Abstract
For children reared in institutions for orphaned or abandoned children, multiple aspects of the early environment deviate from species-typical experiences, which may lead to alterations in neurobehavioral development. Although the effects of early deprivation and early life stress have been studied extensively in animal models, less is known about implications for human brain development. This structural neuroimaging study examined the long-term neural correlates of early adverse rearing environments in a large sample of 12–14 year old children (N = 110) who were internationally adopted from institutional care as young children (median age at adoption = 12 months) relative to a same age, comparison group reared with their biological families in the United States. History of institutional rearing was associated with broad changes in cortical volume even after controlling for variability in head size. Results suggested that prefrontal cortex was especially susceptible to early adversity, with significant reductions in volume (driven primarily by differences in surface area rather than cortical thickness) in post-institutionalized youth. Hippocampal volumes showed an association with duration of institutional care, with later-adopted children showing the smallest volumes relative to non-adopted controls. Larger amygdala volumes were not detected in this sample of post-institutionalized children. These data suggest that this temporally discrete period of early deprivation is associated with persisting alterations in brain morphology even years after exposure. Furthermore, these alterations are not completely ameliorated by subsequent environmental enrichment by early adolescence.
Keywords: post-institutionalized youth, early life stress, early deprivation, early adversity, structural brain development
1. Introduction
Research in both animal and human populations has demonstrated that early, postnatal experiences can have a profound impact on later brain, cognitive, and socioemotional development (Greenough, Black, & Wallace, 1987). The range of experiences known to produce measurable, long-lasting changes in brain structure and function is relatively diverse, encompassing global differences in the early environment such as poverty (Lawson, Duda, Avants, Wu, & Farah, 2013), maltreatment (Hanson et al., 2010), and variations in the prenatal environment (Raznahan, Greenstein, Lee, Clasen, & Giedd, 2012). In many cases, children who experience early adverse environments also experience ongoing adversity during major portions of childhood, thus making it difficult to relate the timing of experience to later indices of brain and behavioral functioning. Recently, researchers have begun examining brain development in children for whom periods of significant adversity are confined to the first years of life by studying children adopted or fostered from institutions (orphanages) into middle- and upper-middle class families.
Institutional rearing environments deviate from species-typical care in multiple ways, leading to potential alterations in both experience-expectant (i.e. functions that develop for all members of a species given a typical environment) and experience-dependent (i.e. functions that develop based on unique interactions between the individual and his/her environment) aspects of brain development (Greenough et al., 1987). The quality of institutional care varies widely, but may include poor and/or inadequate nutrition, exposure to infection due to unsanitary conditions, lack of cognitive and/or perceptual stimulation, high ratios of children to caregivers, and frequent staff turnover, disrupting the formation of infant-caregiver attachment relationships (Nelson, 2007). Furthermore, children currently residing in institutions show biological markers of chronic stress such as altered daily cortisol patterns (Carlson & Earls, 1997), suggesting that the orphanage care environment may alter aspects of brain development that are stress sensitive.
Most post-institutionalized (PI) children exhibit remarkable recovery following adoption into families. However, altered development of specific brain structures, including the limbic system and prefrontal cortex, is of interest given established long-term difficulties in this population with cognitive and socioemotional functions thought to depend on these regions of the brain (Bauer, Hanson, Pierson, Davidson, & Pollak, 2010; Bos, Fox, Zeanah, & Nelson, 2009; Colvert et al., 2008; Hostinar, Stellern, Schaefer, Carlson, & Gunnar, 2012; Rutter & O’Connor, 2004; Rutter, 1998). Although individual variability in neurodevelopmental outcomes exists, longer duration of institutional care is generally associated with poorer outcomes, including reduced physical catch-up growth (Rutter & O’Connor, 2004; Rutter, 1998; van Ijzendoorn & Juffer, 2006), poorer cognitive recovery (Rutter & O’Connor, 2004; Rutter, 1998), and increased risk of psychological disturbance (Rutter & O’Connor, 2004), suggesting a dose-dependent effect of duration of early life deprivation on neurobehavioral development.
1.1 Animal Models of Early Adversity and Brain Development
Several decades of research utilizing animal models has indicated that the prefrontal cortex, responsible for temporal sequencing of goal-directed behaviors, and the brain’s limbic structures associated with forms of memory (i.e. hippocampus) and emotional processing (i.e. amygdala) are highly sensitive to stress, deprivation, and related forms of adversity. In adult rodents and nonhuman primates, repeated mild stress results in reduced dendritic branching, reduced length, and atrophy in both hippocampal and medial prefrontal neurons (e.g. Cook & Wellman, 2004; McEwen, 1999). In contrast, amygdala pyramidal and stellate neurons undergo increased dendritic arborization following stress (e.g. Vyas, Mitra, Shankaranarayana Rao, & Chattarji, 2002).
The majority of animal models investigating the impacts of early adversity employ varying amounts of maternal deprivation, although some now model maltreatment (e.g. see Lutz & Turecki, 2014; Teicher, Tomoda, & Andersen, 2006 for reviews). In most animal models of early adversity, conditions that elicit stress responses are conflated with deprivation of species-typical stimulation, with both aspects of adverse care potentially affecting the developing brain. Early deprivation of parental care is associated with long-term alterations in amygdala (Poeggel et al., 2003) and prefrontal microstructure in juvenile and adult animals (Braun, Lange, Metzger, & Poeggel, 2000; Ovtscharoff & Braun, 2001; Poeggel et al., 2003). Prefrontal microstructure effects are likely reflected at the volumetric level, given that maternal deprivation in non-human primates is associated with long-term changes in medial prefrontal cortex volume (Lyons, Afarian, Schatzberg, Sawyer-Glover, & Moseley, 2002; Spinelli et al., 2009). Interestingly, several studies have reported that the effects of early adverse care on hippocampal development are not observed until animals reach adolescence or adulthood (Andersen & Teicher, 2004; Huot, Plotsky, Lenox, & McNamara, 2002; Karten, Olariu, & Cameron, 2005; Poeggel et al., 2003), although this finding has been somewhat inconsistent (Law et al., 2009; Lyons, Yang, Sawyer-Glover, Moseley, & Schatzberg, 2001; Sánchez, Hearn, Do, Rilling, & Herndon, 1998; Spinelli et al., 2009). Taken together, this growing literature indicates that early adverse experiences alter neuronal microstructure and brain volume in regionally specific ways, perhaps through early disruption of normative neurodevelopmental processes that persists into adolescence and adulthood.
1.2 Preliminary Studies of Brain Development in PI Children
A small number of studies have focused on brain development in children adopted or fostered from institutions, providing preliminary evidence that the effects of early adversity observed in animal models have comparable correlates in humans. Positron emission tomography (PET) studies have demonstrated that PI children show reduced glucose metabolism in limbic regions, including the amygdala and hippocampus (Chugani et al., 2001). However, the current literature on hippocampal and amygdala development following a history of early deprivation is contradictory, potentially due to differences in the ages and ethnic backgrounds of PI children tested in specific samples. PI children have been reported to show increased amygdala volume in comparison to non-adopted controls (Mehta et al., 2009; Tottenham et al., 2010), with greater alterations in later adoptees (Tottenham et al., 2010). However, several studies have failed to detect a difference in hippocampal volume in children with a history of institutional care after controlling for overall brain volume (Mehta et al., 2009; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012; Tottenham et al., 2010).
Prefrontal cortex structure and connectivity are also altered following institutional rearing. Diffusion tensor imaging (DTI) studies indicate that PI children show reduced fractional anisotropy in the uncinate fasiculus (Eluvathingal et al., 2006; Govindan, Behen, Helder, Makki, & Chugani, 2010; Hanson et al., 2013), a white matter tract connecting limbic and frontal lobe regions, as well as more diffuse frontal-striatal projections (Behen et al., 2009) between middle childhood to late adolescence. Multiple studies have found evidence that global white matter volume is reduced in PI children (Eluvathingal et al., 2006; Mehta et al., 2009; Sheridan et al., 2012), that prefrontal white matter organization is disrupted (Behen et al., 2009; Eluvathingal et al., 2006; Govindan et al., 2010; Hanson et al., 2013), and that regions of prefrontal cortex show reduced cortical thickness (McLaughlin et al., 2013) in PI children. However, despite documented deficits within this population on prefrontal-dependent tasks, no study has reported whether organizational differences are accompanied by volumetric alterations in prefrontal cortex, as observed in animal models of early adversity.
1.3 Importance of Brain Development During Childhood and Adolescence
The relationship between early (infantile) experience and brain development during childhood and adolescence is of particular interest for children removed from adversity early and placed in an enriched environment. Given the plasticity of the developing brain and the regionally specific continuation of neurodevelopmental processes including synaptic pruning and myelination (Huttenlocher & Dabholkar, 1997), it is possible that experience in the adoptive home may ameliorate the impacts of early adversity.
Although prefrontal cortex is widely considered to be a “late-developing” region of the brain, developmental changes in structure and function are evident early during the postnatal period, with dramatic increases in glucose metabolism (Chugani & Phelps, 1986) over the first year of life. Extended structural development continues throughout childhood and into adolescence in both the gray and white matter of the frontal lobes, with dorsolateral prefrontal cortex showing a particularly slow rate of structural maturation (Giedd, 2004; Sowell, Thompson, Tessner, & Toga, 2001). In contrast, the brain’s limbic structures mature relatively early in human development. Maximal growth of the hippocampus occurs during the prenatal and early postnatal periods (Pfluger et al., 1999), with slower growth during early childhood (Knickmeyer et al., 2008; Pfluger et al., 1999). The amygdala appears relatively well-developed by the eighth month of gestation in human fetuses (Ulfig, Setzer, & Bohl, 2003), although rapid changes in its volumetric development continue during the early postnatal months in nonhuman primates (Payne, Machado, Bliwise, & Bachevalier, 2010). Although amygdala development appears to be earlier and more rapid in comparison to the hippocampus, structural MRI studies indicate that both regions show continued, subtle changes over childhood and into early adulthood, although these effects may be sex specific, differ by structural sub-regions within each structure, and may show increased individual variability following puberty (Giedd et al., 1996; Lange, Giedd, Castellanos, Vaituzis, & Rapoport, 1997). The early and rapid development of limbic structures may place these regions at heightened vulnerability in the face of adverse early experiences (Tottenham & Sheridan, 2009). Additionally, differences in the continued rate of growth and maturation of limbic and prefrontal regions indicate they may be differentially impacted by early childhood adversity when long-term effects are measured in adolescence.
1.4 Current Study
The present study aimed to investigate whether disruptions in structural brain development in limbic and prefrontal regions would be observed in PI youth during early adolescence, and to determine whether the magnitude of disruption scaled with duration of exposure to early adversity. Unlike previous studies which focused on children adopted primarily from specific geographic regions (e.g. Romania), our diverse sample of PI children was selected to be representative of children with a history of orphanage/institutional care who have entered the United States, improving generalizability of results and broadening implications to more general effects of early adversity in conditions of psychosocial or physical neglect. Second, the use of a large sample of PI children (N = 110) all tested within a narrow age-range (12 – 14 years) provided the opportunity to carefully examine whether the dosage of children’s early experiences (indexed by duration of institutional care) was related to long-term neurodevelopmental outcomes at adolescence. Based on animal models of early adversity and data from previous neuroimaging studies with PI youth, we hypothesized that PI adolescents would show altered prefrontal, hippocampal, and amygdala volumes in comparison to non-adopted youth. Furthermore, our large sample size allowed us to test effects of duration of early adversity on neural development. We hypothesized that earlier adopted children would show more similar patterns of morphological brain development to non-adopted children in comparison to their later adopted peers.
2. Materials and Methods
2.1 Participants
Our sample included 110 post-institutionalized children (PI group: 73 females, 37 males; Mage = 13.01 years, SD = .55, range = 12.04 – 14.15 years) and a comparison group of 62 children raised with their biological families (non-adopted control group: 32 females, 30 males; Mage = 12.12 years, SD =.59, range = 12.12 – 14.22 years). PI children were recruited through a registry of families with internationally adopted children maintained by the International Adoption Project (IAP) at the University of Minnesota. For the ages we examined, this registry reflects approximately 65% of all of the children adopted from orphanages/institutions into our catchment area. All PI children were adopted prior to 78 months of age and spent at least 50% of their pre-adoptive care in an institutional setting, with a total of 3.5 – 60 months of institutional care prior to adoption. PI children were screened to exclude known neurological disorder (e.g. epilepsy), past or current serious medical illness (e.g. cancer treatment with chemotherapy), known diagnosed developmental disorder (i.e. Autism Spectrum Disorder, Asperger’s Syndrome, Pervasive Developmental Disorder – Not Otherwise Specified), known chromosomal or genetic conditions (e.g. Down’s Syndrome, Hurler’s Syndrome), known or suspected Fetal Alcohol Spectrum Disorder, or previous IQ score below 80, or any contraindications for MRI (including braces, permanent retainers, history of claustrophobia, or implanted metal). PI children in the final sample were adopted internationally predominantly from China (30%), Eastern Europe/Russia (41%), and India (15%), and had been living with their adoptive families for an average of 11.73 years (SD = 1.38, range = 7.54 – 13.69 years). Psychological and/or psychiatric disorder was not an exclusionary criterion for PI children; as such, 32 (29%) PI children met diagnostic criteria on the Kiddie-Sads-Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997) for an axis I disorder based on caregiver interviews with a trained clinician, including predominantly Anxiety disorders (N = 11) or Attention Deficit Hyperactivity Disorder (N = 20). PI children were further subdivided into earlier-adopted (EA group; N = 56, 43 females, 13 males) and later-adopted (LA group; N = 54, 30 females, 24 males) sub-groups based on a median split of adoption by 12 months of age. Categorical rather than continuous measures of duration of institutional care were used to allow comparison to non-adopted children who had no history of institutional care. Follow-up analyses tested for effects of the continuous measure of age at adoption within the PI group. Duration of institutional care was conceptualized as a measure for the dosage of children’s early deprivation experience. See Inline Supplementary Table 1 for details about the sample provided by primary caregivers.
Families of comparison (non-adopted) children were recruited from a community volunteer participant pool maintained by the Institute of Child Development at the University of Minnesota. Comparison children met the same screening criteria as the PI children, and were additionally screened for history of birth complications (including known prenatal drug exposure, premature birth, or birth complications resulting in neonatal intensive care unit admission for > 24 hours), and for personal history of diagnosed psychiatric and/or psychological disorder or learning disability. Importantly, the non-adopted and PI groups were similar on demographic variables, including family income and parental education (see Inline Supplementary Table 2).
An additional 41 children beyond the 110 PI and 62 comparison youth participated but were excluded from the final sample for the following reasons: significant health issues not detected during prescreening (1 PI), failure to meet study requirements for duration of institutional care not detected on prescreening (2 PI), MRI scan abnormality requiring clinical follow-up (1 non-adopted, 6 PI), and excess motion or gross segmentation error in structural MRI scan (6 non-adopted, 24 PI). Fifteen subjects are included in the final sample but were excluded only from hippocampal and amygdala analyses due to poor subcortical segmentation (2 non-adopted, 13 PI). Of the 41 children who were excluded from the final sample, PI children were more likely to be excluded than non-adopted controls (p<.035). Within the PI group, excluded children were slightly younger (p<.003), although they did not differ from their peers who provided useable data in sex, age at adoption, IQ score, or the presence of psychopathology on the K-SADS-PL.
2.2 Procedure
Families of children provided both verbal and written consent to participate, children provided verbal and written assent, and both parents and children were compensated for their efforts. Participants were part of a larger study examining learning, attention, executive function, and brain development in internationally adopted youth using behavioral, neuroimaging, and genetic measures. This report describes results from the structural MRI imaging session only. For PI children, MRI scanning took place on the second day of a two-day session. Children selected a movie to watch during the MRI scanning protocol. Study procedures were approved by the University of Minnesota’s Institutional Review Board.
2.3 MRI Acquisition and Processing
Magnetic resonance imaging was performed on a Siemens 3T Trio MRI scanner. A sagittal scout series was first used to verify participant position. A T1-weighted, three-dimensional (3-D) magnetization prepared rapid gradient echo (MPRAGE) series was used to acquire 240 contiguous slices of 1 mm thickness in the sagittal plane (TR = 2530 ms, TE = 3.65 ms, FOV = 256 mm, flip angle = 7°). Cortical reconstruction and volumetric segmentation was performed with the Freesurfer image analysis suite version 5.1.0, which is documented and freely available for download online (http://surfer.nmr.mgh.harvard.edu/). Technical details are described further in the Supplemental Materials and Methods. Freesurfer morphometric procedures generate comparable volumes to hand tracing (Morey et al., 2009), and are valid for use in pediatric populations (Ghosh et al., 2010). Rigorous data quality control mechanisms were used to attempt to match the PI control group on MRI scan quality. Data quality in the original larger group of children was rejected at a higher rate from PI children due to excess head motion as well as higher rates of brain segmentation errors due to deviations from standard brain morphometry (i.e. enlarged and/or asymmetrical ventricles). All image outputs were visually inspected prior to and following segmentation and were rejected for either excess participant motion or specific segmentation errors in regions of interest (hippocampus, amygdala; see Appendix for additional discussion).
2.4 Data Analyses
Previous developmental studies have reported volumetric changes in brain structure associated with age and sex in pediatric populations of this age range (Sowell et al., 2001). Due to differences in the proportion of males versus females in our groups, all analyses reported include age at test and sex as covariates. Discrepancies exist in adult volumetric brain imaging studies about how to best compare differences in regional brain volumes in the context of group differences in whole brain volume or head size (Barnes et al., 2010; O’Brien et al., 2006). Raw volumes are reported in Inline Supplementary Table 3 for comparison to previously published studies and to illustrate the necessity of using a correction factor. We first report group differences in regional volume using estimated individual intracranial volume (ICV) as a covariate. Second, we also report differences in regional volumes covaried for adjusted supratentorial volume (adjSTV). This measure provides a stringent correction specific to cortical and subcortical brain tissue volume, eliminating the impact of potential differences between groups in ventricle size, CSF, or cerebellar volume, and thus improves specificity of results (see Appendix for additional discussion).
Analyses focused on a priori regions of interest, including bilateral prefrontal cortex, hippocampus, and amygdala. Statistical effects of group status for a priori regions of interest used a corrected p-value of p<.02, representing a Bonferonni correction to adjust for multiple comparisons across the three main regions of interest. Planned comparisons between groups were also set to a corrected p-value of p<.02, representing the three potential pairwise group comparisons (comparison vs. PI-EA vs. PI-LA). We fit one-way ANCOVAs within regions of interest using either ICV or adjSTV as a covariate. To investigate specificity of effects, we also fit similar models for other lobar volumes. Additional analyses examining the robustness of volumetric reductions in a priori regions of interest are presented in the Appendix (section A2.2 reports results in a Caucasian subsample; A2.3 reports results following statistical adjustment for IQ).
Given our a priori interest in prefrontal effects, exploratory analyses also investigated changes in volume, surface area, and cortical thickness of prefrontal cortical sub-regions. These exploratory analyses used an uncorrected p<.05 for main effects of group and follow-up comparisons. Volumetric analyses again controlled for ICV or adjSTV; surface area and cortical thickness analyses did not include a statistical control for head size, as surface area and thickness do not necessarily scale linearly with head size, and instead show dynamic interrelations across cortex (Toro et al., 2008; Winkler et al., 2010).
Linear and quadratic effects of age at adoption (log-transformed) within the PI children were assessed in prefrontal cortex, hippocampus, and amygdala again using a corrected p-value of p<.02.
3. Results
3.1 Effects of Early Adverse Care on Cortical Structure
3.1.1 Lobar Volumes
Group differences in raw lobar volumes were present across all four lobes of the brain (Inline Supplementary Table 3), highlighting the necessity of controlling for between-group differences and individual variability in head size. Interestingly, following correction for intracranial volume (ICV), PI children continued to show smaller volumes across all regions of cortex in comparison to non-adopted controls (Inline Supplementary Table 4). This global statistical effect of institutionalization on cortical volume after controlling for variability in head size suggests gross differences in brain tissue growth relative to head size for PI youth.
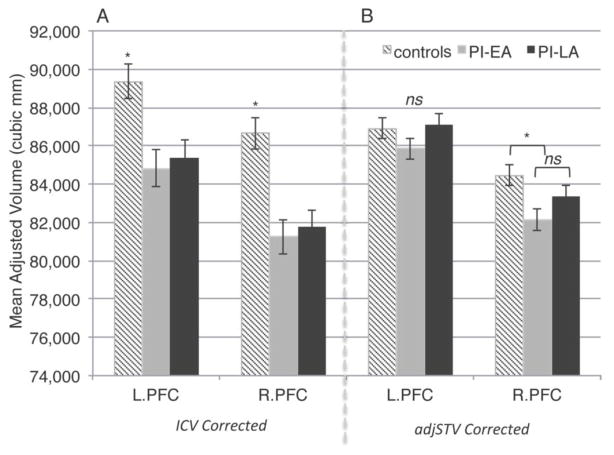
To examine specificity of institutionalization effects on cortical volume, we subsequently ran analyses controlling for adjusted supratentorial volume (adjSTV), which minimizes the impact of potential group differences in ventricular, CSF, brain stem, and cerebellar volumes. Following correction for adjSTV, there was a main effect of group only in the right frontal lobe, which was also present specifically in right prefrontal cortex [F(2, 166) = 4.070, p<.019, ηpartial2 = .047]. After adjusting for age, sex, and ICV, age at adoption (log-transformed) was not a significant linear or quadratic predictor of prefrontal volumes, see Figure 1.
Figure 1. Prefrontal gray matter volume (age and sex adjusted) is reduced in PI youth.
Panel a: ICV corrected (± SEM).
Panel b: adjSTV corrected (± SEM).
3.1.2 Volumetric Sub-Regions of Prefrontal Cortex
Exploratory analyses indicated that history of institutionalization was associated with a broad pattern of volumetric reductions across prefrontal cortical sub-regions, including significant group differences in bilateral superior frontal gyrus, right middle frontal gyrus, bilateral portions of inferior frontal gyrus, bilateral lateral orbitofrontal cortex, and right rostral anterior cingulate cortex. In general, following correction for ICV both PI-EA and PI-LA children had smaller volumes in prefrontal sub-regions in comparison to comparison youth, with no differences between the PI groups (Inline Supplementary Table 5). Group differences in bilateral superior frontal gyrus [left: F(2, 166) = 3.439 p<.034, ηpartial2= .040; right: F(2, 166) = 4.916, p<.018, ηpartial2 = .056] and left pars orbitalis [F(2, 166) = 3.661, p<.028, ηpartial2 = .042] volume survived the stringent correction for adjSTV.
3.1.3 Contributions of Surface Area and Thickness to Changes in Prefrontal Volume
Reductions in prefrontal volume associated with early deprivation could reflect alterations in cortical surface area, cortical thickness, or a combination of both factors. Adult studies report independent genetic contributions to thickness and surface area (Panizzon et al., 2009; Winkler et al., 2010), with some suggestion that individual differences in gray matter volume may be driven more by variability in surface area (Pakkenberg & Gundersen, 1997). We investigated contributions of surface area and thickness to volumetric differences in the prefrontal subregions that demonstrated group differences in volume.
Exploratory analyses indicated that all prefrontal regions that showed volumetric reductions in PI children also showed reduced cortical surface area; both PI-EA and PI-LA children had reduced surface area in comparison to non-adopted youth, with few differences between the PI groups (Inline Supplementary Table 6 and 7). In contrast, only one region in the left inferior frontal gyrus (pars orbitalis; F(2, 166) = 3.904, p<.022, ηpartial2 = .045) showed changes in cortical thickness associated with group status. The PI-LA group showed a reduction in cortical thickness relative to the non-adopted comparison group (p<.006). Overall, these results suggest that volumetric reductions in prefrontal cortex in early adolescent PI youth are driven primarily by changes in surface area rather than cortical thickness.
3.2 Effects of Early Adverse Care on Subcortical Volume
3.2.1 Hippocampal Volume
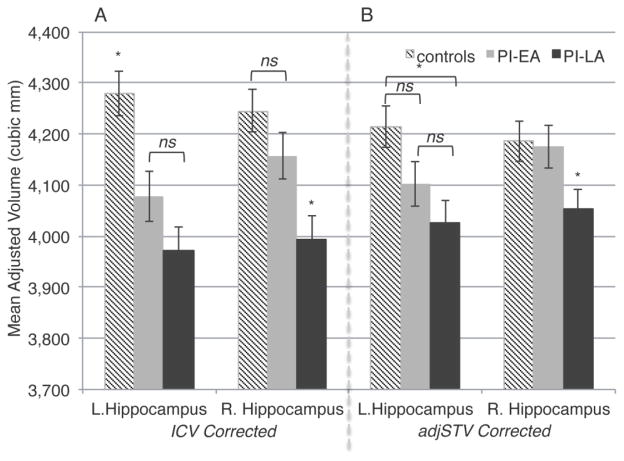
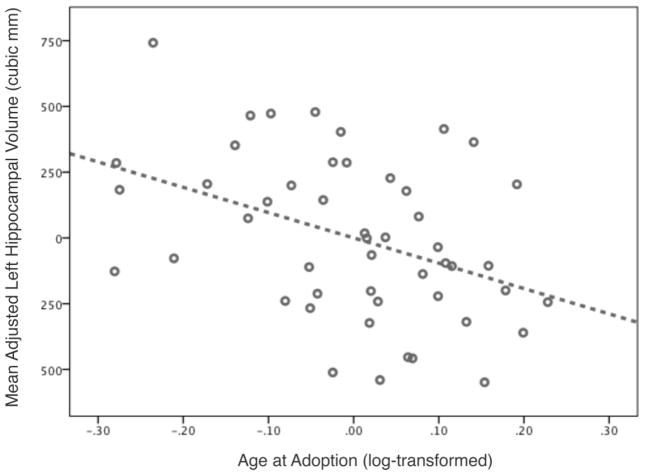
After correction for ICV, PI children had smaller left hippocampal volumes than non-adopted children [F(2, 151) = 11.914, p<.000, ηpartial2= .136], with significantly smaller volumes in both PI-EA (p<.003) and PI-LA (p<.000) children, and no difference between PI groups (p=.123). Although there was an effect of group membership on right hippocampal volumes [F(2, 151) = 8.835, p<.000, ηpartial2= .105], follow-up tests indicated that this effect was specific to PI-LA children, who showed reduced volume in relation to both non-adopted children (p<.000) and PI-EA children (p=.012), with no differences between controls and earlier adoptees (p=.175). PI-LA children had reduced hippocampal volume following the stringent correction for adjSTV, see Figure 2. After adjusting for age, sex, and ICV, age at adoption (log-transformed) was not a significant linear or quadratic predictor of hippocampal volume across all PI children. However, within the PI-EA group alone, age at adoption predicted decreases in bilateral hippocampal volume [left: F(1, 44) = 8.591, p<.005, ηpartial2= .163; right: F(1, 44) = 6.552, p<.014, ηpartial2= .130, see Figure 3].
Figure 2. Duration of early adversity predicts hippocampal volume reduction (age and sex adjusted).
Panel a: ICV corrected (± SEM).
Panel b: adjSTV corrected (± SEM).
Figure 3.
Duration of adversity over first year of life predicts hippocampal volume (age, sex, and ICV adjusted) in early adolescence (right hippocampal effect is equivalent).
3.2.2 Amygdala Volume
After correction for ICV, PI children had smaller left amygdala volumes than non-adopted children [F(2, 151) = 4.175, p<.017, ηpartial2= .052]. This effect was driven by smaller volumes in PI-LA children in comparison to non-adopted children (p<.005), with PI-EA children showing no differences from either non-adopted controls (p=.438) or later-adopted peers (p=.063). An equivalent trend-level effect was found for right amygdala volumes [F(2, 151) = 3.171, p<.045, ηpartial2= .040], with reductions in volume present only in PI-LA children relative to controls (p<.014) and no other differences between groups [PI-LA vs. PI-EA (p=.116); PI-EA vs. controls (p<.460)]. Effects of institutional care on bilateral amygdala volumes were non-significant after controlling for adjSTV, see Inline Supplementary Figure 1. After adjusting for age, sex, and ICV, age at adoption (log-transformed) was not a significant linear or quadratic predictor of amygdala volume.
4. Discussion
The current study reports on long-term correlates of a temporally discrete period of deprivation in infancy and early childhood on structural brain development in regions implicated in both animal models of early adverse care and in previous neuroimaging studies with PI children. These effects are particularly striking given that the PI children in our sample had spent an average of nearly twelve years in their adoptive family by the time of the study, suggesting that adversity limited to early life may have lasting effects on structural brain development despite a substantial period of subsequent development in resource-rich homes.
PI children showed global reductions in lobar grey matter volumes in comparison to non-adopted controls. Interestingly, these effects persisted even after controlling for intracranial volume, suggesting gross differences in brain tissue growth relative to head size for PI youth. It remains unclear what drives this difference in the scaling of cortical volume relative to head size, although it may be related to increases in ventricular or total cerebrospinal fluid volume, alterations in gyrification, or changes in cerebellar volume (Bauer et al., 2010) associated with a history of early adversity.
After more stringent control for brain tissue volume, institutionalization continued to predict smaller right prefrontal cortex volume, suggesting that prefrontal cortex may be particularly vulnerable to early life adversity. This pattern of findings is congruent with previous studies of brain development in PI children reporting altered prefrontal development, including atypical metabolic function (Chugani et al., 2001), reduced prefrontal cortical thickness (McLaughlin et al., 2013) and diffuse white matter organization (Behen et al., 2009; Eluvathingal et al., 2006; Govindan et al., 2010). Exploratory analyses indicated that this effect was consistent in direction across prefrontal cortical sub-regions, with bilateral superior frontal and portions of inferior frontal gyri showing the most robust decreases in volume. Smaller prefrontal volumes associated with institutional care in this adolescent sample reflected decreases in cortical surface area more than cortical thickness. Changes in surface area have not been previously investigated in PI children, but variation in prenatal experience is known to exert a lasting impact on prefrontal cortical surface area (Raznahan et al., 2012). One previous study with PI children has reported cortical thickness differences (McLaughlin et al., 2013), not restricted to frontal lobe, which may reflect overall differences in brain growth following adverse early life experiences.
The biological mechanism by which prefrontal cortex thickness, surface area, and volume are influenced by early adversity remains unclear. Since prefrontal cortex plays a role in some of the more unique and complex aspects of human cognition (Saxe, 2006), it is intuitive that this region is highly sensitive to variations in the early environment. Early and extended sensitivity of prefrontal cortex to environmental experience conveys maximum potential for the developing organism to benefit from environmentally-induced plasticity; however, if the environment is not advantageous and/or if adaptations to the early environment are maladaptive in later contexts, this sensitivity carries risk for long-term neurobehavioral development. Ultimately, the role of stress as a major mechanism by which early experience shapes prefrontal development cannot be ignored. In rodent models, repeated behavioral stress during adulthood is associated with a reduction in the length, branch numbers, spine volume, and total number of apical dendrites present in rat medial prefrontal cortex (Cook & Wellman, 2004; Radley et al., 2004; Radley et al., 2006, 2009), along with impairments in prefrontal-dependent working memory (McEwen & Morrison, 2013). Interestingly, while adult animals show reversibility of medial prefrontal cortex alterations induced by brief periods of stress experienced during adulthood (Radley et al., 2005), it appears that the effects of early life stress are largely resistant to later environmental enrichment in younger animals (Pascual & Zamora-León, 2007). Additionally, in humans the rapid structural and functional development of this region during infancy and toddlerhood (Chugani & Phelps, 1986; Gao et al., 2009; Gilmore et al., 2012; Grossmann, 2013) may additionally explain its heightened sensitivity to the early environment, as well as the presence of long-term prefrontal structure differences even in earlier adopted PI children.
Hippocampal volume measured at adolescence was associated with early adversity in a dose-dependent manner, with increased duration of orphanage care predicting greater hippocampal volume reductions. Although previous structural neuroimaging studies have failed to find evidence of hippocampal reduction in PI children (McLaughlin et al., 2013; Mehta et al., 2009; Sheridan et al., 2012; Tottenham et al., 2010), our result is congruent with animal models reporting that stress either during early life or adulthood can have striking impacts on hippocampal morphology (e.g. Uno, Tarara, Else, Suleman, & Sapolsky, 1989). Several previous studies with PI children have primarily tested children of a younger age than our early-adolescent sample (McLaughlin et al., 2013; Sheridan et al., 2012; Tottenham et al., 2010). Previous studies have also involved smaller numbers of participants, and thus may have been under-powered for detecting associations between duration of deprivation and hippocampal volume. However, both animal and human studies (Andersen & Teicher, 2004; Carrion, Weems, & Reiss, 2007) suggest hippocampal effects may emerge only in adolescence or adulthood. Thus, differences between published samples in pre-adoptive characteristics, age at test, and sample size may explain differential results.
In contrast to two previous studies with PI children (Mehta et al., 2009; Tottenham et al., 2010) we failed to find evidence for amygdala hypertrophy. Instead, we found decreases in ICV adjusted amygdala volumes in later-adoptees, although this effect did not survive correction for the more stringent covariate of adjSTV. Animal work suggests that stress-induced changes in amygdala organization are relatively stable, but studies of amygdala volume in adult and pediatric clinical populations have not produced routinely replicable effects. PI children tested in previous studies (Mehta et al., 2009; Tottenham et al., 2010) were of different ages than our sample of early adolescents. Like the hippocampus, the amygdala is a small region of the brain and is particularly difficult to segment using automated methods (Morey et al., 2009); further consideration of the developmental timing of effects, as well as improvements in amygdala segmentation algorithms may be necessary to determine the impact of early adversity on amygdala structure across the lifespan.
We did not find strong evidence for correlates of duration of early adversity in most of the brain regions examined. Instead, the largest differences in structural brain development were observed between PI children and non-adopted controls. The inability to detect dramatic differences in structural brain development between earlier- and later-adopted PI children is noteworthy given that multiple studies have suggested that longer duration of institutional care is associated with more significant physical, cognitive, and psychosocial deficits Rutter & O’Connor, 2004; Rutter, 1998; van Ijzendoorn & Juffer, 2006). Why does this pattern not appear to hold for structural brain development? Various research groups have defined different age thresholds for early adoption, ranging from children adopted prior to six months of age (Rutter, 1998) to children adopted before 24 months of age (Nelson, 2007). The considerable variation in these definitions of early adoption suggests that there is not a clear cut-off point before which PI children completely recover from the effects of early adversity. Instead, it is likely that different age thresholds exist for different measures of functioning, including the structural development of individual brain regions.
The duration of institutional care experienced by PI children is highly influenced by the adoption policies of individual countries and is therefore typically confounded with ethnic background. Thus, we were unable to assess age at adoption effects as a function of ethnic background. However, our diverse sample of PI children was selected to be representative of children with a history of orphanage/institutional care entering the United States, improving the generalizability of our results. Furthermore, PI children are not randomly assigned to receive orphanage care, and instead may enter the orphanage-rearing environment having experienced a variety of prenatal and/or perinatal risk factors. For example, maternal stress during pregnancy (Lupien, McEwen, Gunnar, & Heim, 2009), pre- and perinatal nutritional deficiencies (Lozoff, Beard, Connor, Felt, & Georgieff, 2006), and/or preterm birth (Nosarti et al., 2008) are known to also affect neurobehavioral development. We were unable to screen PI children for birth complications as we, and their adoptive parents, did not have access to this information. However, PI children were screened for Fetal Alcohol Spectrum Disorder (FAS-D) and major neurological disorders (e.g. cerebral palsy), suggesting they did not have severe pre- or perinatal complications. PI children are also at a higher risk that non-adopted children for developing attention and anxiety disorders during adolescence (Stevens et al., 2008) and for altered intellectual development (Rutter, Kreppner, & O’Connor, 2001), which may also be related to altered structural brain development. It seems likely that higher rates of psychopathology and poorer intellectual functioning in this population are related to structural brain development abnormalities, although we are unable to address the directionality of these effects. Ultimately the confluence of multiple sources of risk in PI children may reduce our ability to detect effects of duration of institutional care on structural brain development.
Assessment of duration effects may also be complicated by the developmental window during which PI children were assessed. The hippocampus, amygdala, and prefrontal cortex show different time courses of normative, volumetric development, which extend into the adolescent years and early adulthood. Normative developmental periods in which there is greater individual variability in brain volume of specific structures may mask potential effects of duration of deprivation. Furthermore, given that later maturing neural systems build on the input of earlier maturing systems, subtle effects of duration of deprivation in brain regions that show prolonged structural maturation (i.e. prefrontal cortex) may not be detectable until later in life. Atypical early experiences may also alter the plasticity of the brain to respond to later positive changes in the environment. However, given rapid neurodevelopmental changes in adolescence, this developmental time period may represent a new opportunity to re-set biological systems to the current environmental context.
Our results complement the extensive animal literature indicating that early adversity has lasting impacts on structural and functional brain development. The current study extends this model to human children who experience a temporally discrete period of deprivation followed by subsequent environmental enrichment. In addition to changes in structural brain development, institutionalization is associated with broad changes in physical growth and pubertal development. A large percentage of PI children show evidence of growth restriction at adoption, which may or may not be accompanied by sparing of head and/or brain growth (Rutter, 1998), along with atypical growth trajectories and altered pubertal timing (Johnson, 2002). The potential for dynamic interactions between growth stunting, rapid catch-up growth, and altered pubertal timing and their relation to structural brain development remains to be investigated in this population. Deviations from typical structural brain development may be an important marker of neurobehavioral functioning in PI children and may mediate difficulties in cognitive and socioemotional domains, particularly on prefrontal-dependent tasks. Describing the plasticity of neural systems in response to early environments and how environmental effects vary based on individual differences in genetic background (Gunnar, Wenner, Thomas, Glatt, & Clark, 2013; Stevens et al., 2009) will be critical in understanding how diverse forms of early adversity impact development across contexts and over time within individuals.
Supplementary Material
Highlights.
We examined brain structure in 110 adolescents adopted from orphanages as children.
Prefrontal cortex volume was reduced in internationally adopted adolescents.
Hippocampal volume was sensitive to the duration of early life adversity.
There was no effect of early life adversity on amygdala volume at adolescence.
Neurodevelopmental correlates of early life adversity persist at adolescence.
Acknowledgments
The authors thank the families of the Minnesota International Adoption Project, the staff of the Cognitive Development & Neuroimaging Lab (especially Jennifer Wenner) and Human Developmental Psychobiology Lab (especially Bonny Donzella) for their help with this project, as well as Byron Mueller for imaging technical support. The research was supported by a NIMH Center grant MH079513 for the Center for Brain, Gene and Behavioral Research Across Development, B.J. Casey (Director), Megan Gunnar and Kathleen Thomas (Co-PIs of project 2). Trainee support was provided by the University of Minnesota’s Center for Cognitive Sciences via a Ruth L. Kirschstein National Research Service Award T32-HD007151 and University of Minnesota Graduate School Fellowships. Additional funding was provided by the University of Minnesota’s Center for Neurobehavioral Development (T32-MH73129) and College of Liberal Arts and the Minnesota Medical Foundation. This work was carried out in part using resources at the University of Minnesota’s Center for Magnetic Resonance Research (P41-RR008079, P41-EB015894, P30-NS076408), and Supercomputing Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SL, Teicher MH. Delayed effects of early stress on hippocampal development. Neuropsychopharmacology. 2004;29(11):1988–93. doi: 10.1038/sj.npp.1300528. [DOI] [PubMed] [Google Scholar]
- Barnes J, Ridgway GR, Bartlett J, Henley SMD, Lehmann M, Hobbs N, Fox NC. Head size, age and gender adjustment in MRI studies: a necessary nuisance? NeuroImage. 2010;53(4):1244–55. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak D. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry. 2010;66(12):1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behen ME, Muzik O, Saporta ASD, Wilson BJ, Hua J, Chugani HT. Abnormal fronto-striatal connectivity in children with histories of early deprivation: A diffusion tensor imaging study. Brain Imaging and Behavior. 2009;3(3):292–297. doi: 10.1007/s11682-009-9071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos KJ, Fox N, Zeanah CH, Nelson CA. Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience. 2009;3(September):16. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun K, Lange E, Metzger M, Poeggel G. Maternal separation followed by early social deprivation affects the development of monoaminergic fiber systems in the medial prefrontal cortex of Octodon degus. Neuroscience. 2000;95(1):309–18. doi: 10.1016/s0306-4522(99)00420-0. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in romania. Annals of the New York Academy of Sciences. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Reiss AL. Stress predicts brain changes in children: a pilot longitudinal study on youth stress, posttraumatic stress disorder, and the hippocampus. Pediatrics. 2007;119(3):509–16. doi: 10.1542/peds.2006-2028. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. NeuroImage. 2001;14(6):1290–301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science (New York, NY ) 1986;231(4740):840–3. doi: 10.1126/science.3945811. [DOI] [PubMed] [Google Scholar]
- Colvert E, Rutter M, Beckett C, Castle J, Groothues C, Hawkins A, Sonuga-Barke EJS. Emotional difficulties in early adolescence following severe early deprivation: findings from the English and Romanian adoptees study. Development and Psychopathology. 2008;20(2):547–67. doi: 10.1017/S0954579408000278. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. Journal of Neurobiology. 2004;60(2):236–48. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhasz C, Muzik O, Maqbool M, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: a diffusion tensor imaging study. Pediatrics. 2006;117(6):2093–100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, Lin W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proceedings of the National Academy of Sciences. 2009;106(16):6790–6795. doi: 10.1073/pnas.0904281106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, Fischl B. Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11years of age. NeuroImage. 2010;53(1):85–93. doi: 10.1016/j.neuroimage.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis aC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. The Journal of Comparative Neurology. 1996;366(2):223–30. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Shen D. Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex. 2012;22(11):2478–85. doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS) Cerebral Cortex. 2010;20(3):561–9. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Development. 1987;58(3):539–59. [PubMed] [Google Scholar]
- Grossmann T. Mapping prefrontal cortex functions in human infancy. Infancy. 2013;18(3):303–324. doi: 10.1111/infa.12016. [DOI] [Google Scholar]
- Gunnar MR, Jennifer AW, Thomas KM, Glatt CE, Clark AG. The BDNF Val66Met polymorphism moderates early deprivation. Development and Psychopathology. 2013;24(4):1215–1223. doi: 10.1017/S095457941200065X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Adluru N, Chung MK, Alexander AL, Davidson RJ, Pollak SD. Early neglect is associated with alterations in white matter integrity and cognitive functioning. Child Development. 2013;84(5):1566–78. doi: 10.1111/cdev.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff Ea, Gee JC, Davidson RJ, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: a tensor-based morphometry investigation of brain structure and behavioral risk. The Journal of Neuroscience. 2010;30(22):7466–72. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Stellern SA, Schaefer C, Carlson SM, Gunnar MR. Associations between early life adversity and executive function in children adopted internationally from orphanages. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(Suppl):17208–12. doi: 10.1073/pnas.1121246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Research. 2002;950(1–2):52–63. doi: 10.1016/s0006-8993(02)02985-2. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology. 1997;387(2):167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Johnson DE. Adoption and the effect on children’s development. Early Human Developmnet. 2002;68:39–54. doi: 10.1016/s0378-3782(02)00017-8. [DOI] [PubMed] [Google Scholar]
- Karten YJG, Olariu A, Cameron HA. Stress in early life inhibits neurogenesis in adulthood. Trends in Neurosciences. 2005;28(4):171–2. doi: 10.1016/j.tins.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao A, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizoprehnia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):908–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. The Journal of Neuroscience. 2008;28(47):12176–82. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N, Giedd JN, Castellanos FX, Vaituzis aC, Rapoport JL. Variability of human brain structure size: ages 4–20 years. Psychiatry Research. 1997;74(1):1–12. doi: 10.1016/s0925-4927(96)03054-5. [DOI] [PubMed] [Google Scholar]
- Law AJ, Pei Q, Walker M, Gordon-andrews H, Shannon C, Feldon J, Harrison PJ. Early parental deprivation in the marmoset monkey produces long-term changes in hippocampal expression of genes involved in synaptic plasticity and implicated in mood disorder. Neuropsychopharmacology. 2009;34(6):1381–1394. doi: 10.1038/npp.2008.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson GM, Duda JT, Avants BB, Wu J, Farah MJ. Associations between children’s socioeconomic status and prefrontal cortical thickness. Developmental Science. 2013;16(5):641–52. doi: 10.1111/desc.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Beard J, Connor J, Felt B, Georgieff M. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutrition Reviews. 2006;64(5):S34–S91. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews. Neuroscience. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Lutz PE, Turecki G. DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience. 2014;264:142–56. doi: 10.1016/j.neuroscience.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons DM, Afarian H, Schatzberg AF, Sawyer-Glover A, Moseley ME. Experience-dependent asymmetric variation in primate prefrontal morphology. Behavioural Brain Research. 2002;136(1):51–9. doi: 10.1016/s0166-4328(02)00100-6. [DOI] [PubMed] [Google Scholar]
- Lyons DM, Yang C, Sawyer-Glover aM, Moseley ME, Schatzberg aF. Early life stress and inherited variation in monkey hippocampal volumes. Archives of General Psychiatry. 2001;58(12):1145–51. doi: 10.1001/archpsyc.58.12.1145. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Reviews Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79(1):16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013:1–10. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SCR, Sonuga-Barke EJS. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2009;50(8):943–51. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Morey RA, Petty CM, Xu Y, Hayes JP, Wagner HR, Lewis DV, McCarthy G. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. NeuroImage. 2009;45(3):855–66. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA. A neurobiological perspective on early human deprivation. Child Development Perspectives. 2007;1(1):13–18. [Google Scholar]
- Nosarti C, Giouroukou E, Healy E, Rifkin L, Walshe M, Reichenberg A, Murray RM. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain : A Journal of Neurology. 2008;131(Pt 1):205–17. doi: 10.1093/brain/awm282. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Ziegler Da, Deutsch CK, Kennedy DN, Goldstein JM, Seidman LJ, Herbert MR. Adjustment for whole brain and cranial size in volumetric brain studies: a review of common adjustment factors and statistical methods. Harvard Review of Psychiatry. 2006;14(3):141–51. doi: 10.1080/10673220600784119. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Braun K. Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience. 2001;104(1):33–40. doi: 10.1016/s0306-4522(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Pakkenberg B, Gundersen HJG. Neocortical Neuron Number in Humans :Effect of Sex and Age. Journal of Comparative Neurology. 1997;384:312–320. [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19(11):2728–35. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual R, Zamora-León SP. Effects of neonatal maternal deprivation and postweaning environmental complexity on dendritic morphology of prefrontal pyramidal neurons in the rat. Acta Neurobiologiae Experimentalis. 2007;67(4):471–9. doi: 10.55782/ane-2007-1663. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20(8):922–35. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger T, Weil S, Weis S, Vollmar C, Heiss D, Egger J, Hahn K. Normative volumetric data of the developing hippocampus in children based on magnetic resonance imaging. Epilepsia. 1999;40(4):414–423. doi: 10.1111/j.1528-1157.1999.tb00735.x. [DOI] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(26):16137–42. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Experimental Neurology. 2005;196(1):199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cerebral Cortex. 2006;16(3):313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Douglas B, Dammann M, Mcewen BS, Hof PR. Repeated Stress Alters Dendritic Spine Morphology. Journal of Comparative Neurology. 2009;507(1):1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher aB, McCall T, Hof PR, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11366–71. doi: 10.1073/pnas.1203350109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Developmental catch-up, and deficit, following adoption after severe global early privation. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39(4):465–476. [PubMed] [Google Scholar]
- Rutter M, O’Connor TG. Are there biological programming effects for psychological development? Findings from a study of Romanian adoptees. Developmental Psychology. 2004;40(1):81–94. doi: 10.1037/0012-1649.40.1.81. [DOI] [PubMed] [Google Scholar]
- Rutter ML, Kreppner JM, O’Connor TG ERA Study Team. Specificity and heterogeneity in children’s responses to profound institutional privation. The British Journal of Psychiatry. 2001;179(2):97–103. doi: 10.1192/bjp.179.2.97. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Research. 1998;812(1–2):38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(32):12927–32. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. The Journal of Neuroscience. 2001;21(22):8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Archives of General Psychiatry. 2009;66(6):658–65. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SE, Kumsta R, Kreppner JM, Brookes KJ, Rutter M, Sonuga-Barke EJS. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: developmental continuities in gene-environment interplay. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics. 2009;150B(6):753–61. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Stevens SE, Sonuga-Barke EJS, Kreppner JM, Beckett C, Castle J, Colvert E, Rutter M. Inattention/overactivity following early severe institutional deprivation: presentation and associations in early adolescence. Journal of Abnormal Child Psychology. 2008;36(3):385–98. doi: 10.1007/s10802-007-9185-5. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, Andersen SL. Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Annals of the New York Academy of Sciences. 2006;1071:313–23. doi: 10.1196/annals.1364.024. [DOI] [PubMed] [Google Scholar]
- Toro R, Perron M, Pike B, Richer L, Veillette S, Pausova Z, Paus T. Brain size and folding of the human cerebral cortex. Cerebral Cortex (New York, NY : 1991) 2008;18(10):2352–7. doi: 10.1093/cercor/bhm261. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare Ta, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3(January):68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Annals of the New York Academy of Sciences. 2003;985:22–23. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Uno H, Tarara R, Else J, Suleman M, Sapolsky R. Hippocampal Damage Associated with Prolonged and Fatal Stress in Primates. Neuroscience. 1989 May;:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ijzendoorn MH, Juffer F. The Emanuel Miller Memorial Lecture 2006: adoption as intervention. Meta-analytic evidence for massive catch-up and plasticity in physical, socio-emotional, and cognitive development. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(12):1228–45. doi: 10.1111/j.1469-7610.2006.01675.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. 20026655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 2010;53(3):1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.