Abstract
Background
All hospitalized patients should be assessed for VTE risk factors and prescribed appropriate prophylaxis. To improve best-practice VTE prophylaxis prescription for all hospitalized patients, we implemented a mandatory computerized clinical decision support (CCDS) tool. The tool requires completion of checklists to evaluate VTE risk factors and contraindications to pharmacologic prophylaxis, and then recommends the risk-appropriate VTE prophylaxis regimen.
Objectives
To examine the effect of a quality improvement intervention on race- and gender-based healthcare disparities across two distinct clinical services.
Research Design
Retrospective cohort study of a quality improvement intervention
Subjects
1942 hospitalized medical patients and 1599 hospitalized adult trauma patients
Measures
Proportion of patients prescribed risk-appropriate, best-practice VTE prophylaxis
Results
Racial disparities existed in prescription of best-practice VTE prophylaxis in the pre-implementation period between black and white patients on both the trauma (70.1% vs. 56.6%, p=0.025) and medicine (69.5% vs. 61.7%, p=0.015) services. After implementation of the CCDS tool, compliance improved for all patients and disparities in best-practice prophylaxis prescription between black and white patients were eliminated on both services: trauma (84.5% vs. 85.5%, p=0.99) and medicine (91.8% vs. 88.0%, p=0.082). Similar findings were noted for gender disparities in the trauma cohort.
Conclusions
Despite the fact that risk-appropriate prophylaxis should be prescribed equally to all hospitalized patients regardless of race and gender, practice varied widely prior to our quality improvement intervention. Our CCDS tool eliminated racial disparities in VTE prophylaxis prescription across two distinct clinical services. Health information technology approaches to care standardization are effective to eliminate healthcare disparities.
Keywords: clinical decision support, venous thromboembolism, disparities
BACKGROUND
Race is shown to be a predictor of healthcare quality and outcomes in the United States.1, 2 Several different mechanisms including institutional, systemic,3, 4 and provider factors have been postulated to explain differences in quality. Many urban hospitals located in economically underprivileged areas serve a greater proportion of minority patients and have generally been associated with a lower quality of care.3, 5, 6 A meta-analysis recently reported that black patients are more likely to experience worse outcomes after trauma.7 Unconscious, or implicit, biases exist among clinicians8 that may influence clinical decision making,9 and may be a root cause of existing disparities in the provision of high quality healthcare.10, 11
Despite widespread recognition of the existence of healthcare disparities, effective solutions to eliminate these disparities have been an elusive goal for many years,12,13 and few, if any, interventions have proven to be beneficial. Health information technology has been proposed as a possible theoretical solution.14–16
Venous thromboembolism (VTE), comprised of deep vein thrombosis and/or pulmonary embolism, is one of the most common causes of mortality among hospitalized patients.17–20 While some VTE events are unavoidable,21, 22 many can be prevented with universal risk assessment and prescription of risk-appropriate prophylaxis.23–28 However, many patients do not receive risk-appropriate prophylaxis.29–31 The Agency for Healthcare Research and Quality has stated that implementing strategies to improve VTE prophylaxis is one of the top patient safety practices that should be implemented32, 33 and the “number one patient safety practice” to prevent in-hospital death.18 Numerous tactics have been developed, with varying degrees of success.34–36
All hospitalized patients are at increased risk of developing VTE.37 Irrespective of clinical condition, race, or gender, all patients should be assessed for VTE risk factors and prescribed risk-appropriate prophylaxis when they are admitted to the hospital. Computerized clinical decision support (CCDS) tools are an objective and reliable method to enhance clinical decision making. Using a mandatory CCDS tool, we have previously demonstrated significant improvements in prescription of risk-appropriate VTE prophylaxis and reduction in potentially preventable VTE.38–41
The purpose of this study is to examine the effect of a quality improvement (QI) intervention on healthcare disparities. We will examine subgroups of patients across two distinct clinical services to determine if our mandatory CCDS tool affects subgroups of patients who are prescribed risk-appropriate VTE prophylaxis.
METHODS
Setting
In December 2007, the Johns Hopkins Hospital implemented a mandatory service-specific CCDS VTE risk assessment tool into the provider order entry (POE) system for adult patients hospital-wide.38 The Johns Hopkins Hospital is an academic medical and state-designated Level 1 trauma center in Baltimore, Maryland. Approval was obtained from the Johns Hopkins Institutional Review Board.
VTE order set
A mandatory, service-specific CCDS tool was developed to improve prescription of best-practice VTE prophylaxis for all hospitalized patients.38, 39 When an admitting provider is writing the admission orders for any patient at our hospital, they must complete short checklists of VTE and bleeding risk factors. The tool then follows an evidence-based, service-specific algorithm to determine the patient’s VTE risk as moderate, high, or very high, with or without contraindications for pharmacological prophylaxis. Based on the individual patient’s risk stratum, the CCDS tool displays the recommended risk-appropriate VTE prophylaxis regimen to the admitting provider. Providers are not required to prescribe the CCDS suggested regimen but may opt-in to the recommendation.
Study Population
Two arms were included in this study, hospitalized adult trauma patients and internal medicine patients, to represent distinct clinical services with minimal overlap in clinical providers. Each arm uses data from previously published cohort studies within the same hospital, 39, 41 employing novel analyses to explore the impact of the intervention on the basis of race and gender.
The first arm included all patients admitted to the adult trauma service. Patients admitted in 2007 served as our pre-implementation group and were compared with the post-implementation group (patients admitted January 1, 2008 through December 31, 2010).39 The second arm included all adult patients admitted to the internal medicine service during the month of November 2007 (the month immediately prior to implementation of the CCDS tool) and during the month of April 2010 (the last month prior to data collection for the study).41
Data collection
For each pre-implementation group, a single data abstracter reviewed patient charts to collect the following VTE-related variables: patient demographic information, VTE risk factors, contraindications to pharmacological prophylaxis, and written orders for prophylaxis (pharmacological and/or mechanical) within 24 hours of admission. For the post-implementation groups, these variables were extracted directly from the POE system and the trauma center registry (Collector Trauma Registry, Digital Innovation, Inc). Compliance with best-practice VTE prophylaxis in both the pre- and post-implementation groups was defined as adherence to our service-specific VTE prevention algorithm.38, 39, 41 Race and gender were determined based on documentation of patient self-identification collected by administrative personnel during hospitalization.
Statistical analysis
We compared prescription of best-practice prophylaxis between races and genders both in the pre-implementation and post-implementation periods using the two-sided chi square test. Mean age was compared using unpaired t-test, categorical variables were compared using two-sided Fisher’s exact test, and median injury severity score, and median LOS were compared using Wilcoxon rank-sum test. A p-value of <0.05 was considered statistically significant. Statistical analyses were performed using STATA version 12.0 (Statacorp, College Station, TX).
RESULTS
1599 hospitalized adult trauma patients and 1942 hospitalized adult internal medicine patients met inclusion criteria. Within the trauma population, there were few Hispanic (n=68), Asian (n=3), Native American (n=1) and unreported ethnicity (n=33) patients. In the medicine cohort, similarly small numbers were noted [Hispanic (n=24), Asian (n=22), and unreported (n=39)]. Due to extremely low numbers we decided to analyze the two most common racial groups, black and white patients, only. The patient populations had a similar age, race, and gender distribution before and after implementation of the CCDS tool (Table 1).
Table 1.
Demographics of included hospitalized adult trauma and internal medicine patients
| Pre- implementation | Post- implementation | p-value | |
|---|---|---|---|
| Trauma | N=374 | N=1120 | |
| Mean age (SD), years | 36.2 (18.1) | 35.9 (17.4) | 0.775 |
| Black, n (%) | 291 (77.8) | 831 (74.1) | 0.168 |
| Male, n (%) | 305 (81.6) | 863 (77.1) | 0.071 |
| Median ISS (IQR) | 9 (4–16) | 9 (4–16) | 0.179 |
| Blunt Trauma, n (%) | 192 (51.3) | 620 (55.4) | 0.187 |
| GCS<15, n (%) | 57 (15.2) | 132 (11.8) | 0.088 |
| Median LOS (IQR), days | 4 (2–8) | 4 (2–7) | 0.009 |
| Any VTE Risk Factor, n (%) | 291 (77.8) | 890 (79.5) | 0.509 |
| Internal Medicine | N=959 | N=898 | |
| Mean age (SD), years | 56.1 (17.1) | 55.2 (16.3) | 0.247 |
| Black, n (%) | 567 (59.1) | 547 (60.9) | 0.448 |
| Male, n (%) | 481 (50.1) | 465 (51.8) | 0.486 |
| Median LOS (IQR), days | 3 (2–6) | 3 (2–6) | 0.249 |
| Any VTE Risk Factor, n (%) | 615 (64.1) | 492 (54.8) | <0.001 |
IQR: interquartile range; ISS: injury severity score; LOS: length of stay; SD: standard deviation; VTE: venous thromboembolism
Trauma Patients
Black trauma patients were younger (32.5 years vs. 46.5 years, p<0.001), disproportionately male (83.9% vs. 61.0%, p<0.001), more likely to present with penetrating trauma (57.5% vs. 9.9%, p<0.001), and less likely to present with one or more risk factors for VTE (77.8% vs. 82.8%, p=0.047) compared with white trauma patients (Table 2). Black trauma patients had a longer length of stay and were more often male in the pre-implementation group than the post-implementation group. There were no differences among white trauma patients before and after implementation (Table 3).
Table 2.
Clinical characteristics of included hospitalized adult trauma and internal medicine patients before and after implementation of the computerized clinical decision support tool, by race
| Pre-implementation | Post-implementation | |||||
|---|---|---|---|---|---|---|
| Black | White | p-value | Black | White | p-value | |
| Trauma | n=291 | n=83 | n=831 | n=289 | ||
| Mean age (SD), years | 32.8 (15.3) | 47.9 (21.8) | <0.001 | 32.4 (14.6) | 46.1 (20.6) | <0.001 |
| Male, n (%) | 256 (88.0) | 49 (59.0) | <0.001 | 685 (82.4) | 178 (61.6) | <0.001 |
| Median ISS (IQR) | 9 (4–16) | 9 (4–17) | 0.58 | 9 (4–16) | 9 (4–16) | 0.730 |
| Blunt Trauma, n (%) | 113 (38.8) | 79 (95.2) | <0.001 | 364 (43.8) | 256 (88.6) | <0.001 |
| GCS<15, n (%) | 48 (16.5) | 9 (10.8) | 0.23 | 105 (12.6) | 27 (9.3) | 0.140 |
| Median LOS (IQR), days | 5 (3–8) | 4 (2–6) | 0.053 | 4 (2–8) | 4 (2–8) | 0.182 |
| Any VTE Risk Factor, n (%) | 226 (77.7) | 65 (78.3) | 0.99 | 647 (77.9) | 243 (84.1) | 0.028 |
| Internal Medicine | n=567 | n=392 | n=547 | n=351 | ||
| Mean age (SD), years | 54.0 (16.4) | 60.4 (16.3) | <0.001 | 54.0 (15.9) | 56.9 (16.7) | 0.009 |
| Male, n (%) | 255 (45.0) | 226 (57.7) | <0.001 | 286 (52.3) | 179 (51.0) | 0.732 |
| Median LOS (IQR), days | 3 (2–6) | 3 (2–7) | 0.625 | 3 (2–6) | 4 (2–6) | 0.853 |
| Any VTE Risk Factor, n (%) | 346 (61.0) | 269 (68.6) | 0.017 | 299 (54.7) | 193 (55.0) | 0.945 |
IQR: interquartile range; ISS: injury severity score; LOS: length of stay; SD: standard deviation; VTE: venous thromboembolism
Table 3.
Clinical characteristics of included hospitalized adult trauma and internal medicine patients, by race before (pre-) and after (post-) implementation
| Black Patients | White Patients | |||||
|---|---|---|---|---|---|---|
| Pre | Post | p-value | Pre | Post | p-value | |
| Trauma | n=291 | n=831 | n=83 | n=289 | ||
| Mean age (SD), years | 32.8 (15.3) | 32.4 (14.6) | 0.691 | 47.9 (21.8) | 46.1 (20.6) | 0.489 |
| Male, n (%) | 256 (88.0) | 685 (82.4) | 0.026 | 49 (59.0) | 178 (61.6) | 0.703 |
| Median ISS (IQR) | 9 (4–16) | 9 (4–16) | 0.197 | 9 (4–17) | 9 (4–16) | 0.727 |
| Blunt Trauma, n (%) | 113 (38.8) | 364 (43.8) | 0.148 | 79 (95.2) | 256 (88.6) | 0.095 |
| GCS<15, n (%) | 48 (16.5) | 105 (12.6) | 0.112 | 9 (10.8) | 27 (9.3) | 0.676 |
| Median LOS (IQR), days | 5 (3–8) | 4 (2–8) | p<0.001 | 4 (2–6) | 4 (2–8) | 0.410 |
| Any VTE Risk Factor, n (%) | 226 (77.7) | 647 (77.9) | 0.935 | 65 (78.3) | 243 (84.1) | 0.248 |
| Internal Medicine | n=567 | n=547 | n=392 | n=351 | ||
| Mean age (SD), years | 54.0 (16.4) | 54.0 (15.9) | 0.99 | 60.4 (16.3) | 56.9 (16.7) | 0.004 |
| Male, n (%) | 255 (45.0) | 286 (52.3) | 0.016 | 226 (57.7) | 179 (51.0) | 0.077 |
| Median LOS (IQR), days | 3 (2–6) | 3 (2–6) | 0.533 | 3 (2–7) | 4 (2–6) | 0.319 |
| Any VTE Risk Factor, n (%) | 346 (61.0) | 299 (54.7) | 0.034 | 269 (68.6) | 193 (55.0) | p<0.001 |
IQR: interquartile range; ISS: injury severity score; LOS: length of stay; SD: standard deviation; VTE: venous thromboembolism
In the pre-implementation period, the proportion of trauma patients prescribed risk-appropriate VTE prophylaxis was significantly higher for black (70.1%) than white (56.6%) patients (p=0.025). After implementation, prescription of risk-appropriate prophylaxis significantly increased for all patients [black (84.5%) and white (85.5%)], and there were no differences between racial groups (p=0.99) (Figure 1A).
Figure 1.
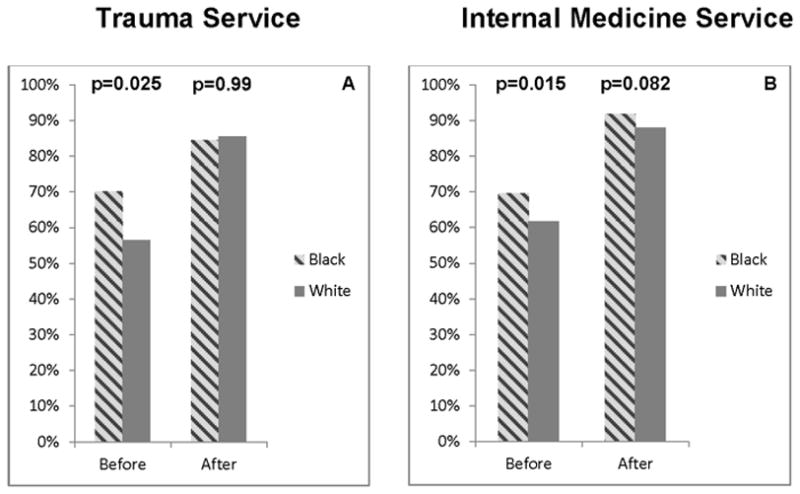
compares the rates for risk-appropriate VTE prophylaxis prescription for black (hashed gray) and white (dark gray) patients before and after implementation of the mandatory computerized provider order entry (CPOE) clinical decision support (CDS) VTE module on the trauma (A) and internal medicine (B) services. The proportion of patients prescribed best-practice VTE prophylaxis increased significantly (p<0.05) within both race categories for both trauma and internal medicine patients.
Before implementation, the proportion of male trauma patients prescribed risk-appropriate VTE prophylaxis was significantly higher (69.5% vs. 55.1%, p=0.045). After implementation, compliance increased significantly for both male (85.7%) and female (81.2%) patients and there were no differences between groups (p=0.078) (Figure 2A).
Figure 2.
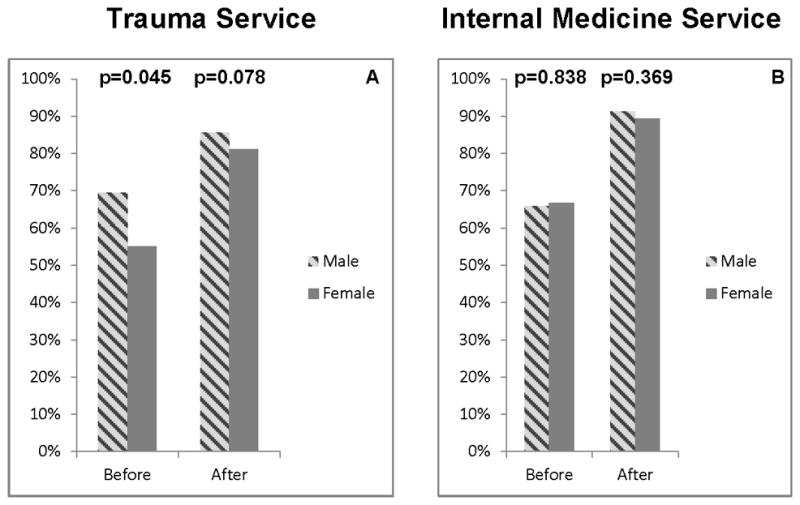
compares the rates for risk-appropriate VTE prophylaxis prescription for female (dark gray) and male (hashed gray) hospitalized patients before and after of the mandatory CPOE clinical decision support VTE module was implemented on the trauma (A) and internal medicine (B) services. The proportion of patients prescribed best-practice VTE prophylaxis increased significantly (p<0.05) within both gender categories for both trauma and internal medicine patients.
Internal Medicine Patients
Between racial groups, black internal medicine patients were younger (54.0 vs. 58.1 years, p<0.001) and less frequently male (48.6% vs. 54.5%, p=0.012). In the pre-implementation period, significantly more white than black patients (68.6% vs. 61.0%, p=0.017) had at least one major VTE risk factor (Table 2). White patients were younger in the pre-implementation group (60.4 vs. 56.9 years, p=0.004). Fewer black (54.7% vs. 61.0%, p=0.034) and white (68.6% vs. 55.0%, p<0.001) patients presented with a major risk factor in the post-implementation period (Table 3).
Before implementation, significantly more black patients were prescribed risk-appropriate VTE prophylaxis than white patients (69.5% vs. 61.7%, p=0.015). After implementation, compliance increased significantly for both black (91.8%) and white (88.0%) patients and there were no differences between races (p=0.082) (Figure 1B). There were no differences in risk-appropriate VTE prophylaxis prescription between genders, before or after implementation (Figure 2B).
DISCUSSION
Implementation of a mandatory CCDS tool eliminated race-based healthcare disparities in risk-appropriate VTE prophylaxis prescription for hospitalized medical and trauma patients. Gender disparities in best-practice VTE prophylaxis prescription were also eliminated by the same CCDS tool. These findings highlight the potential of health information technology approaches to improve the quality of care for all patients and eradicate healthcare disparities. The intended purpose of this QI intervention was to improve the care for all hospitalized patients rather than specifically targeting subgroups of patients who were receiving suboptimal care. We recognize that eliminating disparities in providing best-practice VTE prevention was an unintended consequence of this intervention. However, disparities elimination falls under the general umbrella of QI and has been a goal of health information technologies. Previous studies of QI interventions that have been shown to lessen or eliminate disparities focused narrowly on certain patient populations, such as patients with diabetes42 or myocardial infarction,43 or patients with cancer undergoing surgery.44 Our findings demonstrate the power of broadly applied QI interventions targeting all hospitalized patients and represent another beneficial consequence of QI efforts in healthcare.
It remains unclear which specific factors are most strongly associated with healthcare disparities and may influence disparities in real-world decision making. One possible explanation is that black patients are known to have undiagnosed co-morbidities and be at risk for cardiovascular complications, 45 including VTE. Providers who were making clinical decisions entirely independently, without the use of a standardized mandatory decision support tool, may have chosen to prescribe more aggressive VTE prophylaxis regimens for these black patients to overcompensate for these issues. Providers may not necessarily believe that all patients require VTE prophylaxis and this misguided, subconscious calculation of the risk benefit ratio did not favor prescribing VTE prophylaxis for white patients. A recent study using an Implicit Association Test, and a series of clinical vignettes applied to first-year medical students showed an overall preference for white individuals but the clinical vignette responses were not associated with patient race.11 While these findings are important insights to clinician perceptions, they represent simulated decision making in controlled environments rather than real world clinical decisions for actual hospitalized patients. However, our study demonstrates that a well-integrated CCDS tool transcends those factors, regardless of the causal pathway, and is capable of modifying the decisional behavior that may create healthcare disparities by reducing the impact of bias.
Data show that black patients more commonly receive lower quality care than white patients1, 46 and efforts to reduce healthcare disparities have often failed. Therefore we were somewhat surprised to find that white hospitalized patients were less likely to be prescribed risk-appropriate VTE prophylaxis during the pre-implementation period. However, previous studies have demonstrated better outcomes for black patients compared with white patients undergoing kidney dialysis47 or survival after trauma.48 Similar to what has been reported in these studies, age or clinical condition may be confounding variables which requires further exploration in a larger dataset. Another potential explanation was identified in a study where black hospitalized patients rated their interaction with prescribers as less participatory than whites.49 Consequently, it is possible that shared decision making between white patients and prescribers resulted in suboptimal VTE prophylaxis.
We recognize that our study has several limitations. First, we were not able to evaluate variation among individual types of clinicians (i.e. physicians, nurse practitioners), limiting our ability to evaluate the impact of experience on healthcare disparities. However, our findings were well-conserved across two very different clinical services indicating that these disparities are neither random nor attributable to select prescribers within a single clinical service. Second, our limited sample size did not allow for multivariable analysis to elucidate other associations with the observed disparity. Third, our results were demonstrated using only a single evidence-based practice (VTE prophylaxis) at a single academic medical center. Nevertheless, the CCDS intervention eliminated disparities among a diverse group of medical and surgical patients, proving its effectiveness in a “real-world” setting. Finally, there were differences in the number of patients who presented with major VTE risk factors. However, risk-appropriate prophylaxis is determined on an individual patient basis, so these differences should not have affected the decision making process.
When designing CCDS tools to impact provider behavior, it is important to consider how the tool will be integrated into the clinical and decisional workflow.50 Our mandatory CCDS tool focuses clinician attention on completing a task and forces VTE risk assessment for every patient. Passive CCDS tools that do not require provider action have been shown to be less effective at impacting provider behavior51 and will likely have less impact on eliminating disparities in care delivery.
Despite repeated evidence of the existence of healthcare disparities, we do not know of previous interventions that have been as successful at eliminating these disparities. Mandatory CCDS tools reduce the burden of complex and repetitive decision making, while promoting best practice care for all patients equally.
Contributor Information
Brandyn D. Lau, Email: blau2@jhmi.edu, Division of Acute Care Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD. The Armstrong Institute for Patient Safety, Johns Hopkins Medicine, Baltimore, MD. Division of Health Science Informatics, The Johns Hopkins University School of Medicine, Baltimore, MD.
Adil H. Haider, Email: ahaider1@jhmi.edu, Division of Acute Care Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD. Department of Anesthesiology & Critical Care Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD. Center for Surgical Trials and Outcomes Research (CSTOR), The Johns Hopkins University School of Medicine, Baltimore, MD. Department of Health Policy and Management, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD.
Michael B. Streiff, Email: mstreif@jhmi.edu, Division of Hematology, Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD. The Armstrong Institute for Patient Safety, Johns Hopkins Medicine, Baltimore, MD.
Christoph U. Lehmann, Email: chris.lehmann@vanderbilt.edu, Division of Neonatal-Perinatal Medicine, Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN. Division of Health Science Informatics, The Johns Hopkins University School of Medicine, Baltimore, MD.
Peggy S. Kraus, Email: pkraus2@jhmi.edu, Department of Pharmacy, The Johns Hopkins Hospital, Baltimore, MD.
Deborah B. Hobson, Email: dhobson1@jhmi.edu, Division of Acute Care Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD. The Armstrong Institute for Patient Safety, Johns Hopkins Medicine, Baltimore, MD.
Franca S. Kraenzlin, Email: franca.kraenzlin@gmail.com, Division of Acute Care Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD.
Amer M. Zeidan, Email: azeidan1@jhmi.edu, Division of Hematology, Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD.
Peter J. Pronovost, Email: ppronovo@jhmi.edu, Department of Anesthesiology & Critical Care Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD. Department of Health Policy and Management, The Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD. The Armstrong Institute for Patient Safety, Johns Hopkins Medicine, Baltimore, MD.
Elliott R. Haut, Email: ehaut1@jhmi.edu, Division of Acute Care Surgery, Department of Surgery, The Johns Hopkins University School of Medicine, Baltimore, MD. Department of Anesthesiology & Critical Care Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD. Department of Emergency Medicine, The Johns Hopkins University School of Medicine, Baltimore, MD. Center for Surgical Trials and Outcomes Research (CSTOR), The Johns Hopkins University School of Medicine, Baltimore, MD. The Armstrong Institute for Patient Safety, Johns Hopkins Medicine, Baltimore, MD.
References
- 1.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. 2003 [PubMed] [Google Scholar]
- 2.Haider AH, Chang DC, Efron DT, et al. Race and insurance status as risk factors for trauma mortality. Arch Surg. 2008;143:945–949. doi: 10.1001/archsurg.143.10.945. [DOI] [PubMed] [Google Scholar]
- 3.Hasnain-Wynia R, Baker DW, Nerenz D, et al. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167:1233–1239. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- 4.Baicker K, Chandra A, Skinner JS. Geographic variation in health care and the problem of measuring racial disparities. Perspect Biol Med. 2005;48:S42–53. [PubMed] [Google Scholar]
- 5.Haider AH, Ong’uti S, Efron DT, et al. Association between hospitals caring for a disproportionately high percentage of minority trauma patients and increased mortality: a nationwide analysis of 434 hospitals. Arch Surg. 2012;147:63–70. doi: 10.1001/archsurg.2011.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ly DP, Lopez L, Isaac T, et al. How do black-serving hospitals perform on patient safety indicators? Implications for national public reporting and pay-for-performance. Med Care. 2010;48:1133–1137. doi: 10.1097/MLR.0b013e3181f81c7e. [DOI] [PubMed] [Google Scholar]
- 7.Haider AH, Weygandt PL, Bentley JM, et al. Disparities in Trauma Care and Outcomes in the United States: A Systematic Review and Meta-analysis. J Trauma Acute Care Surg. 2013;74:1195–205. doi: 10.1097/TA.0b013e31828c331d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabin JA, Rivara FP, Greenwald AG. Physician implicit attitudes and stereotypes about race and quality of medical care. Med Care. 2008;46:678–685. doi: 10.1097/MLR.0b013e3181653d58. [DOI] [PubMed] [Google Scholar]
- 9.Green AR, Carney DR, Pallin DJ, et al. Implicit bias among physicians and its prediction of thrombolysis decisions for black and white patients. J Gen Intern Med. 2007;22:1231–1238. doi: 10.1007/s11606-007-0258-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Ryn M, Fu SS. Paved with good intentions: do public health and human service providers contribute to racial/ethnic disparities in health? Am J Public Health. 2003;93:248–255. doi: 10.2105/ajph.93.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haider AH, Sexton J, Sriram N, et al. Association of unconscious race and social class bias with vignette-based clinical assessments by medical students. JAMA. 2011;306:942–951. doi: 10.1001/jama.2011.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haider AH, Pronovost PJ. Health information technology and the collection of race, ethnicity, and language data to reduce disparities in quality of care. Jt Comm J Qual Patient Saf. 2011;37:435–436. doi: 10.1016/s1553-7250(11)37054-7. [DOI] [PubMed] [Google Scholar]
- 13.Fiscella K, Franks P, Gold MR, et al. Inequality in quality: addressing socioeconomic, racial, and ethnic disparities in health care. JAMA. 2000;283:2579–2584. doi: 10.1001/jama.283.19.2579. [DOI] [PubMed] [Google Scholar]
- 14.AHRQ. National Healthcare Disparities Report. 2011;2012:12–0006. [Google Scholar]
- 15.Baron RJ. Quality improvement with an electronic health record: achievable, but not automatic. Ann Intern Med. 2007;147:549–552. doi: 10.7326/0003-4819-147-8-200710160-00007. [DOI] [PubMed] [Google Scholar]
- 16.Graham G. Using Technology to Improve Minority Health. 2009 [Google Scholar]
- 17.Kardooni S, Haut ER, Chang DC, et al. Hazards of benchmarking complications with the National Trauma Data Bank: numerators in search of denominators. J Trauma. 2008;64:273–7. doi: 10.1097/TA.0b013e31816335ae. discussion 277–9. [DOI] [PubMed] [Google Scholar]
- 18.Maynard G, Stein J. Preventing Hospital-Acquired Venous Thromboembolism: A Guide for Effective Quality Improvement. 2008. [Google Scholar]
- 19.Alikhan R, Peters F, Wilmott R, et al. Fatal pulmonary embolism in hospitalised patients: a necropsy review. J Clin Pathol. 2004;57:1254–1257. doi: 10.1136/jcp.2003.013581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandler DA, Martin JF. Autopsy proven pulmonary embolism in hospital patients: are we detecting enough deep vein thrombosis? J R Soc Med. 1989;82:203–205. doi: 10.1177/014107688908200407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streiff MB, Haut ER. The CMS ruling on venous thromboembolism after total knee or hip arthroplasty: weighing risks and benefits. JAMA. 2009;301:1063–1065. doi: 10.1001/jama.301.10.1063. [DOI] [PubMed] [Google Scholar]
- 22.Haut E, Lau B, Streiff M. New oral anticoagulants for preventing venous thromboembolism. BMJ. 2012;344:e3820. doi: 10.1136/bmj.e3820. [DOI] [PubMed] [Google Scholar]
- 23.Cohen AT, Alikhan R, Arcelus JI, et al. Assessment of venous thromboembolism risk and the benefits of thromboprophylaxis in medical patients. Thromb Haemost. 2005;94:750–759. [PubMed] [Google Scholar]
- 24.Guyatt GH, Akl EA, Crowther M, et al. Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers FB, Cipolle MD, Velmahos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group. J Trauma. 2002;53:142–164. doi: 10.1097/00005373-200207000-00032. [DOI] [PubMed] [Google Scholar]
- 26.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med. 1999;341:793–800. doi: 10.1056/NEJM199909093411103. [DOI] [PubMed] [Google Scholar]
- 27.Streiff MB, Lau BD. Thromboprophylaxis in nonsurgical patients. Hematology Am Soc Hematol Educ Program. 2012;2012:631–637. doi: 10.1182/asheducation-2012.1.631. [DOI] [PubMed] [Google Scholar]
- 28.Aboagye JK, Lau BD, Schneider EB, et al. Linking processes and outcomes: a key strategy to prevent and report harm from venous thromboembolism in surgical patients. JAMA Surg. 2013;148:299–300. doi: 10.1001/jamasurg.2013.1400. [DOI] [PubMed] [Google Scholar]
- 29.Goldhaber SZ, Tapson VF DVT FREE Steering Committee. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. The American Journal of Cardiology. 2004;93:259–262. doi: 10.1016/j.amjcard.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 30.Cohen AT, Tapson VF, Bergmann J, et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. The Lancet. 2008;371:387–394. doi: 10.1016/S0140-6736(08)60202-0. [DOI] [PubMed] [Google Scholar]
- 31.US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism. 2008 Available at: http://www.ncbi.nlm.nih.gov/books/NBK44178/ [PubMed]
- 32.Shekelle PG, Pronovost PJ, Wachter RM, et al. The top patient safety strategies that can be encouraged for adoption now. Ann Intern Med. 2013;158:365–368. doi: 10.7326/0003-4819-158-5-201303051-00001. [DOI] [PubMed] [Google Scholar]
- 33.Lau BD, Haut ER. Practices to prevent venous thromboembolism: A brief review. BMJ Qual Saf Mar. 2014;23:187–195. doi: 10.1136/bmjqs-2012-001782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durieux P, Nizard R, Ravaud P, et al. A Clinical Decision Support System for Prevention of Venous Thromboembolism Effect on Physician Behavior. JAMA. 2000;283:2816–21. doi: 10.1001/jama.283.21.2816. [DOI] [PubMed] [Google Scholar]
- 35.Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med. 2005;352:969–77. doi: 10.1056/NEJMoa041533. [DOI] [PubMed] [Google Scholar]
- 36.Maynard GA, Morris TA, Jenkins IH, et al. Optimizing prevention of hospital-acquired venous thromboembolism (VTE): prospective validation of a VTE risk assessment model. J Hosp Med. 2010;5:10–18. doi: 10.1002/jhm.562. [DOI] [PubMed] [Google Scholar]
- 37.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 38.Streiff MB, Carolan H, Hobson DB, et al. Lessons from the Johns Hopkins Multi-Disciplinary Venous Thromboembolism (VTE) Prevention Collaborative. BMJ. 2012;344:e3935. doi: 10.1136/bmj.e3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haut ER, Lau BD, Kraenzlin FS, et al. Improved Prophylaxis and Decreased Preventable Harm with a Mandatory Computerized Clinical Decision Support Tool for Venous Thromboembolism (VTE) Prophylaxis in Trauma Patients. Archives of Surgery. 2012;10:901–907. doi: 10.1001/archsurg.2012.2024. [DOI] [PubMed] [Google Scholar]
- 40.Monn MF, Haut ER, Lau BD, et al. Is venous thromboembolism in colorectal surgery patients preventable or inevitable: one institution’s experience. Journal of the American College of Surgeons. 2013;216:395–401. doi: 10.1016/j.jamcollsurg.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 41.Zeidan AM, Streiff MB, Lau BD, et al. Impact of a venous thromboembolism prophylaxis “smart order set”: Improved compliance, fewer events. Am J Hematol. 2013;88:545–549. doi: 10.1002/ajh.23450. [DOI] [PubMed] [Google Scholar]
- 42.Betancourt JR, Duong JV, Bondaryk MR. Strategies to reduce diabetes disparities: an update. Curr Diab Rep. 2012;12:762–768. doi: 10.1007/s11892-012-0324-1. [DOI] [PubMed] [Google Scholar]
- 43.Cohen MG, Fonarow GC, Peterson ED, et al. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines-Coronary Artery Disease program. Circulation. 2010;121:2294–2301. doi: 10.1161/CIRCULATIONAHA.109.922286. [DOI] [PubMed] [Google Scholar]
- 44.Parsons HM, Habermann EB, Stain SC, et al. What happens to racial and ethnic minorities after cancer surgery at American College of Surgeons National Surgical Quality Improvement Program hospitals? J Am Coll Surg. 2012;214:539–47. doi: 10.1016/j.jamcollsurg.2011.12.024. discussion 547–9. [DOI] [PubMed] [Google Scholar]
- 45.Duron VP, Monaghan SF, Connolly MD, et al. Undiagnosed medical comorbidities in the uninsured: a significant predictor of mortality following trauma. J Trauma Acute Care Surg. 2012;73:1093–8. doi: 10.1097/TA.0b013e31826fc844. discussion 1098–9. [DOI] [PubMed] [Google Scholar]
- 46.Egede LE. Race, ethnicity, culture, and disparities in health care. J Gen Intern Med. 2006;21:667–669. doi: 10.1111/j.1525-1497.2006.0512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucirka LM, Grams ME, Lessler J, et al. Association of race and age with survival among patients undergoing dialysis. JAMA. 2011;306:620–626. doi: 10.1001/jama.2011.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hicks CW, Hashmi ZG, Velopulos C, et al. Association between race and age in survival after trauma. JAMA Surg. 2014;149:642–647. doi: 10.1001/jamasurg.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282:583–589. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 50.Baysari MT, Westbrook JI, Richardson KL, et al. The influence of computerized decision support on prescribing during ward-rounds: are the decision-makers targeted? J Am Med Inform Assoc. 2011;18:754–759. doi: 10.1136/amiajnl-2011-000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller AM, Boro MS, Korman NE, et al. Provider and pharmacist responses to warfarin drug-drug interaction alerts: a study of healthcare downstream of CPOE alerts. J Am Med Inform Assoc. 2011;18 (Suppl 1):i45–50. doi: 10.1136/amiajnl-2011-000262. [DOI] [PMC free article] [PubMed] [Google Scholar]


