Abstract
There are three non-exclusive theoretical explanations for the paradoxical collapse of performance due to large financial incentives. It has been proposed that “choking under pressure” is either due to distraction, interference via an increase in top-down control and performance monitoring, or excessive levels of arousal in the face of large losses. Given the known neural architecture involved in executive control and reward, we used fMRI of human participants during incentivized motor performance to provide evidence to support and/or reconcile these competing models in a visuomotor task. We show that the execution of a pre-trained motor task during neuroimaging is impaired by high rewards. BOLD activity occurring prior to movement onset is increased in dorsolateral prefrontal cortex and functional connectivity between this region and motor cortex is likewise increased just prior to choking. However, the extent of this increase in functional connectivity is inversely related to a participant's propensity to choke, suggesting that a failure in exerting top-down influence on motor control underlies choking under pressure due to large incentives. These results are consistent with a distraction account of choking and suggest that frontal influences on motor activity are necessary to protect performance from vulnerability under pressure.
INTRODUCTION
The phrase “choking under pressure” (hereafter simply “choking”) describes instances where the execution of a well-learned, proceduralized skill fails under high levels of pressure when the desire for superior performance is maximal and produces poorer outcomes than would otherwise be expected (Baumeister, 1984; Baumeister et al., 1986). In controlled laboratory environments, performance decrements under pressure is induced on a wide range of sensorimotor tasks including golf putting (Beilock and Carr, 2001), bimanual coordination (Baumeister, 1984), and a variety of novel motor tasks (Mobbs et al., 2009; Chib et al., 2012). Choking is also observed in various cognitive tasks such as mathematical problem solving, category learning, and tests of fluid intelligence (Beilock and Carr, 2005; Markman et al., 2006; Gimmig et al., 2006; Ariely et al., 2009).
At least three theories can explain the collapse of performance under pressure. Distraction theories, and the related attentional control theory, posit that high pressure situations cause a shift in working memory resources away from the task onto irrelevant internal factors such as worries about failure (Beilock and Carr, 2005; Beilock et al., 2004; Eysenck, et al., 2007). This shift reduces the influence of goal-directed attentional control on task performance and potentially increases the use of bottom-up stimulus driven attentional control (Eysenck, et al., 2007). In contrast, explicit monitoring theories suggest that pressure increases the amount of attention given to the details of the task at hand, but this extra level of control hinders task performance (Lewis and Linder, 1997). In support of this view, attention to execution of the individual steps of a cognitive or motor operation can adversely influence performance (Kimble and Perlmuter, 1970; Masters, 1992; Lewis and Linder, 1997). The third theory of choking under pressure, the over-motivation (or over-arousal) theory, is favored by behavioral economists and posits that as incentive increases, arousal levels also increase. Higher levels of arousal are associated with increased performance up until a point after which increasing levels of arousal begin to degrade performance (Yerkes and Dodson, 1908; Easterbrook, 1959; Mobbs et al., 2009; Ariely et al., 2009). The mechanism by which over-motivation affects behavior is relatively unclear, however proponents of this account often suggest that arousal influences the neural control of goal oriented movement by changes in the scope of attention (e.g. “attentional narrowing”). Alternatively, other researchers have suggested that over-motivation triggers pavlovian withdrawal responses due to loss aversion that interfere with the intended motor plan (Chib et al., 2012).
Previous work has convincingly shown through behavioral manipulations that both distraction and explicit monitoring can potentially account for choking depending on the specific task construction and source of motivation (Decaro, et al. 2011). Human neuroimaging is well suited to provide independent neural evidence above and beyond behavioral analysis that might support and/or dissociate these competing models for a specific skilled motor task given a particular type of performance pressure (e.g. financial incentives). Sufficient neuroimaging evidence is known about human brain systems for cognitive control, monitoring of behavior, and motivation that it is possible to use imaging as supportive evidence for competing models of choking for a given context (e.g. Koechlin, et al., 2003; Ridderinkhof, et al., 2004; Elliott et al., 2000). Additionally, this approach allows an exploration of individual differences in the propensity to choke.
Both the distraction and explicit monitoring theories lead to the prediction that the frontal cortex should be a crucial brain region involved in choking. A large body of literature suggests that the prefrontal cortex (PFC), often in concert with parietal cortex and medial frontal regions such as the anterior cingulate cortex (ACC), is essential for executive control processes such as working memory and the top-down monitoring of performance (Miller and Cohen, 2001; Rushworth et al., 2004). Numerous studies using electrophysiology in nonhuman primates, brain-lesioned patients, and human functional magnetic resonance imaging (fMRI) show that the PFC sends top-down signals that enhance the processing and maintenance of goal-relevant sensorimotor information (Fuster and Alexander, 1971; Miller et al., 1996; Knight et al., 1999; Miller and D'Esposito 2005). Additionally, lateral prefrontal cortex often displays increased activity under divided attention and multi-tasking conditions (Szameitat, et al., 2002; Johnson and Zatorre, 2006). Despite this preponderance of evidence for the role of the fronto-parietal (FP) networks in both distraction and executive control, there have been no direct examinations of its influence on choking and no evidence from the field of cognitive neuroscience of FP involvement in this phenomenon.
In contrast, from the over-motivation theory of choking one would predict greater activity in brain regions known to encode reward or value in those trials where choking occurs. To our knowledge, there have only been two neuroimaging studies of choking (Mobbs et al., 2009; Chib et al., 2012). Both studies viewed the phenomenon through the lens of behavioral economics and over-motivation and focused on reward processing systems in the midbrain and the striatum, demonstrating increased activity in the setting of high levels of financial incentives. While these studies reported changes in brain activation in reward related regions with high value trials, it remains unclear if this activity was simply related to the high level of reward or a motivational state that would lead to choking. Furthermore, these studies leave open the critical question of how a brain state triggered by increased reward pressure in choke trials will specifically alter motor performance.
To understand this interaction between brain state and motor performance, the identification of functional interactions with cortical motor areas is necessary. Whatever the mechanism underlying choking under pressure, it is clear that there must be an influence on the motor system to produce the harmful effects seen on sensorimotor performance. To date, no study has directly examined functional interactions between motor cortex (M1) and other brain regions that might mediate choking under pressure.
The present fMRI study aimed to investigate the interaction between high reward state and failing motor performance directly, by examining activity in motor cortex and PFC. Subjects performed a well learned bimanual motor task that shows vulnerability to high financial pressure. Crucially, we sought to examine the network-level functional connectivity profile of motor cortex during choking in order to best characterize activity that negatively impacts motor performance. The key hypothesis is that if distraction or monitoring is involved in mediating choking, then there should be altered interactions between FP networks and motor cortical areas during choking trials. Prior work has shown that attention to action increases connectivity between PFC and motor regions (Rowe, et al., 2002) while distraction reduces functional connectivity between PFC and primary areas necessary for successful task performance (e.g. Yoon, et al., 2006). From this, we predict that if explicit monitoring leads to choking, functional connectivity between M1 and FP areas known to be involved in top-down control will increase under pressure and that this increase is associated with diminished performance. In contrast if attention is misdirected internally away from the task during choking, there should be a reduction in functional connectivity between M1 and FP regions that is otherwise necessary for the task. Likewise, if motivation and arousal are sufficient theories, then there should be a direct relationship between brain regions important for motivation and arousal with motor cortex.
MATERIALS AND METHODS
Participants
Twenty right-handed individuals (6 males, 14 females; age range, 19-26) participated in the experiment. All participants gave their written informed consent before the study and received monetary compensation for their participation. One subject did not reach a criterion level of accuracy on day two and was removed from the analysis. Due to technical difficulties on day 1 of the experiment, we were unable to collect fMRI data and could not create ROIs for two separate subjects to use for functional connectivity analyses. Therefore this analysis was completed using data from the remaining 17 participants (11 females).
Visuomotor Task
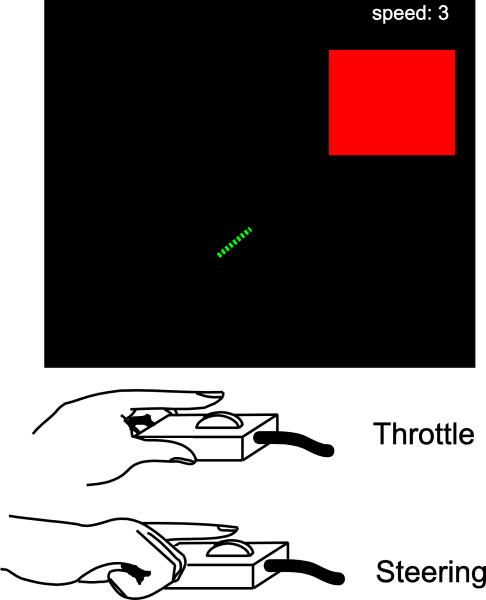
All participants performed a challenging bimanual motor task inspired by the classic arcade video game Snake. In this task, participants use two scroll-click response devices (Current Designs, Philadelphia) to control the movement of a virtual snake on a computer screen (see Figure 1). These response devices are similar to the scroll wheel found on a computer mouse. The response device used by the left hand was used to allow the participant to control the velocity of the snake (in arbitrary units from speeds 1 to 4) by acting as a throttle, while the other response device used by the right hand allowed the participant to steer the snake right and left in 45 degree turns. The snake was pseudo-randomly selected to start at either the left or right edge of the screen and needed to be steered into a square target “apple” on the opposite edge of the screen within 2 s from the start of the trial. There was an additional constraint that the snake must be traveling at speed 2 or less in order to qualify for a “hit”. The time and speed restrictions ensure that participants must coordinate both hands in order to accelerate to reach the target before the time limit, slow the snake down to the appropriate speed before reaching the target, and simultaneously making steering changes at the appropriate time during the trajectory of the snake.
Figure 1. Bimanual Motor Task.
Participants had to steer a virtual “snake” in a square target “apple” with two scrolling devices using the index finger of each hand. The left hand controlled the speed of the snake (from 1-4 in arbitrary units), while the right hand controlled the steering. On each trial, participants had to initially speed up to be able to reach the target before the time limit (~2 s) but then had to slow down again just before reaching the target as entering the target at a speed of 3 or 4 was counted as a miss. The size of the target and the time to reach the target were set on an individual-by-individual basis to ensure an accuracy of ~50% correct.
Experimental Timeline Day 1 – Training with fMRI
All participants underwent an exposure phase of 150 trials to familiarize themselves with the bimanual control of the snake on day 1 of the experiment inside the scanner during the anatomical scan. Immediately following this exposure phase, participants completed an additional set of 300 trials of training during which functional EPI scans were acquired. Difficulty during training was adjusted with a staircase procedure such that each correct trial was followed by a slightly harder trial and each incorrect trial was followed by a slightly easier trial. For the 1st 100 trials of this procedure, difficulty was manipulated by changing the time deadline, after which the deadline was held constant and the size of the target was manipulated. This procedure was used so that participants could adequately learn the motor task while keeping the task relatively challenging. Although participants were given immediate visual feedback about whether they were successful on each trial, there were no monetary rewards during this phase of the experiment. In order to examine how personality traits might interact with one's propensity to choke, participants completed the self-report Barratt Impulsiveness Scale (BIS-11) (Patton, et al., 1995) and a loss aversion task described below following fMRI scanning.
Experimental Timeline Day 2 - Behavioral Calibration and Reward fMRI Task
On day 2 of the experiment, just prior to functional scanning with monetary rewards, participants underwent a short behavioral calibration phase of the experiment consisting of an additional 300 trials in which the size of the target and the time limit to reach the target were adjusted with a staircase protocol such that participants’ level of accuracy on the task was set at ~50% correct. By setting the difficulty of the task on a participant-by-participant basis, we ensured that changes in accuracy due to incentive could be compared across individuals despite differing levels of overall task proficiency. Also, this ensured that participants would have a roughly equivalent number of correct and incorrect trials to allow for an examination of neural activity during errors. This staircase procedure of the experiment occurred while the subject was physically inside of the scanner, however no images were acquired.
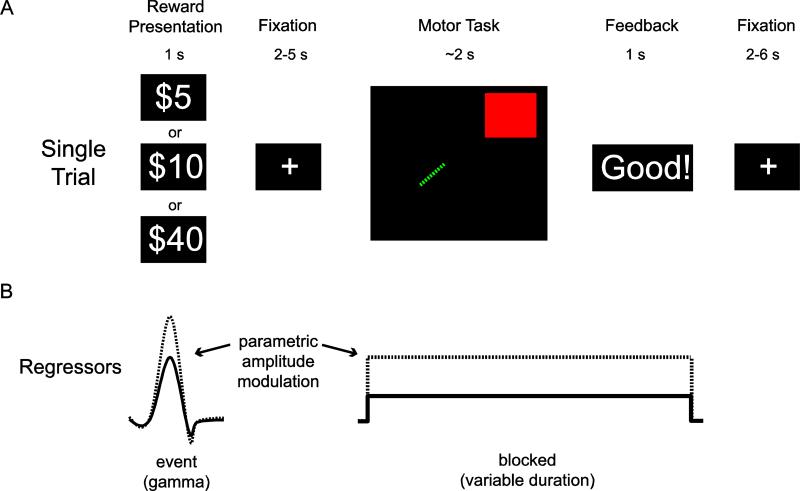
Immediately following staircase adjustments of task difficulty, participants performed 243 trials of the motor task during fMRI scanning (Fig. 2a). Each trial consisted of reward presentation for 1s, followed by a fixation cross (2-5s jittered), motor performance (~ 2s), feedback (~ 1s) and finally a variable inter-trial interval (2-6s). Reward values ($5, $10, or $40) were presented pseudorandomly with the order chosen using an m-sequence to counterbalance the order of presentation (Buracas and Boynton, 2002). Participants were informed that at the end of the experiment one trial would be chosen at random and that if they were successful on that trial they would receive its associated reward. This procedure ensured that the reward for each trial was evaluated independently from other trials. Although the highest reward value used here is less than that used in several previous studies of choking under pressure (e.g. Chib et al., 2012; Ariely, et al. 2009), extensive behavioral piloting with over 70 participants indicated that it is the relative value and not the absolute dollar amount that leads to choking on this task. For example, performance suffers relative to peak performance on infrequent trials worth $20 if that is the highest value trial seen throughout the experiment.
Figure 2. Single Trial Timeline and GLM.
A) Each trial began with a reward cue signaling the incentive for that trial. After a variable duration, participants were shown the start position and the location of the target for 1 s and then had to immediately steer the snake into the target. Immediately upon hitting the target or reaching the time limit, participants were given feedback to indicate whether they hit the target at too high of a speed, went out of bounds, failed to reach the target in time, or correctly navigated to the target within all the constraints. B) In the GLM used for univariate fMRI analyses, the reward cue was modeled as a single event (gamma function). In contrast the entire epoch from when the snake and the target were displayed on the screen, through movement, and ending with feedback was modeled as a block (boxcar convolved with a gamma function) with a variable duration based upon the movement time. For both types of events, additional parameters in the model were included to account for parametric modulation by reward. The resulting parameter estimates capture how the amplitude of the BOLD signal varies as a function of reward.
Functional MRI Acquisition and Pre-processing
MR data were acquired with a Siemens 3.0 T Trio scanner (Berlin/Munich, Germany) with a 12-channel phased-array head coil. Functional data were obtained using a 2-shot T2*-weighted echo-planar imaging (EPI) sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (TR = 2000 msec, TE=30 msec, 192 mm field of view, in-plane resolution 3.0 mm × 3.0 mm). Each functional volume contained 37 contiguous 3.0 mm-thick axial slices separated by a 0.5mm inter-slice gap acquired in an interleaved manner. Whole-brain T1-weighted scans were acquired for anatomical localization. Functional data were realigned to the first volume acquired and spatially smoothed with a 6.0 mm full-width at half-maximum Gaussian kernel.
Behavioral Analysis of Motor Task Accuracy
Mean accuracy rates were computed for each participant for reward level ($5, $10, $40). The effect of reward on behavior was evaluated by a repeated measures analysis of variance (ANOVA) with reward as a within-subject factor. Post-hoc two-tailed t-tests were used to compare results between conditions where appropriate. Behavioral results were similarly examined for the first and the second half of the experiment separately.
Univariate fMRI Analysis
Task-dependent changes in the BOLD signal were modeled in a generalized linear model (GLM) framework with independent regressors for each of the two stages of each correct and incorrect trial of the behavioral task (reward presentation and motor execution) separately (Fig. 2b). The reward regressors were made by convolving a gamma function with a vector containing the onset times separately for each correct and incorrect trial. Motor execution regressors were created by convolving a gamma function with a square wave with a variable duration corresponding to the time from the motor preparation cue, through the actual movement and the feedback for that trial. In addition to regressors for the mean BOLD response to each of the above conditions of interest, separate regressors were included for each condition that varied proportionally with the reward value for the given trial ($5, $10, or $40). These additional regressors allowed us to determine which voxels are both task-active and scale with reward in a linearly proportional manner. Statistical contrasts assessing the interaction of reward value and accuracy were computed as a weighted sum of the estimated beta coefficients for these amplitude modulated regressors (Correct trials minus incorrect trials for each stage of the task). Each run was mean-centered and detrended for linear and polynomial trends using linear least squares. Maps of the parameter estimates (beta values) were computed from the GLM from each session and normalized on a subject-by-subject basis to a standard space (MNI) template. All reported clusters of activity were corrected for multiple comparisons via Monte Carlo simulations using AFNI's 3dClustSim function by ensuring that the size of a given cluster of activation was sufficiently large to rule out discovery by chance (corrected α < 0.05 for an uncorrected p-value of 0.002 and a 40 voxel cluster size).
Functional Connectivity Analysis
Functional connectivity maps were created on a subject-by-subject basis using a beta-series connectivity analysis previously described by Rissman and colleagues (2004). This analysis allows an investigation of which other brain areas display increased functional interaction with cortical motor areas during choking. A separate GLM was run for this analysis. Each reward presentation epoch and each movement execution epoch for each trial at each reward level ($5, $10, $40) received its own beta (β) in the model that corresponds to the amplitude of response for that epoch. The β's were concatenated together to form new time series of betas separately for each reward level. This approach allows us to determine correlations on a trial-by-trial basis between brain regions as opposed to blocks of trials. This analysis produces a whole brain map of Pearson's r values for the seeded ROI for each subject that is subsequently transformed using a Fisher's r-to-z transformation to allow for standard statistical testing for significance. Regions that display a high degree of correlation (p < 0.05 corrected) with the seeded ROI are assumed to be “functionally connected” with that node. To get a measure of functional connectivity between the seeded ROI and other ROIs to evaluate individual differences, the Z-values from the whole brain maps described above were averaged across all voxels within the distal ROI.
Regions of Interest (ROIs)
Motor cortex, supplementary motor area (SMA) and caudate ROIs were functionally defined using data from each participant's first fMRI session intersected with a maximum probability atlas in standard space (MNI) distributed with AFNI (Eickhoff et al. 2007). A separate GLM was run on this data using a blocked design contrasting task vs. rest. Each ROI was created by selecting all voxels that were task-active at an uncorrected p-value < 0.01 within the atlas-space ROI of interest.
Many lateral PFC regions were not active during training when no monetary incentives were given. In order to independently create “choking-related” DLPFC ROIs for each participant, data from the reward task from all other participants was used in a leave-one-subject-out (LOSO) cross-validation procedure. Task-related DLPFC ROIs were created by masking the atlas-space DLPFC ROIs with regions which displayed significantly greater functional connectivity with M1 for $40 trials when compared with $10 trials with one subject iteratively left out to create his or her ROIs. The subsequent analyses extracting data from this ROI is thus independent by essentially using data from all other subjects as an independent localizer.
Brain-behavior correlations
In order to determine whether functional interactions between cortical motor areas and PFC were predictive of choking under pressure and how changes in functional connectivity were related to changes in performance across incentive level, brain-behavior correlation plots were created by plotting the mean z-value (from the connectivity analyses) from each a-priori LOSO ROI for each participant as a function of his difference in mean accuracy for the different reward values ($5, $10, $40). Correlations between connectivity and behavior were explored with Spearman's coefficient rho (ρ).
In a more exploratory whole-brain analysis, each subject's score on the BIS-11 and their choking-related change in accuracy between $40 trials and $10 trials was input as a covariate in statistical analyses comparing whole-brain functional connectivity with M1 across the different reward values. The interaction between these behavioral covariates and the change in connectivity between $40 and $10 reveals regions in which increased connectivity is significantly related to decrements in performance due to choking under pressure and each individual's trait impulsivity.
Loss Aversion Task and Analysis
Prior work has shown that an individual's degree of loss aversion predicts his or her propensity to choke under pressure (Chib et al. 2012). In an attempt to replicate this result, a behavioral loss aversion task based on that used by Tom, et al. (2007) was used to calculate an individual's sensitivity to losses compared to gains. Participants were told that they were likely to earn approximately $100 over the course of the experiment and that they could use these earnings to gamble for extra winnings in this task. They were then asked to either accept or reject a series of coin flip gambles with a 50/50 chance of winning or losing variable amounts of money. Each trial was uniquely chosen from a gains/losses matrix with losses ranging from $5 to $15 in $1 increments and gains ranging from $10 to $30 in $2 increments. Participants were informed that one of the gambles would be randomly chosen at the end of the experiment and if they accepted the chosen gamble it would be played and they would gain or lose the resulting amount. The choice to accept or reject each gamble was indicated via key press. Trials were self-paced.
Logistic regression was performed with the size of the potential gain and loss as independent variables and acceptance/rejection as the dependent variable. This analysis was performed separately for each participant. Behavioral loss aversion (λ) was computed as λ = − βloss/βgain where both βloss and βgain are the unstandardized regression coefficients for the gain and loss variables separately.
RESULTS
Task-based behavioral results
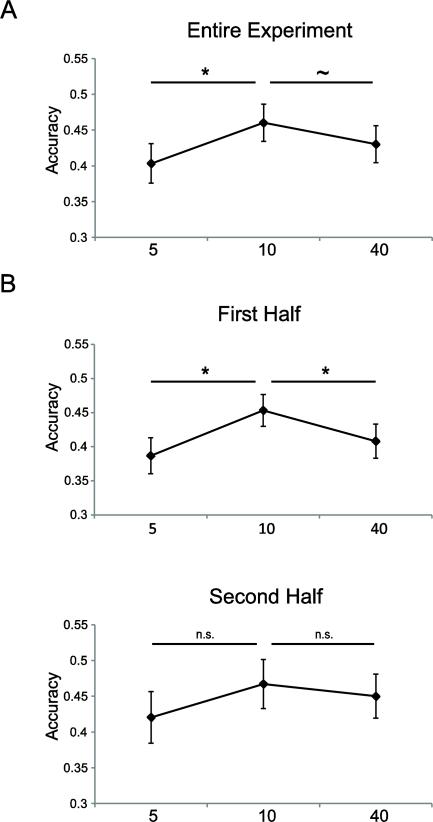
The experiment requires that each subject is capable of performing the task well below any ceiling effect and above any floor effect so that influences of changing reward can be clearly detected. Overall task accuracy across all subjects was 43% (sample standard deviation of 9%) suggesting that staircase adjustments of the task difficulty were successful. A significant main effect of reward on overall accuracy rates suggests that participants were sensitive to the reward values presented at the beginning of each trial (Fig. 3a; F = 5.328, p < 0.01). This effect was negative quadratic rather than linear in nature (quadratic trend – F = 10.915, p < 0.01; linear trend – F = 1.002, p > 0.3), which supports that there is an inflection point after which increasing reward does not contribute to increased accuracy.
Figure 3. Motor Task Accuracy.
A) Across the entire experiment, increasing incentives increased accuracy up to a point ($10 trials) beyond which accuracy began to suffer. B) Participants had significantly increased accuracy on $10 trials relative to both $5 and $40 trials during the first half of the experiment, but there were no significant differences in accuracy for the different levels of incentive during the second half of the experiment. See also Supplementary Table 1.
While a variety of measures quantifying motor performance (e.g. acceleration, proximity to target, etc.) showed improvement due to an increase in incentive from $5 to $10, these same measures did not significantly differ between performance on $10 and $40 trials (see Supplementary Table 1). Despite the evidence for a negative quadratic effect of reward on error rate, there was no other systematic kinematic feature of motor performance that was predictive of the type of error on high value $40 trials compared to $10 trials.
At the onset of the experiment, almost all subjects reported feeling distressed by trials with the highest levels of reward ($40). However, a majority of subjects indicated that they acclimated to the high reward values by the end of the experiment and “no longer felt stressed” in the words of one participant. The effects of performance pressure are often short-lived and can dissipate quickly. Accordingly, performance on $40 trials only suffered relative to $10 trials in the first half of the experiment (t =2.265, p < 0.05) and not in the second half of the experiment (t =0.600, p > 0.5; see Fig. 3b). Additionally, there was no significant difference in accuracy between $5 and $10 trials in the second half of the experiment (t = 1.654, p > 0.1; first half – t = 3.239, p < 0.01), suggesting that the motivational and/or pressure-inducing effects of monetary incentives were weak or absent during the later stage of the experiment. This feature of the experiment allowed us to similarly split the fMRI data and to use the second half of the experiment as a non-choking control in order to compare neural differences in a condition where high value incentive led to performance decrements to when those same dollar values produced no impairments.
Impulsivity, but not loss aversion predicts decrements in performance under high incentive
Behavioral loss aversion was independently measured in each participant using a task in which participants had to accept or reject a series of 50/50 gambles (Tom et al, 2007). The use of this task allowed a calculation of a measure, lambda (λ), which indicates each individual's propensity to weight losses more heavily than gains. Although previous tasks have shown that loss aversion can predict choking under pressure (Chib et al. 2012), we were unable to replicate this result in our paradigm (λ vs. diff in accuracy 40 vs. 10: Spearman's ρ = 0.14, p >0.5). We did find a non-significant positive correlation between loss aversion and overall accuracy suggesting that loss averse individuals tend to perform poorly on the rewarded task irrespective of reward (ρ = −0.38, p > 0.1).
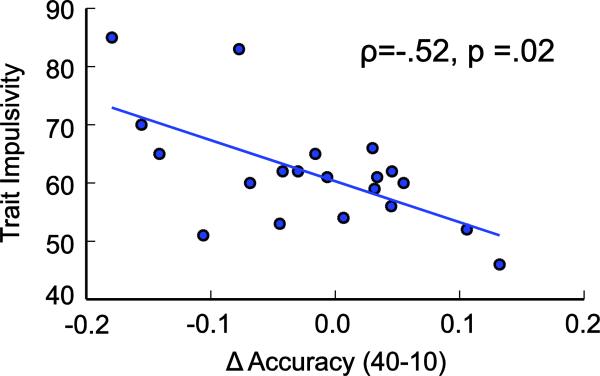
In contrast to loss aversion, we found a significant positive relationship between trait impulsivity as measured by the BIS-11 and choking under pressure whereby the most impulsive individuals were those who had the largest decrement in performance on $40 relative to $10 trials (Fig. 4; ρ = −0.52, p < 0.05). This measure was not related to accuracy on $5 trials (ρ = −0.05, p > 0.5).
Figure 4. Correlation with Impulsivity.
Trait impulsivity was significantly correlated with the individual's propensity to choke under pressure. The accuracy of the most impulsive individuals suffered the most going from a $10 trial to a $40 trial, while the least impulsive individuals displayed improved performance with the highest level of incentive.
Brain response to reward cue
Given the limited temporal resolution of fMRI, it is not possible to differentiate brain activity related to the execution of movement independent of activity related to the detection or correction of movement error. That is, movement related activity during trials where a choking error might have occurred was confounded by error perception, whereas successful non-choke trials were error free. However, we posited that the brain state leading to choking likely begins at the time of the reward cue, prior to movement onset. During this period, there are not any movement- or error-related confounding effects. Therefore, all fMRI results including functional connectivity analyses presented here refer to activity during reward presentation just prior to movement onset. Movement onset occurred several seconds (2-6 s jittered) following reward presentation and during this period subjects were preparing movements. This analysis amounts to a measurement of the state of the participant, who has knowledge of the potential value of the upcoming trial, which will influence motor areas and ultimately lead to successful or unsuccessful performance.
To identify the main effect of incentive, parametric regressors were included as a function of increasing reward values, modeled over the entire experimental data set. Consistent with previous literature, several brain regions showed significant increases in activity in response to increasing reward including motor and premotor cortices, striatum, and parietal cortex (Supplementary Table 2).
FP activity coincides with brain states preceding high value errors
To better characterize neural contributions to choking under pressure (i.e. high-value errors versus low value errors) we examined a contrast that compared activity that scaled with reward for correct trials against those for incorrect trials. Averaging across the entire experiment, the results revealed a large set of regions including a variety of FP areas and midbrain structures, the ventral striatum, ACC, precuneus, and visual cortex that discriminated between high and low value trials on error trials, but not on correct trials. But given that there were separate behavioral profiles for the first and second half of the experiment we sought to determine if the above contrast differed between the two halves of the experiment. In other words, are there regions that are overactive on high value error trials specifically when participants are choking under pressure? To specify this neural signature of a brain state leading to choking under pressure we used a whole-brain t-test to compare this contrast using data from the first half of the experiment, in which participants displayed choking behavior, to the second half, when participants were no longer choking. We continued to identify FP, striatal, and midbrain regions that showed higher levels of activity that coincided with choking (activity that scales with reward on error trials, but not on correct trials, in the first half of the experiment and not the second; see Supplementary Fig. 1, Supplementary Table 3). This suggests that these regions were sensitive to the increased pressure that subjects felt on high value trials and but that this pressure induced by incentive presentation subsided by the end of the experiment.
However, it is important to note that while this univariate analysis reveals regions that show increased activity that coincide with choking it is unclear from this analysis whether this activity actually contributes to impaired performance or is a compensatory recruitment of brain regions to sustain performance. It is equally plausible that in response to the reward cue, participants have an internal appraisal of their likelihood of success and, when failure is likely, the regions implicated in the above analysis are recruited to help improve or maintain successful task performance.
Increased functional connectivity between frontal cortex and motor regions under high incentive
Any mechanism responsible for choking must negatively impact functioning in the motor system in order to produce diminished outcomes. The univariate analysis detailed above implicated regions known to be involved in both top-down control (e.g. dorsolateral prefrontal cortex) and motivation/reward (e.g. striatum and insula). However, if the activity in any of these regions is either deleterious to or essential for performance of the motor task, it is likely that these regions interact with cortical motor areas on choke trials to alter their function. In order to characterize how the network-level activity of motor regions changes under pressure, we performed a functional connectivity analysis on the time series of fMRI data using motor cortex and supplementary motor area as seeds for a beta-series correlation analysis (Rissman et al., 2004) to reveal other brain regions whose activity fluctuated with that of motor cortex during performance with financial incentives. This yielded three separate voxel-wise maps of correlation values, one for each reward value, which reveal distal regions whose activity co-varies with that of the motor ROIs.
Across all three reward conditions and over all trials motor cortex showed significant functional connectivity during the pre-movement period when the reward cue was presented with numerous other brain regions spanning wide swaths of frontal, sensorimotor, visual, and parietal cortex along with subcortical structures in the basal ganglia, the brain stem, and the cerebellum. This network level activity is likely to be due to action preparation and is unsurprising given the visuo-spatial nature of the upcoming motor task.
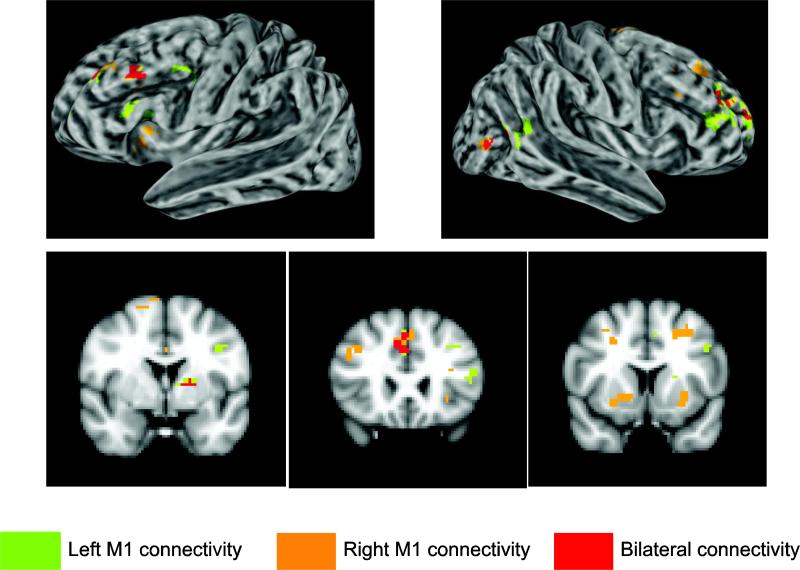
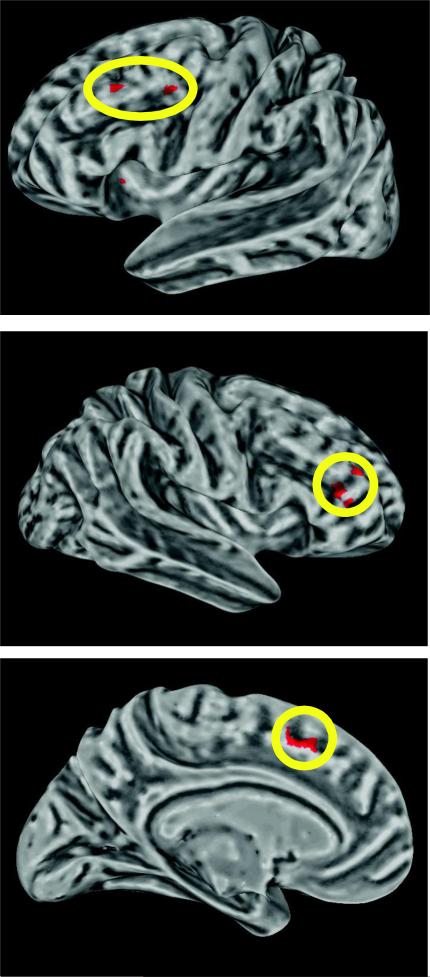
Strikingly, the only regions that displayed higher levels of functional connectivity with bilateral motor cortex for $40 trials when compared to $10 trials (over the course of the 1st half of the experiment when choking occurred) were bilateral dorsolateral prefrontal cortex (DLPFC), ACC, medial orbitofrontal cortex, left putamen, and a posterior portion of the right middle temporal gyrus (Fig. 5, Supplementary Table 4). Critically, because the parameter estimates used in this connectivity analysis were taken from the pre-movement reward presentation period before any movement errors, they are uncontaminated by differences in error rate between $40 and $10 trials. Additionally, a similar connectivity analysis comparing the beta series between $5 and $10 trials does not implicate any of the above regions, suggesting that these changes in functional connectivity are not due to a simple increase in reward value. In the 2nd half of the experiment, when choking was absent, there were no significant differences in functional connectivity at the whole-brain level between $40 and $10 trials. An examination of the overlap between the results from the univariate analyses and the functional connectivity analysis revealed that the only region commonly found to correspond with choking is bilateral middle frontal gyrus (MFG) in DLPFC and ACC (Fig. 6). These results illustrate that even before the onset of movement, there is an over-involvement of frontal regions that are likely influencing motor preparatory activity.
Figure 5. Brain regions who display increased functional connectivity with motor cortex during choking.
Choking under pressure led to increased functional connectivity between motor cortex and regions important for cognitive control (DLPFC), action monitoring (ACC), and reward (striatum). See also Supplementary Table 4.
Figure 6. Brain regions that are both overactive and display increased functional connectivity with motor cortex during choking.
Lateral PFC and ACC activity is increased and is more correlated with motor cortex activity when performance is impaired.
Increased PFC-M1 functional connectivity protects against choking under pressure
The above analyses make clear that functional connectivity between PFC and M1 is increased when choking is likely to occur. However, this finding does not show whether this increased frontal influence is responsible for fragile performance or is recruited in order to compensate for it. In order to examine how this change in functional connectivity contributes to performance, we examined individual differences in functional connectivity and performance across the different reward values. If communication between PFC and M1 is harmful for performance as predicted by explicit monitoring theories, participants with the greatest level of functional connectivity between these two regions should be those that show the largest drop in accuracy between $10 trials and $40 trials. However if an increase in executive control is needed to maintain successful performance under increased pressure, those participants who are able to increase functional connectivity between PFC and M1 should be those that choke the least under pressure.
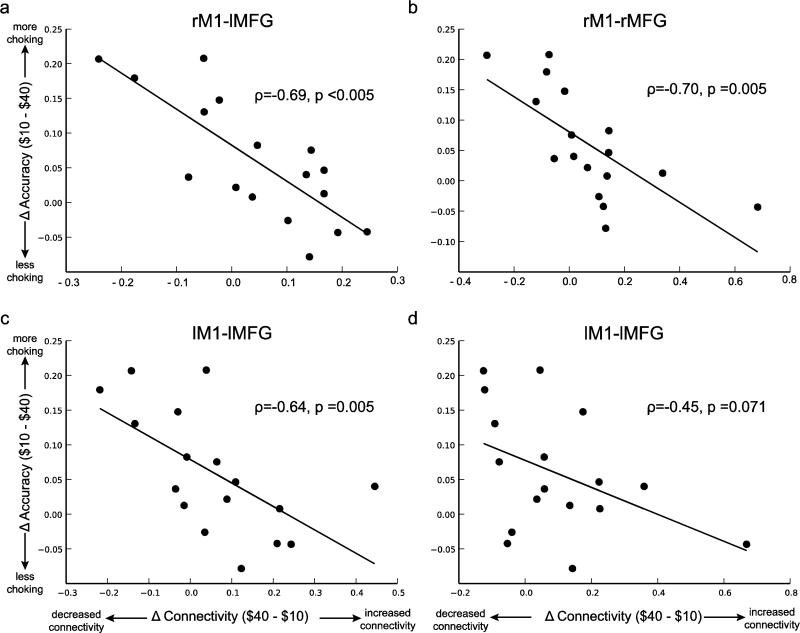
Functional connectivity between DLPFC and M1 was not predictive of accuracy on $40 trials during either half of the experiment. In other words, there was no significant relationship between DLPFC-M1 functional connectivity across $40 trials and accuracy for $40 trials. However, in the first half of the experiment those participants who show the greatest increase in connectivity from $10 to $40 trials are those who choke the least as measured by the difference in accuracy between $10 and $40 trials (Fig. 7; rMFG-rM1 connectivity vs. choking – ρ = −0.70, p < 0.005; lMFG-rM1 – ρ = −0.69, p < 0.005; rMFG-lM1 – ρ = −0.45, p < 0.08; lMFG-LM1 – ρ = −0.64, p < 0.005). These results suggest that increasing functional connectivity between DLPFC and M1 in response to performance pressure protects against choking and only occurs in participants who are able to maintain a relatively high level of performance on $40 trials. This relationship between increased functional connectivity and the difference in accuracy on $40 and $10 trials was not present in the 2nd half of the experiment when participants were no longer choking (range of ρ-values: −0.05 to 0.04; p-values: 0.844-.933).
Figure 7. Correlation between choking under pressure and reward-related increases in DLPFC-M1 functional connectivity.
Consistent with distraction theories of choking under pressure, the ability to increase functional connectivity between DLPFC and M1 for high rewards is predictive of the extent to which participants display stable performance under pressure. See also Supplementary Table 5.
Given that there is no direct anatomical connection between DLPFC and M1, it is likely that the functional connectivity observed in the analysis above is mediated by other premotor regions that bridge DLPFC and M1, such as the supplementary motor area (SMA). To test this, we performed an additional analysis that examined DLPFC-SMA functional connectivity across different levels of reward. The correlation with performance revealed the same pattern of results as was observed between DLPFC and M1. The change in connectivity between $10 and $40 trials is negatively correlated with one's propensity to choke (rMFG-rSMA connectivity vs. choking – ρ = −0.53, p < 0.05; lMFG-rSMA – ρ = −0.57, p < 0.05; rMFG-lSMA – ρ = −0.52, p < 0.05; lMFG-LSMA – ρ = −0.70, p < 0.005). That is, the larger the increases in functional connectivity between DLPFC and SMA on $40 trials relative to $10 trials, the more likely participants were to show maintained task performance under pressure. Conversely, those participants who were unable to increase DLPFC-SMA connectivity on $40 trials were those whose performance suffered the most due to choking under pressure. This suggests there is a general change in prefrontal modulation of activity in concert with premotor regions and motor cortex.
In a follow-up whole-brain analysis, we further examined if any other brain regions show changes in functional connectivity with motor cortex that are related to choking under pressure. This analysis was effectively testing, “Which other regions show a similar correlation between the change in functional connectivity from $10 to $40 trials and the difference in accuracy between these conditions?” In addition to bilateral MFG, several other regions displayed increases in functional connectivity with M1 that was predictive of maintained performance under pressure. These regions include bilateral caudate, bilateral inferior frontal gyrus, right insula, ventromedial prefrontal cortex, and dorsomedial prefrontal cortex and all display the same pattern of results as those seen in Figure 7 (see Supplementary Table 5). . Together with bilateral DLPFC, the degree to which this suite of regions displays increased functional connectivity with bilateral motor cortex on $40 trials in each participant is significantly related to his or her propensity to choke under pressure during the first half of the experiment. No regions significantly displayed this pattern during the second half of the experiment when participants were no longer choking under pressure. Additionally, a similar analysis comparing $5 to $10 trials failed to uncover any regions that displayed significantly increased functional connectivity with M1 for $10 trials when compared to $5 trials or any relationship between functional connectivity and the difference in performance between these two reward levels. Taken together these results suggest that extra top-down resources were recruited specifically in response to performance pressure for $40 trials when choking was likely and that successful recruitment allowed relatively stable task performance.
Trait impulsivity is predictive of reduced PFC-M1 connectivity
To further explore the contribution of trait impulsivity to choking under pressure, a whole-brain exploratory analysis was carried out to examine the relationship between trait impulsivity and PFC-M1 functional connectivity on $40 trials when compared to $10 trials. The only region which displayed a relationship between participants’ trait impulsivity score and the reward-related change in functional connectivity with motor cortex was lateral PFC. Specifically, lower trait impulsivity predicts relatively greater increased connectivity between right M1 and left inferior frontal gyrus (triangularis/orbitalis; × = −42, Y = 30, Z = −15; 51 voxel extent) on $40 trials vs. $10 trials. We must note that though the lateral prefrontal region implicated by this analysis is adjacent to the DLPFC ROIs described in the above section, the region showing the most robust relationship with trait impulsivity is slightly more ventral. However, relaxing the statistical threshold somewhat to p < 0.01 uncorrected extends this activation more dorsally into DLPFC. When combined with the fact that the most impulsive individuals suffer the most from choking under pressure across the entire experiment, this result provides additional evidence that increased functional connectivity between lateral PFC and M1 reflects a compensatory response in the potential choke state. Impulsive individuals are less likely to exhibit this compensatory response and also those who are more likely to choke.
DISCUSSION
Our results provide the first evidence of DLPFC involvement in choking under pressure due to financial incentives in the domain of skilled motor performance. By directly comparing the pre-movement state of participants when they are choking under pressure with trials where the increased stakes no longer hurt performance, a choke-specific link between frontal and motor systems is identified. Activity in PFC and ACC was highly correlated with motor cortex activity specifically when the highest level of reward was at stake. While both explicit monitoring theories and distraction theories would predict PFC involvement in choking under pressure, only distraction theories are consistent with the finding that the inability to increase the extent to which attentional and/or goal-directed activity in prefrontal regions co-varies with motor activity is predictive of impaired performance due to choking.
It is important to again note that the level of functional connectivity between DLPFC and cortical motor areas was not predictive of accuracy on trials of any single reward value nor the increased performance seen on $10 trials relative to $5 trials as would be expected if this functional connectivity simply reflects simple focus on the task. Instead, it is likely that these increases are in response to an internal appraisal of performance pressure signaling a need for increased control, perhaps in the form of increased attention to action. In line with this, several previous studies have shown that attention to action increases both BOLD activity in DLPFC and ACC and the functional connectivity between these frontal regions and cortical motor areas (Jueptner et al., 1997; Rowe et al., 2002; Lau et al., 2004). In our results, these increases do not themselves coincide with diminished performance as might be predicted from explicit monitoring theories of choking. Instead, the fact that failures in the implementation of prefrontal control coincide with choking are in line with distraction theories of choking and specifically the attentional control theory (Eyesenck, et al., 2007), which states that performance anxiety leads to diminished attentional/executive control such that additional compensatory resources are necessary to protect against performance impairments.
Increased prefrontal involvement in those participants with relatively stable task performance on $40 trials relative to $10 trials could signal resistance from distraction. Several previous studies have shown that lateral prefrontal activity is sometimes increased during dual-task performance (Szameitat, et al., 2002; Johnson and Zatorre, 2006; Remy, et al., 2010), although these studies all use extrinsic stimuli during performance to increase cognitive load whereas the stimuli in our task remain constant throughout. Similar to the participants who choke in our study, other work has shown that functional connectivity between DLPFC and cortical areas important for task performance has been shown to decrease during performance failure due to distraction (e.g., Yoon et al., 2006; Clapp et al., 2010).
All fMRI results reported here are from the reward presentation period just prior to the onset of movement which corresponds with the brain state that ultimately leads to a future failure in performance. Although essential for our hypotheses, this design did not allow us to make strong inferences from the movement period itself. During movement, it was not possible to distinguish between error-related BOLD activity due to visual feedback during movement from activity that contributed to making the error itself. Given this, we propose that the pre-movement frontal activity might reflect response selection on an abstract level rather than the monitoring of on-going action. Numerous studies implicate this mid-DLPFC region in the rule-based selection of responses (Rowe, et al., 2000; Bunge, 2004; Badre and Wagner, 2004). In many of these studies, DLPFC activity is responsive to switching response strategies and/or overriding prepotent responses (Bunge, 2004). The increased functional connectivity between motor cortex and ACC likewise points to a transition to a state of increased cognitive control. While prior work has shown that ACC activity is sensitive to conflict monitoring and errors (Botnivinick, et al., 2004), more recent experimental and theoretical work has suggested that the function of the ACC and adjacent medial frontal regions is linked to reward-related action selection and the encoding of the expected value of an upcoming action (Rushworth et al., 2004; Shenhav et al., 2013). In this latter view, dorsal ACC and medial PFC activity would be involved in selecting an action set to be implemented by lateral PFC (Hikosaka and Isoda, 2010).
Similar to the two previous fMRI studies of choking under pressure (Mobbs, et al., 2009; Chib, et al., 2012), we also found evidence that striatal and midbrain regions demonstrate increased activity just before a choke is likely to occur. These prior results have been interpreted as over-arousal or loss aversion signals, however the functional connectivity results presented here suggests this view may be incomplete. Whatever the mechanism underlying choking under pressure, it is clear that there must be an influence on the motor system to produce the harmful effects seen on sensorimotor performance. We propose that activity in striatal regions in response to the reward cue could reflect “over-motivation”, loss aversion, and/or over-arousal signals marking that the upcoming action is especially valuable or salient. This activity then leads to a cascade of medial and lateral frontal activity leading to a change in motor strategy/policy for the upcoming trial. We speculate that failure to implement a new control policy might cause suboptimal on-line control of movement, leading to the diminished performance observed in studies of choking under pressure.
We did not find evidence that loss averse individuals were the most likely to choke at the highest levels of incentive as has been previously reported in the literature (Chib et al, 2012) perhaps because we used a different range of reward values. Instead we found that the most impulsive individuals were those whose performance suffered the most between medium and high levels of reward. Consistent with the fact that impulsivity is usually implicated in deficits in cognitive control, reward processing, and prefrontal function (Cools, et al., 2007; Cools, 2008; Franken, et al., 2008; Sripada, et al., 2011), the most impulsive individuals in our study were those who were the least able to increase functional connectivity between left ventro-lateral PFC and M1 on $40 trials. Additionally, participants in our study who showed the largest choking effect were also those who were unable to enhance functional connectivity between PFC and M1 on $40 trials. Both VLPFC and DLPFC have previously been hypothesized to perform separable cognitive control functions, including stable maintenance of goal-oriented task sets for the former and flexible adaptive control for the latter (Dosenbach, et al. 2007), Although impulsivity was related to deficits specifically in VLPFC-motor functional connectivity, drawing sharp distinctions between lateral prefrontal subregions in choking under pressure is beyond the scope of this study given that more liberal statistical thresholds implicated the entire lateral surface of PFC. It is possible that impulsive individuals could have been more sensitive to high rewards and engaged extra top-down resources to the task, or simply that their use of these resources was less effective than those with lower trait impulsivity. In either case, it seems clear that these participants were unable to recruit compensatory top-down resources to maintain successful performance under pressure. Additionally, our fMRI results implicated striatal and PFC regions that are the source and/or the target of dopaminergic output. Given the relationship between trait impulsivity, dopamine, striatal, and prefrontal function (Cools, 2008), it is tempting to speculate that differences in the propensity to choke under pressure might be due to naturally occurring differences in endogenous dopamine function. In fact, a recent study has shown that individuals with high baseline capacity for dopamine synthesis show impaired performance when motivated by relatively high rewards during a Stroop-like cognitive control task (Aarts et al., 2014). However, we did not measure or manipulate dopaminergic function and our results cannot directly speak this possibility.
Due to the relatively low number of neuroimaging studies examining choking under pressure from an explicit monitoring perspective, selecting a priori frontal ROIs for further analyses was not feasible. However, using a LOSO procedure we were able to independently create DLPFC regions of interest in order to directly examine communication between PFC and motor cortex. Although caution is warranted in over-interpreting the prefrontal cognitive processes contributing to choking under pressure via reverse inference (Poldrack, 2006), our results provide an important step in suggesting that failure of top-down control contributes to choking prior to movement onset. Future fMRI studies using machine learning analyses could reveal neural activity which predicts high pressure errors on a trial-by-trial basis and might elucidate the types of processing responsible for reduced performance.
Although the results presented here support action control theory and distraction theories of choking under pressure in the domain of motor performance and financial incentives, we cannot completely rule out an explicit monitoring account of choking. It remains possible that both are occurring at the same time. The magnitude of increased functional connectivity between DLPFC and M1 in participants whose performance suffered on $40 trials relative to $10 trials was smaller than those participants whose performance remained relatively unchanged. However the “chokers” did still display numerically higher levels of DLPFC-M1 functional connectivity on $40 trials. It is unclear whether these participants implemented a new, but ultimately ineffective, control policy or if they were unable to exert top-down control at all.
Conclusion
The results presented here provide neural evidence that prefrontal activity contributes to choking under pressure due to monetary incentives in the motor domain and supports prior behavioral work suggesting that impaired top-down control can interfere with skilled motor performance. Functional connectivity between prefrontal cortex and motor cortex is greatest just before choking is likely to occur and a relative lack of connectivity is strongly related to an individual's propensity to choke under pressure. We hypothesize that this is a maladaptive response that is highly sensitive to level of training, personality, the nature of the incentives and other factors. While prior efforts to reduce choking have relied primarily on training in the primary task or habituation to the rewards, our results suggest that cognitive training aimed at improving mindfulness or the executive control of attention and performance monitoring are important avenues to mitigate the choke.
Supplementary Material
Highlights.
- Neural mechanism of choking under pressure explored with fMRI
- Increased functional connectivity between PFC and M1 protects against choking
- Trait impulsivity is predictive of poorer performance under performance pressure
- Results are consistent with a distraction account of choking
ACKNOWLEDGEMENTS
This work was supported by PHS grant NS44393 and the Institute for Collaborative Biotechnologies through contract no. W911NF-09-0001 from the U.S. Army Research Office.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aarts E, Wallace DL, Dang LC, Jagust WJ, Cools R, D'Esposito M. Dopamine and the cognitive downside of a promised bonus. sychological science. 2014 doi: 10.1177/0956797613517240. 0956797613517240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariely D, Gneezy U, Loewenstein G, Mazar N. Large stakes and big mistakes. Review of Economic Studies. 2009;76:451–469. [Google Scholar]
- Badre D, Wagner AD. Selection, integration, and conflict monitoring: assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron. 2004;41:473–487. doi: 10.1016/s0896-6273(03)00851-1. [DOI] [PubMed] [Google Scholar]
- Baumeister RF. Choking under pressure: Self-consciousness and paradoxical effects of incentives on skillful performance. Journal of Personality and Social Psychology. 1984;46:610–620. doi: 10.1037//0022-3514.46.3.610. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Showers CJ. A review of paradoxical performance effects: Choking under pressure in sports and mental tests. European Journal of Social Psychology. 1986;16:361–383. [Google Scholar]
- Beilock SL, Carr TH. On the fragility of skilled performance: what governs choking under pressure? Psychological Bulletin. 2001;103:345–366. [PubMed] [Google Scholar]
- Beilock SL, Kulp CA, Holt LE, Carr TH. More on the fragility of performance: choking under pressure in mathematical problem solving. Journal of Experimental Psychology: General. 2004;133:584–600. doi: 10.1037/0096-3445.133.4.584. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH. When high-powered people fail: Working memory and “choking under pressure” in math. Psychological Science. 2005;16:101–105. doi: 10.1111/j.0956-7976.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- Bunge SA. How we use rules to select actions a review of evidence from cognitive neuroscience. Cognitive, Affective, & Behavioral Neuroscience. 2004;4(4):564–579. doi: 10.3758/cabn.4.4.564. [DOI] [PubMed] [Google Scholar]
- Buračas GT, Boynton GM. Efficient design of event-related fMRI experiments using M-sequences. Neuroimage. 2002;16(3):801–813. doi: 10.1006/nimg.2002.1116. [DOI] [PubMed] [Google Scholar]
- Chib VS, De Martino B, Shimojo S, O'Doherty JP. Neural Mechanisms underlying paradoxical performance for monetary incentives are driven by loss aversion. Neuron. 2012;74:582–594. doi: 10.1016/j.neuron.2012.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cerebral Cortex. 2010;20(4):859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D'Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. The Journal of Neuroscience. 2007;27(20):5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. The Neuroscientist. 2008;14(4):381–395. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- DeCaro MS, Thomas RD, Albert NB, Beilock SL. Choking under pressure: Multiple routes to skill failure. Journal of Experimental Psychology: General. 2011;140(3):390. doi: 10.1037/a0023466. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterbrook JA. The effects of emotion on cue utilization and the organization of behavior. Psychological Review. 1959;66:183–202. doi: 10.1037/h0047707. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Elliot R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. The Journal of Neuroscience. 2000;20(16):6159–6166. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Franken IH, van Strien JW, Nijs I, Muris P. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Research. 2008;158(2):155–163. doi: 10.1016/j.psychres.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Fuster JM, Alexander GE. Neuron activity related to short-term memory. Science. 1971;173:652–654. doi: 10.1126/science.173.3997.652. [DOI] [PubMed] [Google Scholar]
- Gimmig D, Huguet P, Caverni JP, Cury F. Choking under pressure and working memory capacity: When performance pressure reduces fluid intelligence. Psychonomic Bulletin and Review. 2006;13:1005–1010. doi: 10.3758/bf03213916. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Isoda M. Switching from automatic to controlled behavior: cortico-basal ganglia mechanisms. Trends in cognitive sciences. 2010;14(4):154–161. doi: 10.1016/j.tics.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JA, Zatorre RJ. Neural substrates for dividing and focusing attention between simultaneous auditory and visual events. Neuroimage. 2006;31(4):1673–1681. doi: 10.1016/j.neuroimage.2006.02.026. [DOI] [PubMed] [Google Scholar]
- Kimble GA, Perlmuter LC. The problem of volition. Psychological Review. 1970;77:361–384. doi: 10.1037/h0029782. [DOI] [PubMed] [Google Scholar]
- Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychologica. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Lau HC, Rogers RD, Haggard P, Passingham RE. Attention to intention. Science. 2004;303(5661):1208–1210. doi: 10.1126/science.1090973. [DOI] [PubMed] [Google Scholar]
- Lewis B, Linder D. Thinking about choking? Attentional processes and paradoxical performance. Personality & Social Psychology Bulletin. 1997;23:937–944. doi: 10.1177/0146167297239003. [DOI] [PubMed] [Google Scholar]
- Markman AB, Maddox WT, Worthy DA. Choking and excelling under pressure. Psychological Science. 2006;17:944–948. doi: 10.1111/j.1467-9280.2006.01809.x. [DOI] [PubMed] [Google Scholar]
- Masters RSW. Knowledge, knerves and know-how: The role of explicit versus implicit knowledge in the breakdown of a complex motor skill under pressure. British Journal of Psychology. 1992;83:343–358. [Google Scholar]
- Miller BT, D'Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48:535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the mcaque. Journal of Neuroscience. 1996;16:5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual review of neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Hassabis D, Seymour B, Marchant JL, Weiskopf N, Dolan RJ, Frith CD. Choking on the money: reward-based performance decrements are associated with midbrain activity. Psychological Science. 2009;20:955–962. doi: 10.1111/j.1467-9280.2009.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS. Factor structure of the Barratt impulsiveness scale. Journal of clinical psychology. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data?. Trends in cognitive sciences. 2006;10(2):59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Rémy F, Wenderoth N, Lipkens K, Swinnen SP. Dual-task interference during initial learning of a new motor task results from competition for the same brain areas. Neuropsychologia. 2010;48(9):2517–2527. doi: 10.1016/j.neuropsychologia.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and cognition. 2004;56(2):129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory. Science. 2000;288(5471):1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Rowe J, Friston K, Frackowiak R, Passingham R. Attention to action: specific modulation of corticocortical interactions in humans. Neuroimage. 2002;17(2):988–998. [PubMed] [Google Scholar]
- Rushworth MFS, Watson ME, Kennerly SW. Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences. 2004;8(9):410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Sakai K, Rowe JB, Passingham RE. Active maintenance in prefrontal area 46 creates distractor-resistant memory. Nature Neuroscience. 2002;5(5):479–484. doi: 10.1038/nn846. [DOI] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrated theory of anterior cingulate function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada CS, Gonzalez R, Luan Phan K, Liberzon I. The neural correlates of intertemporal decision-making: Contributions of subjective value, stimulus type, and trait impulsivity. Human brain mapping. 2011;32(10):1637–1648. doi: 10.1002/hbm.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szameitat AJ, Schubert T, Müller K, Von Cramon DY. Localization of executive functions in dual-task performance with fMRI. Journal of Cognitive Neuroscience. 2002;14(8):1184–1199. doi: 10.1162/089892902760807195. [DOI] [PubMed] [Google Scholar]
- Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relationship of strength of stimulus to rapidity of habit-formation. Journal of comparative neurology of psychology. 1908;18:459–482. [Google Scholar]
- Yoon JH, Curtis CE, D'Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. NeuroImage. 2006;29(4):1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.