Abstract
A general and efficient N–H insertion reaction of rhodium pyridyl carbenes derived from pyridotriazoles was developed. Various N–H containing compounds, including amides, anilines, enamine and aliphatic amines, smoothly underwent the N–H insertion reaction to afford 2-picolylamine derivatives. The developed transformation was further utilized in a facile one-pot synthesis of imidazo[1,2-a]pyridines.
Keywords: N–H insertion, pyridotriazole, rhodium carbene, picolylamine, imidazopyridine, transannulation reaction
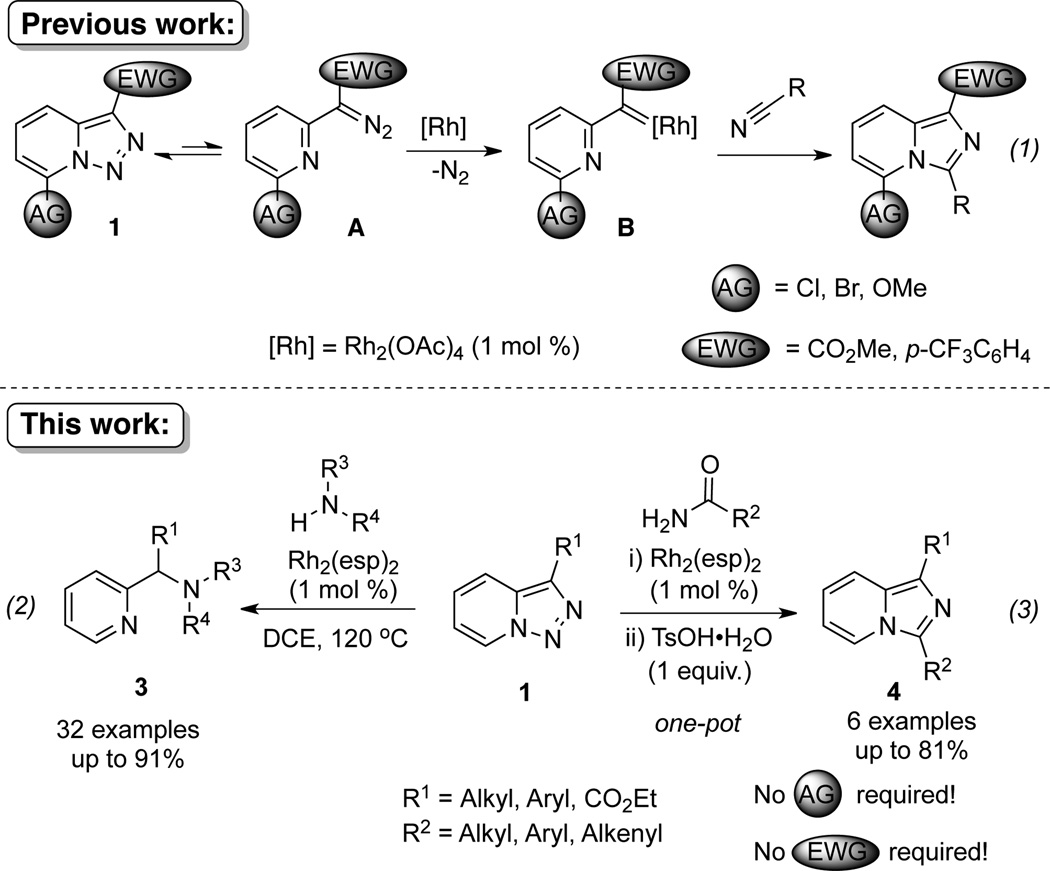
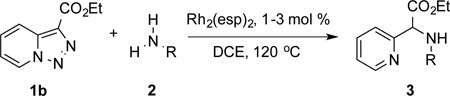
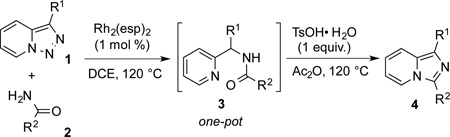
Transition-metal-catalyzed denitrogenative transannulation of pyridotriazoles[1] is a powerful method for synthesis of nitrogencontaining heterocycles.[2–4] As a convenient progenitor of metal carbene species, pyridotriazole 1 exists in the equilibrium with diazo-form A, which can be trapped with Rh(II) to form the reactive pyridyl carbene intermediate B (Scheme 1, eq. 1). In 2007, our group reported the transannulation reaction of pyridotriazoles based on the reaction of intermediate B with nitriles. It was shown that Cl, Br or OMe substituents at C-7 position (AG, activating group), as well as electron-withdrawing (EWG) groups at C-3 position, were requisite for efficient formation of the imidazo[1,2-a]pyridines (eq. 1).[1a] Naturally, we were interested in expanding the scope of imidazo[1,2-a]pyridines which can be accessed via transannulation reaction of pyridotriazoles. Herein, we report a general rhodiumcatalyzed N–H insertion reaction of pyridylcarbenes B derived from pyridotriazoles 1 to afford valuable picolylamine derivatives 3 (eq. 2);[5] and their application in a one-pot synthesis of imidazo[1,2-a]pyridines 4 (eq. 3).[6] This new method toward imidazo[1,2-a]pyridines features much broader scope, where the presence of AG and EWG in starting pyridotriazole 1 no longer required.
Scheme 1.
Transannulation reactions of pyridotriazoles.
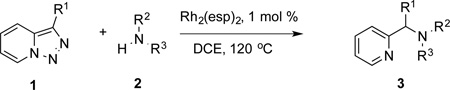
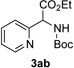
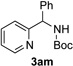
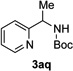
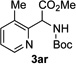
In continuation of our studies on application of diazocompounds for the synthesis of nitrogen-containing heterocycles,[7] we investigated the reaction of pyridotriazoles with primary amides as a potential route to imidazo[1,2-a]pyridines (vide infra). 7-Cl-substituted triazole 1a, which was proved to be an effective carbene precursor,[1] was tested in the Rh-catalyzed N–H insertion reaction first.[8] Indeed, the reaction of 1a with BocNH2 in the presence of Rh2(esp)2 catalyst at room temperature produced the corresponding piclolyl amine 3aa in 74% yield (Table 1, entry 1).[9] Attempts to employ 7-unsubstituted pyridotriazole 1b under these reaction conditions failed. However, we were pleased to find that at 120 °C it underwent insertion reaction to furnish picolylamine 3ab in 90% yield (Entry 2).[10]
Table 1.
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Triazole 1 | Product 3 | Yield/% | Entry | Triazole 1 | Product 3 | Yield/% |
| 1 | 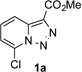 |
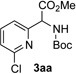 |
74[c] | 11 | 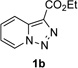 |
 |
66 |
| 2 | 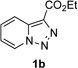 |
 |
90 | 12 | 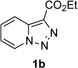 |
 |
75 |
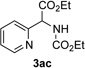
| 3 | 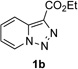 |
 |
91 | 13 | 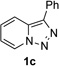 |
 |
89 |
| 4 | 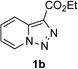 |
 |
65 | 14 | 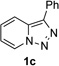 |
 |
75 |
| 5 | 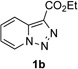 |
 |
85 | 15 | 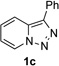 |
 |
81 |
| 6 | 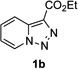 |
 |
87 | 16 | 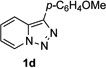 |
 |
77 |
| 7 | 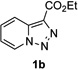 |
 |
76 | 17 | 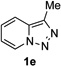 |
 |
88 |
| 8 | 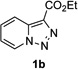 |
 |
85 | 18 | 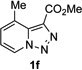 |
 |
66 |
| 9 | 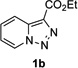 |
 |
75 | 19 | 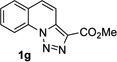 |
 |
63 |
| 10 | 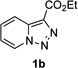 |
 |
68[d] | 20 | 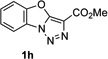 |
 |
91 |
Conditions: triazole 1 (0.20 mmol), N–H compounds 2 (1.5 equiv.), and Rh2(esp)2 (1.0 mol %) were heated in 2 ml of dry DCE at 120 °C until completion.
Isolated yields.
Performed at room temperature.
3.0 mol % Rh2(esp)2.
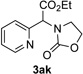
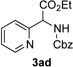
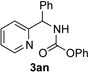
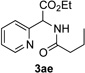
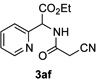
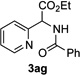
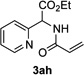
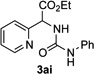
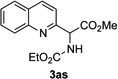
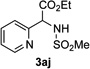
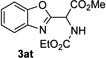
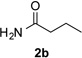
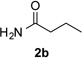
Next, we examined the scope of this N−H insertion reaction (Table 1). Thus, alkyl carbamates, such as t-BuOCONH2, EtOCONH2, and BnOCONH2 produced picolyl amines 3ab–3ad in high yields (entries 2–4). The reaction also worked efficiently with alkyl and aryl amides (entries 5–7), as well as with alkenyl amide (entry 8). Notably, cyano-group and alkenyl moiety, which normally react with metal carbenes, stayed intact under these reaction conditions (entries 6,8). Moreover, we found that phenyl urea and sulfonamide could also participate in this transformation to produce insertion products 3ai and 3aj (entries 9,10). Secondary amides, such as oxazolidin-2-one (entry 11) and 3(2-H)-pyridazinone (entry 12), were also competent reaction partners. Notably, the reaction also efficiently proceeded with pyridotriazoles containing different substituents at the C-3 poisition. Thus, 3-aryl pyridotriazoles (entries 13–16) and even 3-methyl pyridotriazole (entry 17) reacted smoothly to produce the desired N–H insertion products. In addition, 4-methyl pyridotriazole (entry 18), N-fused quinolinotriazole (entry 19) and benzoxazolotriazole (entry 20) also underwent an efficient N–H insertion reaction to afford the corresponding amides.
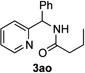
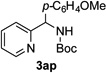
After developing the N–H insertion reaction with various amides, we turned our attention to more challenging aromatic and aliphatic amines, which, due to their high basicity, may potentially deactivate Rh(II) catalyst. To our delight, reasonable to good yields in the reaction of 1b with anilines were achieved upon raising catalyst loading to 3 mol % (Table 2, entries 1–9). Thus, anilines bearing functional groups, such as halogen (entries 3 and 8), CF3 (entries 4 and 7), and CO2Me (entry 5), efficiently underwent the reaction with pyridotriazole 1b to produce the insertion products. Moreover, sterically hindered 2,6-dichloro, and 2,6-diisopropylaniline reacted smoothly to give the corresponding insertion products in reasonable yield (entries 8,9). In addition, enamine also underwent the N–H insertion reaction to form the corresponding product 3bj (entry 10). Among aliphatic amines, α-CF3-substituted alkyl amines could undergo N–H insertion reaction, which was demonstrated by the reactions of 1b with 2,2,2-trifluoro-1-phenylethane-1-amine (entry 11). Notably, the successful N–H insertion reaction with CF3-amino acid (entry 12) opens access to fluorinated opine derivatives (i.e. 3bl).[11]
Table 2.
Substrate Scope for the Rh(II)-Catalyzed Reaction of Pyridotriazoles with Anilines and Aliphatic Amines.[a,b]
 | |||||
|---|---|---|---|---|---|
| Entry | Product | Yield, % |
Entry | Product | Yield, % |
| 1 | 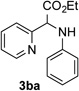 |
88 | 7 | 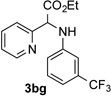 |
71 |
| 2 | 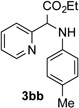 |
63 | 8 | 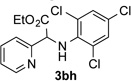 |
90 |
| 3 | 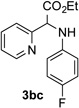 |
80 | 9 | 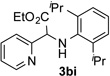 |
47 |
| 4 | 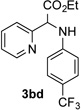 |
86 | 10 | 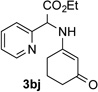 |
91[c] |
| 5 | 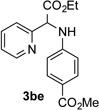 |
72 | 11 | 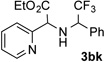 |
87 |
| 6 | 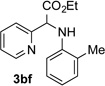 |
76 | 12 | 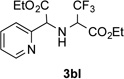 |
82 |
Conditions: triazole 1 (0.20 mmol), N–H compounds 2 (1.5 equiv.), and Rh2(esp)2 (3.0 mol %) were heated in 2 ml of dry DCE at 120 °C until completion.
Isolated yields.
1.0 mol % of Rh2(esp)2.
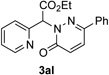
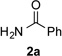
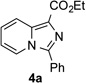
Along the line of our studies on the development of new transformations toward heterocyclic molecules, we envisioned that the obtained picolylamides 3 could be cyclized into imidazopyridines 4 via a nucleophilic attack of the pyridine nitrogen at a suitably activated amide group (Table 3).[12] Accordingly, we developed a formal one-pot transannulation reaction of pyridotriazoles with primary amides that proceeds via the Rh-catalyzed N–H insertion reaction followed by a cyclization into imidazo[1,2-a]pyridines (Table 3). Noteworthy, this transannulation reaction of pyridotriazoles 1 with amides has much broader scope compared to the previously developed transannulation reaction of 1 with nitriles (Scheme 1, eq. 1). Thus, the activating group AG is not necessary for the successful reaction, as well as substituent at C-3 position is notlimited to an electron-withdrawing group. Generally, the developed transannulation reaction is allowed for an efficient synthesis of imidazo[1,2-a]pyridines containing aryl, alkenyl and alkyl substituents (entries 1–6).
Table 3.
 | ||||
|---|---|---|---|---|
| Entry | Triazole 1 | Amide 2 | Product 4 | Yield/% |
| 1 | 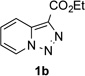 |
 |
 |
70 |
| 2 | 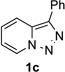 |
 |
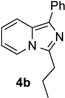 |
77 |
| 3 | 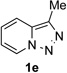 |
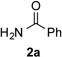 |
 |
73 |
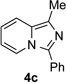
| 4 |  |
 |
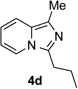 |
81 |
| 5 | 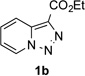 |
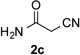 |
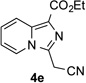 |
58 |
| 6 | 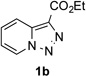 |
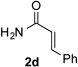 |
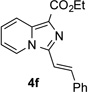 |
78 |
Conditions: triazole 1 (0.20 mmol), amides 2 (1.5 equiv.), and Rh2(esp)2 (1.0 mol %) were heated in 2 ml of dry DCE at 120 °C until completion. Then TsOH•H2O (1.0 equiv.) and Ac2O (0.2 ml) were added and the reaction mixture was heated at 120 °C.
Isolated yields.
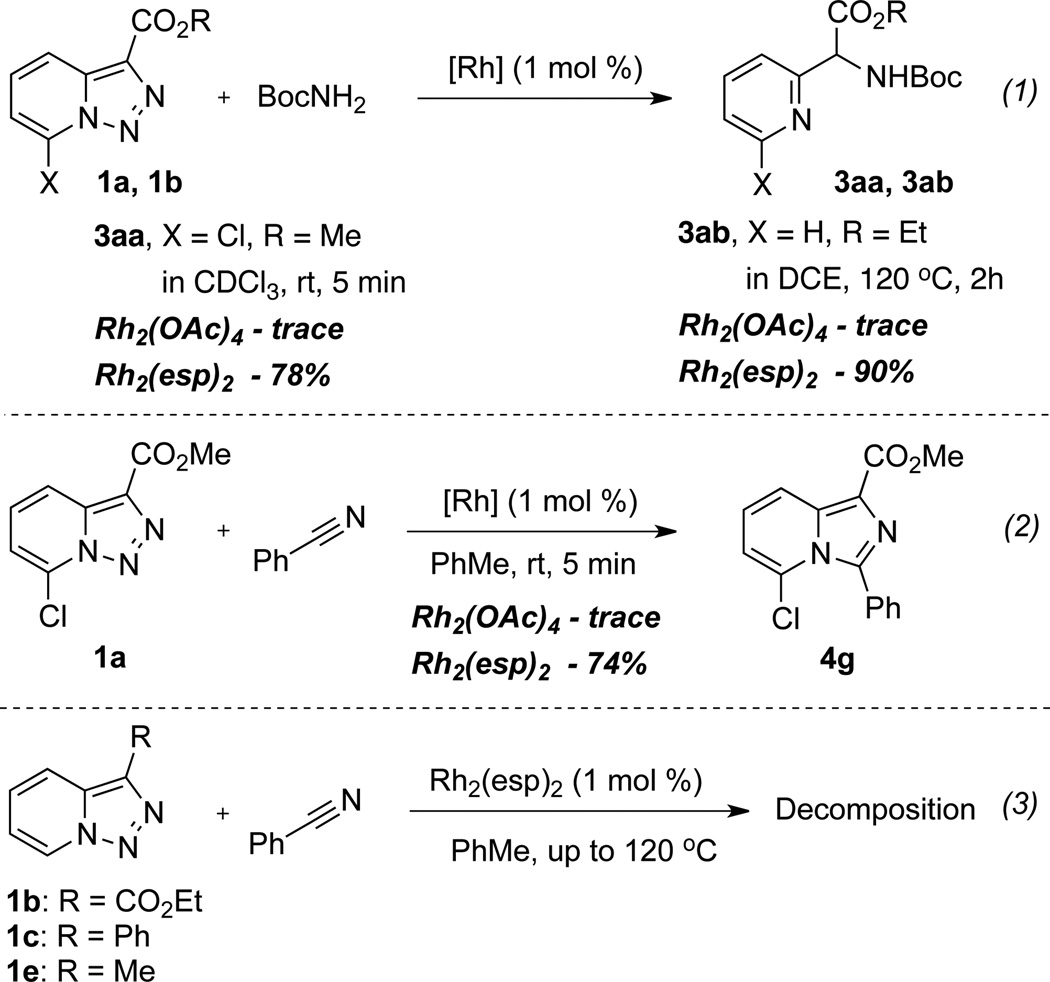
In order to understand the superior efficiency of the newly developed reaction of pyridotriazoles with amines over the previously reported reaction with nitriles, we performed reactions of pyridotriazoles 1a,b with BocNH2 and PhCN in the presence of different rhodium catalysts (Scheme 2). Thus, it was found that Rh2(esp)2, indeed, is a superior catalyst over the previously used Rh2(OAc)2 for reactions of pyridotriazole, both with amides (eq. 1) and nitriles (eq. 2). It was also verified that amides showed higher reactivity towards Rh-pyridocarbene (i.e. B, Scheme 1) over nitriles, since even Rh2(esp)2 catalyst was not efficient for transannulation of unactivated pyridotriazoles 1b,c,e with nitriles (Scheme 2, eq. 3). It is believed that the N-H insertion reaction of pyridotriazoles, analogously to that of phenyldiazoacetates, proceeds via an ylide mechanism.[13,14] However, it requires higher temperatures to produce sufficient amounts of a reactive diazo-form (i.e. B, Scheme 1).[15] Overall, we believe that a superior efficiency of the newly developed reaction of pyridotriazoles with amines and amides over the previously reported reaction with nitriles is due to a combination of an increased potency of the Rh-catalyst and a higher reactivity of amines and amides over that of nitriles.
Scheme 2.
Reactions of pyridotriazoles with amides and nitriles.
In conclusion, we have developed a general and efficient Rhcatalyzed reaction of pyridotriazoles with amides and amines producing valuable picolylamine derivatives. The subsequent cyclization provides an expeditious access to various disubstituted imidazopyridines in a one-pot manner. The developed protocol is allowed for the synthesis of polysubstituted imidazopyridines, which were not accessible via previously reported transannulation reaction of pyridotriazoles with nitriles. Further studies on the unique reactivity of pyridotriazoles are currently underway in our lab.
Experimental Section
An oven-dried 3.0 mL V-vial equipped with a stirring bar was charged with Rh2(esp)2 (1–3 mol %), pyridotriazole (0.2 mmol), amide or amine (1.5 equiv.) and DCE (2 mL) under N2 atmosphere. The reaction vessel was capped with Mininert syringe valve and the reaction mixture was stirred at 120 °C for 3 hours. Upon completion, the reaction mixture was cooled to room temperature, concentrated under reduced pressure, and the crude product was purified by column chromatography to afford the corresponding N–H insertion products.
Supplementary Material
Acknowledgements
The support of the National Institutes of Health (GM 64444) and National Science Foundation (CHE-1362541) is gratefully acknowledged.
References
- 1. Chuprakov S, Hwang F, Gevorgyan V. Angew. Chem. 2007;119:4841–4843. doi: 10.1002/anie.200700804. Angew. Chem. Int. Ed.2007, 46, 4757–4759; Chuprakov S, Gevorgyan V. Org. Lett. 2007;9:4463–4466. doi: 10.1021/ol702084f. For review, see: Chattopadhyay B, Gevorgyan V. Angew. Chem. 2012;124:886–896. doi: 10.1002/anie.201104807. Angew. Chem. Int. Ed.2012, 51, 862–872.
- 2.For reviews on reactions of metallocarbenes derived from N-sulfonyl 1,2,3-triazoles, see: Davies HML, Alford JS. Chem. Soc. Rev. 2014;43:5151–5162. doi: 10.1039/c4cs00072b. Gulevich AV, Gevorgyan V. Angew. Chem. 2013;125:1411–1413. doi: 10.1002/anie.201209338. Angew. Chem. Int. Ed.2013, 52, 1371–1373;
- 3.For transannulation reactions of N-sulfonyl triazoles, see: Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. J. Am. Chem. Soc. 2008;130:14972–14974. doi: 10.1021/ja805079v. Miura T, Yamauchi M, Murakami M. Chem. Commun. 2009:1470–1471. doi: 10.1039/b819162j. Chattopadhyay B, Gevorgyan V. Org. Lett. 2011;13:3746–3749. doi: 10.1021/ol2014347. Parr BT, Green SA, Davies HML. J. Am. Chem. Soc. 2013;135:4716–4718. doi: 10.1021/ja401386z. Spangler JE, Davies HML. J. Am. Chem. Soc. 2013;135:6802–6805. doi: 10.1021/ja4025337. Alford JS, Spangler JE, Davies HML. J. Am. Chem. Soc. 2013;135:11712–11715. doi: 10.1021/ja405043g. Parr BT, Davies HML. Angew. Chem. 2013;125:10228–10231. Angew. Chem. Int. Ed.2013, 52, 10044–10047; Zibinsky M, Fokin VV. Angew. Chem. 2013;125:1547–1550. doi: 10.1002/anie.201206388. Angew. Chem. Int. Ed.2013, 52, 1507–1510; Chuprakov S, Kwok SW, Fokin VV. J. Am. Chem. Soc. 2013;135:4652–4655. doi: 10.1021/ja400350c. Miura T, Tanaka T, Hiraga K, Stewart SG, Murakami M. J. Am. Chem. Soc. 2013;135:13652–13655. doi: 10.1021/ja407166r. Miura T, Hiraga K, Biyajima T, Nakamuro T, Murakami M. Org. Lett. 2013;15:3298–3301. doi: 10.1021/ol401340u. Schultz EE, Sarpong R. J. Am. Chem. Soc. 2013;135:4696–4699. doi: 10.1021/ja401380d. Shi Y, Gevorgyan V. Org. Lett. 2013;15:5394–5396. doi: 10.1021/ol4027655. Miura T, Funakoshi Y, Murakami M. J. Am. Chem. Soc. 2014;136:2272–2275. doi: 10.1021/ja412663a. Kim C, Park S, Eom D, Seo B, Lee PH. Org. Lett. 2014;16:1900–1903. doi: 10.1021/ol500718s. Yang J, Zhu C, Tang X, Shi M. Angew. Chem. 2014;126:5242–5246. Angew. Chem. Int. Ed.2014, 53, 5142–5146; Shang H, Wang Y, Tian Y, Feng J, Tang Y. Angew. Chem. 2014;126:5768–5772. doi: 10.1002/anie.201400426. Angew. Chem. Int. Ed.2014, 53, 5662–5666; Chen K, Zhu Z-Z, Zhang Y-S, Tang X-Y, Shi M. Angew. Chem. 2014;126:6763–6767. Angew. Chem. Int. Ed.2014, 53, 6645–6649; Rajagopal B, Chou C-H, Chung C-C, Lin P-C. Org. Lett. 2014;16:3752–3755. doi: 10.1021/ol501618z. Ran R-Q, He J, Xiu SD, Wang K-B, Li C-Y. Org. Lett. 2014;16:3704–3707. doi: 10.1021/ol501514b. Medina F, Besnard C, Lacour J. Org. Lett. 2014;16:3232–3235. doi: 10.1021/ol5012532.
- 4.For other reactions of N-sulfonyl triazoles, see: Grimster N, Zhang L, Fokin VV. J. Am. Chem. Soc. 2010;132:2510–2511. doi: 10.1021/ja910187s. Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J. Am. Chem. Soc. 2009;131:18034–18035. doi: 10.1021/ja908075u. Selander N, Worrell BT, Chuprakov S, Velaparthi S, Fokin VV. J. Am. Chem. Soc. 2012;134:14670–14673. doi: 10.1021/ja3062453. Chuprakov S, Worrell BT, Selander N, Sit RK, Fokin VV. J. Am. Chem. Soc. 2014;136:195–202. doi: 10.1021/ja408185c. Chuprakov S, Malik JA, Zibinsky M, Fokin VV. J. Am. Chem. Soc. 2011;133:10352–10355. doi: 10.1021/ja202969z. Alford JS, Davies HML. Org. Lett. 2012;14:6020–6023. doi: 10.1021/ol3029127. Muira T, Biyajima T, Fujii T, Murakami M. J. Am. Chem. Soc. 2012;134:194–196. doi: 10.1021/ja2104203. Miura T, Tanaka T, Biyajima T, Yada A, Murakami M. Angew. Chem. 2013;125:3975–3978. doi: 10.1002/anie.201209603. Angew. Chem. Int. Ed.2013, 52, 3883–3886. Miura T, Funakoshi Y, Morimoto M, Biyajima T, Murakami M. J. Am. Chem. Soc. 2012;134:17440–17443. doi: 10.1021/ja308285r. Selander N, Worrell BT, Fokin VV. Angew. Chem. 2012;124:13231–13234. doi: 10.1002/anie.201207820. Angew. Chem. Int. Ed.2012, 51, 13054–13057; Boyer A. Org. Lett. 2014;16:1660–1663. doi: 10.1021/ol500309x. Jung DJ, Jeon HJ, Kim JH, Kim Y, Lee S. Org. Lett. 2014;16:2208–2211. doi: 10.1021/ol500723s. Yadagiri D, Anbarasan P. Org. Lett. 2014;16:2510–1513. doi: 10.1021/ol500874p. Miura T, Funakoshi Y, Tanaka T, Murakami M. Org. Lett. 2014;16:2760–2763. doi: 10.1021/ol5010774. Miura T, Nakamuro T, Hiraga K, Murakami M. Chem. Commun. 2014;50:10474–10477. doi: 10.1039/c4cc04786a.
- 5.For selected reports on 2-pycolylamine-containing bioactive molecules, see: Tsuboi K, Bachovchin DA, Speers AE, Spicer TP, Fernandez-Vega V, Hodder P, Rosen H, Cravatt BF. J. Am. Soc. Chem. 2011;133:16605–16616. doi: 10.1021/ja2066972. Wood MR, Schirripa KM, Kim JJ, Kuduk SD, Chang RK, DiMarco CN, DiPardo RM, Wan B-L, Murphy KL, Ransom RW, Chang RSL, Holahan MA, Cook JJ, Lemaire W, Mosser SD, Bednar RA, Tang C, Prueksaritanont T, Wallace AA, Mei Q, Yu J, Bohn DL, Clayton FC, Adarayn ED, Sitko GR, Leonard YM, Freidinger RM, Pettibone DJ, Bock MG. Bioorg. Med. Chem. Lett. 2008:716–720. doi: 10.1016/j.bmcl.2007.11.050.
- 6.For reports on the biological activity of imidazopyridines, see: Kim D, Wang L, Hale JJ, Lynch CL, Budhu RJ, MacCoss M, Mills SG, Malkowitz L, Gould SL, DeMartino JA, Springer MS, Hazuda D, Miller M, Kessler J, Hrin RC, Carver G, Carella A, Henry K, Lineberger J, Schleif WA, Emini EA. Bioorg. Med. Chem. Lett. 2005;15:2129–2134. doi: 10.1016/j.bmcl.2005.02.030. Nakahara S, Kubo A, Mikami Y, Ito J. Heterocycles. 2006;68:515–520. Kamal A, Ramakrishna G, Ramaiah MJ, Viswanath A, Bao AVS, Bagul C, Mukhopadyay DVL, Pushpavalli SNC, Pal-Bhadra M. Bioorg. Med. Chem. 2013;4:697–703.
- 7.a) Gulevich AV, Helan V, Wink DJ, Gevorgyan V. Org. Lett. 2013;15:956–959. doi: 10.1021/ol400148r. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kuznetsov A, Gulevich AV, Wink DJ, Gevorgyan V. Angew. Chem. 2014;126:9167–9171. doi: 10.1002/anie.201404352. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2014;53:9021–9025. doi: 10.1002/anie.201404352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For a 1,3- N−H insertion reaction of iminocarbenes with non-basic N−H groups, see: Chuprakov S, Worrell BT, Selander N, Sit RK, Fokin VV. J. Am. Chem. Soc. 2014;136:195–202. doi: 10.1021/ja408185c.
- 9.Other rhodium catalysts, such as Rh2(OAc)4 and Rh2(Oct)4, are also capable of catalyzing the reaction of pyridotriazole 1a with BocNH2.
- 10.The reaction of pyridotriazole 1b with BocNH2 in the presence of other rhodium catalysts, such as Rh2(OAc)4 and Rh2(Oct)4, did not give any product.
- 11.For selected reports on bioactive iminodicarboxylic acids and opines, see: Moore LW, Chilton WS, Canfield ML. Appl. Environ. Microbiol. 1997;63:201–207. doi: 10.1128/aem.63.1.201-207.1997. Chilton WS, Petit A, Chilton M-D, Dessaux Y. Phytochemistry. 2001;58:137–142. doi: 10.1016/s0031-9422(01)00166-2.
- 12.Kovtun YP, Prostota YA. Chem. Heterocycl. Compd. 2000;36:557–559. [Google Scholar]
- 13.For selected reviews on the Rh-catalyzed N-H insertion reactions of diazocompounds, see: Gillingham D, Fei N. Chem. Soc. Rev. 2013;42:4918–4931. doi: 10.1039/c3cs35496b. Zhang Z, Wang J. Tetrahedron. 2008;64:6577–6605. Doyle MP, McKervey MA, Ye T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides. New York: Wiley; 1998.
- 14.Analogously to pyridotriazole 1a, phenyldiazoacetate quantitatively reacts with BocNH2 in the presence of Rh2(esp)2 catalyst at room temperature.
- 15.Test experiments indicated no N-H insertion reaction of 1b with BocNH2 and PhNH2 occurred under thermal conditions in the absence of Rh catalyst
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.