to hypercapnia (Fig. 7A). This effect reversed when the slice was restored to normocapnic aCSF (Fig. 7A). The firing rate of LC neurons in response to hypercapnia (5% to 15% CO2) in older neonates (>P10) increased from control values of 0.28 ± 0.04 Hz (n=12) in the absence of H89 to 1.33 ± 0.43 Hz (n=5) in the presence of H89 (significant difference; unpaired t-test) . Thus, it appears that PKA has very low activity in control LC neurons but that hypercapnia activates PKA.
Figure 7.
The effects of PKA inhibition and HEPES-buffered aCSF on the magnitude of the firing rate response of LC neurons from neonatal rats older than P10. (A) The bottom trace shows an integrated firing rate of an LC neuron to 3 challenges of hypercapnia (going from 5% to 15% CO2). Note the modest increase in firing rate in the first and the third challenge, both done in the absence of the PKA-inhibitor H89, but the larger firing rate response in the middle challenge, which is in the presence of H89. The top trace shows a record of individual action potentials at a very slow time trace to show the whole record. (B) The bottom trace shows the integrated firing rate for a neuron that has gone from HEPES-buffered medium at pH 7.45 to an acidified HEPES-buffered medium (6.9). Not the reversible increase in firing rate induced by the acidified HEPES-buffered solution. The top trace shows a record of individual action potentials at a very slow time trace to show the whole record.
We also hypothesized that acidified HEPES, in the nominal absence of CO2/HCO3−, would not activate the braking pathway (no activation of sAC) and would thus result in a large firing rate response of LC neurons to hypercapnia. Indeed, a large increase in the firing rate response to acidification was seen when a LC neuron (from a neonatal rat older than P10) in HEPES-buffered aCSF (pH 7.4) was exposed to HEPES-buffered aCSF acidified to pH 6.9 (Fig. 7B). In comparison to the firing rate response to hypercapnia (5% to 15% CO2) of LC neurons from older neonates, which was 0.28 ± 0.04 Hz (n=12), the response of similar neurons to acidified HEPES solution was significantly (unpaired t-test) larger, amounting to 1.43 ± 0.32 Hz (n=5). These data are consistent with the proposed HCO3− dependence of the braking pathway in LC neurons from older neonatal rats.
Our results support our hypothesis that a HCO3−/sAC/PKA-dependent pathway leads to the chemosensitive activation of Ca2+ channels, resulting in a BK channel-dependent braking mechanism on the firing rate response of LC neurons to hypercapnia.
4. DISCUSSION
In this study we report on our findings of the presence of a HCO3−-dependent pathway, involving sAC and PKA, which mediates the activation of L-type Ca2+ channels by hypercapnia in LC neurons. This pathway is consistent with our previous demonstration of a HCO3−dependence to Ca2+ channel activation in LC neurons (Imber & Putnam, 2012). This is the first demonstration of such a pathway being active in chemosensitive neurons, adds a novel pathway by which the magnitude of the chemosensitive response could be regulated in LC neurons, and emphasizes a potential role for Ca2+ in central chemosensitivity.
4.1 Hypercapnic-activation of L-type Ca2+ channels through a HCO3—dependent pathway involving sAC, cAMP and PKA
We have demonstrated the presence of sAC in the cytoplasm of neonatal LC neurons (Fig. 4). sAC differs from transmembrane adenylyl cyclases by being insensitive to G-proteins and to forskolin and by its regulation by HCO3− (Zippin et al., 2001; Summers et al., 2002). In LC neurons, it is likely that sAC is activated by the increase in intracellular HCO3− induced by hypercapnia. Most studies of the cellular signaling pathways in chemosensitive neurons emphasize changes in pH and the role of pH-sensitive ion channels (Pineda & Aghajanian, 1997; Xu et al., 2000; Bayliss et al., 2001; Wiemann & Bingmann, 2001; Bradley et al., 2002; Filosa et al., 2002; Putnam et al., 2004; Cui et al., 2011; Li and Putnam, 2013). Our findings suggest that changes of intracellular HCO3− are an additional important signal associated with the chemosensitive response to hypercapnia.
We have previously demonstrated that hypercapnia activates an L-type Ca2+ current in LC neurons (Filosa et al., 2002) and that this activation is pH-independent and develops over the ages P3-P16 (Imber et al., 2012; Imber & Putnam, 2012). The current study helps to define the precise mechanism of this activation. When db-cAMP was added to the superfusate, the IV plot for Ca2+ currents was enhanced in LC neurons from neonatal rats older than P10 (Fig. 1), resulting in increased levels of intracellular Ca2+ (Fig. 2). The ability of db-cAMP to increase intracellular Ca2+ levels and the Ca2+ current amplitude and voltage sensitivity in the presence of normocapnia (5% CO2) is analogous to the effects of hypercapnia on Ca2+ currents and intracellular Ca2+ levels in LC neurons (Imber et al., 2012). These findings implicate increased cAMP in the hypercapnia-induced pathway of activation of Ca2+ channels in LC neurons. We have further shown that in the nominal absence of CO2/HCO3−, using an acidified HEPES-buffered aCSF, Ca2+ currents recorded from LC neurons are decreased (Fig. 3). These results support the previous finding of a strong correlation between intracellular HCO3− and Ca2+ channel activity (Imber & Putnam, 2012).
Taken together, the above findings imply a HCO3−-dependent pathway that leads to the production of intracellular cAMP. It seems possible that such a pathway should involve sAC. Our demonstration of the presence of sAC in LC neurons (Fig. 4) is consistent with its involvement in the activation of Ca2+ channels by hypercapnia in LC neurons. Further, the involvement of increased cAMP suggests activation of PKA in LC neurons by hypercapnia and activation of Ca2+ channels by PKA-mediated phosphorylation.
4.2 Reduction of the chemosensitive brake in LC neurons by inhibitors of the Ca2+ channel activation pathway
Our previous results have implicated hypercapnia-activated Ca2+ channels and increased intracellular Ca2+ in a braking pathway, mediated by activation of BK channels (Imber et al., 2012). We have used this braking phenomenon to further investigate the pathway by which hypercapnia activates Ca2+ channels. We reasoned that if an inhibitor blocks part of the Ca2+ channel activation mechanism, it would also block the braking pathway in LC neurons from older neonates. Using such an approach, two inhibitors of sAC, 2HE and KH7, resulted in a significant increase in the firing rate response to hypercapnia of LC neurons from older neonates (Figs. 5 and 6). The hypercapnia-induced firing rate increases in the presence of 2HE or KH7 were similar to those recorded in the presence of an inhibitor of BK channels (Imber et al., 2012). We also found that the PKA inhibitor H89 resulted in a significant increase in the firing rate response to hypercapnia of LC neurons from older neonates (Fig. 7A). Once again, PKA inhibition led to increases in hypercapnia-induced firing rate that were similar to the increases resulting from inhibition of BK channels. These data are consistent with a role for a HCO3−-sAC-cAMP-PKA pathway in the activation of Ca2+ currents in LC neurons in response to hypercapnia causing a concurrent decrease of the chemosensitive response of LC neurons from neonatal rats older than P10.
In our study we use a high concentration of CO2 (15%) in order to determine if a HCO3−-dependent pathway exists for activation of Ca2+ channels and the braking phenomenon. In preliminary data we have observed the braking phenomenon in response to 10% CO2 and a small but significant phenomenon in response to 7.5% CO2. Further, we have evidence from in vivo experiments that inhibition of BK channels in LC neurons increases the hypercapnic ventilatory response. These findings suggest that the pathway proposed here is involved in the chemosensitive response of LC neurons and in ventilatory control. However, given the strong response to high levels of CO2 it is also possible that the pathway described here is involved in other responses to hypercapnia mediated by the LC such as anxiety and panic disorders (Sullivan et al., 1999; Griez and Schruers, 2003). Further study will be required to clearly define the role of this HCO3−-dependent pathway in the response of LC neurons to hypercapnia.
4.3 Significance
There are several significant findings to this study. Our findings suggest a role for HCO3−as a chemosensitive signal in LC neurons and describe the first role for a sAC-cAMP-PKA pathway in a central chemosensitive neuron. Further, that this pathway leads to the activation of Ca2+ channels and increased intracellular Ca2+ points to a previously nearly unexplored potential role of calcium in central chemosensitive signaling. There are several possible ways in which calcium could contribute to central chemosensitivity. The activation of Ca2+ channels should depolarize and therefore activate chemosensitive neurons. In fact, the inhibition of L-type Ca2+ channels by nifedipine decreased the chemosensitive response in LC neurons from young neonatal rats (P1-P9) (Filosa & Putnam, 2003). This could reflect a Ca2+-dependent activation of chemosensitive LC neurons from young neonates. Alternatively, since L-type Ca2+ channel inhibition can also diminish synaptic input, it is possible that the effects of nifedipine inhibition on LC neuron chemosensitivity is not due to depolarization of Vm by activated Ca2+ channels, but rather due to the inhibition of synaptic input. It is clear that in LC neurons from older neonates (>P10), increased intracellular Ca2+ plays a role as a brake on the chemosensitive response due to activation of BK channels (Imber et al., 2012).
Our work raises some interesting unanswered questions as well. Elevated intracellular Ca2+ could alter the activity of any number of channels or intracellular signaling pathways, all of which could affect the chemosensitive response of LC neurons, but such a possibility remains largely unexplored. It is also currently unclear to what extent sAC or the activation of Ca2+ channels is involved in the hypercapnic response of chemosensitive neurons from other areas of the medulla and pons, although there has been evidence for hypercapnic Ca2+ signaling in astrocytes near the region of the retrotrapezoid nucleus (Gourine et al., 2010; Huckstepp et al., 2010; Wenker et al., 2010). In addition, pH-induced inhibition of tonically active KCa channels (possibly by inhibition of Ca2+ channels) in cultured medullary neurons has been suggested to be part of the pathway by which hypercapnia activates these neurons (Wellner-Kienitz et al., 1998). It is clear, therefore, that there is a need to better characterize pathways involving Ca2+ and central chemoreceptive control.
HIGHLIGHTS.
Hypercapnia-induced Increased HCO3- activates Ca2+ channels in LC neurons
This pathway involves activation of sAC, increased cAMP and activation of PKA
Intracellular HCO3- can be a chemosensitive signaling molecule in LC neurons
Intracellular Ca2+ can play a role in central chemosensitivity in LC neurons
Altered sAC function could contribute to breathing and/or panic disorders
ACKNOWLEDGEMENTS
This work was supported by National Heart, Lung and Blood Institute Grant R01 HL-56683 (to RWP), an American Heart Association Great Rivers Affiliate Pre-doctoral Fellowship (to ANI) and a Research Challenge Augmentation Grant from Wright State University (to RWP). JMS is supported by NSF IOS Grant 1257338 (P.I. Dr. Lynn K. Hartzler, Wright State University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive ‘leak’ K+ channel expressed in brainstem respiratory neurons. Respir. Physiol. 2001;129:159–174. doi: 10.1016/s0034-5687(01)00288-2. [DOI] [PubMed] [Google Scholar]
- Biancardi V, Bicego KC, Alameda MC, Gargaglioni LH. Locus coeruleus noradrenergic neurones and CO2 drive to breathing. Eur. J. Physiol. 2008;455:1119–1128. doi: 10.1007/s00424-007-0338-8. [DOI] [PubMed] [Google Scholar]
- Bitterman JL, Ramos-Espiritu L, Diaz A, Levin LR, Buck J. Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J. Pharmacol. Exp. Ther. 2013;347:589–598. doi: 10.1124/jpet.113.208496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley SR, Pieribone VA, Wang W, Severson CA, Jacobs RA, Richerson GB. Chemosensitive serotonergic neurons are closely associated with large medullary arteries. Nat. Neurosci. 2002;5:401–402. doi: 10.1038/nn848. [DOI] [PubMed] [Google Scholar]
- Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates EL, Li A, Nattie EE. Widespread sites of brain stem ventilatory chemoreceptors. J. Appl. Physiol. 1993;75:5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J. Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui N, Zhang X, Tadepalli JS, Yu L, Gai H, Petit J, Pamulapati RT, Jin X, Jiang C. Involvement of TRP channels in the CO2 chemosensitivity of locus coeruleus neurons. J. Neurophysiol. 2011;105:2791–2801. doi: 10.1152/jn.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S, Hall DD, Hell JW. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 2009;89:411–452. doi: 10.1152/physrev.00029.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam M, Yao T, Thoren P, Svensson TH. Hypercapnia and hypoxia: chemoreceptor-mediated control of locus coeruleus neurons and splanchnic, sympathetic nerves. Brain Res. 1981;222:373–381. doi: 10.1016/0006-8993(81)91040-4. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J. Physiol. 2002;541:493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am. J. Physiol. Cell Physiol. 2003;284:C145–155. doi: 10.1152/ajpcell.00346.2002. [DOI] [PubMed] [Google Scholar]
- Gargaglioni LH, Hartzler LK, Putnam RW. The locus coeruleus and central chemosensitivity. Respir. Physiol. Neurobiol. 2010;173:264–273. doi: 10.1016/j.resp.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griez E, Schruers K. Mechanisms of CO2 challenges. J. Psychopharmacol. 2003;203:260–262. doi: 10.1177/02698811030173003. [DOI] [PubMed] [Google Scholar]
- Hartzler LK, Dean JB, Putnam RW. The chemosensitive response of neurons from the locus coeruleus (LC) to hypercapnic acidosis with clamped intracellular pH. Adv. Exp. Med. Biol. 2008;605:333–337. doi: 10.1007/978-0-387-73693-8_58. [DOI] [PubMed] [Google Scholar]
- Hille B., 3rd . Ion Channels of Excitable Membranes. Sunderland, Mass: Sinauer; 2001. [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J. Physiol. 2010;588:3901–3920. doi: 10.1113/jphysiol.2010.192088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imber AN, Graham CD, Putnam RW. The role of Ca2+ and BK channels in the firing rate response of locus coeruleus neurons to CO2: controlling the chemosensitive gain. FASEB J. 2012;26:8. Program No. 894. [Google Scholar]
- Imber AN, Putnam RW. Postnatal development and activation of L-type Ca2+ currents in locus coeruleus neurons: implications for a role for Ca2+ in central chemosensitivity. J. Appl. Physiol. 2012;112:1715–1726. doi: 10.1152/japplphysiol.01585.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SM, Haxhiu MA, Richerson GB. GFP-expressing locus coeruleus neurons from Prp57 transgenic mice exhibit CO2/H+ responses in primary culture. J. Appl. Physiol. 2008;105:1301–1311. doi: 10.1152/japplphysiol.90414.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie EE. Catecholamine neurons in rats modulate sleep, breathing, central chemoreception, and breathing variability. J. Physiol. 2006;570:385–396. doi: 10.1113/jphysiol.2005.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K-Y, Putnam RW. Transient outwardly rectifying A currents are involved in the firing rate response to altered CO2 in chemosensitive locus coeruleus neurons from neonatal rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R780–R792. doi: 10.1152/ajpregu.00029.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Allen KT, Bonanno JA. Soluble adenylyl cyclase mediates bicarbonate-dependent corneal endothelial cell protection. Am. J. Physiol. Cell Physiol. 2011;300:C368–C374. doi: 10.1152/ajpcell.00314.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. eLife. 2013:e01213. doi: 10.7554/eLife.01213. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes ARSM, Monteiro EC, Johnson SM, Gauda EB. Bicarbonate-regulated soluble adenylyl cyclase (sAC) mRNA expression in peripheral and central chemoreceptors. FASEB J. 2008;22 doi: 10.1007/978-90-481-2259-2_27. Program No. 171. [DOI] [PubMed] [Google Scholar]
- Nunes AR, Holmes APS, Sample V, Kumar P, Cann MJ, Monteiro EC, Zhang J, Gauda EB. Bicarbonate-sensitive soluble and transmembrane adenylyl cyclases in peripheral chemoreceptors. Resp. Physiol. Neurobiol. 2013;188:83–93. doi: 10.1016/j.resp.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda J, Aghajanian GK. Carbon dioxide regulates the tonic activity of locus coeruleus neurons by modulating a proton- and polyamine-sensitive inward rectifier potassium current. Neuroscience. 1997;77:723–743. doi: 10.1016/s0306-4522(96)00485-x. [DOI] [PubMed] [Google Scholar]
- Putnam RW. CO2 chemoreception in cardiorespiratory control. J. Appl. Physiol. 2010;108:1796–1802. doi: 10.1152/japplphysiol.01169.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am. J. Physiol. Cell Physiol. 2004;287:C1493–1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Dean JB, Putnam RW. Somatic vs. dendritic responses to hypercapnia in chemosensitive locus coeruleus neurons from neonatal rats. Am. J. Physiol. Cell Physiol. 2005;289:C1094–1104. doi: 10.1152/ajpcell.00329.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicker C, Rauch A, Hess KC, Kachholz B, Levin LR, Buck J, Steegborn C. Structure-based development of novel adenylyl cyclase inhibitors. J. Med. Chem. 2008;51:4456–4464. doi: 10.1021/jm800481q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Figourov A, De Groat WC. Voltage-dependent potentiation of neuronal L-type calcium channels due to state-dependent phosphorylation. Am. J. Physiol. Cell Physiol. 1995;269:C725–C732. doi: 10.1152/ajpcell.1995.269.3.C725. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- Shah MJ, Meis S, Munsch T, Pape H. Modulation by extracellular pH of low- and high-voltage-activated calcium currents of rat thalamic relay neurons. J. Neurophysiol. 2001;85:1051–1058. doi: 10.1152/jn.2001.85.3.1051. [DOI] [PubMed] [Google Scholar]
- Sullivan GM, Coplan JD, Kent JM, Gorman JM. The noradrenergic system in pathological anxiety: a focus on panic with relevance to generalized anxiety and phobias. Biol. Psychiatry. 1999;46:1205–1218. doi: 10.1016/s0006-3223(99)00246-2. [DOI] [PubMed] [Google Scholar]
- Summers BA, Overholt JL, Prabhakar NR. CO2 and pH independently modulate L-Type Ca2+ current in rabbit carotid body glomus cells. J. Neurophysiol. 2002;88:604–612. doi: 10.1152/jn.2002.88.2.604. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Somjen GG. Differential sensitivity to intracellular pH among high- and low-threshold Ca2+ currents in isolated rat CA1 neurons. J. Neurophysiol. 1997;77:639–653. doi: 10.1152/jn.1997.77.2.639. [DOI] [PubMed] [Google Scholar]
- Wellner-Kienitz MC, Shams H, Scheid P. Contribution of Ca2+-activated K+ channels to central chemosensitivity in cultivated neurons of fetal rat medulla. J. Neurophysiol. 1998;79:2885–2894. doi: 10.1152/jn.1998.79.6.2885. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ. Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc. Natl. Acad. Sci. 2004;101:3621–3626. doi: 10.1073/pnas.0308753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J. Neurophysiol. 2010;104:3042–3052. doi: 10.1152/jn.00544.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann M, Bingmann D. Ventrolateral neurons of medullary organotypic cultures: intracellular pH regulation and bioelectric activity. Respir. Physiol. 2001;129:57–70. doi: 10.1016/s0034-5687(01)00282-1. [DOI] [PubMed] [Google Scholar]
- Xu H, Cui N, Yang Z, Qu Z, Jiang C. Modulation of kir4.1 and kir5.1 by hypercapnia and intracellular acidosis. J. Physiol. 2000;524:725–735. doi: 10.1111/j.1469-7793.2000.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, 3rd, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin JH, Levin LR, Buck J. CO2/HCO3-responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol. Metab. 2001;12:366–370. doi: 10.1016/s1043-2760(01)00454-4. [DOI] [PubMed] [Google Scholar]