Abstract
Postnatal glucocorticoids (GCs) are widely used in the prevention of chronic lung disease in premature infants. Their pharmacologic use is associated with neurodevelopmental delay and cerebral palsy. However, the effect of GC dose and preparation (dexamethasone versus betamethasone) on short and long-term neurological outcomes remains undetermined, and the mechanisms of GC-induced brain injury are unclear. We hypothesized that postnatal GC would induce hypomyelination and motor impairment in a preparation- and dose-specific manner, and that GC receptor (GR) inhibition might restore myelination and neurological function in GC-treated animals. Additionally, GC-induced hypomyelination and neurological deficit might be transient. To test our hypotheses, we treated prematurely delivered rabbit pups with high (0.5 mg/kg/day) or low (0.2 mg/kg/day) doses of dexamethasone or betamethasone. Myelin basic protein (MBP), oligodendrocyte proliferation and maturation, astrocytes, transcriptomic profile, and neurobehavioral functions were evaluated. We found that high-dose GC treatment, but not low-dose, reduced MBP expression and impaired motor function at postnatal day 14. High-dose dexamethasone induced astrogliosis, betamethasone did not. Mifepristone, a GR antagonist, reversed dexamethasone-induced myelination, but not astrogliosis. Both GCs inhibited oligodendrocyte proliferation and maturation. Moreover, high-dose dexamethasone altered genes associated with myelination, cell-cycle, GR, and Mitogen-activated protein kinase. Importantly, GC-induced hypomyelination, gliosis, and motor-deficit, observed at day 14, completely recovered by day 21. Hence, high-dose, but not low-dose, postnatal GC causes reversible reductions in myelination and motor functions. GC treatment induces hypomyelination by GR-dependent genomic mechanisms, but astrogliosis by non-genomic mechanisms. GC-induced motor impairment and neurodevelopmental delay might be transient and recover spontaneously in premature infants.
Keywords: glucocorticoids, betamethasone, dexamethasone, myelination, astrocytes, oligodendrocytes
Introduction
Postnatal glucocorticoids (GCs) are used in premature infants for the treatment and prevention of chronic lung disease. However, the use of postnatal GCs is associated with cerebral palsy and neurodevelopmental delay in preschool children. (Shinwell et al., 2000; Vermont Oxford Network Steroid Study, 2001; Yeh et al., 2004). Indeed, the American Academy of Pediatrics (AAP) has stated that high-dose postnatal GCs should not be used in preterm infants and that neonatologists should use their clinical judgment even when using low-dose GCs in these patients (Watterberg, 2010). The AAP also noted that there was insufficient evidence to make recommendations regarding GC dose and preparation (Watterberg, 2010). Despite these, postnatal GCs are frequently used in a large number of neonatal intensive care units in both the United States and Europe (Taylor et al., 2002; Truffert et al., 2003). Importantly, long term neurocognitive outcome (beyond school age) of preterm infants treated with postnatal GC remains unclear and mechanisms underlying GC-induced brain injury are poorly understood. Hence, there is considerable controversy about the use of postnatal GC in preterm infants.
Postnatal GC treatment in premature infants is associated with reduced head circumference, neuro-developmental impairment, and substantial diminution in the volume of the cerebral cortex (Bakker et al., 2001; Barrington, 2001; Murphy et al., 2001). GC-exposed children exhibit impaired neuromotor and cognitive function, and disabilities at school age (Barrington, 2001; Yeh et al., 2004). Conversely, a few studies show that dexamethasone treatment may not account for long term neuro-cognitive impairments (Crotty et al., 2012; Jones and Collaborative Dexamethasone Trial Follow-up, 2005; Yates and Newell, 2012). Recent meta-analyses suggest that early (<8 d) postnatal treatment with dexamethasone increases the risk of cerebral palsy, but not the delayed (>7 d) treatment (Doyle et al., 2014a, b). In animal experiments, treatment with high doses of postnatal steroids results in reduced brain weight, decreased myelination, and neuronal apoptosis in the hippocampus and cerebellum (Baud et al., 2005; Duksal et al., 2009; Maloney et al., 2011). Apoptosis of oligodendrocytes (OL) and suppression of genes for myelin basic protein (MBP) and proteolipid protein (PLP) appears to contribute to GC-induced hypomyelination in newborn rats (Kim et al., 2013). However, the effect of GCs on the proliferation and maturation of OL lineage, and transcriptional network regulating myelination remains unknown.
Effects of GCs are preparation-, dose-, and context-dependent (Grier and Halliday, 2003; Uno et al., 1994). Dexamethasone and betamethasone are the two GCs commonly used in neonatal and perinatal medicine (DeCastro et al., 2009; Grier and Halliday, 2003). They differ with respect to pharmacokinetics, penetration of the blood brain barrier, and effect on neural cells (Baud et al., 1999; Cambonie et al., 2008; Trenque et al., 1994). Dexamethasone is the most commonly used postnatal GC to prevent chronic lung disease in premature infants, whereas postnatal betamethasone has been used in some neonatal units (DeCastro et al., 2009; Watterberg, 2010). Large randomized controlled trials of postnatal dexamethasone both in high (0.5 mg/kg/d for 7 d) and low doses (0.2 mg/kg/d for 7-14 d) have shown efficacy in facilitating extubation and improving pulmonary function in preterm infants (McEvoy et al., 2004; Walther et al., 2003; Watterberg, 2010). OL culture experiments show that GC induced myelination-delays are mediated by microglia and astrocytes, but not by the direct effect on OL (Jenkins et al., 2014). Studies in animal models and neuronal cultures show that low doses of GCs are neuroprotective, whereas higher doses are neurodegenerative (Abraham et al., 2000; Uno et al., 1994; Uno et al., 1990). The genomic effect of these GCs are mediated by GR and the non-genomic effect is carried out by membrane and cytoplasmic proteins (Stahn and Buttgereit, 2008). Based on these considerations, we hypothesized that postnatal GCs would suppress myelination and induce motor impairment in a preparation (dexamethasone versus betamethasone) and dose (high vs. low) dependent manner, and that GC receptor inhibition might restore myelination and facilitate neurological recovery in GC-treated animals. We also postulated that the GC-induced hypomyelination, and neurological deficit might be transient. To test our hypotheses, we employed a preterm rabbit pup model (embryonic day 29, term= 32 d). Rabbits, similar to humans, exhibit perinatal brain growth and a gyrencephalic brain, and the cerebral blood supply is from carotid and vertebral arteries (Coulter et al., 1984). Our study highlights the mechanistic basis of GC-induced white matter injury and reveals the safety of low-dose GCs relative to high-dose and the optimism that GC-induced motor impairment and delayed neurodevelopment might be transient in preterm infants, if GCs are used in an appropriate dosing regimen.
Material and Methods
Animal Experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee of New York Medical College, Valhalla, NY, which is in compliance with the 8th Edition of the Guide for the Care and Use of Laboratory Animals (Guide), NRC 2011 and the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes, Council of Europe (ETS 123). Timed pregnant New Zealand rabbits were purchased from Charles River Laboratories, Inc. (Wilmington, Mass., USA). The premature pups were delivered by C-section at d 29 of gestational age (term= 32 d). Pups of either sex were dried with pre-warmed towels and placed in infant incubator at 35°C. The pups were gavage-fed with puppy formula (Esbilac, Petag, IL) in a volume of 2 ml every 12 h (100ml/kg/d) starting at 2 h age. Subsequently, feeds were advanced to 120, 150, 180, 220, 250, and 280 ml/kg at 3, 5, 7, 10, 14, and 21 d respectively. The pups were euthanized at 3, 7, 14, and 21 d. We processed the brain tissues as described before (Ballabh et al., 2007). Briefly, forebrains were sectioned into 2 mm slices starting from the rostral end. Brain slices at the level of mid-septal nucleus were used for immuno-histochemistry, Western blot analyses, microarray analyses, and real-time qPCR. For immunohistochemistry, the samples were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS) overnight and were then cryoprotected by immersing into a 20% sucrose solution in PBS. The tissues were frozen after embedding them into optimum cutting temperature compound (Sakura, Japan). Frozen coronal blocks were cut in 30 μm sections. For Western blot analyses and qPCR, brain slices were frozen immediately on dry ice.
Glucocorticoid treatment
Dexamethasone (West-Ward, Eatontown, NJ) was administered in two doses—high (0.25 mg/kg twice daily) and low (0.1 mg/kg twice daily)—for a maximum duration of 5 d or until euthanized. Similarly, betamethasone (Schering, Kenilworth, NJ) was used either in high (0.5 mg/kg once daily for 5 d) or low (0.2 mg/kg once daily for 5 d) doses. Betamethasone and dexamethasone used had aqueous base and they were diluted in saline. Hence, saline treatment was used as in control animals. The dose of GCs was based on its use in premature infants at risk of chronic lung disease in neonatal intensive care units (Barrington, 2001; Durand et al., 2002; McEvoy et al., 2004; Walther et al., 2003). To block GC receptors, we used mifepristone (RU486, Sigma-Aldrich, St. Louis, MO) in a dose of 200 mg/kg once daily for five days. This dose was based on the fact that 1 mg of dexamethasone antagonizes 400 mg of mifepristone (DeBattista and Belanoff, 2006). Mifepristone was given in the morning at the same time as dexamethasone while dexamethasone was given alone in the evening. Mifepristone was dissolved in DMSO and accordingly, vehicle consisted of DMSO. All medications were administered intramuscularly (IM).
Immunohistochemistry
The primary antibodies used in the experiments included rabbit monoclonal Ki67 (Catalog # 275R-14, Cell Marque, Rocklin, CA), goat polyclonal Olig2 (Catalog # AF-2418, R & D, Minneapolis, MN), mouse monoclonal glial fibrillary acidic protein (GFAP, catalog #G6171, St Louis, MO), rat monoclonal myelin basic protein (Catalog # AB7439, Abcam, Cambridge, MA), mouse monoclonal myelin associated glycoprotein (MAG, Catalog # ab89780, Abcam, Cambridge, MA), mouse monoclonal APC antibody (Catalog # OP80, EMD Chemicals, Gibbstown, NJ), mouse monoclonal 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase (CNPase, Catalog # SMI91R, Covance, Dedham, MA), goat polyclonal Iba 1 antibody (catalog #ab5076, Abcam Cambridge, MA), mouse monoclonal Ki67 (catalog #M7240; Dako, CA), and O4 antibody (generous gift from Dr. Rashmi Bansal). The secondary antibodies used were Alexa 488 donkey anti-mouse, Alexa 647 donkey anti-goat, and Alexa 594 donkey anti-rabbit (Invitrogen, Grand Island, NY). Briefly, sections were hydrated in 0.01M PBS, blocked in 5% normal donkey serum, and then incubated with the primary antibodies diluted in PBS at 4°C overnight. After washing in PBS, the sections were incubated with secondary antibody diluted in 1% normal donkey serum in PBS at room temperature for 60 minutes. Finally, after washing the sections in PBS, we mounted them with Slow Fade Light Antifade reagent (Molecular Probes, Invitrogen, CA). The stained sections were visualized under a confocal microscope (Nikon Instruments, Japan). Stereology was performed using a fluorescent microscope (Axioskop 2 plus, Carl Zeiss Inc) with motorized specimen stage for automated sampling (ASI, Eugene, OR), CCD color video camera (Microfire, Optronics, Goleta, CA), and stereology software (Stereologer, SRC, Baltimore, MD).
Stereological analysis of myelin and glia
All stereological analyses were performed at our stereology workstation which is equipped with a Zeiss Axioscope-2 microscope, motorized stage, an Optronics Microfire digital camera and computerized software system (Stereologer, SRC, MD). We quantified myelination (myelin load) and gliosis (GFAP+ astrocytes cell bodies and fibers) in the corpus callosum and corona radiata of the brain section taken at the level of mid-septal nucleus. Briefly, coronal sections were cut on cryostat at a setting of 30 μm thickness with a section sampling interval of three (90 μm) to achieve at least 6 sections at the level of mid-septal nucleus. The sections were triple-labeled with GFAP and MBP antibodies along with DAPI (nuclear stain). The reference spaces (corona radiata, corpus callosum) were first outlined on the section under 5× objective. The volume of the outlined area (reference space) was quantified using a point counting probe (frame 25 μm × 25 μm; guard zone 2 μm, inter-frame interval = 300 μm). The total volume fraction (load) of myelin labeled by antibodies to MBP through a defined reference space was quantified using the object area fraction probe under 60× oil lens. For the area fraction probe (frame 25 μm × 25 μm; guard zone 2 μm, interframe interval 400 μm), the user clicked on the grid points that touched myelin fibers in sections stained with MBP. The area fraction of myelination was quantified as the ratio of product of the area per point and number of points hitting reference area [a(point)· ΣPref] over the product of the area per point and number of points hitting the sampled area [a(point)· ΣPsamp], as reported (Buser et al., 2012; Mouton et al., 2009). Sampling continued until the coefficient of error (CE) was less than 0.10. To assess gliosis, we determined total volume fraction of astrocyte cell bodies and fibers (Mouton et al., 2009). Volume fraction of astrocytes was quantified in a similar fashion as for myelin.
Western blot analyses
We homogenized the frozen brain tissue, collected as 2 mm slice at the level of mid-septal nucleus, in sample buffer (3% SDS, 10% glycerol, and 62.5 mMol Tris-HCl) using a mechanical homogenizer and boiled the samples immediately for 5 minutes. The protein concentration in the supernatant was determined using BCA Protein Assay Kit (Pierce catalog # 23227, Thermo Scientifics, IL). Total protein samples were separated by SDS-PAGE according to the previously described method (Ballabh et al., 2007). Equal amounts of protein (10-20 μg) were loaded into a 4-20% gradient precast gel (Bio-Rad, CA). The separated proteins were transferred onto polyvinylidene difluoride (PVDF) membrane by electro-transfer. The membranes were then incubated with primary antibodies. We detected target proteins with chemiluminescence ECL system (Amersham Biosciences Inc., NJ) by using secondary antibodies conjugated with horseradish peroxidase (Jackson Immunoresearch, PA). We next stripped the blots with stripping buffer (Pierce) and incubated with β actin primary antibody followed by secondary antibody and detection with chemiluminescence ECL system. As described previously (Ballabh et al., 2007), the blots from each experiment were densitometrically analyzed using ImageJ. We used pre-calibrated optical density step tablet to calibrate on image J (rsbweb.nih.gov/ij/) and then measured the optical density of the bands. The optical density values were normalized by taking the ratio of the target protein and β actin. A couple of times, same blot was used to probe for two markers, such as CNPase and MAG, and this followed actin detection.
Quantification of proliferation and maturation of oligodendrocytes
Three groups comprising high-dose betamethasone, dexamethasone, and vehicle were evaluated for a) OL proliferation (Olig2+Ki67+) and b) OL maturation—Olig2+, O4+, and O4+APC+ count. Brain section were double labeled with Olig2 and Ki67 antibodies at 3 d and 7 d and double stained with O4 and APC antibodies at 7 d (Dummula et al.). Five 20 μm thick coronal sections from each brain were used for immunolabeling. Counting was performed under the confocal microscope using 60× lens (Nikon Instruments, Japan) by a blinded investigator in an unbiased fashion and random basis. Cells were counted in the periventricular corpus callosum, and corona radiata. We countedv objects in ~30 images (6 images × 5 coronal sections) per brain region for each pup (n = 5-6 pups each group) for every parameter. The density of Iba1+ microglia in the coronal radiata and corpus callosum (white matter) was assessed in high dose dexamethasone treated pups vs. controls, in a similar manner as OL progenitors.
Fluorescent in situ detection of DNA fragmentation (TUNEL)
We performed TUNEL staining on fixed brain sections as described previously (Dummula et al.) on three groups of preterm rabbit pups, including high-dose betamethasone, dexamethasone, and vehicle at d 3. We counted Olig2+ OL co-labeled with TUNEL+ nuclei. Briefly, tissue sections were air dried on slides, hydrated in 0.01 M PBS, and permeabilized for 5 min in 1:1 ethanol: acetic acid. An ApopTag-fluorescein in situ DNA fragmentation detection kit (catalog # S7110; Millipore) was used to visualize TUNEL-labeled nuclei. TUNEL+Olig2+ cells were counted in the periventricular corona radiata and corpus callosum in a similar manner as OL, which is discussed in the previous paragraph.
Microarray
“Two-Color Microarray-Based Gene Expression Analysis” protocol (www.agilent.com) was employed as previously described (Iacobas et al., 2008). The samples (n = 4 each, 2 mm coronal slice at mid-septal nucleus level at d 3) were hybridized to an Agilent rabbit gene expression microarray (Catalog # G2519F-020908, one glass slide formatted with four high-definition 44K arrays) at 65°C for 17 h. The hybridized chip was washed at room temperature with gene expression wash pack, stabilization solution, and drying solution. We next scanned the chip using an Agilent G2565A dual laser scanner at 5 μm resolution. The tiff images, obtained by Scan Control 8.3 (Agilent), were analyzed with (Agilent) Feature Extraction 10.7.3.1. All spots affected by local corruption or with the median foreground fluorescence less than twice the median background fluorescence were removed from the analysis (Iacobas et al., 2008),
Transcriptome analysis
We employed “Better Bunny” software for augmented gene annotation, orthologue identification, and integrated functional analysis (http://cptweb.cpt.wayne.edu/BB/index.php) (Craig et al., 2012) We completed annotations of 12,118 unigenes probed from 43,603 array spots and to identify the human putative orthologs. The software was also used to quantify genes into various functional categories including cell location, molecular function, and biological process. Detection of significantly regulated genes relied on both absolute (> 1.5×) fold-change and statistical significance (P < 0.05, heteroscedastic t-test with Bonferroni correction) between means (Iacobas et al., 2008).
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (RT-PCR) was performed as before (Ballabh et al., 2007). Briefly, total RNA were isolated form 1 mm thick slice taken at the level of mid-septal nucleus of the forebrain using Mini RNA isolation kit (Roche, NJ). RNA was reverse-transcribed using Superscript II RT (Invitrogen, CA). Real-time reverse transcriptase-PCR was used to analyze mRNA expression using the Applied Biosystems 9700 (USA). Quantification was performed using the efficiency-corrected ΔΔCT method. The primers used for qRT-PCR are in Table 1.
Table 1.
Primers used in the study
| Gene | Accession number |
Forward primer | Reverse primer |
|---|---|---|---|
| Olig1 | XM_002716810.1 | 5′-CAGCAGCAGCAACTAAGG-3′ | 5′-GAGTAGGGCAGGATGACC-3′ |
| Olig2 | NM_005806 | 5′-GTGCGGATGCTTATTATAG-3′ | 5′-ATCTGGATGCGATTTGAG-3′ |
| ID4 | NM_001546 | 5′-GCATAATGGCAAATCCTTCAAG-3′ | 5 ′-CACAAGAGATGGGACAGTAG C-3′ |
| Sox10 | XM_002723532 | 5′-AAGCCTTTCTGTCTGGCTCACT-3′ | TCAGGTCCTGGATAGAGGGTCATT |
| MBP | XM_002723002.1 | 5′-GCAACCTGTACAAGGACCA-3′ | 5′-GAAGAAGTGGACTACAGGGTTT-3 |
| GFAP | NG_008401 | 5′-ACTCAATGCTGGCTTCAAGGAGAC-3′ | 5′-ATGTAGCTGGCAAAGCGGTCATTG-3′ |
| GAPDH | NM_001082253.1 | 5′-CAAGAAGGTGGTGAAGCAGGCA-3′ | 5′-CTTCACAAAGTGGTCATTGAGGG-3′ |
Neurobehavioral assessment
Neurobehavioral assessment was performed by two blinded physicians using previously described scoring system at d 14 and d 21 (Dummula et al., 2011). Cranial nerves were tested by smell (aversive response to ethanol), sucking, and swallowing (formula delivered by a plastic pipette). The response were scored on a scale of 0–3, 0 being the no response, and 3 normal response. We assessed motor activity by tone (flexion and extensions of extremities), righting reflex (ability and quick turning from supine to prone position), locomotion at 30° angle, and gait. Grading of tone, and locomotion at 30° angle are i llustrated in the footnote of Table 2. We performed two tests to evaluate sensory system, which included touch on face (touching face with cotton swab) and extremities and mild pin prick on limbs for pain assessment. We checked the coordination and muscle strength of pups by measuring the latency to slip down the slope after placing them on the upper end of a ramp pitched at a slope of 60°. Gait was assessed as grade 0 (no locomotion), 1 (crawls with trunk touching the ground for few steps and then rolls over), 2 (walks taking alternate steps, trunk low and cannot walk on inclined surface), 3 (walks taking alternate steps, cannot propel its body using the hind legs synchronously, but walks on 30° inclined surface), 4 (walks, runs, and jumps without restriction, propels the body using synchronously the back legs, but limitation in speed, balance, and coordination manifesting as clumsiness in gait), or 5 (normal walking).
Table 2.
Neurobehavioral evaluation of GCs-treated pups compared to saline-treated controls at postnatal day 14
| System | Tests | Saline Control (n = 12) |
Dexa Low-dose (n = 7) |
Beta Low-dose (n = 7) |
Dexa High-dose (n = 8) |
Beta High-dose (n = 9) |
High dose Dexa and Mifepristone (n = 8) |
|---|---|---|---|---|---|---|---|
| Cranial Nerve | Aversive response to alcohol | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) |
| Sucking and Swallowing | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | |
| Vision | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | |
| Motor | Motor activity: Head |
3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) |
| Fore legs | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (2,3) | 3 (2,3) | 3 (3,3) | |
| Hind legs | 3 (3,3) | 3 (3,3) | 3 (3,3) | 2 (2,3)*† | 2 (2,3)*† | 3 (3,3) | |
| Righting reflexa | 5 (5,5) | 4 (4,5) | 5 (4,5) | 3.5 (2.5,5)* | 4(2.7,4)*** | 4 (4,5) | |
| Locomotion on 30°inclinationb | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (1.5,3) | 3 (2,3) | 3 (3,3) | |
| Tone in forelimbc | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | |
| Tone in hind limbc | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | 0 (0,0) | |
| Ability to hold their position at 60°inclination (Average latency to slip down the slope, in seconds) |
22.1 | 18.9 | 18.1 | 11***,†† | 11 4***†† | 20## | |
| Distance walked in inches in 60s |
111.8 | 103.9 | 105.1 | 67.6 ***,†† | 56.8 ***,†† | 101.6## | |
| Posture | 4 (4,4) | 4 (4,4) | 4 (4,4) | 3 (2.2,4)* | 3 (3,4)* | 3.5 (4,4) | |
| Gait | 4 (4,4) | 4 (4,4) | 4 (4,4) | 3(1.5,4)* | 2.5 (2,4)*,*** | 4 (3,4.4) | |
| Motor impairment (%)d | 0% | 0% | 0% | 37% | 44% | 0% | |
| Sensory | Facial touch | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) |
| Pain | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) | 3 (3,3) |
Values are median and interquartile range. Zero is the worst response, and 3 is the best response.
Score (range, 1–5): no. of times turns prone within 2 s when placed in supine out of five tries.
Score (range, 0–3): 0, does not walk; 1, takes a few steps (less than 8 inches); 2, walks for 9–18 inches; 3, walks very well beyond 18 inches.
Score (range, 1–3): 0, no increase in tone; 1, slight increase in tone; 2, considerable increase in tone; 3, limb rigid in flexion or extension.
Motor impairment was defined as weakness in either fore- or hind-legs, score for gait 2 or less, and distance walked less than 50 inches in 60 seconds. Values are expressed as %.
P<0.05,
P<0.001 for high-dose dexa (dexamethasone) or Beta (betamethasone) vs. control
P<0.05,
P<0.01, high-dose dexa vs. low-dose dexa, and high-dose beta vs. low-dose beta
P<0.01 for high-dose dexa vs. dexa with mifepristone.
Statistics and Analysis
Data are expressed as means ± standard error of the mean (s.e.m.). For Western blot analyses, real time qPCR, and data obtained by stereology, 3 group comparisons were done using one-way ANOVA. All post hoc comparisons to test for differences between means were done using Tukey multiple comparison test at the 0.05 significance level. For two group comparisons, either t-test or Mann Whitney U test was performed, as applicable.
Results
High dose GC, but not low dose, treatment reduces myelination
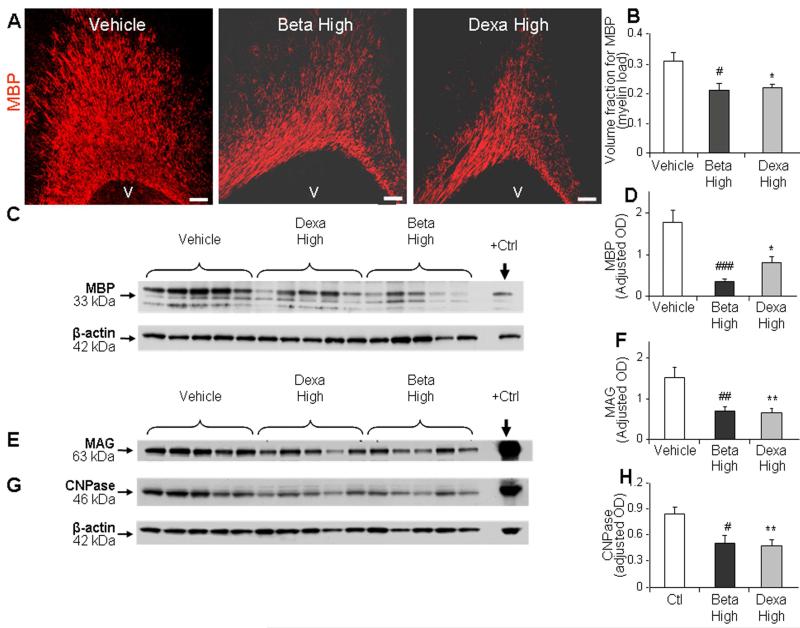
To determine whether high-dose postnatal GCs reduce myelination in premature rabbit pups, we performed stereological quantification of MBP in immunolabeled brain sections and Western blot analyses on forebrain homogenates of dexamethasone-, betamethasone- and vehicle-treated pups at postnatal d 14. These pups were treated with dexamethasone (0.5 mg/kg/d IM), betamethasone (0.5 mg/kg/d IM), or vehicle for 5 d starting at 24 h age. Stereological analyses depicted that volume fraction (load) of MBP in immuno-stained sections was significantly reduced in dexamethasone and betamethasone treated pups compared to vehicle controls in the periventricular white matter (P=0.047 and 0.03 respectively, Fig. 1A, 1B). Accordingly, Western blot analyses revealed that dexamethasone and betamethasone treatment reduced the levels of MBP (P<0.01and 0.001, respectively, Fig. 1C, 1D). Similarly, other components of myelin—myelin associated glycoprotein (MAG) and CNPase—were also significantly reduced in dexamethasone and betamethasone treated pups compared to vehicle treated controls (MAG: P < 0.01 and 0.004; CNPase: P < 0.01 and 0.02 for dexamethasone and betamethasone, respectively; Fig. 1E-1H). The expression of MBP, MAG, and CNPase was comparable between dexamethasone- and betamethasone-treated pups in Western blot analyses. Together, both the GCs–dexamethasone and its diastereoisomer betamethasone—in high-dose almost equally inhibited myelination of the white mater in preterm rabbit pups.
Fig. 1. High-dose GC treatment reduced myelination at d 14.
A, B) Representative immunofluorescence of cryosections from periventricular corona radiata labeled with MBP antibody in rabbit pups treated with dexamethasone, betamethasone, or vehicle. Scale bar, 100 μm. V= ventricle. Data are mean ± SEM (n = 5 each group). Volume fraction of MBP+ labeling was significantly less in GC treated pups compared with controls on stereological analyses. C, D) Representative Western blot analyses for MBP in the forebrain of pups at d 14. Adult rat brain was positive control (arrow). Graph shows mean ± SEM (n = 5 each group). Values were normalized to β-actin. The MBP levels were reduced in the forebrain of pups treated with dexamethasone or betamethasone relative to controls. E, F) Representative Western blot analyses for MAG in the forebrain homogenates of pups as indicated. Graph shows mean ± SEM (n = 5 each). MAG levels were reduced in pups treated with GC compared to controls. β-actin blot for both MAG and CNPase is the same. G, H) Western blot analyses were performed for CNPase in the forebrain homogenates of pups as indicated. The bar charts are mean ± SEM (n = 5 each). CNPase levels were attenuated in pups treated with dexamethasone and betamethasone vs. controls. *P<0.05, **P<0.01 high-dose dexamethasone vs. vehicle. #P<0.05, ††P<0.01, ‡‡P<0.001 high-dose betamethasone vs. vehicle. Dexa, dexamethasone; Beta, betamethasone. OD=Optical density
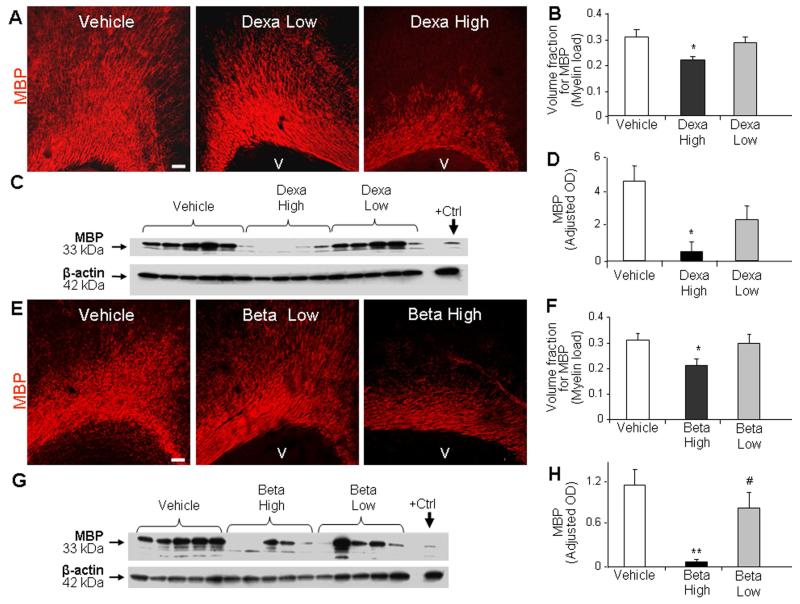
Since both in vivo and in vitro studies have shown that the effect of GCs on neurons is dose-dependent (Abraham et al., 2000; Uno et al., 1994; Uno et al., 1990), we hypothesized that GCs would inhibit myelination in a dose dependent manner. To this end, we compared the effect of high-dose dexamethasone (0.5 mg/kg/d), low-dose dexamethasone (0.2 mg/kg/d), and vehicle treatment on myelination. Stereological quantification in immuno-labeled sections revealed that high-dose dexamethasone, but not the low-dose, reduced volume fraction of MBP compared to the vehicle controls (P=0.045, Fig. 2A, 2B). Accordingly, Western blot analyses showed that MBP levels were reduced in high-dose dexamethasone treated pups compared to vehicle controls (P=0.008, Fig. 2C, 2D), and that low-dose dexamethasone did not affect MBP expression. We next compared the effects of high- (0.5 mg/kg/d) and low-dose (0.2 mg/kg/d) betamethasone on myelination. Stereological analyses of immunolabeled sections revealed lower MBP expression in high-dose betamethasone compared to vehicle treated pups (P=0.044, Fig. 2E, 2F). MBP levels were comparable between low-dose betamethasone and vehicle treated pups. Western blot analyses revealed reduced MBP levels in high-dose betamethasone treated pups relative to vehicle controls and low-dose betamethasone treated pups (P=0.003, 0.028; Fig. 2G, 2H). Together, both dexamethasone and betamethasone in high-dose significantly reduced myelination, but not in low-dose.
Fig. 2. Low-dose GCs did not reduce myelination.
A, B) Representative immunofluorescence of cryosections from corona radiata stained for MBP from pups treated with high or low dose of dexamethasone. MBP expression was more abundant in the pups treated with low-dose than high-dose dexamethasone. Scale bar, 100 μm. V=ventricle. Volume fraction of MBP was reduced in high-dose dexamethasone-treated pups than controls. The bars are mean±SEM (n = 5 each). C, D) Western blot analyses for MBP were performed in the forebrain homogenates of pups at d 14. Adult rat brain was positive control (arrow). Graph shows mean ± SEM (n = 5 each). MBP levels were less in high-dose dexamethasone treated pups than vehicle controls. E, F) Representative immunofluorescence of cryosections from betamethasone-treated (d 14) pups labeled with MBP antibody. Stereological analyses revealed reduced volume fraction of MBP in high-dose than vehicle controls. The bars are mean ± s.e.m (n = 5 each). G, H) Western blot analyses for MBP were performed in the forebrain homogenates of rabbit pups at d 14. The bar chart shows mean ± SEM (n = 5 each). MBP level was less in high-dose betamethasone than vehicle controls and low-dose betamethasone treated pups. *P<0.05, **P<0.01 high-dose GC vs. vehicle. #P<0.05, high vs. low-dose GC. OD=Optical density
Dexamethasone, but not betamethasone, induces astrogliosis
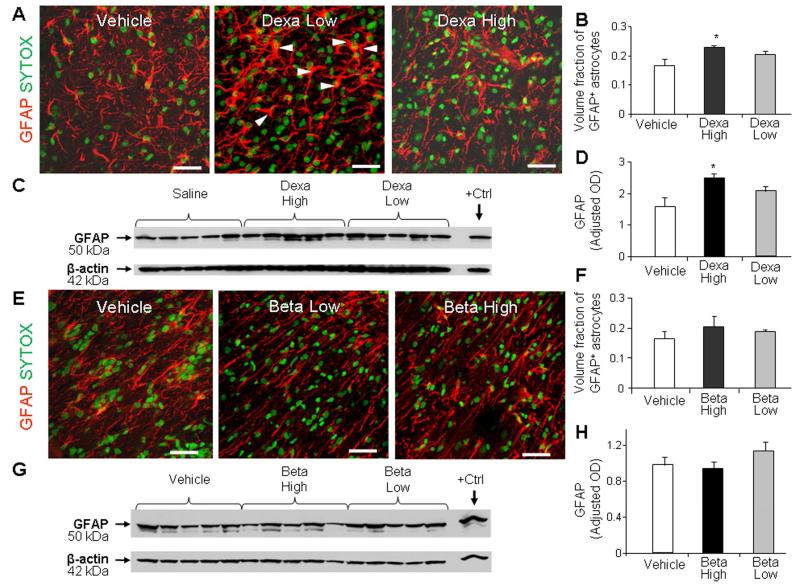
To evaluate the effect of GCs on astrogliosis, we compared the expression of glial fibrillary acidic protein (GFAP) in high-dose dexamethasone, low-dose dexamethasone, and vehicle-treated pups at d 14. Stereological analyses revealed that the high-dose of dexamethasone raised the volume fraction of GFAP+ astroglial cell bodies and fibers compared to vehicle controls (P=0.03, Fig. 3A, 3B). However, low-dose of dexamethasone did not significantly affect astrocytes. Accordingly, Western blot analyses demonstrated that high-dose, but not low-dose, dexamethasone elevated GFAP levels compared to vehicle controls (P<0.05, Fig. 3C, 3D).
Fig. 3. High-dose dexamethasone induced gliosis, but not the low dose.
A, B) Representative immunofluorescence of cryosections from pup (d 14) treated with dexamethasone labeled with GFAP antibody and sytox (nuclear staining). Note abundant hypertrophic astrocytes in pups treated with high-dose compared to low-dose dexamethasone. Scale bar, 20 μm. Graph shows mean ± SEM (n = 5 each). High-dose, not the low-dose, dexamethasone treatment increased volume fraction of astroglial fibers compared to controls on stereological-analyses. C, D) Western blot analyses for GFAP were performed in forebrain homogenates of pups (14 d). Adult rat brain was positive-control. Graph shows mean ± SEM (n = 5 each). Values were normalized to β-actin. High-dose dexamethasone, not low-dose, increased the GFAP levels compared to vehicle controls. *P < 0.05 for high-dose dexamethasone vs. vehicle controls. E, F) Representative immunolabeling of cryosections from pups (14 d) treated with betamethasone stained with GFAP antibody sytox (nuclear staining). Note astrocyte morphology and density of astroglial fibers are similar in high-dose and low-dose betamethasone treated pups. Scale bar, 20μm. Bar chart shows mean ± SEM (n = 5 each). Neither high-nor low-dose betamethasone treatment affected the volume fraction of astroglial fibers on stereological-analyses. G, H) Western blot analyses for GFAP were performed in the forebrain homogenates. Adult rat brain was positive-control (arrow). Graph shows mean ± SEM (n = 5 each). Betamethasone did not affect GFAP levels.
We next assessed the effect of betamethasone on astrogliosis. We noted that neither high- nor low-dose betamethasone treatment significantly increased GFAP expression on stereological evaluation (Fig. 3E, 3F). Similarly, Western blot analyses revealed that GFAP levels were not affected by high or low dose of betamethasone treatment (Fig. 3G, 3H).
To further assess the effect the high-dose dexamethasone on glia, we evaluated coronal sections from the forebrain (d 3), which were triple stained with GFAP, Iba1 (microglia) and Ki67 (proliferation marker) antibodies. We found that proliferation of GFAP+ astrocytes or Iba1+ microglia was rare in the corona radiata and corpus callosum of both GC and vehicle treated pups. In addition, the density of Iba1+ microglia did not differ between the two groups (Supplemental Fig. 1A, 1B). Together, dexamethasone induced astrogliosis only in high doses, whereas betamethasone did not stimulate gliosis at either high or low doses. In addition, high-dose dexamethasone did not affect microglial density.
Glucocorticoid receptors mediate GC-induced hypomyelination, but not the GC-induced astrogliosis
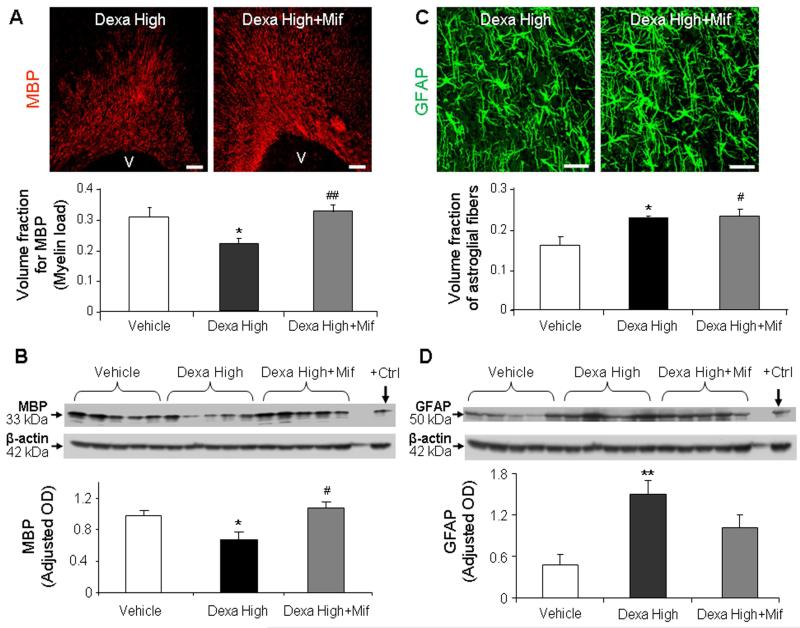
The genomic effects of GCs are mediated by GR (Stahn and Buttgereit, 2008). To determine whether reduced myelination in GC treated pups is a genomic effect, we compared three sets of preterm rabbit pups at d 14: a) high-dose dexamethasone treatment, b) high-dose dexamethasone plus mifepristone treatment, and c) vehicle controls. Stereological analysis of immunolabeled sections and Western blot analyses revealed that MBP expression was higher in pups treated with a combination of mifepristone and dexamethasone compared with only dexamethasone (P=0.002 and 0.015 respectively; Fig. 4A, 4B) MBP levels were comparable between pups receiving dexamethasone with mifepristone and vehicle treatment. Mifepristone blocks both GR and progesterone receptor. And progesterone inhibition does not enhance, but might reduce, myelination (Ghoumari et al., 2003). Therefore, Mifepristone-induced restoration in myelination appears predominantly to be the effect of GR inhibition rather than progesterone receptor antagonism. Collectively, co-administration of dexamethasone and GR antagonist demonstrates that GR-mediated hypomyelination is GR-dependent.
Fig. 4. Dexamethasone-induced hypomyelination was reversed by mifepristone treatment, but not dexamethasone-induced gliosis.
A) Representative immunofluorescence of cryosections from pups (d 14) treated with high-dose dexamethasone and high-dose dexamethasone plus mifepristone, labeled with MBP antibody. Note more abundant immunoreactivity for MBP in pups receiving dexamethasone with mifepristone compared to dexamethasone alone. Scale bar, 100 μm. Graph shows mean ± SEM (n = 5 each). B) Western blot analyses for MBP were performed in the forebrain homogenates of pups as indicated. Adult rat brain was positive-control (arrow). Graph shows mean ± SEM (n = 5 each). MBP levels were less in dexamethasone-treated pups compared with vehicle or dexamethasone plus mifepristone treatment. C) Cryosections from high-dose dexamethasone and high-dose dexamethasone with mifepristone treated pups were immunolabeled with GFAP antibody. Note comparable immunoreactivity for GFAP in dexamethasone, and dexamethasone with mifepristone treated pups. Scale bar, 20μm. Graph shows mean±SEM (n = 5 each). D) Western blot analyses for GFAP were performed in forebrain homogenates of pups. Graph shows mean ± SEM (n = 5 each). GFAP levels were comparable between pups receiving dexamethasone with mifepristone and dexamethasone alone. For Fig. A and B: *P < 0.05 high-dose dexamethasone vs. vehicle. #P < 0.05 high-dose dexamethasone (dexa high) vs. dexamethasone + mifepristone (Mif). For Fig C and D: *P < 0.05 for high-dose dexamethasone vs. vehicle. #P<0.05, for vehicle vs. dexamethasone + mifepristone.
We next postulated that GC-induced gliosis is mediated by GR. To this end, we quantified GFAP expression in the above 3 sets of pups by stereological quantification of immunolabeled sections and Western blot analyses. Stereological quantification showed that the volume fraction of GFAP+ glial fibers were higher in both dexamethasone and dexamethasone with mifepristone treated pups relative to vehicle controls (P=0.03 and 0.04, Fig. 4C). In addition, GFAP expression was comparable between dexamethasone and dexamethasone plus mifepristone treated animals. Accordingly, Western blot analyses revealed that GFAP levels were comparable between dexamethasone and dexamethasone with mifepristone treated pups, but reduced in controls relative to the dexamethasone treated pups (P<0.01 each, Fig. 4D). Hence, blocking GR by mifepristone did not decrease GFAP expression in dexamethasone treated pups, suggesting that dexamethasone-induced gliosis is mediated by GR-independent non-genomic mechanism(s).
High-dose dexamethasone affects cell cycle, MAP kinase, and GR associated genes
To determine overall transcriptomic changes after dexamethasone treatment, we performed microarray analyses on high-dose dexamethasone and vehicle treated pups (coronal slice taken at the level of midseptal nucleus) at d 3. The gene expression data complying with the “Minimum Information about Microarray Experiments” (MIAME) were deposited at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44610. The “Better Bunny” software selected 43 myelin, 244 cell cycle regulation, 114 MAP kinase signaling associated, and 16 GC signaling unigenes that were adequately quantified in all samples. Of these, a number of genes involved in cell death, cell proliferation, and MAP kinase signaling were significantly altered in high-dose dexamethasone treated pups compared to vehicle controls (Suppl. Table 1). Annexin A1 (ANXA1), a pro-apoptotic gene activated in inflammation, was upregulated, whereas subunit 12 and 17 of caspase-3 (CASP3) were reduced. Genes linked with progression of cell cycle, including ribosomal protein S6 kinase beta 1 (KS6B1), polo-like kinase 2 (PLK2), ribosomal RNA processing 8 (PRP8), and tumor protein D52 like 1 (TPD52L1) were elevated, while CDC28 protein kinase 2 (LOC100349119) was reduced in dexamethasone treated pups compared to vehicle controls. However, a number of genes connected with cell cycle arrest, including excision repair cross-complementing rodent deficiency complementation group 3 (ERCC3), multi drug resistance P-glycoprotein −1 (MDR-1), sestrin 1 (SESN1, aka PA26), telomeric repeat binding factor (NIMA-interacting)1 (TERF1), tumor susceptibility gene 101 (TSG101), and ubiquitin specific peptidase 47(USP47) were also elevated. Hence, the combinatorial effect of changes in the expression of these genes could affect the neural cell proliferation and maturation.
We next assessed genes involved in MAP kinase signaling. MAP kinase kinase 4 (MAP2K4), angiotensinogen (AGT), zinc finger protein 622 (ZNF622), and neudesin neurotropic factor (NENF) were elevated in pups treated with dexamethasone compared to controls; and these molecules are linked with activation of MAP kinase signaling. Nevertheless, caveolin-1 (CAV1), expressed in the blood brain barrier and an inhibitor of MAP kinase signaling, was also elevated in GC treated animals. Bone morphogenetic protein 4 (BMP4) expression was elevated, whereas that of GR was reduced in GC treated pups. GFAP was unaltered in dexamethasone treated pups. In conclusion, dexamethasone treatment alters a wide-array of genes related to cell cycle, MAP-kinase signaling, myelination, gliosis, and programmed cell death; the biological relevance of these changes will depend upon the collective effect of these altered pathways.
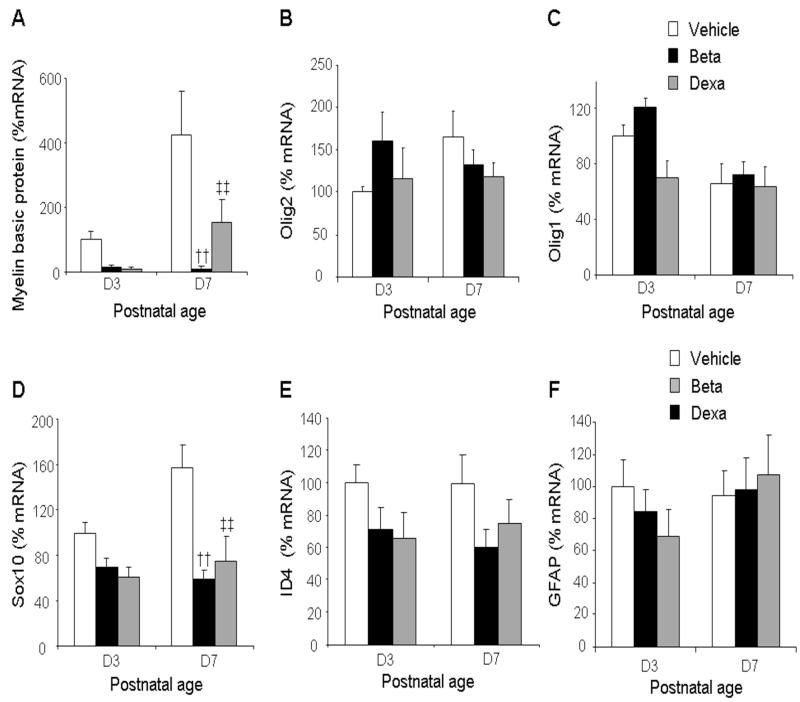
High-dose GCs reduce transcription of MBP and Sox10 gene, but not of GFAP gene
To confirm whether GC-induced hypomyelination is a genomic action and gliosis a non-genomic action, we performed real time qPCR to compare mRNA accumulation of genes controlling myelination--MBP, Olig1/2, Id4 and Sox10—and gliosis (GFAP) between GC treated pups and vehicle controls. We found that mRNA levels for MBP were significantly reduced in both betamethasone and dexamethasone treated pups compared to vehicle controls at d 7 (P=0.001 both; Fig 5), but not at d3. Similarly, mRNA expression of Sox10 was significantly reduced in both betamethasone and dexamethasone treated pups relative to controls at 7d (P=0.001 both; Fig 5). The mRNA accumulation for other bHLH transcription factors—Olig1/2 and ID4--was comparable between GC and vehicle treated pups. Moreover, GFAP mRNA expression did not differ between GC-treated pups and vehicle controls. GC-induced increase in GFAP protein without an associated elevation in gene expression can be attributed to either reduced degradation of GFAP or to alteration(s) in the post translational modifications of GFAP. Hence, high-dose dexamethasone or betamethasone reduced transcription of MBP and Sox10, suggesting that suppression of myelination on GC treatment was mediated at transcription level via GC receptors.
Fig. 5. High-dose GCs reduced transcription of MBP and Sox10 genes.
Data are mean ± SEM (n = 4-6 pups, each group). MBP mRNA expression was reduced in both dexamethasone and betamethasone treated pups compared to controls at d 7 (A). Olig1, Olig2 and Id4 mRNA levels were similar between the three groups (B, C, E). Sox10 mRNA levels were reduced in both dexamethasone- and betamethasone-treated pups at d 7 relative to controls. GFAP mRNA accumulation was similar among the three groups at both d 3 and 7 (F). †† P < 0.001 for high-dose betamethasone vs. vehicle at d 7. ‡‡P < 0.001for high-dose dexamethasone vs. vehicle at d 7
High-dose GC treatment, not low-dose, induces motor impairment; and mifepristone alleviates motor weakness in GC treated pups
To determine whether GC treatment induces neurological impairment as a function of dose, we performed neurobehavioral evaluation on 5 sets of preterm pups at d 14, based on a previously described protocol (Chua et al., 2009): a) high-dose dexamethasone, b) low-dose dexamethasone, c) high-dose betamethasone, d) low-dose betamethasone, and e) vehicle treatment. We observed significant motor impairment in 37% of high-dose dexamethasone and 44% of high-dose betamethasone treated pups, whereas low-dose GC treated pups and vehicle controls did not exhibit any motor weakness (Table 2). Motor activity in the hind limb—capability to lift the trunk and pelvis--was also significantly reduced in high-dose dexamethasone and betamethasone treated pups compared to controls (P<0.05 both, Table 2), while there was no difference between low-dose GC and vehicle treated pups for this parameter. Similarly, scores for posture and righting reflex were inferior for high-dose dexamethasone and betamethasone treated pups relative to controls (P<0.05 all).The latency to slip down a ramp pitched at a slope of 60° was significantly shor ter in duration for high-dose dexamethasone and betamethasone compared to their low-dose counterparts and controls (P<0.01 each). The distance walked in 60 seconds was also less in high-dose GC treated pups compared to low-dose GC treated pups and controls (P<0.001 and 0.01 for both dexamethasone and betamethasone). Scores for gait were worse for high-dose betamethasone treated pups compared to low-dose betamethasone and vehicle treated pups (P<0.05 each). High-dose dexamethasone treated pups also scored significantly less for gait compared to vehicle controls (P<0.05), but not low-dose dexamethasone treated pups.
To determine the effect of GR inhibition on neuro-behavioral scores in dexamethasone treated pups, we compared high-dose dexamethasone and high-dose dexamethasone with mifepristone treated pups. We found that scores for distance walked over 60 seconds, and the ability to hold their position at 60° inclin ation was significantly better in pups treated with dexamethasone with mifepristone compared to dexamethasone alone. In conclusion, neurobehavioral scores were superior in low-dose GC treated pups compared to high-dose GC treated pups and mifepristone treatment improved the motor performance of the high-dose dexamethasone treated pups.
High-dose GC administration leads to poor weight gains
Previous studies in both preterm infants and newborn animals have shown that GC treatment results in poor weight gain (Tsuneishi et al., 1991; Watterberg). Therefore, we asked whether weight was reduced in high or low dose GC treated pups compared to controls. We found that weight was significantly reduced in high-dose dexamethasone and betamethasone treated pups compared to controls at d 14. [106±6.3 (control) vs. 74.5 ± 4.9 (betamethasone) vs. 85 ± 3.4 g (dexamethasone); P=0.002 and 0.01]. The mean weights of low-dose dexamethasone and betamethasone treated pups showed a trend toward decrease relative to controls [106 ± 6.3 (control) vs. 91.9 ± 4.9 (dexamethasone) vs. 83.9 ± 5.3 g (betamethasone)]; however the comparison was not significant. The difference in weight between high-dose dexamethasone with mifepristone and high-dose dexamethasone treated pups was also not significant (106.63 ± 87.8 vs. 87.2 ± 7.0 g). At d 21, the dexamethasone treated pups were still smaller in weight compared to vehicle controls (122.3 ± 8.3 vs. 163.6 ± 9.6 g, P = 0.026). In addition, the GC treated pups were less active and their hair growth was delayed compared to vehicle controls.
GC treatment reduces oligodendrocyte proliferation, but does not affect apoptosis
Since prenatal betamethasone induces apoptosis in fetal white matter in both rabbits and sheep (Malaeb et al., 2009; Vinukonda et al., 2010), we asked whether postnatal GCs would result in increased programmed cell death in the periventricular white matter. To this end, we performed TUNEL staining in high-dose betamethasone, high-dose dexamethasone, and vehicle treated pups at d 3. We noted that neither postnatal dexamethasone nor betamethasone increased TUNEL+ apoptosis in periventricular white matter (corona radiata and corpus callosum) compared to vehicle controls (20.6±1.4 vs. 19.4±1.5 vs. 16.6±2.1 per mm2, respectively).
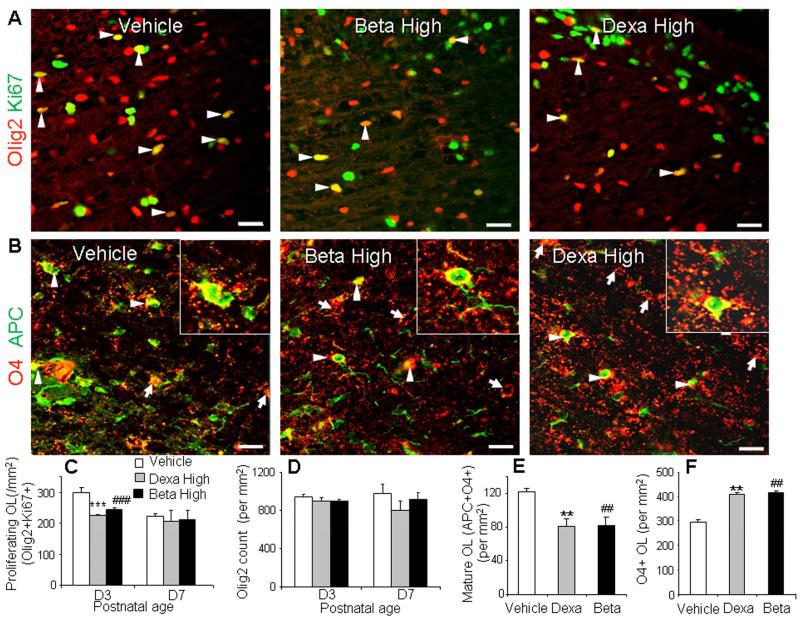
Since dexamethasone has reduced proliferation of OL progenitors in culture experiments (Barres et al., 1994), we compared proliferation of OL lineage cells between high-dose betamethasone, high-dose dexamethasone, and vehicle treated pups at both d 3 and d 7. To this end, we quantified proliferating Olig2+ cells in sections double-labeled with Ki67 and Olig2 antibodies. Olig2 labels both OL progenitors and mature OL. The proliferating Olig2+ (Olig2+KI67+) cells were reduced in the corona radiata and corpus callosum of high-dose betamethasone and dexamethasone treated pups compared with vehicle controls at d 3 (P < 0.01 each), but not at d 7 (Fig. 6A, 6C). Together, high-dose GC administration suppressed proliferation of cells of OL lineage at d 3, but did not impact apoptosis.
Fig. 6. High-dose GC treatment suppressed both proliferation and maturation of OLs.
A) Representative immunofluorescence of cryosections from 3 d old pups double-labeled with Olig2 and Ki67 specific antibody. Note lower density of Olig2+Ki67+ (proliferating OL, arrowheads) OL in dexamethasone-, betamethasone-treated animals compared with vehicle controls. B) Cryosections were double labeled with O4 (arrow) and APC antibodies. Note reduced number of cells co-labeled with O4 and APC (arrowhead) in dexamethasone and betamethasone treated pups compared to vehicle controls. C) Graph shows mean ± SEM (n = 5 each). Proliferating OL (Olig2+Ki67+) were reduced in density in GCs treated pups compared with vehicle controls at d 3, not at d 7. D) Graph shows mean ± SEM (n = 5 each). Olig2 count displayed similar abundance in dexamethasone, betamethasone and control pups at 3 d and d 7. E) The bar chart shows mean ± SEM (n = 5 each). O4+APC+ OL were reduced in density in dexamethasone, betamethasone treated pups relative to controls at 7d. F) Graph shows mean ± SEM (n = 5 each). O4+ OL were higher in density in dexamethasone, betamethasone compared to vehicle controls at d 7. ***P < 0.001, **P < 0.01 for high-dose dexamethasone vs. vehicle. ###P < 0.001, ##P < 0.01 for high dose betamethasone vs. controls. All scale bars, 20 μm. OL, oligodendrocyte.
GC treatment reduces oligodendrocyte maturation
The findings, that GC treatment suppressed myelination and did not induce apoptosis of OLs, led us to the question of whether GCs inhibit maturation of OLs. To this end, we labeled brain sections from high-dose GC treated and vehicle treated pups with a) Olig2 antibody at d 3 and d 7, and b) APC (labels mature OLs) and O4 (stains pre-OLs and mature OLs) specific antibodies at d 7. We found that the density of Olig2+ cells in the corona radiata and corpus callosum was comparable between dexamethasone, betamethasone, and vehicle treated groups at both d 3 and 7 (Fig. 6D).
Evaluation of sections double labeled with APC and O4 antibody revealed that mature OLs (O4+APC+) in the white matter were significantly reduced in both betamethasone and dexamethasone treated pups compared to vehicle controls (P = 0.007, 0.008, Fig. 6B, 6E). O4+ OLs in the corona radiata and corpus callosum were increased in dexamethasone and betamethasone treated pups compared to vehicle controls (P < 0.001 each, Fig. 6F). This suggests that GC treatment suppresses the density of mature OLs, thus increasing the number of OL progenitors. Together, GC contributes to hypomyelination by inhibiting maturation of OL progenitors.
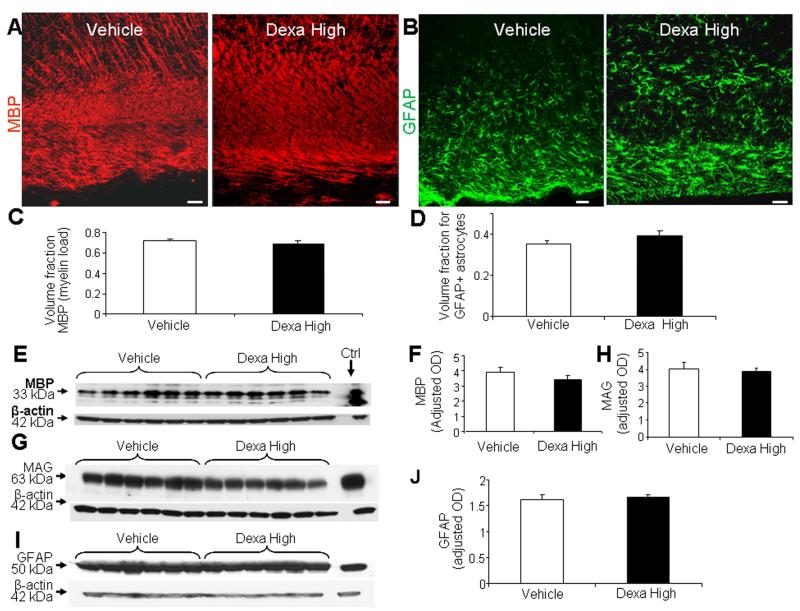
Effects of high-dose GCs over a longer developmental period
To evaluate the long term effect of GCs on myelination, we performed stereological quantification of MBP in immunolabeled brain sections and Western blot analyses on forebrain homogenates of dexamethasone-, and vehicle-treated pups at d 21. Stereological analyses depicted that the volume fraction of myelin (load) was similar in dexamethasone treated pups and vehicle controls (Fig. 7A, 7C). Western blot analyses using MBP specific antibody confirmed this finding (Fig. 7E, 7F). In addition, MAG expression was similar between the two groups on Western blot analyses (Fig. 7G, 7H). We then performed neurobehavioral evaluation of dexamethasone and vehicle treated animals, which showed no difference between the two groups (Suppl. Table 2). A comparison between betamethasone and vehicle treated animals at d 21 also showed no difference in the MBP and MAG expression, as well as the neurobehavioral functions between groups (data not shown).
Fig. 7. High-dose dexamethasone does not affect myelination and GFAP+ astrocytes in d 21 pups.
A, C) Representative immunofluorescence of cryosections from periventricular white matter labeled with MBP antibody from dexamethasone- and vehicle-treated pups at d 21. Note MBP+ immunoreactivity is similar in dexamethasone- and vehicle-treated pups. Scale bar, 50 μm. V = ventricle. Data are mean ± SEM (n = 5 each). Volume fraction of MBP+ labeling was comparable between the groups on stereological-analyses. B, D) Representative immunofluorescence of cryosections labeled with GFAP antibody from pups (d 21) treated with dexamethasone or vehicle. Note similar morphology and distribution of astrocytes. Scale bar, 50 μm. Graph shows mean ± SEM (n = 6 each). Volume fraction of astroglial fibers was similar between groups. E, F) Representative Western blot analyses for MBP in forebrain of pups treated with dexamethasone, and vehicle at d 21. Graph shows mean ± SEM (n = 5 each). MBP levels were similar between groups. G, H) Representative Western blot analyses for MAG in the forebrain of pups treated with dexamethasone, or vehicle. Adult rat brain was positive-control (arrow). Graph shows mean ± SEM (n = 6 each). The MAG levels were comparable between dexamethasone- and vehicle-treated pups. I, J) Western blot analyses for GFAP on pups (d 21) as indicated. Graph shows mean ± SEM (n = 6 each). GFAP levels were similar between high-dose dexamethasone- and vehicle-treated pups.
We next compared GFAP levels between dexamethasone and vehicle treated pups. GFAP expression was assessed by stereology in immunostained sections (Fig. 7 B, 7D) and by Western blot analyses on forebrain homogenates (Fig. 7I, 7J). GFAP expression was comparable between dexamethasone treated and vehicle treated pups at d 21. Together, the GC-induced hypomyelination, gliosis, and neurological impairment, observed at d 14, spontaneously recovered by d 21.
DISCUSSION
The incidence of prematurity is over 10% worldwide. The neurologic sequelae in the survivors of prematurity are major public health concerns because of increased survival of the extremely preterm infants (<28 gestational weeks, survival of 80%) and their high rate (>30%) of neurodevelopmental impairment (Lorch et al., 2012; Wilson-Costello et al., 2005). A large number of these premature infants are ventilator-dependent for prolonged periods, develop chronic lung disease, and have an extended stay in the neonatal intensive care unit. Neonatologists use GCs to facilitate extubation, and prevent chronic lung disease, which expedites discharge of the preterm neonates. Since the use of GCs for this purpose in premature infants is also associated with developmental delay and cerebral palsy, it is exceedingly important to determine the effect of GC dose and preparation on myelination, gliosis, and both short and long-term neurological outcomes. In the present study, we found that high doses of both postnatal dexamethasone and betamethasone induced hypomyelination and motor impairment at d 14, but low doses of GC did not. More importantly, high-dose GC induced hypomyelination and neurological deficits reversed spontaneously by d 21. Hypomyelination in GC treated pups was attributed to genomic mechanisms, while the dexamethasone-specific gliosis was ascribed to non-genomic mechanisms.
One of the key findings of the present study was that treatment with high-dose postnatal GCs—dexamethasone and betamethasone--suppressed both maturation and proliferation of OL lineage cells in preterm rabbits. We evaluated both dexamethasone and betamethasone because they are commonly used in neonatal units and they differ with respect to the blood brain permeability and pharmacokinetic properties (Trenque et al., 1994). Specifically, the total number of mature OLs (APC+O4+) was reduced, while OL progenitors (APC-O4+) were increased in density in GC treated pups relative to controls at 7d. This suggested that GC treatment arrested the maturation of OLs, thereby increasing the population of OL progenitors and diminishing the density of mature OLs. A recent study has reported that postnatal dexamethasone in neonatal rats induces apoptotic changes in the pre-OLs resulting in reduction in their density and hypomyelination of the corpus callosum (Kim et al., 2013). In contrast, we did not find any evidence of apoptosis, but noted reduced proliferation and maturation of OL in premature rabbit pups. The discrepancy between these two sets of data can be attributed to different species of animals used, which exhibit dissimilar phases of OL maturation and susceptibility to GC (Clancy et al., 2007). Maturation of OLs is transcriptionally regulated; and basic helix-loop-helix (bHLH) transcription factors—Olig1, Olig2, Id4 and Sox10— are involved in OL lineage specification and in progressive stages of maturation including myelination (Gokhan et al., 2005; Stolt et al., 2002). We noted significant reduction in MBP and Sox10 mRNA expression in GC-treated pups compared to vehicle controls. However, the other transcription factors--Olig1, Olig2, and Id4--were not affected by GC treatment. Since the MBP promoter contains a binding sequence for Sox10, and because Sox10 activates the MBP promoter leading to terminal differentiation of OLs, a reduction in Sox10 expression might hinder myelination (Gokhan et al., 2005), (Stolt et al., 2002). In addition, Sox10 diverts multipotent neural progenitor cells toward the OL lineage during development (Pozniak et al., 2010). Hence, a downregulation of Sox10 after GC treatment would reduce both the commitment of precursors to OL lineage and maturation of OLs. Taken together, high-dose postnatal GC treatment suppresses transcription of MBP and Sox10 gene, and inhibits both maturation and proliferation of OL lineage, thereby reducing myelination of the white matter.
Several effects of GC treatment are dose-dependent (Duksal et al., 2009; Powell et al., 2006). Accordingly, we observed that high-dose GC treatment led to hypomyelination, but not the low-dose of either dexamethasone or betamethasone. Both in vivo and in vitro studies have evaluated the effects of GC dose on neuronal degeneration. Prenatal administration of dexamethasone in rhesus monkeys at d 135 and 162 of gestation results in degeneration of hippocampal neurons in fetuses in a dose-dependent manner (Uno et al., 1994; Uno et al., 1990). In a rat model of NMDA-induced neuro-degeneration, high concentrations of corticosterone increases and low level of corticosterone decreases cholinergic fiber loss, exhibiting a U-shaped dose-response correlation. High-dose dexamethasone treatment also results in neuronal apoptosis in the hippocampus and reduction of brain weight, in contrast to the low-dose therapy (Duksal et al., 2009). To our knowledge, we are the first to demonstrate in animal models that hypomyelination is a manifestation of high-dose GC treatment, but not low-dose. A few clinical studies have also shown that low-dose GC (0.2 mg/kg × 5 d) induces recovery of pulmonary function, similar to high doses GC (McEvoy et al., 2004; Walther et al., 2003; Watterberg, 2010). Data from recent meta-analyses on postnatal dexamethasone studies have indicated a need for further studies on low-dose systemic corticosteroids in preterm infants (> 7 d age) at high risk of developing chronic lung disease (Doyle et al., 2014a, b). Hence, low doses of GC might be relatively safe in preterm infants compared to high-dose, however, this needs to be confirmed on a larger multicenter clinical trial.
One of the important findings of the present study was that GC-induced hypomyelination and neurological deficits noted at d 14 were spontaneously reversible at d 21. This delay in myelination and neurological function on GC treatment has been found in previous studies. Repeated prenatal GC treatment in fetal sheep delays myelination in the corpus callosum; and postnatal steroid decelerates myelination of the optic nerve (Bohn and Friedrich, 1982; Huang et al., 2001). Importantly, the weight of dexamethasone treated pups were significantly less compared to vehicle controls at d 21, but the motor performance of the d 21 pups was similar between the two group of pups. This suggests that reduction in weight did not significantly confound the motor function of the pups. The preterm rabbits received postnatal GC in the first 5 days of life, developed hypomyelination at d 14, and showed recovery at d 21. It is plausible that discontinuation of GC after a short period of treatment led to a reversal in genomic and morphological (proliferation and maturation of OL) changes. However, long-duration GC regimen during the major phase of OL maturation and myelination might lead to permanent hypomyelination and neurological deficit. Although, premature infants treated with GC exhibit neurodevelopmental delay and motor impairment at school age, the effects of postnatal GC beyond school age and during adolescence showed no significant difference between GC treated and untreated subjects (Barrington, 2001; Doyle et al., 2014a; Jones and Collaborative Dexamethasone Trial Follow-up, 2005; Yeh et al., 2004). However, the available systematic- and meta-analyses of clinical trials on postnatal GCs are flawed by inclusion of studies that were too heterogeneous with respect to the dosing regimen, duration of treatment, and timing of the initiation of treatment (Crotty et al., 2012; Jones and Collaborative Dexamethasone Trial Follow-up, 2005; Watterberg, 2010). While conventional clinical wisdom raises concern about the impact of GC on development, the available clinical evidence is not definite. Some studies show that dexamethasone treated children exhibit poor motor-skills and visual-motor integration at school age, while others conclude that dexamethasone treated children display better or similar behavioral outcomes during childhood and adolescence compared to untreated controls (Crotty et al., 2012; Gross et al., 2005; Halliday et al., 2003). This underscores the need for additional randomized control trials to determine the long-term outcome of postnatal GCs. Hence, the neurological deficit induced by high-dose of GC might be transient and recover spontaneously.
We demonstrated that GC-induced hypomyelination and motor impairment in rabbit pups were reversed by blocking GRs, suggesting the effect of GCs on myelination to be GR-mediated. GRs are expressed in the cytoplasm of OLs and exert genomic effects either by transactivation or transrepression (Stahn and Buttgereit, 2008; Vielkind et al., 1990). We performed microarray analyses to assess the genomic effects of GCs. We found that a number of molecules linked with cell cycle regulation, MAP kinase pathway, GR signaling, and apoptosis were significantly altered. These pathways control OL survival and maturation, and disturbances in these cascades in GC treated infants would impact myelination (Dummula et al., 2011; Ishii et al., 2012). We found that genes linked with cell-cycle arrest, including BMP4, PA26, and others were elevated in high-dose dexamethasone treated pups compared to controls. Hence, GC-induced BMP4 and PA26 upregulation might have contributed to reduced proliferation and arrested maturation of OLs, leading to myelination failure (Dummula et al., 2011; Velasco-Miguel et al., 1999). We also noted elevation in both pro-apoptotic and anti-apoptotic genes. However, their combinatorial effect did not result in increased apoptosis in GC-treated pups. Consistent with previous studies, dexamethasone treatment reduced transcription of the GR gene (Okret et al., 1986). Together, GC treatment activates GR resulting in significant changes in a wide array of genes and more importantly, GCs induce hypomyelination via GR-dependent genomic mechanisms.
Interestingly, dexamethasone treatment induced astrogliosis, whereas betamethsone did not affect the morphology of astrocytes. Intriguingly, dexamethasone-induced gliosis was not blocked by a GR antagonist, consistent with an effect of dexamethasone mediated by GR-independent non-genomic mechanisms. The non-genomic mechanism may be a direct result of GCs or an indirect effect via an agonist, impacting cell membrane lipids, intracytoplasmic proteins, or through GC transporters (Stahn and Buttgereit, 2008). Previous in vivo and in vitro studies have shown that dexamethasone reduces astrocyte number, which is in inconsistent with the present study (Claessens et al., 2012; Unemura et al., 2012). However in those studies, the dose of dexamethasone used in P1 rat pups was lower and shorter in duration compared to our study. In addition, preterm rabbits (E29) may exhibit dissimilar timing of telencephalon maturation as P1 rats (Claessens et al., 2012). We were intrigued to find that dexamethasone induced gliosis, but not betamethasone. Since, these two GCs are diastereoisomers that exhibit different pharmacodynamics and penetration of the blood brain barrier (Trenque et al., 1994), they might display dissimilar non-genomic actions. Collectively, dexamethasone-induced gliosis is a novel non-genomic function of dexamethasone, which is not observed on betamethasone treatment.
Conclusion
Two commonly used GCs in neonatal-perinatal medicine—dexamethasone and betamethasone—in high doses induced hypomyelination and motor impairment in preterm rabbit pups at d 14, which was reversible at d 21. Myelination failure in high-dose GC treated pups was attributed to reduced transcription of MBP and Sox10 genes, and suppressed proliferation along with arrested maturation of OL progenitors. Hypomyelination was mediated by GR-dependent genomic mechanisms, whereas astrogliosis was induced by GR-independent non-genomic mechanisms upon dexamethasone treatment. Since low-dose GCs induce neither hypomyelination nor motor deficit in rabbit pups, we speculate that low-dose GCs used for the treatment of chronic lung disease are less likely to induce developmental delay or cerebral palsy in preterm infants. A long term follow up of high-dose GC treated infants beyond school age is important to determine neurological recovery in these infants, if any. Hence, there is an unmet need for large multicenter randomized control trials to determine the neurodevelopment outcome of GC treatment in both low and high doses in preterm infants at risk of chronic lung disease so that beneficial effects of GC might be exploited to enhance the quality of life of premature infants.
Supplementary Material
HIGHLIGHTS.
High-dose, but not low-dose, dexamethasone and betamethasone delay myelination
High-dose dexamethasone induces astrogliosis, betamethasone does not
High-dose GCs, not low-dose, delay motor function
GCs inhibit proliferation and maturation of oligodendrocytes
GCs induce hypomyelination by genomic and astrogliosis by non-genomic mechanisms
Acknowledgements
This work was supported by NIH-NINDS Grants RO1 NS071263 (P.B.) and R01NS083947 (P.B.), and a scientist development grant from the American Heart Association (G.V.). We thank Joanne Abrahams for the assistance with images.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abraham I, Harkany T, Horvath KM, Veenema AH, Penke B, Nyakas C, Luiten PG. Chronic corticosterone administration dose-dependently modulates Abeta(1-42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol. 2000;12:486–494. doi: 10.1046/j.1365-2826.2000.00475.x. [DOI] [PubMed] [Google Scholar]
- Bakker JM, van Bel F, Heijnen CJ. Neonatal glucocorticoids and the developing brain: short-term treatment with life-long consequences? Trends Neurosci. 2001;24:649–653. doi: 10.1016/s0166-2236(00)01948-2. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13:477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- Barres BA, Lazar MA, Raff MC. A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development. 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. doi: 10.1186/1471-2431-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baud O, Foix-L’Helias L, Kaminski M, Audibert F, Jarreau PH, Papiernik E, Huon C, Lepercq J, Dehan M, Lacaze-Masmonteil T. Antenatal glucocorticoid treatment and cystic periventricular leukomalacia in very premature infants. N Engl J Med. 1999;341:1190–1196. doi: 10.1056/NEJM199910143411604. [DOI] [PubMed] [Google Scholar]
- Baud O, Verney C, Evrard P, Gressens P. Injectable dexamethasone administration enhances cortical GABAergic neuronal differentiation in a novel model of postnatal steroid therapy in mice. Pediatr Res. 2005;57:149–156. doi: 10.1203/01.PDR.0000148069.03855.C4. [DOI] [PubMed] [Google Scholar]
- Bohn MC, Friedrich VL., Jr. Recovery of myelination in rat optic nerve after developmental retardation by cortisol. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1982;2:1292–1298. doi: 10.1523/JNEUROSCI.02-09-01292.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, Gong X, Nguyen T, Nelson K, Luo NL, Ren J, Struve J, Sherman LS, Miller SP, Chau V, Hendson G, Ballabh P, Grafe MR, Back SA. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Annals of neurology. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambonie G, Mesnage R, Milesi C, Pidoux O, Veyrac C, Picaud JC. Betamethasone impairs cerebral blood flow velocities in very premature infants with severe chronic lung disease. J Pediatr. 2008;152:270–275. doi: 10.1016/j.jpeds.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 2009;40:3369–3377. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claessens SE, Belanoff JK, Kanatsou S, Lucassen PJ, Champagne DL, Ronald de Kloet E. Acute effects of neonatal dexamethasone treatment on proliferation and astrocyte immunoreactivity in hippocampus and corpus callosum: towards a rescue strategy. Brain Res. 2012;1482:1–12. doi: 10.1016/j.brainres.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Clancy B, Luo L, Thomas JE. Observation of nearly perfect irrotational flow in normal and superfluid strongly interacting Fermi gases. Phys Rev Lett. 2007;99:140401. doi: 10.1103/PhysRevLett.99.140401. [DOI] [PubMed] [Google Scholar]
- Coulter DM, LaPine T, Gooch WM., 3rd Intraventricular hemorrhage in the premature rabbit pup. Limitations of this animal model. Journal of neurosurgery. 1984;60:1243–1245. doi: 10.3171/jns.1984.60.6.1243. [DOI] [PubMed] [Google Scholar]
- Craig DB, Kannan S, Dombkowski AA. Augmented annotation and orthologue analysis for Oryctolagus cuniculus: Better Bunny. BMC Bioinformatics. 2012;13:84. doi: 10.1186/1471-2105-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty KC, Ahronovich MD, Baron IS, Baker R, Erickson K, Litman FR. Neuropsychological and behavioral effects of postnatal dexamethasone in extremely low birth weight preterm children at early school age. Journal of perinatology: official journal of the California Perinatal Association. 2012;32:139–146. doi: 10.1038/jp.2011.62. [DOI] [PubMed] [Google Scholar]
- DeBattista C, Belanoff J. The use of mifepristone in the treatment of neuropsychiatric disorders. Trends Endocrinol Metab. 2006;17:117–121. doi: 10.1016/j.tem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- DeCastro M, El-Khoury N, Parton L, Ballabh P, LaGamma EF. Postnatal betamethasone vs dexamethasone in premature infants with bronchopulmonary dysplasia: a pilot study. Journal of perinatology: official journal of the California Perinatal Association. 2009;29:297–304. doi: 10.1038/jp.2008.194. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. The Cochrane database of systematic reviews. 2014a;5:CD001146. doi: 10.1002/14651858.CD001146.pub4. [DOI] [PubMed] [Google Scholar]
- Doyle LW, Ehrenkranz RA, Halliday HL. Late (> 7 days) postnatal corticosteroids for chronic lung disease in preterm infants. The Cochrane database of systematic reviews. 2014b;5:CD001145. doi: 10.1002/14651858.CD001145.pub3. [DOI] [PubMed] [Google Scholar]
- Duksal F, Kilic I, Tufan AC, Akdogan I. Effects of different corticosteroids on the brain weight and hippocampal neuronal loss in rats. Brain Res. 2009;1250:75–80. doi: 10.1016/j.brainres.2008.10.051. [DOI] [PubMed] [Google Scholar]
- Dummula K, Vinukonda G, Chu P, Xing Y, Hu F, Mailk S, Csiszar A, Chua C, Mouton P, Kayton RJ, Brumberg JC, Bansal R, Ballabh P. Bone morphogenetic protein inhibition promotes neurological recovery after intraventricular hemorrhage. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:12068–12082. doi: 10.1523/JNEUROSCI.0013-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics. 2002;109:262–268. doi: 10.1542/peds.109.2.262. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. Journal of neurochemistry. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Gokhan S, Marin-Husstege M, Yung SY, Fontanez D, Casaccia-Bonnefil P, Mehler MF. Combinatorial profiles of oligodendrocyte-selective classes of transcriptional regulators differentially modulate myelin basic protein gene expression. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2005;25:8311–8321. doi: 10.1523/JNEUROSCI.1850-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier DG, Halliday HL. Corticosteroids in the prevention and management of bronchopulmonary dysplasia. Seminars in neonatology: SN. 2003;8:83–91. doi: 10.1016/s1084-2756(02)00189-6. [DOI] [PubMed] [Google Scholar]
- Gross SJ, Anbar RD, Mettelman BB. Follow-up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics. 2005;115:681–687. doi: 10.1542/peds.2004-0956. [DOI] [PubMed] [Google Scholar]
- Halliday HL, Ehrenkranz RA, Doyle LW. Moderately early (7-14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. The Cochrane database of systematic reviews. 2003:CD001144. doi: 10.1002/14651858.CD001144. [DOI] [PubMed] [Google Scholar]
- Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2001;19:415–425. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- Iacobas DA, Iacobas S, Urban-Maldonado M, Scemes E, Spray DC. Similar transcriptomic alterations in Cx43 knockdown and knockout astrocytes. Cell Commun Adhes. 2008;15:195–206. doi: 10.1080/15419060802014222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii A, Fyffe-Maricich SL, Furusho M, Miller RH, Bansal R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2012;32:8855–8864. doi: 10.1523/JNEUROSCI.0137-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SI, Pickard MR, Khong M, Smith HL, Mann CL, Emes RD, Chari DM. Identifying the cellular targets of drug action in the central nervous system following corticosteroid therapy. ACS chemical neuroscience. 2014;5:51–63. doi: 10.1021/cn400167n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Collaborative Dexamethasone Trial Follow-up, G. Randomized, controlled trial of dexamethasone in neonatal chronic lung disease: 13-to 17-year follow-up study: I. Neurologic, psychological, and educational outcomes. Pediatrics. 2005;116:370–378. doi: 10.1542/peds.2004-1818. [DOI] [PubMed] [Google Scholar]
- Kim JW, Kim YJ, Chang YP. Administration of dexamethasone to neonatal rats induces hypomyelination and changes in the morphology of oligodendrocyte precursors. Comp Med. 2013;63:48–54. [PMC free article] [PubMed] [Google Scholar]
- Lorch SA, Baiocchi M, Ahlberg CE, Small DS. The differential impact of delivery hospital on the outcomes of premature infants. Pediatrics. 2012;130:270–278. doi: 10.1542/peds.2011-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaeb SN, Hovanesian V, Sarasin MD, Hartmann SM, Sadowska GB, Stonestreet BS. Effects of maternal antenatal glucocorticoid treatment on apoptosis in the ovine fetal cerebral cortex. Journal of neuroscience research. 2009;87:179–189. doi: 10.1002/jnr.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney SE, Noguchi KK, Wozniak DF, Fowler SC, Farber NB. Long-term Effects of Multiple Glucocorticoid Exposures in Neonatal Mice. Behav Sci (Basel) 2011;1:4–30. doi: 10.3390/behavsci1010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy C, Bowling S, Williamson K, McGaw P, Durand M. Randomized, double-blinded trial of low-dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia. Pediatr Pulmonol. 2004;38:55–63. doi: 10.1002/ppul.20037. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BP, Inder TE, Huppi PS, Warfield S, Zientara GP, Kikinis R, Jolesz FA, Volpe JJ. Impaired cerebral cortical gray matter growth after treatment with dexamethasone for neonatal chronic lung disease. Pediatrics. 2001;107:217–221. doi: 10.1542/peds.107.2.217. [DOI] [PubMed] [Google Scholar]
- Okret S, Poellinger L, Dong Y, Gustafsson JA. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci U S A. 1986;83:5899–5903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K, Kerkering KW, Barker G, Rozycki HJ. Dexamethasone dosing, mechanical ventilation and the risk of cerebral palsy. J Matern Fetal Neonatal Med. 2006;19:43–48. doi: 10.1080/14767050500363519. [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Langseth AJ, Dijkgraaf GJ, Choe Y, Werb Z, Pleasure SJ. Sox10 directs neural stem cells toward the oligodendrocyte lineage by decreasing Suppressor of Fused expression. Proc Natl Acad Sci U S A. 2010;107:21795–21800. doi: 10.1073/pnas.1016485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinwell ES, Karplus M, Reich D, Weintraub Z, Blazer S, Bader D, Yurman S, Dolfin T, Kogan A, Dollberg S, Arbel E, Goldberg M, Gur I, Naor N, Sirota L, Mogilner S, Zaritsky A, Barak M, Gottfried E. Early postnatal dexamethasone treatment and increased incidence of cerebral palsy. Arch Dis Child Fetal Neonatal Ed. 2000;83:F177–181. doi: 10.1136/fn.83.3.F177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nature clinical practice. Rheumatology. 2008;4:525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M, Bartsch U, Wegner M. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev. 2002;16:165–170. doi: 10.1101/gad.215802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C, Shah PS, Dunn MS. Meta-analysis of postnatal steroid use challenged. Pediatrics. 2002;109:716–717. doi: 10.1542/peds.109.4.716. author reply 716-717. [DOI] [PubMed] [Google Scholar]
- Trenque T, Lamiable D, Vistelle R, Millart H, Leperre A, Choisy H. Comparative pharmacokinetics of two diastereoisomers dexamethasone and betamethasone in plasma and cerebrospinal fluid in rabbits. Fundamental & clinical pharmacology. 1994;8:430–436. doi: 10.1111/j.1472-8206.1994.tb00822.x. [DOI] [PubMed] [Google Scholar]
- Truffert P, Empana JP, Breart G, Saugstad OD, Goelz R, Halliday HL, Anceschi M. Treatment strategies for bronchopulmonary dysplasia with postnatal corticosteroids in Europe: the EURAIL survey. Acta Paediatr. 2003;92:948–951. [PubMed] [Google Scholar]
- Tsuneishi S, Takada S, Motoike T, Ohashi T, Sano K, Nakamura H. Effects of dexamethasone on the expression of myelin basic protein, proteolipid protein, and glial fibrillary acidic protein genes in developing rat brain. Brain Res Dev Brain Res. 1991;61:117–123. doi: 10.1016/0165-3806(91)90121-x. [DOI] [PubMed] [Google Scholar]
- Unemura K, Kume T, Kondo M, Maeda Y, Izumi Y, Akaike A. Glucocorticoids decrease astrocyte numbers by reducing glucocorticoid receptor expression in vitro and in vivo. J Pharmacol Sci. 2012;119:30–39. doi: 10.1254/jphs.12047fp. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Velasco-Miguel S, Buckbinder L, Jean P, Gelbert L, Talbott R, Laidlaw J, Seizinger B, Kley N. PA26, a novel target of the p53 tumor suppressor and member of the GADD family of DNA damage and growth arrest inducible genes. Oncogene. 1999;18:127–137. doi: 10.1038/sj.onc.1202274. [DOI] [PubMed] [Google Scholar]
- Vermont Oxford Network Steroid Study, G. Early postnatal dexamethasone therapy for the prevention of chronic lung disease. Pediatrics. 2001;108:741–748. doi: 10.1542/peds.108.3.741. [DOI] [PubMed] [Google Scholar]
- Vielkind U, Walencewicz A, Levine JM, Bohn MC. Type II glucocorticoid receptors are expressed in oligodendrocytes and astrocytes. Journal of neuroscience research. 1990;27:360–373. doi: 10.1002/jnr.490270315. [DOI] [PubMed] [Google Scholar]
- Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, Ungvari Z, Ballabh P. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41:1766–1773. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther FJ, Findlay RD, Durand M. Adrenal suppression and extubation rate after moderately early low-dose dexamethasone therapy in very preterm infants. Early Hum Dev. 2003;74:37–45. doi: 10.1016/s0378-3782(03)00082-3. [DOI] [PubMed] [Google Scholar]
- Watterberg K. Evidence-based neonatal pharmacotherapy: postnatal corticosteroids. Clin Perinatol. 39:47–59. doi: 10.1016/j.clp.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Watterberg KL. Policy statement--postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126:800–808. doi: 10.1542/peds.2010-1534. [DOI] [PubMed] [Google Scholar]
- Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- Yates HL, Newell SJ. Postnatal intravenous steroids and long-term neurological outcome: recommendations from meta-analyses. Arch Dis Child Fetal Neonatal Ed. 2012;97:F299–303. doi: 10.1136/adc.2010.208868. [DOI] [PubMed] [Google Scholar]
- Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, Tsai CH. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. 2004;350:1304–1313. doi: 10.1056/NEJMoa032089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.