Abstract
Recently published findings indicate that a knockout (KO) of soluble adenylyl cyclase (sAC, also known as AC-10) gene expression in mice leads to defective glucoregulation that is characterized by reduced pancreatic insulin secretion and reduced intraperitoneal glucose tolerance. Summarized here are current concepts regarding the molecular basis for this phenotype, with special emphasis on the potential role of sAC as a determinant of glucose-stimulated insulin secretion. Highlighted is new evidence that in pancreatic beta cells, oxidative glucose metabolism stimulates mitochondrial CO2 production that in turn generates bicarbonate ion (HCO3−). Since HCO3− binds to and directly stimulates the activity of sAC, we propose that glucose-stimulated cAMP production in beta cells is mediated not simply by transmembrane adenylyl cyclases (TMACs), but also by sAC. Based on evidence that sAC is expressed in mitochondria, there exists the possibility that beta-cell glucose metabolism is linked to mitochondrial cAMP production with consequent facilitation of oxidative phosphorylation. Since sAC is also expressed in the cytoplasm, sAC catalyzed cAMP production may activate cAMP sensors such as PKA and Epac2 to control ion channel function, intracellular Ca2+ handling, and Ca2+-dependent exocytosis. Thus, we propose that the existence of sAC in beta cells provides a new and unexpected explanation for previously reported actions of glucose metabolism to stimulate cAMP production. It seems possible that alterations of sAC activity might be of importance when evaluating new strategies for the treatment of type 2 diabetes (T2DM), or when evaluating why glucose metabolism fails to stimulate insulin secretion in patients diagnosed with T2DM.
Keywords: Soluble adenylyl cyclase, bicarbonate ion, cAMP, glucose, insulin secretion
1. Introduction
Pancreatic beta cells secrete insulin in response to the increase of blood glucose concentration that occurs after a meal [1]. Released insulin acts at insulin receptors on liver, fat, and muscle to promote uptake of glucose from the blood, while also suppressing hepatic release of glucose into the circulation [2]. As levels of blood glucose drop in response to insulin, this glucose-stimulated insulin secretion (GSIS) is terminated so that circulating levels of insulin will return to levels that are characteristic of the fasting state. When glucose fails to stimulate insulin secretion, or when tissues with insulin receptors become resistant to insulin, there can exist chronic hyperglycemia, as is the case for individuals with type 2 diabetes mellitus (T2DM) [3]. Since a loss of GSIS can occur prior to the development of insulin resistance in T2DM [4], there is at the present time great interest to understand the molecular defects of GSIS that occur in the context of T2DM.
2. Pancreatic beta-cell stimulus-secretion coupling
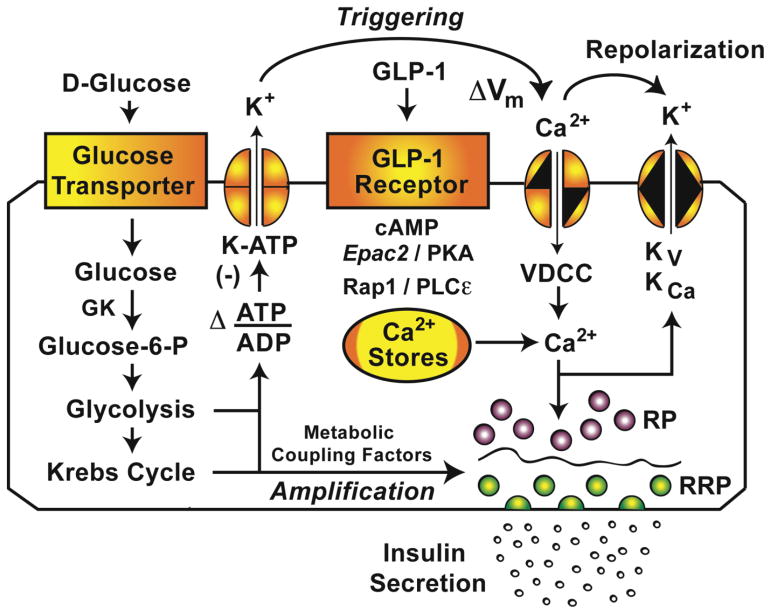
When considering how glucose stimulates insulin secretion from pancreatic beta cells, attention has focused on the “triggering” and “amplification” mechanisms of beta-cell stimulus-secretion coupling (Fig. 1) [5,6]. These two interdependent mechanisms of GSIS require beta-cell glucose uptake that is mediated by a facilitative glucose transporter (Glut1 for humans, Glut2 for rodents) [7,8]. However, the rate-limiting step in glucose sensing is governed by glucokinase (GK) [9], a type IV hexokinase that catalyzes conversion of glucose to glucose-6-phosphate (G6P) [10]. Oxidative glycolytic and mitochondrial metabolism of G6P stimulates an increase of the cytosolic ATP/ADP concentration ratio [11], and this key metabolic signal is directly responsible for the closure of ATP-sensitive K+ channels (K-ATP), depolarization, and Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs) [11].
Figure 1.
Triggering and amplification pathways of GSIS.
Glucose-stimulated Ca2+ influx through VDCCs constitutes a “triggering” mechanism for the Ca2+-dependent exocytosis of dense core secretory granules containing insulin (Fig. 1). More specifically, this triggering mechanism targets two distinct pools of secretory granules that undergo Ca2+-dependent fusion with the plasma membrane. These pools are a readily releasable pool (RRP) and a reserve pool (RP) of secretory granules. The RRP and RP are distinguishable because exocytosis of the RRP is not constrained by a cytoskeletal cortical actin barrier, whereas exocytosis of the RP can only occur after glucose-induced remodeling of the cortical actin barrier [12,13]. Therefore, the RRP of secretory granules located at or near the plasma membrane undergoes exocytosis quickly in order to generate an immediate 1st phase of GSIS, whereas the RP of secretory granules located deeper within the cytoplasm undergoes exocytosis with a delay in order to generate a 2nd phase of GSIS. Importantly, neither 1st nor 2nd phase GSIS can occur in the absence of the Ca2+ signal that triggers exocytosis.
The “amplification” mechanism of GSIS allows glucose metabolism to increase the efficiency with which Ca2+ triggers exocytosis of secretory granules located in the RRP and the RP (Fig. 1) [5,6]. Although glucose metabolism promotes remodeling of the cortical actin barrier to enhance secretory granule transit to the plasma membrane [12,13], this remodeling may not explain how glucose metabolically amplifies insulin secretion [14–16]. Instead, glycolytic and/or mitochondrial metabolites may be of primary importance [5,6]. As discussed below, the second messenger adenosine-3′,5′-cyclic monophosphate (cAMP) is another potential coupling factor linking glucose metabolism to amplification. It is generated in response to glucose metabolism, and its synthesis is catalyzed by transmembrane adenylyl cyclases (TMACs) and a soluble adenylyl cyclase (sAC) expressed in beta cells.
When considering the relative importance of “triggering” and “amplification” to the control of insulin secretion by cAMP, there is evidence that in healthy beta cells, the amplification mechanism predominates. Thus, in healthy beta cells, glucose metabolism is capable of generating the cytosolic Ca2+ signal that triggers exocytosis. Under such conditions, cAMP amplifies soluble NSF attachment protein receptor (SNARE) complex-mediated actions of Ca2+ to trigger the release of a relatively small number of secretory granules located within the RRP [17]. Simultaneously, cAMP amplifies the Ca2+-dependent exocytosis of a much larger pool of secretory granules located in the RP [17]. These amplifying actions of cAMP are detectable using methods of live-cell imaging in which it is demonstrated that cAMP enhances the translocation of so-called “restless newcomer” secretory granules from the cytoplasm to the plasma membrane [18]. Restless newcomer secretory granules undergo immediate fusion with the plasma membrane, and their exocytosis is a major factor that underlies the ability of cAMP to potentiate 1st and 2nd phase GSIS.
A different scenario exists when one considers how cAMP stimulates insulin secretion in patients with T2DM. In certain forms of this disorder, beta-cell glucose sensing is defective, possibly as a consequence of altered glycolytic and/or mitochondrial metabolism [19,20]. Under such conditions, glucose metabolism fails to produce the increase of cytosolic ATP/ADP concentration ratio that normally leads to K-ATP channel closure and depolarization-induced Ca2+ influx that triggers insulin exocytosis (Fig. 1). Remarkably, beta-cell glucose sensing is restored after the administration of glucagon-like peptide-1 (GLP-1), a cAMP-elevating agent that facilitates glucose-dependent ATP production, while also raising the [Ca2+]i and potentiating GSIS [21,22]. For GLP-1, Holz et al. define this restoration of glucose sensing as the induction of beta-cell “glucose competence” [23–25].
Pictured are metabolic, ionic, and GPCR signaling pathways in a pancreatic beta-cell that mediate stimulatory actions of glucose and glucagon-like peptide-1 (GLP-1) on insulin secretion. A readily releasable pool (RRP) of secretory granules is positioned immediately beneath the plasma membrane, whereas a reserve pool (RP) of secretory granules is located deeper in the cytoplasm behind the cortical actin barrier (wavy line). The triggering pathway of GSIS links glucose metabolism to depolarization-induced Ca2+ influx that triggers exocytosis of the RRP and RP. The amplification pathway allows metabolic coupling factors to increase the efficiency of Ca2+ as a stimulus for exocytosis. These actions of glucose are reinforced by GLP-1 acting at its GPCR to stimulate cAMP production with consequent activation of the cAMP sensors PKA and Epac2. Abbreviations: Kv, KCa, voltage and Ca2+ dependent K+ channels; ΔVm, depolarization; PLCε, phospholipase C-epsilon.
3. Defective insulin secretion in T2DM
GSIS can be evaluated in vivo using a protocol in which glucose is administered by intravenous infusion. When the glucose concentration is stepped from a low to a high level, the 1st and 2nd phase kinetic components of GSIS are measurable in healthy individuals, whereas there is a characteristic loss of 1st and 2nd phase GSIS in T2DM [4]. One way to explain this loss of GSIS is that in T2DM there are reduced numbers of beta cells [27]. Alternatively, there might be a molecular defect of exocytosis that prevents GSIS [28,29]. Differentiating between these two possibilities is not possible in vivo, but the results of post-mortem investigations indicate that in T2DM, the loss of GSIS is not explained simply by a decrease in the total number of beta-cells in the islets [4]. Thus, when considering a molecular defect of GSIS as a potential contributing factor to T2DM, it is surprising that the process of exocytosis involving fusion of secretory granules with the plasma membrane is reported not to be disturbed in beta cells from donors with T2DM [30]. Such findings suggest that in certain forms of T2DM, beta-cell stimulus-secretion coupling is disrupted at an early step of glucose sensing [31]. For example, there might be defective coupling of glucose metabolism to K-ATP channel closure and Ca2+ influx that triggers exocytosis. If so, it is understandable that T2DM patients can be treated with sulfonylureas (e.g., tolbutamide) that directly inhibit K-ATP channels, or with GLP-1 receptor agonists that stimulate cAMP production in order to restore glucose sensing and K-ATP channel closure. In this regard, there is great interest concerning the identification of beta-cell cAMP-elevating agents that will restore defective GSIS, while also lowering levels of blood glucose in patients with T2DM [34–38]. Ideally, these cAMP-elevating agents will not stimulate excessive insulin secretion, and will not induce hypoglycemia, as can be the case for sulfonylureas [33].
4. sAC is a bicarbonate-stimulated adenylyl cyclase potentially important to GSIS
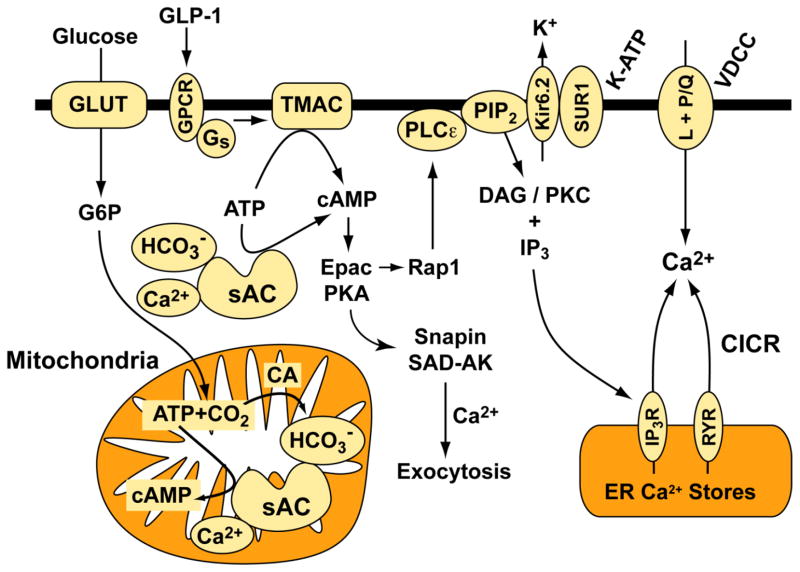
Here we advance the hypothesis that soluble adenylyl cyclase (sAC) might constitute a new molecular target for pharmacological intervention in the treatment of T2DM. More specifically, we propose that in T2DM, the coupling of glucose metabolism to cAMP production is diminished, and that activators of sAC will restore levels of cAMP so that defective GSIS is repaired. Our hypothesis is based on the finding that sAC is a HCO3− and Ca2+ stimulated adenylyl cyclase that also serves as a physiological ATP sensor [39–46]. Our hypothesis is also consistent with the established fact that oxidative metabolism of glucose generates CO2 and ATP while also promoting an increase of [Ca2+]i in beta cells [47]. In our model (Fig. 2), CO2 generated by glucose metabolism will be converted by carbonic anhydrase (CA) to carbonic acid (H2CO3) that will dissociate at physiological pH to generate HCO3−. Bicarbonate ion generated in this manner will stimulate sAC to generate cAMP that in turn potentiates GSIS.
Figure 2.
Hypothetical role of sAC in GSIS.
What evidence is there in support of this hypothesis? One finding of potential significance is that GSIS is reduced when rat islets are equilibrated in saline to which no HCO3− is added [48,49]. This experimental finding obtained in the 1970’s is understandable since the balance of CA-catalyzed HCO3− production is influenced by HCO3− transporters that are responsive to intracellular and extracellular HCO3−. Potentially, live-cell imaging techniques using cAMP biosensors such as Epac1-camps can be employed in order to determine if sAC-catalyzed cAMP production is sensitive to alterations of extracellular HCO3− concentration. In this regard, it might be possible to generate recombinant fusion proteins of sAC and Epac1-camps so that levels of cAMP can be monitored directly at the site of sAC-catalyzed cAMP production.
It is interesting to note that the CA inhibitor acetazolamide suppresses GSIS from rat islets [50,51]. Potentially, this action of acetazolamide is a consequence of its ability to block HCO3− production that normally couples sAC activation to the stimulation of insulin secretion (Fig. 2). Alternatively, acetazolamide might act non-specifically as a Ca2+ channel blocker to suppress Ca2+ influx that triggers insulin secretion. In fact, acetazolamide is reported to reduce glucose-stimulated Ca2+ influx in rat islets [51]. Unfortunately, interpretation of these findings is complicated by the lack of electrophysiological data with which to evaluate potential Ca2+ channel blocking actions of acetazolamide. Still, it should be noted that prior patch clamp studies demonstrate an ability of cAMP to facilitate glucose-dependent Ca2+ influx in beta cells [52–55]. Thus, it could be that acetazolamide indirectly inhibits Ca2+ influx by interfering with sAC-catalyzed cAMP production. Appropriate electrophysiological studies are needed to test whether beta-cell Ca2+ channel activity is directly or indirectly inhibited by acetazolamide.
Since beta cells express mitochondrial carbonic anhydrase V (CA-V) [50], and since sAC activity in mitochondria facilitates oxidative phosphorylation in some cell types [56–58], it could be that sAC elevates levels of cAMP in beta-cell mitochondria, thereby allowing oxidative glucose metabolism to more efficiently stimulate insulin secretion (Fig. 2). This concept is consistent with the established importance of mitochondrial metabolism to GSIS [59]. Conceivably, prior studies of islets underestimated the effectiveness with which glucose stimulates cAMP production since measurements of cAMP content in whole islet lysates do not reflect the true concentration of cAMP in mitochondria. Live-cell imaging studies using cAMP reporters targeted to the mitochondria should resolve these issues.
If mitochondrial sAC activity is of importance to GSIS, a pharmacological inhibitor of sAC should uncouple glucose metabolism from cAMP production and insulin secretion. Unfortunately, the most commonly used sAC inhibitor (KH7) is now recognized to exert non-specific inhibitory effects on glucose metabolism [60] and mitochondrial respiration [61]. Such non-specific effects of KH7 render it difficult to interpret prior reports that KH7 blocks cAMP production and insulin secretion in response to glucose [44,62]. Established alternatives to the use of KH7 include catechol ester-mediated inhibition of sAC catalytic activity [62], or siRNA-mediated knockdown of sAC expression [62]. Still, neither of these two alternative approaches allows selective targeting of mitochondrial sAC in the absence of any effect at cytosolic sAC. The availability of more specific inhibitors would be useful in order to test for a potential functional interaction of mitochondrial sAC and TMACs. For example, if mitochondrial sAC activation upregulates glucose-dependent mitochondrial ATP production, the resultant depolarization-induced Ca2+ influx might have the capacity to activate the type 8 TMAC (AC-8) that is under the control of Ca2+/calmodulin in rodent beta cells [63–65].
Since sAC expression is not restricted to mitochondria [56], it is easy to imagine a scenario in which glucose-stimulated mitochondrial CO2 production generates HCO3− that stimulates cytosolic sAC to activate cAMP-dependent protein kinase A (PKA) located in the vicinity of secretory granules. If so, this signaling cascade might explain prior reports that PKA mediates the action of glucose to stimulate insulin secretion [66], possibly by promoting phosphorylation of Snapin [67], by increasing the Ca2+ sensitivity of exocytosis [68], or by activating SAD-A kinase (SAD-AK) to stimulate cytoskeletal remodeling that enables secretory granule exocytosis during 2nd phase GSIS [69,70]. Since sAC and PKA are both found within nuclei of cells [56,71], sAC might also mediate stimulatory effects of glucose to elevate nuclear levels of cAMP, and to activate beta-cell gene transcription that is PKA and CREB regulated [71].
Interestingly, sAC-stimulated cAMP production also has the capacity to activate Rap1 GTPase in PC12 cells [72]. Rap1 is the immediate downstream effector of the cAMP-regulated guanine nucleotide exchange factors Epac1 and Epac2 [73,74], and Rap1 links cAMP production to the PKA-independent stimulation of insulin secretion [1,14,75–83]. Thus, it is remarkable that a knockout (KO) of Epac1 gene expression leads to defective GSIS and a loss of glucoregulation in mice [84]. Furthermore, a KO of Epac2 gene expression in mice leads to a failure of glucose to stimulate insulin secretion under pathophysiological conditions of diet-induced insulin resistance [85,86]. Thus, there is good reason to believe that Epac proteins mediate stimulatory effects of glucose on insulin secretion, and that sAC might participate in this process by raising levels of cAMP. These possibilities are testable using newly described synthetic small molecules that selectively inhibit Epac proteins [87–89].
To understand the potential roles of sAC and Epac2 in the control of GSIS, it is important to note that when beta cells are stimulated with glucose, Epac2 is recruited to the plasma membrane by activated Ras GTPase [90]. This recruitment is mediated by binding of Ras to the Ras-association (RA) domain of Epac2, and it requires that Epac2 be activated by cAMP [90]. Remarkably, recruitment of Epac2 to active Ras is also Ca2+-dependent [90]. This finding is understandable if glucose metabolism stimulates Ca2+ influx that activates a Ca2+-sensitive and Ras-specific guanine nucleotide exchange factor such as Ras-GRF1 [91]. Using TIRF microscopy to monitor the inner surface of the plasma membrane, Idevall-Hagren et al. find that glucose induces oscillations of cAMP and Ca2+ that are synchronized with Ras and Rap1 activation, and that correlate with reversible translocation of Epac2 to the plasma membrane [90]. Although these oscillations are blocked by an inhibitor of TMACs [90], it is possible that glucose metabolism activates mitochondrial sAC to generate cAMP and Ca2+ signals that support this oscillatory activity. For example, if sAC-catalyzed cAMP production in mitochondria facilitates glucose-dependent oxidative phosphorylation, the resultant increase of ATP/ADP concentration ratio will provide the metabolic signal for K-ATP channel closure and depolarization-induced Ca2+ influx. This Ca2+ signal will activate Ras-GRF1 to sequentially activate Ras, while also acting at the AC-8 TMAC to further stimulate cAMP production. The net effect will be periodic recruitment of Epac2 to the plasma membrane, and this periodicity will be dictated by oscillations of [Ca2+]i that are initiated by oxidative glucose metabolism under the control of sAC.
When evaluating the above-summarized beta-cell cAMP signaling mechanisms controlling insulin secretion, it is important to note that permissive PKA activity supports GSIS from rodent and human islets, even in the absence of any added cAMP-elevating agent [66,92]. Such findings indicate that it will be important in future studies to ascertain whether the permissive PKA activity that supports GSIS is generated by sAC and/or TMACs. Just as important, it will be of interest to determine if sAC or TMAC activity is altered in donor islets of T2DM patients. Prior studies using rat models of T2DM indicate that islets of these rats have defective coupling of glucose metabolism to cAMP production [93,94].
Glucose metabolism raises levels of HCO3−, Ca2+, and ATP in pancreatic beta cells to stimulate sAC-catalyzed production of cAMP in the mitochondria and in the cytoplasm. cAMP in the mitochondria enhances oxidative phosphorylation that acts as a stimulus for insulin secretion. cAMP in the cytoplasm activates PKA to promote phosphorylation of substrate proteins (e.g., Snapin) that regulate secretory granule exocytosis. Cytoplasmic cAMP also activates an Epac, Rap1, and PLCε signal transduction “module” that mobilizes Ca2+ from endoplasmic reticulum (ER) Ca2+ stores at which IP3 receptors (IP3R) and ryanodine receptors (RYR) are located [102,136–138]. In human beta cells, L-type VDCCs participate in action potential generation, whereas P/Q-type VDCCs participate in insulin exocytosis. Additional abbreviations: CICR, Ca2+-induced Ca2+ release; DAG, diacylglycerol; GLUT, glucose transporter; GPCR, G protein-coupled receptor; Kir6.2, pore-forming subunit of K-ATP channels; PKC, protein kinase C; SAD-AK, synapses of amphids defective kinase-A; SUR1, sulfonylurea receptor-1 subunit of K-ATP channels.
5. Investigation of sAC activity using an insulin-secreting cell line
Ramos et al. report that a step-wise increase of glucose concentration from 2.5 to 16 mM leads to cAMP production in rat INS-1E insulin-secreting cells [62]. The response appears slowly (peak at 15 min) and is measurable under conditions in which cells are treated with the cyclic nucleotide phosphodiesterase (PDE) inhibitor IBMX. Despite interpretive problems associated with the lack of specificity of sAC inhibitor KH7 (see above) Ramos et al. also find that the cAMP-elevating action of glucose is suppressed by KH7, whereas a P-site inhibitor of TMACs (ddAdo) is ineffective. In contrast, the action of GLP-1 to raise levels of cAMP is unaffected by KH7 but is blocked by ddAdo. Immunoprecipitation, western blot, and immunocytochemical analyses also demonstrate expression of an ca. 50 kDa isoform of sAC in these INS-1E cells. Knockdown of sAC expression using sAC-specific siRNA reduces levels of sAC protein while also reducing the action of glucose to stimulate cAMP production [44,62]. Interestingly, agents that interfere with depolarization-induced Ca2+ influx (diazoxide, verapamil) or that buffer intracellular Ca2+ (EGTA-AM) eliminate the action of glucose to raise levels of cAMP [62]. Collectively, these findings indicate that for INS1-E cells, the binding of Ca2+ to a TMAC (i.e., AC-8) or sAC is of significance to glucose-stimulated cAMP production. However, it is not yet possible to decipher which of these two adenylyl cyclases plays a dominant role in the overall process of glucose-stimulated cAMP production. Finally, the utility of INS-1E cells for studies of this type is further emphasized by the finding that KH7, but not the TMAC inhibitor ddAdo, uncouples glucose metabolism from cAMP-dependent activation of ERK1/2 mitogen-activated protein kinases [62]. Moreover, the sAC inhibitors KH7 and catechol estrogen block glucose-stimulated insulin secretion from INS-1E cells [44].
6. Defective glucoregulation in sAC knockout mice
Using a new strain of sAC-C1 mice in which there is a global KO of sAC gene expression, Zippin et al. report that sAC participates in the in vivo control of blood glucose homeostasis [44]. This finding is established by comparing glucoregulation in homozygous sAC-C1 −/− KO mice as compared with heterozygous sAC-C1 +/− mice containing a single wild-type sAC allele. Thus, in an intraperitoneal (i.p.) glucose tolerance (IPGT) test that reveals direct stimulatory effects of glucose at the endocrine pancreas, levels of blood glucose rise to higher levels after administration of glucose to sAC −/− KO mice. Furthermore, i.p. administration of glucose to sAC −/− KO mice stimulates only a small increase of plasma insulin in comparison with sAC +/− mice. Such findings indicate that sAC expression is necessary in order for glucose tolerance and insulin secretion to be homeostatically regulated under conditions of i.p. glucose administration.
Zippin et al. also report remarkable findings concerning assays of 1st and 2nd phase GSIS using isolated islets of sAC-C1 KO mice [44]. By comparing insulin secretion from islets of sAC +/+ wild-type mice or sAC −/− KO mice, Zippin et. al. demonstrate that for the sAC KO, there is a reduction of both 1st and 2nd phase GSIS, as measured when the glucose concentration is stepped from 5 mM to 30 mM. Although a detailed analysis of islet insulin content and beta-cell mass has yet to be provided, histology demonstrates that the homozygous sAC-C1 KO mice have normal pancreatic morphology, normal islet structure, and normal islet size [44]. However, one important caveat to the interpretation of these studies is that the KO of sAC gene expression is not restricted to beta cells in sAC-C1 KO mice. Therefore, it will be important to assess how glucoregulation is altered in beta-cell specific sAC KO mice.
7. Is there a role for sAC in metabolic processes of beta-cell compensation?
When considering future avenues of research regarding sAC, it will be especially important to assess whether sAC participates in processes of beta-cell compensation that serve to maintain GSIS under conditions of metabolic stress. For example, it could be that the beta-cell cAMP signaling “network” exhibits plasticity under conditions of metabolic stress, and that sAC-stimulated cAMP production explains enhanced GSIS that serves to counteract insulin resistance induced by metabolic stress. A hint that this might be the case is provided by the study of Song et al. in which metabolic stress induced by a high fat diet (HFD) reveals a prominent role for Epac2 in the control of GSIS [85]. The HFD induces insulin resistance, and under these conditions, mouse beta cells undergo functional compensation to upregulate GSIS, whereas this compensation is lost in beta cells of Epac2 KO mice [85]. Based on the findings of Song et al., HFD-induced beta-cell compensation in mice appears to involve an increased capacity of glucose metabolism to signal through Epac2 to increase levels of cytosolic Ca2+ [85]. Importantly, this compensation is measureable in assays of GSIS using isolated islets, and it occurs in the absence of an added GPCR agonist [85]. Since Epac2 activation in normal wild-type beta cells promotes Ca2+ influx while also mobilizing an intracellular source of Ca2+ [95–102], the coupling of sAC activity to Epac2 activation and Ca2+ handling seems like a fruitful area for future investigation. Potentially, sAC-C1 KO mice can be evaluated in order to determine if processes of beta-cell compensation important to GSIS are disrupted under conditions of the HFD. Since cAMP-elevating agents stimulate beta-cell proliferation [103–105], while protecting against beta-cell death [106], sAC activity might also maintain beta-cell mass under conditions of metabolic stress.
8. Regulation of beta-cell cAMP production by GPCRs and TMACs
sAC-catalyzed cAMP production in response to glucose is complemented by TMAC-catalyzed cAMP production that is initiated by agonist binding to G protein-coupled receptors (GPCRs). The GPCRs that stimulate cAMP production in beta cells include members of the Family B GPCRs that specifically bind glucagon, GLP-1, GIP, and PACAP [34,35,107]. Traditionally, it is thought that these GPCRs mediate the intra-islet (glucagon), hormonal (GLP-1, GIP) and neural (PACAP) stimulation of insulin secretion. However, recent findings indicate that GLP-1 released from intestinal L-cells also activates vagal-vagal reflexes that stimulate insulin secretion [35,108]. For the treatment of T2DM, synthetic GLP-1 receptor agonists exist, and they include exenatide and liraglutide [35].
Signal transduction crosstalk of sAC and TMACs might exist in beta cells because sAC-catalyzed cAMP production in mitochondria is predicted to enhance the action of glucose to promote depolarization-induced Ca2+ influx, thereby allowing Ca2+ to directly stimulate TMAC activity. Multiple isoforms of TMACs are expressed in beta cells [63], and in this regard AC-8 is especially important for rodent beta cells due to the fact that it serves as a molecular coincidence detector for the cAMP-dependent control of GSIS [64,65]. Coincidence detection at AC-8 occurs because the activity of AC-8 is stimulated by heterotrimeric Gs proteins and also by Ca2+/CaM that binds directly to AC-8 [109,110]. For example, we propose that binding of GLP-1 to its GPCR stimulates AC-8, and that this effect is reinforced by glucose metabolism that is under the control of sAC and that elevates levels of Ca2+ in order to further stimulate AC-8. Synergistic activation of AC-8 is achieved when GLP-1 is paired with glucose, and under these conditions oscillations of cAMP and Ca2+ are generated so that GSIS is powerfully stimulated [111–116].
An additional noteworthy finding is the recent report of Hodson et al. that human islet GSIS is conditional on expression of the TMAC isoform 5 (AC-5) in beta cells [117]. In studies of human islets subjected to an shRNA-mediated knockdown of AC-5, it is possible to demonstrate that reduced AC-5 expression leads to a modest but significant reduction of GSIS under conditions in which the glucose concentration is stepped from 3 to 11 mM. In contrast, a knockdown of AC-5 does not alter the action of GLP-1 to stimulate insulin secretion under conditions in which the glucose concentration is fixed at 11 mM. By imaging the fluorescent cAMP reporter Epac2-camps, or the fluorescent Ca2+ reporter fluo-2, in single human islets or single human beta cells, it is also possible to demonstrate that glucose is a less effective stimulus for cAMP production and Ca2+ influx in islets subjected to the knockdown of AC-5. Such findings are surprising in view of the fact that AC-5 was previously considered to be an adenylyl cyclase that is inhibited by Ca2+. However, Hodson et al. point out that AC-5 is inhibited by concentrations of Ca2+ that are in excess of that normally found in the cytosol [117]. Taken as a whole, such findings concerning AC-5 are consistent with the prior finding of Chepurny et al. demonstrating a role for permissive PKA activity in support of GSIS [92], as well as the findings of Hatakeyama, Takahashi, Kasai, and co-workers who propose that beta-cell glucose metabolism is tightly linked to TMAC-stimulated cAMP production [66,118,119]. One particularly interesting outgrowth of this line of investigation is the apparent role of AC-5 as a signal transducer linking human beta-cell glucose metabolism to depolarization-induced Ca2+ influx [117]. Although it remains to be demonstrated, cAMP generated in response to glucose metabolism might be linked to Epac2 activation with consequent K-ATP channel closure that generates beta-cell depolarization [95,96].
9. Conclusion
Over forty years have elapsed since the first reports documenting an ability of glucose to raise levels of cAMP in the islets of Langerhans [120–131]. With time, these studies were discounted due to the relatively small change of cAMP concentration induced by glucose. Since such prior studies used biochemical assays to detect levels of cAMP in whole islets, we propose that these studies need to be reinterpreted because the methods that were used cannot detect compartmentalized cAMP production that is potentially sAC-mediated. Importantly, earlier studies found that the time course of 1st phase GSIS matched that of the glucose-stimulated increase of cAMP concentration in whole islets [121,126,127]. In retrospect, this temporal coincidence is understandable if 1st phase GSIS results from exocytosis that is at least in part cAMP-stimulated [14]. In this regard, we wish to emphasize that cAMP is not the only stimulus for GSIS, but that additional metabolic coupling factors derived from glucose metabolism also participate. However, since genetically-encoded cAMP biosensors are able to detect glucose-stimulated changes of cAMP concentration in single beta-cells and whole islets [90,112,113,132,133), there exists a new appreciation for actions of glucose to stimulate insulin secretion in a cAMP-mediated manner [134]. In fact, a capacity of cAMP to directly stimulate beta-cell exocytosis is convincingly documented in the report of Ammala et al. published in 1993 [135], at which time the functional significance of sAC was not yet appreciated. However, since the cloning of sAC from a rat testis cDNA library in the late 1990’s [136], the significance of sAC to cAMP signaling in many cell types is increasingly apparent. With the availability of sAC KO mice in which there is defective glucoregulation accompanied by reduced GSIS [44], it is anticipated that the full significance of sAC to beta-cell biology, and possibly T2DM, will become readily apparent.
Highlights.
sAC KO mice have reduced glucose tolerance and defective insulin secretion.
sAC may link glucose metabolism to cAMP production in pancreatic beta-cells.
cAMP generated by sAC may facilitate glucose-stimulated insulin secretion.
Acknowledgments
This work was supported by NIH/NIDDK R01-DK069575 awarded to G.G.H., and by American Diabetes Association Basic Science Awards to G.G.H. (7-12-BS-077) and C.A.L. (1-12-BS-109). O.G.C. acknowledges the support of SUNY Upstate Medical University. G.G.H. serves as the guarantor of this work.
Abbreviations
- AC-5/8
adenylyl cyclase isoforms 5 or 8
- ddAdo
2′,5′-dideoxyadenosine
- CA
carbonic anhydrase
- cAMP
adenosine-3′,5′-cyclic monophosphate
- CREB
cAMP response element-binding protein
- Epac1/2
exchange proteins activated by cyclic AMP isoforms 1/2
- GIP
glucose-dependent insulinotropic polypeptide
- GK
glucokinase
- GLP-1
glucagon-like peptide-1
- Glut1/2
glucose transporter isoforms 1/2
- GPCR
G protein-coupled receptor
- GSIS
glucose-stimulated insulin secretion
- HFD
high fat diet
- K-ATP
ATP-sensitive K+ channel
- KO
knockout
- Kir6.2
inward rectifier pore-forming subunit
- Kv
voltage-dependent K+ channel
- KCa
calcium-activated K+ channel
- PACAP
pituitary adenylyl cyclase-activating polypeptide
- PDE
cyclic nucleotide phosphodiesterase
- PKA
protein kinase A
- PLCε
phospholipase C-epsilon
- RA
Ras-association domain
- RP
reserve pool
- RRP
readily releasable pool
- sAC
soluble adenylyl cyclase
- SAD-AK
SAD-A kinase
- SUR1
sulfonylurea receptor type1
- T2DM
type 2 diabetes mellitus
- TMAC
transmembrane adenylyl cyclase
- VDCC
voltage-dependent Ca2+ channel
Footnotes
AUTHOR CONTRIBUTIONS
G.G.H. wrote the manuscript. O.G.C. and C.A.L. edited the manuscript. G.G.H. created Figure 1. C.A.L created Figure 2.
The participating authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121:2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerich JE. Control of glycaemia. Baillieres Clin Endocrinol Metab. 1993;7:551–586. doi: 10.1016/s0950-351x(05)80207-1. [DOI] [PubMed] [Google Scholar]
- 3.Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383:1068–1083. doi: 10.1016/S0140-6736(13)62154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51(Suppl 1):S117–S121. doi: 10.2337/diabetes.51.2007.s117. [DOI] [PubMed] [Google Scholar]
- 5.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- 6.Henquin JC. The dual control of insulin secretion by glucose involves triggering and amplifying pathways in β-cells. Diabetes Res Clin Pract. 2011;93(Suppl 1):S27–S31. doi: 10.1016/S0168-8227(11)70010-9. [DOI] [PubMed] [Google Scholar]
- 7.Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and β-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 8.De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koranyi LI, Tanizawa Y, Welling CM, Rabin DU, Permutt MA. Human islet glucokinase gene. Isolation and sequence analysis of full-length cDNA. Diabetes. 1992;41:807–811. doi: 10.2337/diab.41.7.807. [DOI] [PubMed] [Google Scholar]
- 10.Matschinsky FM. Regulation of pancreatic β-cell glucokinase: from basics to therapeutics. Diabetes. 2002;51(Suppl 3):S394–S404. doi: 10.2337/diabetes.51.2007.s394. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald PE, Joseph JW, Rorsman P. Glucose-sensing mechanisms in pancreatic β-cells. Philos Trans R Soc Lond B Biol Sci. 2005;360:2211–2225. doi: 10.1098/rstb.2005.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–9546. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Thurmond DC. Mechanisms of biphasic insulin-granule exocytosis-roles of the cytoskeleton, small GTPases and SNARE proteins. J Cell Sci. 2009;122:893–903. doi: 10.1242/jcs.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mourad NI, Nenquin M, Henquin JC. Metabolic amplifying pathway increases both phases of insulin secretion independently of beta-cell actin microfilaments. Am J Physiol Cell Physiol. 2010;299:C389–398. doi: 10.1152/ajpcell.00138.2010. [DOI] [PubMed] [Google Scholar]
- 15.Mourad NI, Nenquin M, Henquin JC. Metabolic amplification of insulin secretion by glucose is independent of β-cell microtubules. Am J Physiol Cell Physiol. 2011;300:C697–706. doi: 10.1152/ajpcell.00329.2010. [DOI] [PubMed] [Google Scholar]
- 16.Mourad NI, Nenquin M, Henquin JC. cAMP-mediated and metabolic amplification of insulin secretion are distinct pathways sharing independence of β-cell microfilaments. Endocrinology. 2012;153:4644–4654. doi: 10.1210/en.2012-1450. [DOI] [PubMed] [Google Scholar]
- 17.Rorsman P, Renström E. Insulin granule dynamics in pancreatic beta cells. Diabetologia. 2003;46:1029–1045. doi: 10.1007/s00125-003-1153-1. [DOI] [PubMed] [Google Scholar]
- 18.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiederkehr A, Wollheim CB. Impact of mitochondrial calcium on the coupling of metabolism to insulin secretion in the pancreatic β-cell. Cell Calcium. 2008;44:64–76. doi: 10.1016/j.ceca.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Mudler H, Ling C. Mitochondrial dysfunction in pancreatic β-cells in type 2 diabetes. Mol Cell Endocrinol. 2009;297:34–40. doi: 10.1016/j.mce.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA. Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells. Biochem J. 2003;369:287–299. doi: 10.1042/BJ20021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodson DJ, Tarasov AI, Gimeno Brias S, Mitchell RK, Johnston NR, Haghollahi S, Cane MC, Bugliani M, Marchetti P, Bosco D, Johnson PR, Hughes SJ, Rutter GA. Incretin-modulated beta cell energetics in intact islets of Langerhans. Mol Endocrinol. 2014 doi: 10.1210/me.2014-1038. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holz GG, Habener JF. Induction of glucose competence in pancreatic beta cells by glucagon-like peptide-1(7-37) Transactions of the Association of American Physicians. 1992;105:260–267. [PubMed] [Google Scholar]
- 24.Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: pancreatic beta-cells and the glucose competence concept. Trends Biochem Sci. 1992;17:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holz GG, 4th, Kühtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7-37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holz GG. New insights concerning the glucose-dependent insulin secretagogue action of glucagon-like peptide-1 in pancreatic β-cells. Horm Metab Res. 2004;36:787–794. doi: 10.1055/s-2004-826165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 28.Kwan EP, Gaisano HY. Rescuing the subprime meltdown in insulin exocytosis in diabetes. Ann N Y Acad Sci. 2009;1152:154–164. doi: 10.1111/j.1749-6632.2008.03990.x. [DOI] [PubMed] [Google Scholar]
- 29.Ivanova A, Kalaidzidis Y, Dirkx R, Sarov M, Gerlach M, Schroth-Diez B, Müller A, Liu Y, Andree C, Mulligan B, Münster C, Kurth T, Bickle M, Speier S, Anastassiadis K, Solimena M. Age-dependent labeling and imaging of insulin secretory granules. Diabetes. 2013;62:3687–3696. doi: 10.2337/db12-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, Zhang E, Almgren P, Ladenvall C, Axelsson AS, Edlund A, Pedersen MG, Jonsson A, Ramracheya R, Tang Y, Walker JN, Barrett A, Johnson PR, Lyssenko V, McCarthy MI, Groop L, Salehi A, Gloyn AL, Renström E, Rorsman P, Eliasson L. Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes. 2012;61:1726–1733. doi: 10.2337/db11-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashcroft F, Rorsman P. Type 2 diabetes mellitus: not quite exciting enough? Hum Mol Genet. 2004;13(Spec No 1):R21–R31. doi: 10.1093/hmg/ddh066. [DOI] [PubMed] [Google Scholar]
- 32.Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- 33.Groop LC. Sulfonylureas in NIDDM. Diabetes Care. 1992;15:737–754. doi: 10.2337/diacare.15.6.737. [DOI] [PubMed] [Google Scholar]
- 34.Holz GG, Chepurny OG. Glucagon-like peptide-1 synthetic analogs: new therapeutic agents for use in the treatment of diabetes mellitus. Curr Med Chem. 2003;10:2471–2483. doi: 10.2174/0929867033456648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nadkarni P, Chepurny OG, Holz GG. Regulation of glucose homeostasis by GLP-1. Prog Mol Biol Transl Sci. 2014;121:23–65. doi: 10.1016/B978-0-12-800101-1.00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clardy-James S, Chepurny OG, Leech CA, Holz GG, Doyle RP. Synthesis, characterization and pharmacodynamics of vitamin-B12-conjugated glucagon-like peptide-1. Chem Med Chem. 2013;8:582–586. doi: 10.1002/cmdc.201200461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. Eur J Intern Med. 2014 doi: 10.1016/j.ejim.2014.03.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Ahrén B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat Rev Drug Discov. 2009;8:369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, Levin LR, Buck J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 40.Zippin JH, Levin LR, Buck J. CO2/HCO3− -responsive soluble adenylyl cyclase as a putative metabolic sensor. Trends Endocrinol Metab. 2001;12:366–370. doi: 10.1016/s1043-2760(01)00454-4. [DOI] [PubMed] [Google Scholar]
- 41.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–15926. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 42.Steegborn C, Litvin TN, Levi LR, Buck J, Wu H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol. 2005;12:32–37. doi: 10.1038/nsmb880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman N, Buck J, Levin LR. pH sensing via bicarbonate-regulated “soluble” adenylyl cyclase (sAC) Front Physiol. 2013;4:343. doi: 10.3389/fphys.2013.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zippin JH, Chen Y, Straub SG, Hess KC, Diaz A, Lee D, Tso P, Holz GG, Sharp GW, Levin LR, Buck J. CO2/HCO3− - and calcium-regulated soluble adenylyl cyclase as a physiological ATP sensor. J Biol Chem. 2013;288:33283–33291. doi: 10.1074/jbc.M113.510073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinboelting S, Diaz A, Moniot S, van den Heuvel J, Weyand M, Levin LR, Buck J, Steegborn C. Proc Natl Acad Sci USA. 2014;111:3727–3732. doi: 10.1073/pnas.1322778111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA. 2003;100:10676–10681. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Newgard CB, McGarry JD. Metabolic coupling factors in pancreatic β-cell signal transduction. Annu Rev Biochem. 1995;64:689–719. doi: 10.1146/annurev.bi.64.070195.003353. [DOI] [PubMed] [Google Scholar]
- 48.Henquin JC, Lambert AE. Extracellular bicarbonate ions and insulin secretion. Biochim Biophys Acta. 1975;381:437–442. doi: 10.1016/0304-4165(75)90251-2. [DOI] [PubMed] [Google Scholar]
- 49.Henquin JC, Lambert AE. Bicarbonate modulation of glucose-induced biphasic insulin release by rat islets. Am J Physiol. 1976;231:713–721. doi: 10.1152/ajplegacy.1976.231.3.713. [DOI] [PubMed] [Google Scholar]
- 50.Parkkila AK, Scarim AL, Parkkila S, Waheed A, Corbett JA, Sly WS. Expression of carbonic anhydrase V in pancreatic beta cells suggests role for mitochondrial carbonic anhydrase in insulin secretion. J Biol Chem. 1998;273:24620–24623. doi: 10.1074/jbc.273.38.24620. [DOI] [PubMed] [Google Scholar]
- 51.Sener A, Jijakli H, Zahedi Asl S, Courtois P, Yates AP, Meuris S, Best LC, Malaisse WJ. Possible role of carbonic anhydrase in rat pancreatic islets: enzymatic, secretory, metabolic, ionic, and electrical aspects. Am J Physiol Endocrinol Metab. 2007;292:E1624–E1630. doi: 10.1152/ajpendo.00631.2006. [DOI] [PubMed] [Google Scholar]
- 52.Leech CA, Holz GG, Habener JF. Pituitary adenylate cyclase-activating polypeptide induces the voltage-independent activation of inward membrane currents and elevation of intracellular calcium in HIT-T15 insulinoma cells. Endocrinology. 1995;136:1530–1536. doi: 10.1210/endo.136.4.7895663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holz GG, 4th, Leech CA, Habener JF. Activation of a cAMP-regulated Ca2+ -signaling pathway in pancreatic β-cells by the insulinotropic hormone glucagon-like peptide-1. J Biol Chem. 1995;270:17749–17757. [PMC free article] [PubMed] [Google Scholar]
- 54.Leech CA, Holz GG, Habener JF. Signal transduction of PACAP and GLP-1 in pancreatic β cells. Ann N Y Acad Sci. 1996;805:81–92. doi: 10.1111/j.1749-6632.1996.tb17475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leech CA, Dzhura I, Chepurny OG, Kang G, Schwede F, Genieser HG, Holz GG. Molecular physiology of glucagon-like peptide-1 insulin secretagogue action in pancreatic β cells. Prog Biophys Mol Biol. 2011;107:236–247. doi: 10.1016/j.pbiomolbio.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zippin JH, Chen Y, Nahirney P, Kamenetsky M, Wuttke MS, Fischman DA, Levin LR, Buck J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 2003;17:82–84. doi: 10.1096/fj.02-0598fje. [DOI] [PubMed] [Google Scholar]
- 57.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valsecchi F, Ramos-Espiritu LS, Buck J, Levin LR, Manfredi G. cAMP and mitochondria. Physiology (Bethesda) 2013;28:199–209. doi: 10.1152/physiol.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiederkehr A, Wollheim CB. Mitochondrial signals drive insulin secretion in the pancreatic β-cell. Mol Cell Endocrinol. 2012;353:128–137. doi: 10.1016/j.mce.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 60.Tian G, Sandler S, Gylfe E, Tengholm A. Glucose- and hormone-induced cAMP oscillations in α- and β-cells within intact pancreatic islets. Diabetes. 2011;60:1535–1543. doi: 10.2337/db10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Benedetto G, Scalzotto E, Mongillo M, Pozzan T. Mitochondrial Ca2+ uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell Metab. 2013;17:965–975. doi: 10.1016/j.cmet.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Ramos LS, Zippin JH, Kamenetsky M, Buck J, Levin LR. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J Gen Physiol. 2008;132:329–338. doi: 10.1085/jgp.200810044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leech CA, Castonguay MA, Habener JF. Expression of adenylyl cyclase subtypes in pancreatic β-cells. Biochem Biophys Res Commun. 1999;254:703–706. doi: 10.1006/bbrc.1998.9906. [DOI] [PubMed] [Google Scholar]
- 64.Delmeire D, Flamez D, Hinke SA, Cali JJ, Pipeleers D, Schuit F. Type VIII adenylyl cyclase in rat beta cells: coincidence signal detector/generator for glucose and GLP-1. Diabetologia. 2003;46:1383–1393. doi: 10.1007/s00125-003-1203-8. [DOI] [PubMed] [Google Scholar]
- 65.Roger B, Papin J, Vacher P, Raoux M, Mulot A, Dubois M, Kerr-Conte J, Voy BH, Pattou F, Charpentier G, Jonas JC, Moustaïd-Moussa N, Lang J. Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia. 2011;54:390–402. doi: 10.1007/s00125-010-1955-x. [DOI] [PubMed] [Google Scholar]
- 66.Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–282. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song WJ, Seshadri M, Ashraf U, Mdluli T, Mondal P, Keil M, Azevedo M, Kirschner LS, Stratakis CA, Hussain MA. Snapin mediates incretin action and augments glucose-dependent insulin secretion. Cell Metab. 2011;13:308–319. doi: 10.1016/j.cmet.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skelin M, Rupnik M. cAMP increases the sensitivity of exocytosis to Ca2+ primarily through protein kinase A in mouse pancreatic beta cells. Cell Calcium. 2011;49:89–99. doi: 10.1016/j.ceca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Nie J, Sun C, Faruque O, Ye G, Li J, Liang Q, Chang Z, Yang W, Han X, Shi Y. Synapses of amphids defective (SAD-A) kinase promotes glucose-stimulated insulin secretion through activation of p21-activated kinase (PAK1) in pancreatic β-Cells. J Biol Chem. 2012;287:26435–26444. doi: 10.1074/jbc.M112.378372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nie J, Lilley BN, Pan YA, Faruque O, Liu X, Zhang W, Sanes JR, Han X, Shi Y. SAD-A potentiates glucose-stimulated insulin secretion as a mediator of glucagon-like peptide 1 response in pancreatic β cells. Mol Cell Biol. 2013;33:2527–2534. doi: 10.1128/MCB.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zippin JH, Farrell J, Huron D, Kamenetsky M, Hess KC, Fischman DA, Levin LR, Buck J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J Cell Biol. 2004;164:527–534. doi: 10.1083/jcb.200311119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stessin AM, Zippin JH, Kamenetsky M, Hess KC, Buck J, Levin LR. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J Biol Chem. 2006;281:17253–17258. doi: 10.1074/jbc.M603500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holz GG. Epac: A new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic β-cell. Diabetes. 2004;53:5–13. doi: 10.2337/diabetes.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 76.Eliasson L, Ma X, Renström E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic B-cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279:7956–7961. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 78.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 79.Liu G, Jacobo SM, Hilliard N, Hockerman GH. Differential modulation of Cav1.2 and Cav1.3-mediated glucose-stimulated insulin secretion by cAMP in INS-1 cells: distinct roles for exchange protein directly activated by cAMP 2 (Epac2) and protein kinase A. J Pharmacol Exp Ther. 2006;318:152–160. doi: 10.1124/jpet.105.097477. [DOI] [PubMed] [Google Scholar]
- 80.Kwan EP, Xie L, Sheu L, Ohtsuka T, Gaisano HY. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes. 2007;56:2579–2588. doi: 10.2337/db06-1207. [DOI] [PubMed] [Google Scholar]
- 81.Seino S, Takahashi H, Fujimoto W, Shibasaki T. Roles of cAMP signalling in insulin granule exocytosis. Diabetes Obes Metab. 2009;11(Suppl 4):180–188. doi: 10.1111/j.1463-1326.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- 82.Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, Eliasson L. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2009;297:E452–E461. doi: 10.1152/ajpendo.90585.2008. [DOI] [PubMed] [Google Scholar]
- 83.Park JH, Kim SJ, Park SH, Son DG, Bae JH, Kim HK, Han J, Song DK. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic β-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology. 2012;153:574–582. doi: 10.1210/en.2011-0259. [DOI] [PubMed] [Google Scholar]
- 84.Kai AK, Lam AK, Chen Y, Tai AC, Zhang X, Lai AK, Yeung PK, Tam S, Wang J, Lam KS, Vanhoutte PM, Bos JL, Chung SS, Xu A, Chung SK. Exchange protein activated by cAMP 1 (Epac1)-deficient mice develop β-cell dysfunction and metabolic syndrome. FASEB J. 2013;27:4122–4135. doi: 10.1096/fj.13-230433. [DOI] [PubMed] [Google Scholar]
- 85.Song WJ, Mondal P, Li Y, Lee SE, Hussain MA. Pancreatic β-cell response to increased metabolic demand and to pharmacologic secretagogues requires EPAC2A. Diabetes. 2013;62:2796–2807. doi: 10.2337/db12-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holz GG, Chepurny OG, Leech CA. Epac2A makes a new impact in β-cell biology. Diabetes. 2013;62:2665–2666. doi: 10.2337/db13-0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsalkova T, Mei FC, Li S, Chepurny OG, Leech CA, Liu T, Holz GG, Woods VL, Jr, Cheng X. Isoform-specific antagonists of exchange proteins directly activated by cAMP. Proc Natl Acad Sci USA. 2012;109:18613–18618. doi: 10.1073/pnas.1210209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen H, Tsalkova T, Chepurny OG, Mei FC, Holz GG, Cheng X, Zhou J. Identification and characterization of small molecules as potent and specific EPAC2 antagonists. J Med Chem. 2013;56:952–962. doi: 10.1021/jm3014162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Courilleau D, Bisserier M, Jullian JC, Lucas A, Bouyssou P, Fischmeister R, Blondeau JP, Lezoualc’h F. Identification of a tetrahydroquinoline analog as a pharmacological inhibitor of the cAMP-binding protein Epac. J Biol Chem. 2012;287:44192–44202. doi: 10.1074/jbc.M112.422956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Idevall-Hagren O, Jakobsson I, Xu Y, Tengholm A. Spatial control of Epac2 activity by cAMP and Ca2+ -mediated activation of Ras in pancreatic β cells. Sci Signal. 2013;6:S1–S6. doi: 10.1126/scisignal.2003932. [DOI] [PubMed] [Google Scholar]
- 91.Cullen PJ. Decoding complex Ca2+ signals through the modulation of Ras signaling. Curr Opin Cell Biol. 2006;18:157–61. doi: 10.1016/j.ceb.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 92.Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–33. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dachicourt N, Serradas P, Giroix MH, Gangnerau MN, Portha B. Decreased glucose-induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. Am J Physiol. 1996;271:E725–732. doi: 10.1152/ajpendo.1996.271.4.E725. [DOI] [PubMed] [Google Scholar]
- 94.Dolz M, Movassat J, Bailbé D, Le Stunff H, Giroix MH, Fradet M, Kergoat M, Portha B. cAMP-secretion coupling is impaired in diabetic GK/Par rat β-cells: a defect counteracted by GLP-1. Am J Physiol Endocrinol Metab. 2011;301:E797–806. doi: 10.1152/ajpendo.00652.2010. [DOI] [PubMed] [Google Scholar]
- 95.Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J Physiol. 2006;573:595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of KATP channel ATP sensitivity in human pancreatic β-cells and rat INS-1 cells. J Physiol. 2008;586:1307–1319. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, Holz GG. Facilitation of β-cell KATP channel sulfonylurea sensitivity by a cAMP analog selective for the cAMP-regulated guanine nucleotide exchange factor Epac. Islets. 2010;2:72–81. doi: 10.4161/isl.2.2.10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β-cells. J Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kang G, Holz GG. Amplification of exocytosis by Ca2+-induced Ca2+ release in INS-1 pancreatic β cells. J Physiol. 2003;546:175–189. doi: 10.1113/jphysiol.2002.029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic β-cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dzhura I, Chepurny OG, Kelley GG, Leech CA, Roe MW, Dzhura E, Afshari P, Malik S, Rindler MJ, Xu X, Lu Y, Smrcka AV, Holz GG. Epac2-dependent mobilization of intracellular Ca2+ by glucagon-like peptide-1 receptor agonist exendin-4 is disrupted in β-cells of phospholipase C-ε knockout mice. J Physiol. 2010;588:4871–4889. doi: 10.1113/jphysiol.2010.198424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hussain MA, Porras DL, Rowe MH, West JR, Song WJ, Schreiber WE, Wondisford FE. Increased pancreatic beta-cell proliferation mediated by CREB binding protein gene activation. Mol Cell Biol. 2006;26:7747–7759. doi: 10.1128/MCB.02353-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song WJ, Schreiber WE, Zhong E, Liu FF, Kornfeld BD, Wondisford FE, Hussain MA. Exendin-4 stimulation of cyclin A2 in beta-cell proliferation. Diabetes. 2008;57:2371–2381. doi: 10.2337/db07-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cornu M, Modi H, Kawamori D, Kulkarni RN, Joffraud M, Thorens B. Glucagon-like peptide-1 increases beta-cell glucose competence and proliferation by translational induction of insulin-like growth factor-1 receptor expression. J Biol Chem. 2010;285:10538–10545. doi: 10.1074/jbc.M109.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holz GG, Habener JF. Black widow spider alpha-latrotoxin: a presynaptic neurotoxin that shares structural homology with the glucagon-like peptide-1 family of insulin secretagogic hormones. Comp Biochem Physiol B Biochem Mol Biol. 1998;121:177–184. doi: 10.1016/s0305-0491(98)10088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith EP, An Z, Wagner C, Lewis AG, Cohen EB, Bailing L, Parinaz M, Sandoval D, Perez-Tilve D, Tamarina N, Philipson LH, Stoffers DA, Seeley RJ, D’Alessio DA. The role of beta-cell glucagon-like peptide-1 signaling in glucose regulation and responses to diabetes drugs. Cell Metabol. 2014 doi: 10.1016/j.cmet.2014.04.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fridlyand LE, Harbeck MC, Roe MW, Philipson LH. Regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach. Am J Physiol Cell Physiol. 2007;293:C1924–C1933. doi: 10.1152/ajpcell.00555.2006. [DOI] [PubMed] [Google Scholar]
- 110.Holz GG, Heart E, Leech CA. Synchronizing Ca2+ and cAMP oscillations in pancreatic β-cells: a role for glucose metabolism and GLP-1 receptors? Focus on “regulation of cAMP dynamics by Ca2+ and G protein-coupled receptors in the pancreatic β-cell: a computational approach”. Am J Physiol Cell Physiol. 2008;294:C4–C6. doi: 10.1152/ajpcell.00522.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic β-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7-37) J Biol Chem. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 β-cell line. J Biol Chem. 2005;280:31294–31302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dyachok O, Idevall-Hagren O, Sågetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjärvi G, Gylfe E, Tengholm A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 114.Idevall-Hagren O, Barg S, Gylfe E, Tengholm A. cAMP mediators of pulsatile insulin secretion from glucose-stimulated single β-cells. J Biol Chem. 2010;285:23007–23018. doi: 10.1074/jbc.M109.095992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, Zhang J. Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol. 2011;7:34–40. doi: 10.1038/nchembio.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Takeda Y, Amano A, Noma A, Nakamura Y, Fujimoto S, Inagaki N. Systems analysis of GLP-1 receptor signaling in pancreatic β-cells. Am J Physiol Cell Physiol. 2011;301:C792–C803. doi: 10.1152/ajpcell.00057.2011. [DOI] [PubMed] [Google Scholar]
- 117.Hodson DJ, Mitchell RK, Marselli L, Pullen TJ, Brias SG, Semplici F, Everett KL, Cooper DM, Bugliani M, Marchetti P, Lavallard V, Bosco D, Piemonti L, Johnson PR, Hughes SJ, Li D, Li WH, Shapiro AM, Rutter GA. ADCY5 couples glucose to insulin secretion in human islets. Diabetes. 2014 doi: 10.2337/db13-1607. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takahashi N, Kadowaki T, Yazaki Y, Ellis-Davies GC, Miyashita Y, Kasai H. Post-priming actions of ATP on Ca2+-dependent exocytosis in pancreatic beta cells. Proc Natl Acad Sci USA. 1999;19:760–765. doi: 10.1073/pnas.96.2.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kasai H, Suzuki T, Liu TT, Kishimoto T, Takahashi N. Fast and cAMP-sensitive mode of Ca2+-dependent exocytosis in pancreatic beta-cells. Diabetes. 2002;51(Suppl 1):S19–24. doi: 10.2337/diabetes.51.2007.s19. [DOI] [PubMed] [Google Scholar]
- 120.Grill V, Cerasi E. Activation by glucose of adenyl cyclase in pancreatic islets of the rat. FEBS Lett. 1973;33:311–314. doi: 10.1016/0014-5793(73)80218-2. [DOI] [PubMed] [Google Scholar]
- 121.Charles MA, Fanska R, Schmid FG, Forsham PH, Grodsky GM. Adenosine 3′,5′-monophosphate in pancreatic islets: glucose-induced insulin release. Science. 1973;179:569–571. doi: 10.1126/science.179.4073.569. [DOI] [PubMed] [Google Scholar]
- 122.Grill V, Cerasi E. Stimulation by D-glucose of cyclic adenosine 3′:5′-monophosphate accumulation and insulin release in isolated pancreatic islets of the rat. J Biol Chem. 1974;249:4196–4201. [PubMed] [Google Scholar]
- 123.Grill V, Cerasi E. Glucose-induced cyclic AMP accumulation in rat islets of Langerhans: preferential effect of the alpha anomer. FEBS Lett. 1975;54:80–83. doi: 10.1016/0014-5793(75)81073-8. [DOI] [PubMed] [Google Scholar]
- 124.Grill V, Asplund K, Hellerström C, Cerasi E. Decreased cyclic AMP and insulin response to glucose in isolated islets of neonatal rats. Diabetes. 1975;24:746–752. doi: 10.2337/diab.24.8.746. [DOI] [PubMed] [Google Scholar]
- 125.Suzuki S, Oka H, Yasuda H, Yamashita K, Kaneko T, Oda T. Effect of glucose on adenosine 3′, 5′-monophosphate levels in rat pancreatic islets. Endocrinol Jpn. 1975;22:479–482. doi: 10.1507/endocrj1954.22.479. [DOI] [PubMed] [Google Scholar]
- 126.Zawalich WS, Karl RC, Ferrendelli JA, Matschinsky FM. Factors governing glucose induced elevation of cyclic 3′5′ AMP levels in pancreatic islets. Diabetologia. 1975;11:231–235. doi: 10.1007/BF00422327. [DOI] [PubMed] [Google Scholar]
- 127.Charles MA, Lawecki J, Pictet R, Grodsky GM. Insulin secretion. Interrelationships of glucose, cyclic adenosine 3:5-monophosphate, and calcium. J Biol Chem. 1975;250:6134–6140. [PubMed] [Google Scholar]
- 128.Grill V, Cerasi E. Enhancement by D2O of glucose-induced cyclic AMP accumulation in rat islets of Langerhans. FEBS Lett. 1976;68:165–169. doi: 10.1016/0014-5793(76)80428-0. [DOI] [PubMed] [Google Scholar]
- 129.Rabinovitch A, Grill V, Renold AE, Cerasi E. Insulin release and cyclic AMP accumulation in response to glucose in pancreatic islets of fed and starved rats. J Clin Invest. 1976;58:1209–1216. doi: 10.1172/JCI108574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schauder P, Arends J, Schindler B, Ebert R, Frerichs H. Permissive effect of glucose on the glucagon-induced accumulation of cAMP in isolated rat pancreatic islets. Diabetologia. 1977;13:171–175. doi: 10.1007/BF00745146. [DOI] [PubMed] [Google Scholar]
- 131.Tsumura Y, Kobayashi K, Yoshida K, Kagawa S, Matsuoka A. Dynamics of insulin and cyclic adenosine 3′,5′-monophosphate release from the perifused rat islets of Langerhans under a slow-rise stimulation with D-glucose and its anomers. Endocrinol Jpn. 1979;26:245–253. doi: 10.1507/endocrj1954.26.245. [DOI] [PubMed] [Google Scholar]
- 132.Kim JW, Roberts CD, Berg SA, Caicedo A, Roper SD, Chaudhari N. Imaging cyclic AMP changes in pancreatic islets of transgenic reporter mice. PLoS One. 2008;3:e2127. doi: 10.1371/journal.pone.0002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Harbeck MC, Chepurny O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Simultaneous optical measurements of cytosolic Ca2+ and cAMP in single cells. Sci STKE. 2006;(353):pl6. doi: 10.1126/stke.3532006pl6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chepurny OG, Leech CA, Cheng X, Holz GG. cAMP sensor Epac and gastrointestinal function. In: Johnson L, Ghishan F, Kaunitz JM, Said H, Wood J, editors. Physiology of the Gastrointestinal Tract. 5. Elsevier; 2012. pp. 1849–1861. [Google Scholar]
- 135.Ammälä C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta cells. Nature. 1993;363:356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- 136.Buck J, Sinclair ML, Schapal L, Cann MJ, Levin LR. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc Natl Acad Sci USA. 1999;96:79–84. doi: 10.1073/pnas.96.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leech CA, Chepurny OG, Holz GG. Epac2-dependent rap1 activation and the control of islet insulin secretion by glucagon-like peptide-1. Vitam Horm. 2010;84:279–302. doi: 10.1016/B978-0-12-381517-0.00010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dzhura I, Chepurny OG, Leech CA, Roe MW, Dzhura E, Xu X, Lu Y, Schwede F, Genieser HG, Smrcka AV, Holz GG. Phospholipase C-ε links Epac2 activation to the potentiation of glucose-stimulated insulin secretion from mouse islets of Langerhans. Islets. 2011;3:121–128. doi: 10.4161/isl.3.3.15507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Smrcka AV, Brown JH, Holz GG. Role of phospholipase Cε in physiological phosphoinositide signaling networks. Cell Signal. 2012;24:1333–1343. doi: 10.1016/j.cellsig.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]