Abstract
Renal dysfunction (RD) is associated with increased mortality in heart failure (HF). The aim of this study was to identify whether worsened or improved renal function during mid-term follow-up is associated with worsened outcomes in chronic HF patients. 892 participants from a multicenter cohort study of chronic HF were followed over 3.1±1.9 years of enrollment. Worsened and improved renal function were tested with multivariable models as independent predictors of HF hospitalization and mortality. While 12% of subjects experienced a ≥25% decrease in estimated glomerular filtration rate (eGFR), 17% experienced a ≥25% increase in eGFR, and there was stability of kidney function observed in the cohort as a whole. The quartile with the worst RD at any point in time had increased risk of HF hospitalization and mortality. Worsened eGFR was associated with HF outcomes in the unadjusted (HR=1.71 (95%CI 1.04-2.81), p=0.035), but not the adjusted analysis. Improvement in eGFR was not associated with outcome (p=0.453). In chronic HF, the severity of RD predicts risk of poor outcome better than changes in renal function during mid-term follow-up. This suggests that in patients with appropriately treated chronic HF, worsening renal function in itself does not yield useful prognostic information and may not reflect poor outcome.
Keywords: worsening, improved, renal function, hospitalization, death
Introduction
Heart failure (HF) affects approximately 6 million people in the United States.1 Comorbidities clearly impact HF prognosis. Over the last two decades, the number of comorbidities and medications in the average HF patient has increased substantially, renal failure being among those.2 Given the high cost of HF hospitalization, identifying risk factors that increase its likelihood is useful. Renal function is considered to be a sensitive marker of decreased organ perfusion and is commonly thought to deteriorate in HF due to chronic hypoperfusion.3 Recently, several studies have reported an association between worsening renal function (WRF) during inpatient treatment for acute decompensated HF and poor clinical outcomes. 4-11 In chronic HF, reduced renal perfusion may occur over a long period, and patients may experience few symptoms related to the declining renal function.3 Several studies have found an association of WRF with mortality in the ambulatory setting. 12-17 Most studies have included only patients with heart failure with reduced ejection fraction (HFrEF), and follow-up has typically been short, investigating changes in renal function over no more than a 6 month interval from baseline. Our aim was to assess how kidney function changed during mid-term follow-up in HF patients, and whether WRF predicts all-cause mortality and HF hospitalization in patients medically treated for chronic HF. We also examined risk factors for WRF and whether improvement in renal function was associated with improved outcomes.
Methods
Subjects were enrolled in the multicenter Penn Heart Failure Study (PHFS). The PHFS began in 2003 at the University of Pennsylvania and subsequently expanded into a multi-center study. This is a prospective observational cohort study of over 2,000 subjects with heart failure followed in HF specialty clinics. The study was approved by institutional review committees and the subjects gave informed consent. Detailed patient information was collected at baseline and patients followed every six months to measure predefined endpoints (hospitalization, change in therapy and death). Patients were either seen in clinic or called at six month intervals. Inclusion criteria in this analysis were an available baseline measurement of creatinine (at time of enrollment) and at least one follow-up value. At the beginning of the study follow-up kidney function was not routinely collected, and therefore only the subset of patients in whom this information was available was included in this analysis. The primary outcome measures were death or HF hospitalization (primary composite outcome) and death alone. Ten subjects underwent heart transplantation and were counted in the death endpoint. This was done since the assumed outcome without transplantation is death. HF hospitalization was based on primary discharge diagnosis. Patients with a clinical diagnosis of HF were considered to have HFrEF based on an EF <=40% as defined in current guidelines.18 The remaining patients were classified as HF with preserved EF (HFpEF).
eGFR was calculated using the Modification of Diet in Renal Disease (MDRD) equation.19 Change in eGFR was calculated by subtracting the most recent follow-up eGFR from baseline eGFR. For patients with a primary outcome, the most recent eGFR prior to reaching the primary outcome was used. We used previously defined criteria for WRF: a ≥25% decrease in eGFR20,21 or an increase in SCr≥0.3 mg/dL.22-24 Improvement in renal function was defined as a ≥ 25% increase in eGFR or a decrease in SCr≥0.3 mg/dL.
Participants were divided into quartiles of baseline eGFR. Comparisons between baseline eGFR groups were made with one-way ANOVA, Kruskal-Wallis tests, or Chi-square tests based on distribution and normality assumptions. Univariate Cox proportional hazards (Cox PH) models were used to assess the relationship between time to a primary outcome and baseline or follow-up eGFR/SCr. WRF status and time to primary composite outcome were also assessed with univariate Cox proportional hazards model. Similarly, univariate Cox models were used for the mortality outcome. To assess for linearity in the coefficients of the Cox model over the entire range of follow-up SCr and eGFR, each group was divided into quartiles and hazard ratios calculated using the lowest SCr quartile and highest eGFR quartile as the reference.
Multivariate Cox proportional hazards models were developed by compiling a list of 39 baseline variables of clinical importance and that did not have large numbers of missing values. Univariate Cox models of each baseline variable were created for time to primary composite outcome. Candidate variables were considered to be baseline variables that had chi-square p-values less than 0.05. Backwards and forwards stepwise models of the candidate variables were run to determine final variables for inclusion in multivariate models. To test for robustness, models were rerun excluding variables that might over-correct the model, such as New York Heart Association (NYHA) Class. This led to no significant changes in the predictive value of the variables in the model, so the best iteration is presented. The above analysis was repeated for HFrEF and HFpEF separately.
Results
The analysis cohort included 892 patients. Fifty-two (5.7%) of the 892 subjects were missing data for at least one candidate variable in the multivariate analysis. Table 1 illustrates baseline characteristics across eGFR quartiles. The average age was 56 years and 2/3 were men. HFrEF was present in 61% of patients. Most patients had NYHA Class II or III symptoms. More than a third (36%) of the study population had experienced a hospitalization in the 12 months prior to enrollment into PHFS. Older patients, those with an ischemic etiology, and those with comorbidities such as diabetes, hypertension, and stroke were more likely to have lower baseline eGFR. NYHA class and the Minnesota Living with Heart Failure Questionnaire score25 were greater in patients with lower baseline eGFR, indicating higher symptom burden. Mean EF (37%) was not different between groups, nor were blood pressure, heart rate, BMI, or serum sodium. Loop diuretics, aldosterone antagonists, aspirin, hydralazine, long acting nitrates, and statins were more commonly prescribed in patients with worse baseline renal function. ACE inhibitor use was more common in those with better baseline renal function.
Table 1. Baseline characteristics by baseline eGFR quartiles.
| Quartile | Total Cohort (n=892) | P | ||||
|---|---|---|---|---|---|---|
| Variable | 1 (n=223) |
2 (n=223) |
3 (n=223) |
4 (n=223) |
||
| eGFR (mL*min-1*1.73m-2) | ||||||
| median (minimum, maximum) | 95 (85, 628) | 76 (69, 84) | 62 (53, 69) | 40 (6, 53) | 69 (6, 628) | … |
| Age (years) | 48 (14) | 54 (13) | 59 (14) | 64 (12) | 56 (15) | < 0.01 |
| Male | 154 (69%) | 138 (62%) | 135 (61%) | 128 (57%) | 555 (62%) | 0.07 |
| White Race | 155 (71%) | 165 (76%) | 182 (83%) | 151 (70%) | 653 (73%) | < 0.01 |
| Black Race | 58 (27%) | 50 (23%) | 32 (15%) | 58 (27%) | 198 (22%) | |
| Ischemic origin | 33 (15%) | 47 (21%) | 70 (32%) | 78 (36%) | 228 (26%) | < 0.01 |
| Systolic heart failure | 114 (52%) | 130 (58%) | 121 (55%) | 121 (55%) | 486 (55%) | 0.57 |
| Hospitalization in prior 12 months | 68 (30%) | 83 (37%) | 84 (38%) | 91 (41%) | 326 (37%) | 0.14 |
| Diabetes mellitus | 44 (20%) | 49 (22%) | 49 (22%) | 86 (39%) | 228 (26%) | < 0.01 |
| Hypertension | 11 (50%) | 121 (54%) | 131 (59%) | 166 (74%) | 529 (59%) | < 0.01 |
| Stroke | 3 (1%) | 16 (7%) | 10 (4%) | 21 (9%) | 50 (6%) | < 0.01 |
| Follow-up time, years, median (IQR) | 3.0 (1.6, 5.0) | 2.9 (1.8, 4.8) | 3.0 (1.7, 4.4) | 2.5 (1.3, 3.7) | 2.9 (1.5, 4.5) | < 0.01 |
| New York Heart Association Class | ||||||
| II | 122 (55%) | 123 (55%) | 116 (52%) | 113 (52%) | 472 (53%) | |
| III | 31 (14%) | 43 (19%) | 45 (20%) | 71 (33%) | 190 (22%) | < 0.01 |
| IV | 1 (0%) | 0 (0%) | 6 (3%) | 6 (3%) | 13 (1%) | |
| Ejection Fraction (%) | 37 (16) | 36 (17) | 38 (17) | 39 (18) | 37 (17) | 0.53 |
| Body Mass Index (kg/m2) | 30 (7) | 30 (8) | 31 (7) | 32 (9) | 31 (8) | 0.11 |
| Heart rate (beats per minute) | 73 (13) | 72 (13) | 73 (14) | 72 (13) | 72 (13) | 0.78 |
| Systolic blood pressure (mm Hg) | 117 (21) | 116 (21) | 117 (22) | 119 (25) | 118 (22) | 0.59 |
| MLHFQ* score, median (IQR) | 19 (2, 51) | 18 (4, 45) | 24 (6, 50) | 34 (9, 59) | 24 (4, 52) | 0.01 |
| Serum creatinine (mg/dL) | 0.9 (0.1) | 1.0 (0.1) | 1.2 (0.2) | 2.1 (1.4) | 1.3 (0.9) | < 0.01 |
| Serum sodium (mEq/L) | 139 (3) | 140 (2) | 139 (3) | 139 (4) | 139 (3) | 0.19 |
| Potassium-sparing diuretics | 2 (1%) | 3 (1%) | 7 (3%) | 2 (1%) | 14 (2%) | 0.25 |
| Loop diuretics | 118 (53%) | 135 (61%) | 138 (62%) | 168 (75%) | 559 (63%) | < 0.01 |
| ACE inhibitors | 161 (72%) | 163 (73%) | 149 (67%) | 138 (62%) | 611 (68%) | 0.04 |
| Aldosterone antagonist | 59 (26%) | 64 (29%) | 61 (27%) | 87 (39%) | 271 (30%) | 0.01 |
| Angiotensin receptor blockers | 48 (22%) | 50 (22%) | 59 (26%) | 56 (25%) | 213 (24%) | 0.58 |
| Aspirin | 114 (51%) | 122 (55%) | 123 (55%) | 145 (65%) | 504 (57%) | 0.02 |
| β-Blockers | 195 (87%) | 198 (89%) | 194 (87%) | 198 (89%) | 785 (88%) | 0.91 |
| Digoxin | 63 (28%) | 58 (26%) | 57 (26%) | 70 (31%) | 248 (28%) | 0.5 |
| Hydralazine | 14 (6%) | 9 (4%) | 9 (4%) | 36 (16%) | 68 (8%) | < 0.01 |
| Long acting nitrate | 19 (9%) | 21 (9%) | 21 (9%) | 54 (24%) | 115 (13%) | < 0.01 |
| Statin | 89 (40%) | 127 (57%) | 128 (57%) | 143 (64%) | 487 (55%) | < 0.01 |
Continuous variables are reported as mean (SD) unless otherwise noted.
Categorical variables are reported as frequency (%).
P-values for continuous variables are from one-way ANOVA or Kruskal-Wallis tests.
P-values for categorical variables are from Pearson chi-square test or Fisher's exact test.
Minnesota Living with Heart Failure Questionnaire (MLHFQ).
There are 2767 patient-years of follow up in the cohort, with a median follow up time of 2.9 years, and mean follow-up of 3.1±1.9 years. A regression analysis of creatinine values versus time was created for each subject. The mean change in creatinine over time was 0.0074±0.43 mg/dL increase in creatinine per year; the slope of this regression line was not statistically different from zero. Similarly, estimated GFR did not deteriorate over time in the cohort as a whole. Stage 3 or greater CKD was present at baseline in 309 (35%) of the 892 subjects. 322 (36%) subjects had Stage 3 or greater CKD at the most recent follow-up visit or just prior to reaching a primary outcome. A total of 109 (12%) subjects experienced WRF during follow-up using eGFR, 110 (12%) using SCr. A total of 152 (17%) subjects experienced improved eGFR; 108 (12%) had improved SCr. A total of 674 (76%) subjects had stable eGFR. Mean baseline SCr in the worsening eGFR group was 1.56±1.18mg/dL and was 1.27±0.81mg/dL in the stable group (p=0.015). There was a trend toward WRF in patients with lower baseline eGFR, but this did not reach statistical significance (p=0.076). 110 subjects (12%) had a primary outcome; 26 (2.9%) died. Of 840 subjects with complete data in the multivariate analysis, 107 (12%) had a primary outcome, of whom 26 (3.1%) died, and of whom 10 (1.2%) underwent heart transplant.
Table 2 includes the univariate analysis results for the 14 candidate variables and 4 pre-selected variables (age, sex, diabetes, and ischemic status). Of the pre-selected variables, only sex did not have a statistically significant association with the primary composite outcome of death or HF hospitalization (p=0.906). As illustrated in Table 3, baseline and follow-up renal function demonstrated significant associations with the primary composite outcome in both the unadjusted and adjusted analysis. Separate analyses of HFrEF (HR=1.05 (95%CI 1.00-1.09) and 1.21 (95%CI 1.10– 1.32), p=0.05, <0.001 for baseline SCr and eGFR respectively) and HFpEF (HR=1.09 (95%CI 1.02- 1.16) and 1.20 (95%CI 1.01–1.42), p=0.009, 0.038 for baseline SCr and eGFR respectively) showed the same association with the primary outcome in unadjusted analysis but not in the adjusted analysis. Figure 1 demonstrates that risk of the primary outcome rises markedly in the group with most impaired renal function at baseline. The association between follow-up renal function and the primary composite outcome was even stronger.
Table 2. Baseline variables associated with primary outcome.
| Baseline characteristic | Hazard Ratio | 95% CI | P-value | Number (%) | Events (%) |
|---|---|---|---|---|---|
| New York Heart Association class | 882 (99) | 109 (99.1) | |||
| I | reference | -- | -- | ||
| II | 3.21 | 1.46 - 7.09 | 0.004 | ||
| III | 9.89 | 4.47 - 21.91 | < 0.001 | ||
| IV | 13.47 | 3.93 - 46.2 | < 0.001 | ||
| Race | 867 (97.3) | 108 (98.2) | |||
| Caucasian | reference | -- | -- | ||
| Black | 1.63 | 1.07 - 2.48 | 0.022 | ||
| Other | 0.54 | 0.07 - 3.86 | 0.536 | ||
| Gender | 892 (100) | 110 (100) | |||
| Female | reference | -- | -- | ||
| Male | 0.94 | 0.64 - 1.40 | 0.747 | ||
| Age (years) | 1.019 | 1.01 - 1.03 | 0.007 | 892 (100) | 110 (100) |
| Ischemic origin | 1.47 | 0.98 - 2.21 | 0.060 | 876 (98.3) | 109 (99.1) |
| Hospitalization in prior 12 months | 1.22 | 1.14 - 1.32 | < 0.001 | 892 (100) | 110 (100) |
| Diabetes | 1.52 | 1.01 - 2.28 | 0.043 | 892 (100) | 110 (100) |
| Hypertension | 2.07 | 1.37 - 3.12 | 0.001 | 892 (100) | 110 (100) |
| Hyperlipidemia | 1.65 | 1.12 - 2.42 | 0.010 | 892 (100) | 110 (100) |
| Ejection fraction | 0.973 | 0.96 - 0.99 | < 0.001 | 886 (99.4) | 110 (100) |
| Heart rate, beats per minute | 1.017 | 1.00 - 1.03 | 0.021 | 877 (98.4) | 108 (98.2) |
| Systolic blood pressure (mm Hg) | 0.989 | 0.98 - 1.00 | 0.026 | 884 (99.2) | 110 (100) |
| MLHFQ score | 1.017 | 1.01 - 1.02 | < 0.001 | 892 (100) | 110 (100) |
| Loop diuretic | 2.82 | 1.78 - 4.47 | < 0.001 | 892 (100) | 110 (100) |
| Aldosterone antagonist | 2.08 | 1.42 - 3.03 | < 0.001 | 892 (100) | 110 (100) |
| Hydralazine | 3.13 | 1.76 - 5.55 | < 0.001 | 892 (100) | 110 (100) |
| Long acting nitrate | 3.04 | 1.96 - 4.71 | < 0.001 | 892 (100) | 110 (100) |
| Digoxin | 1.57 | 1.07 - 2.31 | 0.020 | 892 (100) | 110 (100) |
Univariate Cox proportional hazard modes were used to assess the relationship between 14 candidate variables and the 4 pre-selected variables to control for (age, sex, diabetes and ischemic status) with primary outcome. Frequency for each variable is reported by number and % out of 892 subjects studied.
Table 3. Relationship between renal parameters and primary outcome.
| Unadjusted Analysis | Adjusted Analysis* | |||||
|---|---|---|---|---|---|---|
| Primary Composite | HR† | 95% CI | P | HR† | 95% CI | P |
| Baseline SCr | 1.06 | 1.02 – 1.10 | 0.002 | 1.04 | 0.99 – 1.09 | 0.091 |
| Baseline eGFR | 1.20 | 1.11 – 1.30 | < 0.001 | 1.11 | 1.02 – 1.21 | 0.017 |
| Follow-Up SCr | 1.05 | 1.02 – 1.08 | 0.002 | 1.05 | 1.00 – 1.10 | 0.040 |
| Follow-Up eGFR | 1.24 | 1.15 – 1.33 | < 0.001 | 1.16 | 1.07 – 1.26 | < 0.001 |
| Stable SCr | ref. | |||||
| ≥ 0.3 mg/dL ↓SCr‡ | 2.02 | 1.20 – 3.40 | 0.008 | 1.11 | 0.64 – 1.93 | 0.713 |
| ≥ 0.3 mg/dL ↑SCr‡ | 2.21 | 1.36 – 3.57 | 0.001 | 1.27 | 0.76 – 2.13 | 0.368 |
| Stable eGFR | ref. | |||||
| ≥ 25% ↑eGFR‡ | 1.32 | 0.81 – 2.15 | 0.269 | 0.88 | 0.53 – 1.47 | 0.624 |
| ≥ 25% ↓eGFR‡ | 1.71 | 1.04 – 2.81 | 0.035 | 0.92 | 0.53 – 1.58 | 0.759 |
|
| ||||||
| Death | HR | 95% CI | P | HR | 95% CI | P |
|
| ||||||
| Baseline SCr | 1.03 | 0.95 - 1.13 | 0.424 | 0.96 | 0.85 - 1.07 | 0.433 |
| Baseline eGFR | 1.21 | 1.05 - 1.39 | 0.009 | 1.05 | 0.90 - 1.23 | 0.514 |
| Follow-Up SCr | 1.05 | 1.00 – 1.11 | 0.052 | 1.03 | 0.94 - 1.14 | 0.477 |
| Follow-Up eGFR | 1.33 | 1.17 - 1.52 | < 0.001 | 1.23 | 1.04 - 1.44 | 0.014 |
| Stable SCr | ref. | |||||
| ≥ 0.3 mg/dL ↓SCr‡ | 1.92 | 0.72 - 5.14 | 0.192 | 0.81 | 0.28 - 2.31 | 0.689 |
| ≥ 0.3 mg/dL ↑SCr‡ | 3.1 | 1.41 - 6.82 | 0.005 | 1.48 | 0.61 - 3.57 | 0.387 |
| Stable eGFR | ref. | |||||
| ≥ 25% ↑eGFR‡ | 1.03 | 0.39 - 2.73 | 0.956 | 0.53 | 0.19 - 1.52 | 0.239 |
| ≥ 25% ↓eGFR‡ | 2.26 | 1.00 – 5.10 | 0.050 | 1.03 | 0.42 - 2.55 | 0.941 |
Cox proportional hazards model controlling for the following baseline characteristics: age, sex, race, ejection fraction, NYHA class, ischemic etiology, systolic blood pressure, long acting nitrate, hypertension, MLHFQ, diabetes, and loop diuretic.
Hazard ratio (HR) calculated per 0.3 mg/dL increments in serum creatinine (SCr) and per 10 mL/min decrements in eGFR.
Improved renal function, defined as a decrease in SCr ≥ 0.3 mg/dL; and an increase in eGFR ≥ 25%.
Worsening renal function, defined as an increase in SCr ≥ 0.3 mg/dL; and a decrease in eGFR ≥ 25%.
Figure 1.
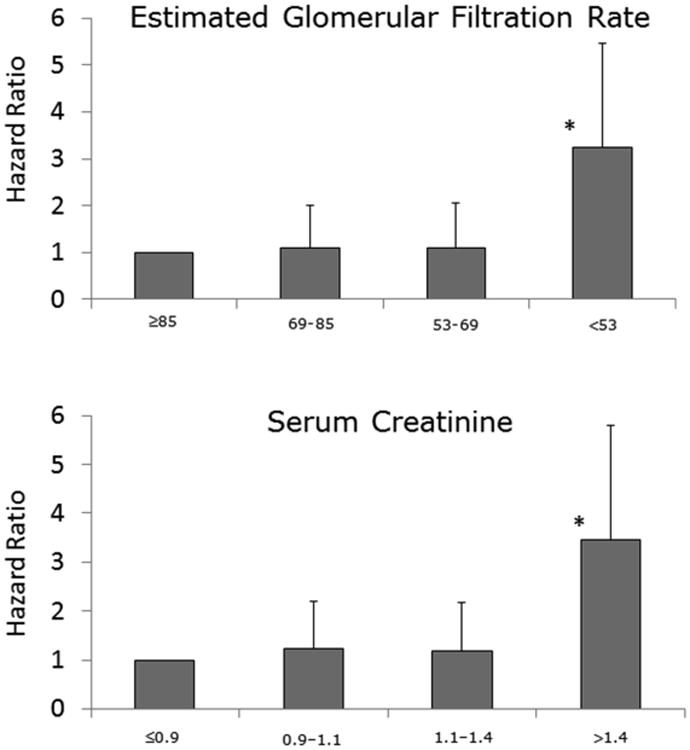
The relation between baseline renal function, expressed as estimated glomerular filtration rate (mL/min/1.73m2) or creatinine (mg/dL), and the hazard ratio for death or heart failure hospitalization. The risk of the outcome rises markedly in the group with the most impaired renal function at baseline. * = p < 0.001.
There was an association between WRF and the primary composite outcome and death alone in the unadjusted analysis, however not in the adjusted analysis. Improvement in renal function showed no association with primary composite outcome in the adjusted analysis. As shown in table 4, diabetes and age were predictors of WRF (using either SCr or eGFR). Loop diuretic and hydralazine use were predictors of worsening SCr but not change in eGFR. Importantly, EF and NYHA class did not predict WRF.
Table 4. Multivariable analysis of factors associated with worsening renal function.
| ≥ 0.3 mg/dL ↑SCr | Stable† (n=782) | Worsened‡ (n=109) | Odds Ratio* | 95% CI | P-value |
|---|---|---|---|---|---|
| Age – 5 year increments | 55.5 (14.5) | 60.8 (14) | 1.12 | 1.04 – 1.21 | 0.005 |
| Coronary artery disease | 17 (2.2%) | 9 (8.3%) | 3.13 | 1.27 - 7.28 | 0.01 |
| Diabetes | 185 (23.7%) | 43 (39.4%) | 1.59 | 1.02 – 2.46 | 0.04 |
| Baseline creatinine | 1.27 (0.81) | 1.56 (1.19) | 1.22 | 1.00 - 1.46 | 0.04 |
| Hydralazine use | 50 (6.4%) | 18 (16.5%) | 2.53 | 1.34 – 4.62 | 0.003 |
| Loop diuretic use | 473 (60.5%) | 85 (78%) | 1.82 | 1.12 – 3.04 | 0.02 |
|
| |||||
| ≥ 25% ↓eGFR | Stable (n=783) | Worsened‡ (n=109) | Odds Ratio | 95% CI | P-value |
|
| |||||
| Age – 5 year increments | 55.7 (14.7) | 59.3 (13.6) | 1.08 | 1.00 – 1.17 | 0.05 |
| Coronary artery disease | 17 (2.2%) | 9 (8.3%) | 3.23 | 1.32 - 7.42 | 0.01 |
| Baseline eGFR | 69.5 (31.3) | 70.0 (25.5) | 1.01 | 1.00 - 1.01 | 0.08 |
| Diabetes | 186 (23.8%) | 42 (38.9%) | 1.71 | 1.09 – 2.64 | 0.007 |
| Loop diuretic use | 477 (60.9%) | 82 (75.2%) | 1.77 | 1.13 – 2.87 | 0.02 |
Odds ratio from logistic regression model while controlling for the other factors in each respective list.
Reported as group mean (SD) or frequency (%).
Worsened renal failure, defined as an increase in SCr ≥ 0.3 mg/dL; and a decrease in eGFR ≥ 25% from baseline to last creatinine follow-up or last creatinine follow-up prior to composite event.
Discussion
The results of this study highlight significant facts about kidney function in an aggressively treated cohort of outpatients with heart failure. Our study confirmed the high prevalence of chronic renal dysfunction in chronic HF patients. However we did not find any evidence of deterioration of kidney function over time in participants, despite over 2700 patient-years of follow-up. There was a statistically significant independent association between kidney function at multiple time points and the primary composite outcome of HF hospitalization or death. Follow-up measures of renal function were most strongly associated with outcomes, suggesting that perhaps optimization of hemodynamics with therapy in HF clinics unmasked intrinsic renal dysfunction and thus predicted outcomes most effectively. It has previously been suggested that severity of renal dysfunction rather than its change over time appeared to be the most important determinant of outcome,12 similar to our findings.
In this analysis, 12% of patients had WRF (using eGFR). While the degree of renal insufficiency measured at single points in time showed associations with the primary outcome variables in this study, the change in renal function over time was not related to the primary outcome in the multivariate analysis. This suggests that worsening renal function in a heart failure patient may be a marker of progression of HF, but is not independently prognostic.
Several studies have shown an association of WRF with increased mortality in patients with chronic heart failure.12-17,19-22 A recent meta-analysis showed that in chronic HF, WRF occurring without treatment strongly correlated with poor outcome but in other clinical settings may not.26 WRF in a cohort of elderly patients receiving 6 months of intensive medical therapy was associated with mortality only when SCr increased by ≥ 0.5 mg/dL.17 These elderly patients received high doses of loop diuretics and spironolactone. The authors suggested that the higher doses of loop diuretics may have played a causal role instead of being a surrogate marker of more severe HF. They also proposed that WRF in the elderly may reflect initiation of aggressive treatment. Two studies assessing WRF in the setting of ACE inhibitors did not demonstrate a correlation with poor outcome.12,27 Measuring WRF over shorter periods may be misleading, especially during initiation of medical treatment. ACE inhibitors are known to cause an acute decline in GFR while preserving kidney function over time.28 At the time of enrollment, 92% of patients in this cohort were on an ACE inhibitor or ARB. It is thus useful to measure WRF over longer follow-up periods to determine whether an association with outcomes exists in chronic HF. Renal dysfunction attributable to normal aging in the HFrEF population has been shown to have limited risk on mortality.29 These findings suggest that the clinical setting in which WRF occurs may be important in evaluating its significance. Our results expand previous findings in chronic HF patients to include HFpEF. Although we suffered from loss of power, the separated analyses in HFrEF and HFpEF demonstrated qualitatively similar results, with WRF not associated with outcome in either group.
Table 4 shows that mean baseline SCr and eGFR were significantly higher in the WRF group compared to the stable group, indicating that the presence of intrinsic renal dysfunction was associated with WRF. Age, coronary artery disease, diabetes, loop diuretic and hydralazine use (SCr definition only) were also independently associated with WRF. The WRF with hydralazine may reflect the absence of renin-angiotensin system blockade in this group, but may also be confounding by indication, as ACE inhibitors are routinely withheld from those with elevated creatinine
In this analysis, more patients (17% using eGFR) had improvement in renal function over time than had WRF. One study has reported decreased risk of all-cause mortality with improved SCr over the initial 6 month follow-up period (HR 0.8, CI 0.6-1.0).12 In contrast, in our study, improved renal function was not associated with improved long-term outcome in either the univariate or the multivariate analysis. This was unexpected as we had hypothesized that improved renal function with medication optimization would result in fewer HF hospitalizations and better outcomes. This may suggest that improvement in renal function accompanying HF therapy may result in improved volume status but does not appear to alter disease trajectory.
WRF due to low cardiac output is rare. Our study suggests that, while commonly coexisting, heart failure and renal failure are two separate processes. Patients with heart failure who have significant intrinsic renal disease have a poorer prognosis than those without renal disease. Having heart failure in and of itself does not worsen kidney function in HF outpatients. Clinicians should not alter management of chronic HF patients based solely on change in renal function. An inexorable decline in kidney function is not an obligatory accompaniment to a HF diagnosis suggesting that WRF should alert providers of coexistent significant kidney disease.
Our cohort was comprised of patients seen in tertiary HF clinics. Patients in this study were younger, had more systolic dysfunction, more severe symptoms, and less CAD than chronic HF patients in population-based studies.1 It is notable that the referral nature of this cohort provides both strengths and limitations. The population spanned a full spectrum of diseases, heart failure etiologies, and severity. This permits us to evaluate findings from such subgroups using data from three US centers in order to make interferences about differences in disease pathophysiology and outcome. Interferences are likely to be generalizable to similar populations but may not be extrapolated to the general heart failure population. Additionally, HFrEF are more likely to be referred to tertiary specialty centers than HFpEF, thus resulting in a higher proportion of HFrEF patients in this cohort than in the general population. The HFpEF group was too small to make meaningful conclusions however there were not substantial qualitative differences between the two groups when analyzed separately. More study is needed to determine the true differences between HFrEF and HFpEF with regard to change in kidney function with outpatient treatment.
Acknowledgments
The authors thank David Birtwell, Erin McIntosh, Allie Galati at University of Pennsylvania, Kimberly Huck at Case Western Reserve University and Todd Forsythe at University of Wisconsin, for their help in data acquisition for this study. This work was supported by National Institutes of Health [grant number R01HL088577] and the University of Wisconsin Department of Medicine.
Footnotes
Disclosures:The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failurehospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong CY, Chaudhry SI, Desai MM, Krumholz HM. Trends in comorbidity, disability, and polypharmacy in heart failure. Am J Med. 2011;124:136–143. doi: 10.1016/j.amjmed.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ljungman S, Laragh JH, Cody RJ. Role of the kidney in congestive heart failure. Relationship of cardiac index to kidney function. Drugs. 1990;39(Suppl 4):10–21. doi: 10.2165/00003495-199000394-00004. discussion 22-24. [DOI] [PubMed] [Google Scholar]
- 4.Smith GL, Lichtman JH, Bracken MB, Shlipak MG, Phillips CO, DiCupa P, Krumholz HM. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol. 2006;47:1987–1996. doi: 10.1016/j.jacc.2005.11.084. [DOI] [PubMed] [Google Scholar]
- 5.Hillege HL, Nitsch D, Pfeffer MA, Swedberg K, McMurray JJ, Yusuf S, Granger CB, Michelson EL, Ostergren J, Cornel JH, de Zeeuw D, Pocock S, van Veldhuisen DJ. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation. 2006;113:671–678. doi: 10.1161/CIRCULATIONAHA.105.580506. [DOI] [PubMed] [Google Scholar]
- 6.Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. 2007;13:422–430. doi: 10.1016/j.cardfail.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- 8.Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- 9.McAlister FA, Ezekowitz J, Tonelli M, Armstrong PW. Renal insufficiency and heart failure: prognostic and therapeutic implications from a prospective cohort study. Circulation. 2004;109:1004–1009. doi: 10.1161/01.CIR.0000116764.53225.A9. [DOI] [PubMed] [Google Scholar]
- 10.Blair JE, Pang PS, Schrier RW, Metra M, Traver B, Cook T, Campia U, Ambrosy A, Burnett JC, JR, Grinfield L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Kromstam MA, Gheorghiade M. Changes in renal function during hospitalization and soon after discharge in patients admitted for worsening heart failure in the placebo group of the EVEREST trial. Eur Heart J. 2011;32:2563–2572. doi: 10.1093/eurheartj/ehr238. [DOI] [PubMed] [Google Scholar]
- 11.Damman K, Jaarsma T, Voors AA, Navis G, Hillege HL, van Veldhuisen DJ. Both in- and out-hospital worsening of renal function predict outcome in patients with heart failure: results from the Coordinating Study Evaluating Outcome of Advising and Counseling in Heart Failure (COACH) Eur J Heart Fail. 2009;11:847–854. doi: 10.1093/eurjhf/hfp108. [DOI] [PubMed] [Google Scholar]
- 12.de Silva R, Nikitin NP, Witte KK, Rigby AS, Goode K, Bhandari S, Clark AL, Cleland JG. Incidence of renal dysfunction over 6 months in patients with chronic heart failure due to left ventricular systolic dysfunction: contributing factors and relationship to prognosis. Eur Heart J. 2006;27:569–581. doi: 10.1093/eurheartj/ehi696. [DOI] [PubMed] [Google Scholar]
- 13.Damman K, Navis G, Voors AA, Asselbergs FW, Smilde TD, Cleland JG, van Veldhusien DJ, Hillege HL. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Khan NA, Ma I, Thompson CR, Humphries K, Salem DN, Sarnak MJ, Levin A. Kidney function and mortality among patients with left ventricular systolic dysfunction. J Am Soc Nephrol. 2006;17:244–253. doi: 10.1681/ASN.2005030270. [DOI] [PubMed] [Google Scholar]
- 15.Jose P, Skali H, Anavekar N, Tomson C, Krumholz HM, Rouleau JL, Moye L, Pfeffer MA, Solomon SD. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17:2886–2891. doi: 10.1681/ASN.2006010063. [DOI] [PubMed] [Google Scholar]
- 16.Waldum B, Westheim AS, Sandvik L, Flønaes B, Grundtvig M, Gullestad L, Hole T, Os I. Renal function in outpatients with chronic heart failure. J Card Fail. 2010;16:374–380. doi: 10.1016/j.cardfail.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Maeder MT, Rickli H, Pfisterer ME, Muzzarelli S, Ammann P, Fehr T, Hack D, Weilenmann D, Dieterle T, Kiencke S, Estlinbaum W, Brunner-La Rocca HP. Incidence, clinical predictors, and prognostic impact of worsening renal function in elderly patients with chronic heart failure on intensive medical therapy. Am Heart J. 2012;163:407–414. doi: 10.1016/j.ahj.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Weinfeld MS, Chertow GM, Stevenson LW. Aggravated renal dysfunction during intensive therapy for advanced chronic heart failure. Am Heart J. 1999;138:285–290. doi: 10.1016/s0002-8703(99)70113-4. [DOI] [PubMed] [Google Scholar]
- 21.Maeder M, Klein M, Fehr T, Rickli H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. 2004;44:1763–1771. doi: 10.1016/j.jacc.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 22.Gottlieb SS, Abraham W, Butler J, Forman DE, Loh E, Massie BM, O'connor CM, Rich MW, Stevenson LW, Young J, Krumholz HM. The prognostic importance of different definitions of worsening renal function in congestive heart failure. J Card Fail. 2002;8:136–141. doi: 10.1054/jcaf.2002.125289. [DOI] [PubMed] [Google Scholar]
- 23.Klein L, Massie BM, Leimberger JD, O'Connor CM, Piña IL, Adams KF, JR, Califf RM, Gheorghiade M. Admission or changes in renal function during hospitalization for worsening heart failure predict postdischarge survival: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) Circ Heart Fail. 2008;1:25–33. doi: 10.1161/CIRCHEARTFAILURE.107.746933. [DOI] [PubMed] [Google Scholar]
- 24.Pitt B, Segal R, Martinez FA, Meurers G, Cowley AJ, Thomas I, Deedwania PC, Ney DE, Snavely DB, Change PI. Randomised trial of losartan versus captopril in patients over 65 with heart failure (Evaluation of Losartan in the Elderly Study, ELITE) Lancet. 1997;349:747–752. doi: 10.1016/s0140-6736(97)01187-2. [DOI] [PubMed] [Google Scholar]
- 25.Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: reliability and validity during a randomized, double-blind, placebo-controlled trial of pimobendan. Pimobendan Multicenter Research Group. Am Heart J. 1992;124:1017–1025. doi: 10.1016/0002-8703(92)90986-6. [DOI] [PubMed] [Google Scholar]
- 26.Damman K, Valente MA, Voors AA, O'Connor CM, van Veldhuisen DJ, Hillege HL. Renal impairment, worsening renal function and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J. 2014;35:455–469. doi: 10.1093/eurheartj/eht386. [DOI] [PubMed] [Google Scholar]
- 27.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin- converting enzyme inhibitor therapy in patiens with cardiac dysfunction. Circ Heart Fail. 2011;4:685–691. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Testani JM, Brisco MA, Han G, Laur O, Kula AJ, Cheng SJ, Tang WH, Parikh CR. Influence of age-related versus non-age-related renal dysfunction on survival in patients with left ventricular dysfunction. Am J Cardiol. 2014;113:127–131. doi: 10.1016/j.amjcard.2013.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


