Abstract
Uric acid and purines (such as adenosine) regulate mood, sleep, activity, appetite, cognition, memory, convulsive threshold, social interaction, drive, and impulsivity. A link between purinergic dysfunction and mood disorders was first proposed a century ago. Interestingly, a recent nationwide population-based study showed elevated risk of gout in subjects with bipolar disorder (BD), and a recent meta-analysis and systematic review of placebo-controlled trials of adjuvant purinergic modulators confirmed their benefits in bipolar mania. Uric acid may modulate energy and activity levels, with higher levels associated with higher energy and BD spectrum. Several recent genetic studies suggest that the purinergic system particularly the modulation of P1 and P2 receptor subtypes—plays a role in mood disorders, lending credence to this model. Nucleotide concentrations can be measured using brain spectroscopy, and ligands for in vivo positron emission tomography (PET) imaging of adenosine (P1) receptors have been developed, thus allowing potential target engagement studies. This review discusses the key role of the purinergic system in the pathophysiology of mood disorders. Focusing on this promising therapeutic target may lead to the development of therapies with antidepressant, mood stabilization, and cognitive effects.
Keywords: bipolar disorder, depression, purines, uric acid, mania, treatment
Introduction
The purinergic system encompasses signaling pathways of the neurotransmitter adenosine triphosphate (ATP) as well as the neuromodulator adenosine. The notion that this system might be indirectly involved in mood regulation and dysfunction was proposed nearly a century ago, and recent evidence supports this hypothesis. The present review discusses the key role of the purinergic system in the pathophysiology of mood disorders, including: an overview of the physiology of the purinergic system; a summary of the literature to date focusing on molecular studies of purinergic receptors, animal behavioral studies, and human genetic and clinical studies in mood disorders; and potential future therapies with antidepressant, mood stabilization, and cognitive effects that target the purinergic system.
A literature search was conducted using the following Medline PubMed keywords: purinergic, uric acid, adenosine (receptor), ATP (receptor), P receptors, A receptors, P2 receptors, P2X7, guanosine, mood, behavior, brain, bipolar disorder, depression, mood disorders, and affective disorders. All articles reporting results in subjects with mood disorders were included in the present review, as were preclinical models on the interface between mood disorders and the purinergic system.
1. The Purinergic System: General Overview
Adenosine triphosphate (ATP) is widely used in multiple cell types as a coenzyme for energy transfer. The purinergic system includes transmembrane receptors, named P1 and P2 based on their pharmacological properties of activation by adenosine or nucleotides, respectively. In 1972, ATP was found to act as a neurotransmitter, originating the concept of “purinergic” nerves (Burnstock, 1972). Subsequent studies showed the presence of both ATP and adenosine signaling in the central nervous system (CNS) (Phillis and Kostopoulos, 1975; Wu and Phillis, 1978), as well as ATP storage and release by neurons (Burnstock, 1977). The cloning of various receptor subtypes, together with functional data, has rapidly expanded the study of purinergic signaling in neurotransmission.
The purinergic system—which comprises various receptor subtypes and ectoenzymes that degrade ATP into adenosine and inosine—is present in numerous brain areas including the cerebral cortex, hypothalamus, basal ganglia, hippocampus, and other limbic areas (Burnstock, 2008). ATP is prevalent in neuronal and non-neuronal cells, and is synthesized in the mitochondria during oxidative phosphorylation. ATP is stored in the cytoplasm of nerve terminals. In addition to directly participating in neurotransmission as a co-transmitter, the purinergic system serves as a communication link between neuronal and glial cells in which ATP generates intercellular calcium wave signaling that contributes to synapse formation and neuronal plasticity (Fields and Burnstock, 2006; Ulrich et al., 2012). When activated, axons and synapses release ATP to activate purinergic receptors on glial cells, which causes shifts in intracellular calcium concentrations and cyclic adenosine monophosphate (cAMP), leading to glial release of ATP. This signaling is involved in glial proliferation, survival, differentiation, motility, and myelination. Purinergic effects can impact the activity of other neurotransmitters, including the dopaminergic, gamma aminobutyric acid (GABA)-ergic, glutamatergic, and serotonergic systems; notably, all are involved in the pathophysiology of mood disorders (Machado-Vieira et al., 2002). Both adenosine and ATP, as well as some of their metabolites, can induce downstream effects in the CNS by activating distinct purinergic receptor types (Burnstock, 2008). Furthermore, purinergic receptors serve as a method of communication between the CNS and outside systems such as the immune, vascular, and neuronal systems (Fields and Burnstock, 2006).
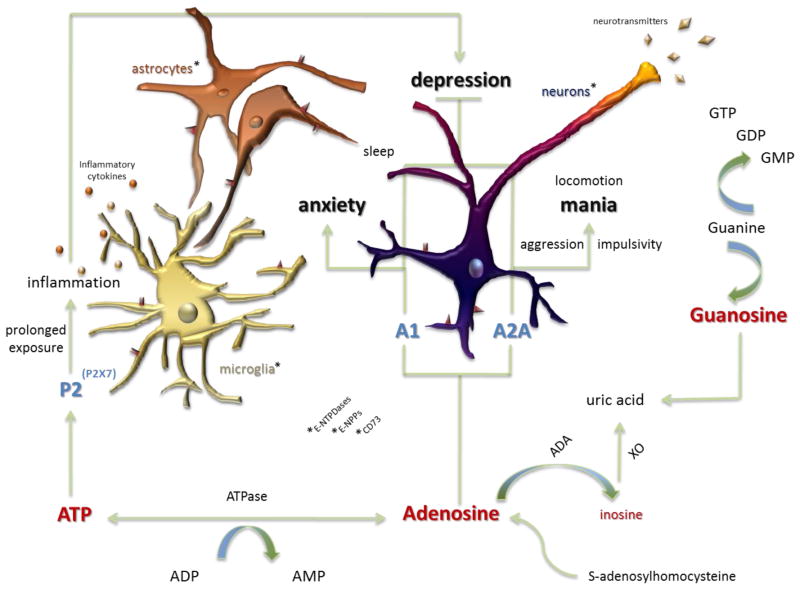
The availability of extracellular nucleotides is regulated by a set of enzymes that include ectonucleoside triphosphate diphosphohydrolases (E-NTPDases), nucleotide pyrophosphatase/phosphodiesterase (NPPs), alkaline phosphatases (ALPs), and ecto-5′-nucleotidase (e5NT or CD73). These enzymes catalyze the complete nucleotide breakdown to produce adenosine (Figure 1). Adenosine can also be produced from the cleavage of S-adenosyl homocysteine (SAH). Adenosine can also be taken up by nerve terminal transporters (Sperlagh et al., 2012) and broken down into inosine by adenosine deaminase and by xanthine oxidase (XO) activity to produce its downstream product, uric acid (Herken et al., 2007). Increased uric acid levels accelerate purinergic transformation and decrease adenosinergic transmission (Burnstock, 2008). Metabolites of the purinergic system may serve an important role as additional signaling molecules or markers of purinergic system activation in various conditions.
Figure 1. The purinergic system and signaling in mood disorders.
Extracellular nucleotides and nucleosides, including adenosine triphosphate (ATP), uridine triphosphate (UTP), and guanosine triphosphate (GTP), their metabolites, and their associated purinergic receptors, have been linked to mood disorders. Most of the existing evidence points to an association between ATP and adenosine signaling and mood dysregulation. Adenosine is a purine nucleoside produced in part through the metabolism of ATP by ATPase and 5′-nucleotidase enzymatic activity. Adenosine acts through purinergic P1 receptors, whereas ATP acts through purinergic P2 receptors. The availability of extracellular nucleotides is regulated by enzymes, including ectonucleoside triphosphate diphosphohydolases (e-NTPDases), nucleotide pyrophosphatase / phosphodiesterase (NPPs), ecto-alkaline phosphatases (ecto-ALPs), and ecto-5′-nucleotidase (e5NT). These enzymes catalyze the complete nucleotide breakdown to yield adenosine. Adenosine can also be produced from the cleavage of S-adenosyl-L-homocysteine (SAH). Further breakdown of purines through enzymes, including adenosine deaminase (ADA) and xanthine oxidase (XO), yields metabolites such as inosine and uric acid. Purinergic signaling occurs through adenosine, and potentially inosine, at P1 receptors (both A1 and A2A subtypes). Signaling also occurs by way of P2 receptors, including the P2X7 subtype, in the context of behavior and mood dysregulation. These receptors are found on neuronal and non-neuronal cells including microglia and astrocytes. Signaling at these sites is thought to disturb other neurobiological systems including neuroinflammation and neurotransmitter modulation. Dysfunction at any number of these aspects of purinergic signaling—either at a genetic, biochemical, or functional level—is thought to lead to altered behavior (impulsivity, aggression, changes in locomotor activity, and sleep patterns) and mood (depression, anxiety, and mania). This dysfunction, therefore, suggests a link between purinergic dysfunction and the etiology of mood disorders
1.1 P1 (adenosine) receptors
P1 receptors are G-protein coupled receptors with an extracellular binding site for adenosine and have seven transmembrane domains as well as an intracellular COOH terminus (Burnstock, 2007). The P1 adenosine receptor family includes the A1, A2A, A2B, and A3 subtypes; A1 and A2A receptors are the most studied in CNS pathophysiology, and adenosine binds to A1 receptors with the highest affinity (Wei et al., 2011).
Adenosine receptors can be found in diverse brain areas including the basal ganglia, striatum, and forebrain and on glutamatergic and dopaminergic neurons (Cunha et al., 2008). Presynaptic A1 receptor activation reduces calcium influx and, consequently, limits neurotransmitter and neuropeptide release. In contrast, postsynaptic A1 activation leads to hyperpolarization, thus inhibiting neuronal signaling (Machado-Vieira et al., 2002; Paul et al., 2011). The A1 receptor binds to adenosine following Gi protein activation. The subsequent signal transduction results in decreased cAMP levels, increased IP3 levels, and altered calcium levels (Machado-Vieira et al., 2002). The versatility of the A1 adenosine receptor allows it to interact with various G-protein subtypes under different conditions, providing a possible explanation for adenosine’s striking diversity of biochemical and behavioral effects (Cordeaux et al., 2004; Baker and Hill, 2007). At the same time, the A2A receptor is prominently distributed in the cortex and striatum (El Yacoubi et al., 2001), and acts as a G-protein coupled receptor linked to the activation of adenylyl cyclase (Zezula and Freissmuth, 2008). For more information about adenosinergic signaling in the brain, we refer the interested reader to the excellent review article by Dias and colleagues (Dias et al., 2013).
1.2 P2 (ATP) receptors
P2 receptors are further divided into P2X and P2Y families, ligand-gated ion channels, and G-protein coupled receptors, respectively. P2Y receptors are metabotropic, which vary in their agonist properties and coupling to G-proteins. P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 subtypes have been identified in mammalian tissues. They differ in their agonist properties in responding to stimulation by ATP, adenosine diphosphate (ADP), uridine triphosphate (UTP), and uridine diphosphate (UDP)-glucose (for a comprehensive review, see (Weisman et al., 2012)).
P2X receptors are also widely distributed throughout various tissues, but are more prevalent throughout the nervous system than P2Y receptors (Burnstock, 2007). The P2X family comprises seven receptor subunits. They are ligand-gated ion channels formed as trimeric receptors, assembled as homo- or heteromeric receptors from P2X1-P2X7 subunits. Intracellular sites allow the regulation of receptor activity by interactions with enzymes such as protein kinases (Burnstock, 2007). P2X receptors allow for the passage of cations (Burnstock, 2007) and are, therefore, responsible for fast excitatory transmission induced by the flux of sodium, calcium, and potassium (Machado-Vieira et al., 2002). Though ionotropic signaling is transient, prolonged stimulation results in further dilation of the molecular channel (Skaper et al., 2010). For example, prolonged stimulation of the P2X7 receptor may result in the activation of the inflammatory nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathway and the release of larger molecules such as inflammatory cytokines (Ferrari et al., 1999; Potucek et al., 2006). The P2X7 receptor is prominently distributed on immune cells and glia but, within the CNS, is also prevalently expressed by astrocytes as well as neurons in lesser amounts (Burnstock, 2007; Majumder et al., 2007; Skaper et al., 2010).
1.3 Guanine-binding receptors
Although guanine is known to be involved in G-protein pathway signaling (Lin et al., 2001; Schmidt et al., 2007), evidence suggests that, synaptically, it may also function like traditional neurotransmitters (Santos et al., 2006). Evidence also supports its activity as an external molecular signal in the CNS. Signaling may occur through guanine-based nucleotides such as guanosine triphosphate (GTP), guanosine diphosphate (GDP), and guanosine monophosphate (GMP), or the nucleoside guanosine (Schmidt et al., 2007). Guanosine may be produced extracellularly by ectonucleotidases from the metabolism of guanine-based nucleotides. Guanosine affects astrocytes, neuronal cells, and microglia and has been particularly studied in the cortex (Schmidt et al., 2007).
1.4 Physiology overview
Purinergic activity depends on neuroanatomical location, cell type, and signaling mechanisms. Actions of extracellular ATP through P2X and P2Y receptors in the brain range from the regulation of membrane potential and electrical activity to neurotransmitter and hormone release (reviewed by (Vavra et al., 2012)). This diversity may account for the fact that the purinergic system has been implicated in a variety of pathologic disorders including trauma, stroke, ischemia, neurodegenerative disorders, migraine, seizure disorders, and neuropsychiatric conditions (Burnstock, 2008). The ubiquitous presence of purinergic receptors in the CNS led to the hypothesis that ATP serves as a neuroprotective modulator (Inoue, 1998; Franke et al., 2006). After release associated with trauma due to ischemic damage or mechanical strain, it can initiate signaling responses (Franke et al., 2006; Burnstock, 2013), but also acts as a classic neurotransmitter when released from packed secretory granules as a result of stimulation by action potentials (Machado-Vieira et al., 2002).
The concept of classic ATP-mediated postsynaptic currents—for instance, in the hippocampus—has come into question due to several studies showing that ATP produces neuromodulatory responses by promoting neurotransmitter release (including glutamate and GABA) rather than acting as a neurotransmitter itself. The modulation of extracellular glutamate, GABA, norepinephrine, and adrenaline/noradrenaline concentrations are major features in mood disorders such as major depressive disorder (MDD) (Northoff, 2013), underscoring the importance of purinergic receptors in this context. In addition to regulating neurotransmitter concentrations via presynaptic P2 receptors, the mechanisms of depolarization and calcium flux also lead to potentiation of hormone secretion in the pituitary glands. These hormones are important for various cellular functions including stress response (reviewed by (Burnstock, 2014)). A neurohormonal basis of mood disorders has been postulated, whereby the hypothalamic pituitary axes interact with the brain monoaminergic system (reviewed by (Spinelli, 2005)). Studies have found that purinergic receptors promoted estrogen liberation and lowered dopamine and catecholamine levels, which can contribute to the pathogenesis of depression. Steroid control of cerebrospinal GABA levels and GABAergic synapses may also provide an important mechanism promoted by purinergic receptors (Spinelli, 2005; Herzog, 2007).
Since the 1980s, studies have linked the purinergic system in the CNS to abnormal movements, seizure disorders, and sleep (Caporali et al., 1987; Popoli et al., 1988; Smolen and Smolen, 1991). Around this time, other studies emerged elucidating the involvement of the centrally acting purinergic system in behavior (Lekic, 1988; Ushijima et al., 1989). P1 and P2 receptors have been extensively studied in relationship to CNS physiology and pathology (for reviews of adenosinergic and ATP signaling in the normal brain and brain disease see (Majumder et al., 2007; Dias et al., 2013)). For instance, presynaptic inhibition of adenosine-induced signal transmission in the hippocampus was explored as a pharmaceutical target for anti-epileptic drugs (Williams-Karnesky et al., 2013). Furthermore, inhibition of purinergic gliotransmission, principally mediated by P2Y1 receptor activation, was found to reduce transmission of calcium waves as a possible strategy against epileptic effects (Kumaria et al., 2008). P2X7 receptors appear to be related to seizure induction and cell death in epilepsy conditions due to activation of excitotoxic pathways; inhibition of P2X7 receptor activation resulted in reduced electric and clinical seizure severity and less seizure-induced cell death in the neocortex (Jimenez-Pacheco et al., 2013). In addition, because of the common expression of P2X7 receptors by supportive cells of the CNS, these receptors have been associated with the pathology of mood disorders (Burnstock, 2008).
2. The Purinergic System: Neurobiological Findings in Mood Disorders
2.1 Overview
Kraeplin was the first to propose that the purinergic system might be indirectly involved in the pathology of mood disorders; his observations suggested that excretion of uric acid was decreased during altered mood states (Kraepelin, 1921). Indeed, the purinergic system has been linked to many symptoms of MDD, bipolar disorder (BD), and anxiety disorders, including dysfunctional sleep (Huang et al., 2011), anhedonia (Csolle et al., 2013), changes in appetite, energy levels, and motor function (Salamone and Correa, 2009), cognitive impairment (Salamone and Correa, 2009), psychomotor agitation, and severity of emotional symptoms (Wei et al., 2011). The purinergic system has also been linked to the etiology of mood disorders via dysfunctional ATP and adenosine signaling and through inherited patterns and the neuroinflammatory hypothesis. In the 1970s, Brooks and colleagues studied peripheral uric acid in relationship to specific mood symptoms, based on observations that peripheral and urinary clearance of uric acid resulted from significant purine turnover in the brain (Brooks et al., 1978). In that study, uric acid levels correlated with hallucinations and suicidality, and trended to correlate with mania. Ten years ago, our group first described the role of purinergic dysfunction as a possible therapeutic intervention for mood disorders (Machado-Vieira et al., 2008).
The preclinical involvement of the purinergic system in mood disorders has been studied in vitro and in animal models. In vitro studies suggested that ATP and adenosine are involved in pathophysiological mechanisms and potential therapeutics in mood disorders. For example, it was recently found that allopurinol and febuxostat—potent purine XO inhibitors that block urate accumulation—had antidepressant effects in the forced swim test animal model of depression (Karve et al., 2013). The effects exerted by these compounds were comparable to those of fluoxetine. This and other studies point at the need to clarify the biochemical mechanisms underlying purinergic dysfunction in mood states as a means of identifying therapeutic targets.
While preclinical and in vitro studies aim to understand the specific molecular mechanisms of purinergic dysfunction in behavioral states, human studies have assessed genetic variations related to purinergic function in patients with mood disorders. Human studies include neuroimaging techniques that explore phosphorous metabolism, such as positron emission tomography (PET) imaging studies of ATP turnover using specific purinergic receptor ligands (Kato et al., 1995; Bauer and Ishiwata, 2009). Human studies have also analyzed peripheral tissue samples for purinergic receptor expression in leukocytes and circulating levels of potential purinergic biomarkers, such as uric acid levels (Brooks et al., 1978; Iacob et al., 2013). Notably, diverse comorbid medical conditions have been described in patients suffering from BD, including gout (Chung et al., 2010). These findings suggest that the purinergic system may be a viable target for identifying novel therapeutics to treat mood disorders.
2.2 ATP & its Receptors
Metabolic dysfunction driven by ATP and its receptors (P2 receptors) has been associated with mood disorders. Several studies have demonstrated that ATP is an essential modulator of astrocyte involvement in depressive-like behaviors (Kirshenbaum et al., 2011; Cao et al., 2013). Much of the action of purinergic signaling in the brain seems to occur in non-neuronal cells of the CNS including astrocytes and microglia. Animals exposed to repeated social defeat demonstrate reduced brain levels of ATP, and replenishing ATP in mice with transgenic blockage of vesicular gliotransmission-induced astrocytic ATP release resulted in antidepressant-like effects such as decreased immobility time in the forced swim test (Cao et al., 2013).
The overactivity of P2 receptors has been associated with depression. A recent study revealed antidepressant- and anti-anxiety-like effects in mice treated with the P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS) (Pereira et al., 2013). As a result, P2X receptor antagonism has been proposed as a therapeutic strategy for mood disorders and has been further evaluated in preclinical models (particularly examining the P2X7 subtype). Astrocyte activation by ATP led to a positive feedback loop through the P2X7 receptor involving other neurotransmitters, indicating that slight imbalances in extracellular ATP concentrations may lead to widespread signal changes in the brain and, potentially, to behavioral modifications (Bennett, 2007). P2X7 receptor activation on microglia also contributed to the release of inflammatory markers linked to depressive behavior (Basso et al., 2009; Skaper et al., 2010; Furuyashiki, 2012).
P2X7 receptor antagonism had antidepressant (forced swim test) and anxiolytic-like effects in mice (Pereira et al., 2013). Recently, a novel high affinity P2X7 receptor antagonist, JNJ-4795567, demonstrated permeability to the CNS (Bhattacharya et al., 2013). In mice, P2X7 receptor knockout had antidepressant-like effects (Basso et al., 2009; Boucher et al., 2011; Csolle et al., 2013); compared to wild-type animals, these P2X7 knockout mice also displayed increased resilience in the repeated forced swim test (Boucher et al., 2011), and decreased anhedonia-like behavior in the sucrose preference test (Csolle et al., 2013). P2X receptors are also thought to play a role in mood disorders, given that ivermectin, a potent positive allosteric modulator of the P2X4 receptor, induced depressive-like behaviors (Bortolato et al., 2013).
Another receptor of possible therapeutic importance is the P2Y1 receptor. This receptor, which is expressed by astrocytes, provides a key mechanism for calcium wave stimulation in astrocytes (Hamilton et al., 2008; Ulrich et al., 2012) as well as in presynaptic facilitation of dopamine and glutamate release, which are then responsible for motivation-related responses to stimuli (Burnstock and Kennedy, 2011). Moreover, ATP release is thought to occur from neurons and glial cells during electroconvulsive therapy (ECT), resulting in the initial activation of P2 receptors and subsequent activation of P1 receptors following degradation of ATP into adenosine. Sadek and colleagues proposed that the antidepressant effects of this therapy would result from the co-release of ATP with dopamine (Sadek et al., 2011). On the one hand, the released ATP could stimulate P2X7 receptors, thereby affecting depressive symptoms (Sadek et al., 2011). On the other hand, P2Y1 receptors expressed by neuronal cells have also been associated with both anxiolytic and antidepressant effects (Kittner et al., 2003; Taayedi, 2007). The cited anxiolytic-like effects were reversed in the presence of the selective P2Y1 receptor antagonist MRS 2179 (Kittner et al., 2003). The importance of the P2Y1 receptor was further studied in animal models of motivation and adaptive behaviors (Krugel et al., 2004). A chronic succession of starvation and feeding periods altered P2Y1 receptor expression, supporting a role for altered sensitivity in responding to environmental stimuli (Krugel et al., 2004). Phase I clinical trials are needed to investigate the therapeutic potential of purinergic modulators in the treatment of mood disorders.
2.3 Adenosine & its Receptors
Evidence from animal studies supports dysfunctional adenosine (P1) receptor signaling, mostly by A1 and A2A subtypes, in the pathophysiology of mood disorders. Adenosine is produced from ATP by a cascade of cell surface transmembrane enzymes, including E-NTPDases and e5NT (Figure 1). Enzymes of this cascade function in mood and somatic symptoms. e5NT knockout mice lack non-REM sleep responses to sleep deprivation, but no changes in anxiety-like behaviors were evident in these knock-out mice (Zlomuzica et al., 2013). Lithium, used to treat BD, inhibits ectonucleotidase activity (Oliveira Rda et al., 2011). Given that the activity of such enzymes would otherwise lead to the accumulation of adenosine, lithium directly targets the adenosinergic system.
Adenosine receptor knockout animals had prolonged immobility in behavioral models of depression though no motor function effects were seen (Fedorova et al., 2003). Increased A1 and A2A adenosine receptor binding in rat hippocampus and striatum was associated with depressive-like behaviors after exposure to chronic mild stress (Crema et al., 2013). Interestingly, adenosine and its metabolite inosine reduced immobility time in the forced swim test and tail suspension test in mice (Kaster et al., 2004; Kaster et al., 2013). The antidepressant effects of adenosine may involve interactions with potassium channel activity, opioid and serotonin receptors, and nitric oxide signaling (Kaster et al., 2005b; Kaster et al., 2005c; Kaster et al., 2005a; Kaster et al., 2007b; Kaster et al., 2007a).
In other models, the antidepressant effects of sleep deprivation and ECT augmented brain adenosine levels (Lewin and Bleck, 1981; Savelyev et al., 2012). Similarly, tricyclic antidepressants (TCAs) increased inhibition of cortical neuron firing and potentiated the effects of adenosine on cortical neurons (Stone and Taylor, 1978; Phillis, 1984). Aggressive behavior induced by clonidine was overcome by adenosine receptor antagonism (Ushijima et al., 1989). The synthetic A1 and A2A receptor agonists, CHA (N6-cyclohexyladenosine) and DMPA (N6-[2-(3,5-dimethoxyphenyl)-2-(methylphenyl)ethyl]adenosine), respectively, had antidepressant effects in the forced swim test in mice (Kaster et al., 2004). In another study, the A2A receptor antagonists preladenant and SCH 412348 had similar antidepressant effects in the tail suspension test (Hodgson et al., 2009). The adenosine deaminase inhibitor EHNA (erythro-9-(2-hydroxy-3nonyl) adenine) had antidepressant-like effects in the forced swim test (Kaster et al., 2013) as did inosine, an adenosine metabolite. These effects were inhibited by adenosine receptor antagonists.
Evidence indicates that the effects of purinergic molecules go beyond the phenotype of depression. For example, inosine and hypoxanthine stimulation also induced anxious behaviors in rats by acting at the benzodiazepine receptor (Wagner and Katz, 1983). Theacrine, which, like caffeine, binds to both A1 and A2A receptors, increased locomotor activity when administered to rats in the nucleus accumbens (Feduccia et al., 2012). However, studies using the electric shock avoidance test, which serves as an animal model of helplessness, found that adenosine induced depressive-like behaviors in animals (Minor et al., 1994; Woodson et al., 1998; Shen and Chen, 2009). This suggests that purinergic systems may mitigate depressive and anxiety symptoms as well as psychomotor agitation, but further research is needed to further elucidate the mechanisms and specific targets involved in such effects.
2.3.1 A1 receptor
Animal studies targeting the A1 receptor have been conducted in an attempt to elucidate the potential mechanisms of adenosine that may underlie mood regulation. Sleep deprivation in rats increased the number of A1 receptors in the brain (Elmenhorst et al., 2009). Some studies found that A1 receptor knockout or antagonism increased anxiety (Gimenez-Llort et al., 2002; Lang et al., 2003; Prediger et al., 2004); in contrast, A2A blockade did not (El Yacoubi et al., 2001). A1 receptors seem only indirectly involved in caffeine increases in locomotion, as their antagonism alone was not necessary to elicit these effects. Rather, it is hypothesized that A1 antagonism exacerbates the effects seen through A2A receptor antagonism (Halldner et al., 2004). It is possible, however, that A1 receptors play a role in a variety of symptoms and phenotypes other than depression in patients with mood disorders. For example, A1 receptor knockout animals experienced hyperalgesia (Johansson et al., 2001).
One study found that the mood stabilizer carbamazepine upregulated A1 receptor expression by astrocytes in vitro (Biber, 1999). Notably, in an attempt to harness these mechanisms for synthesis of novel therapeutic agents, A1 adenosine receptor agonists have been synthesized and demonstrate potential for binding to brain tissue in vitro (Maillard et al., 1994) and in animal models of mood disorders. However, these compounds have only been assessed for potential anti-convulsive (Tosh et al., 2012) and ischemic protective effects (Von Lubitz et al., 1996) and have not yet been applied to animal models of mood and behavior. Given these findings, studies are being pursued using pharmacologic agents that work through the A1 receptor.
2.3.2 A2A receptors
Several preclinical studies also support the involvement of A2A receptors in mood disorders and related symptoms. For example, various adenosine receptor knockout studies, mostly specific to the A2A receptor, support adenosinergic involvement in neuroplasticity, the sleep-wake cycle, motor function, cognition, and emotion-related behaviors (reviewed by (Wei et al., 2011)). A2A receptor knockout mice were less likely to display depressive behaviors in the tail suspension test than control animals (El Yacoubi et al., 2001). A2A receptor antagonism and knockout increased motor motivational and goal-directed behavior, respectively (El Yacoubi et al., 2001; O’Neill and Brown, 2006; Short et al., 2006; Pardo et al., 2013). In addition, A2A receptor antagonism was found to be responsible for the arousal effects of caffeine (El Yacoubi et al., 2003; Lazarus et al., 2011). Interestingly, A2A receptor knockout effects may be specific to brain region. For instance, A2A knockout to the striatum affected habit formation and increased psychomotor activity (Shen et al., 2008), but activity was decreased in extrastriatal or forebrain A2A knockouts (Shen et al., 2008; Yu et al., 2009).
Studies also suggest A2A involvement in mania. A2A agonism decreased amphetamine-induced motor activity and behavioral unrest in animal models (reviewed in (Fredholm et al., 2005)). In contrast, A2A antagonism both increased motor behavior in mice and overcame the reduction of motor activity induced by dopamine receptor antagonism (Pardo et al., 2013). A2A antagonism also countered the activity of clozapine (an antipsychotic medication) (Pinna et al., 1999), and A2A knockout animals showed modulations of dopaminergic effects (Seeman et al., 2006). A2A receptor knockout animals also showed blunted response to psychostimulants (Chen et al., 2000). In addition, interrelationships between A2A and dopamine D1 receptor activity have been reported in motivational behavior (Short et al., 2006). These findings, which implicate the purinergic system and its interaction with dopaminergic modifications, should be considered in the development of antipsychotic and antimanic therapies.
2.4 Guanine
Some models have suggested that the purine guanine may play a role in mood disorders. Its signaling effects were classified in three broad categories: glutamatergic inhibition (particularly in astrocytes), impact on memory and behavior, and trophic effects on neuronal cells (Schmidt et al., 2007). Guanine is believed to have neuroprotective effects due to glutamate inhibition (Porciuncula et al., 2002). Evidence for memory impairment comes from animal studies, which, among other findings, demonstrated that guanine inhibits recall of an avoidance task in rodents (Vinade et al., 2004). This mechanism may account for the cognitive and memory impairment symptoms seen in various mood disorders. Guanine may also be involved in the motor symptoms of mood disorders such as BD. However, these effects are likely independent of A1 and A2A receptor activity. Caffeine, an adenosine antagonist, did not antagonize the effects of guanosine (a guanine-based purine) in mice with induced seizures or in an amnesic state (Schmidt et al., 2007). When guanine-monophosphate was administered to rats, they showed evidence of increased anxiety-like behaviors when presented with behavioral tasks, including an elevated plus maze task and exposure to light and open field (Almeida et al., 2010).
Taken together, these findings suggest that P2 receptor activation and decreased adenosine concentrations could contribute to the development of a mood disorder. Consequently, therapeutic interventions might be aimed at inhibiting P2 receptor activity, while inhibition of adenosine deaminase would result in beneficial adenosine accumulation and adenosine A1 receptor activation (see also Table 1).
Table 1.
Purinergic Therapeutic Targets Identified via Clinical and Preclinical Studies
| Target | Mechanism | Clinical | Preclinical | Study design | Authors | |
|---|---|---|---|---|---|---|
| Antidepressant/Anxiolytic Agents | ||||||
| A1 receptor | Agonism | X | a | Transgenic mice | (Gimenez-Llort et al., 2002; Lang et al., 2003) | |
| A1 receptor | Agonism | a | Chemical agonism in mice | (Prediger et al., 2004) | ||
| A2A receptor | Antagonism | X | Chemical antagonism in mice | (El Yacoubi et al., 2001; El Yacoubi et al., 2003; Hodgson et al., 2009) | ||
| P2X7 receptor | Antagonism | X | Chemical antagonism in mice | (Pereira et al., 2013) | ||
| Adenosine deaminase (ADA) | Inhibition | X | Chemical antagonism in mice | (Kaster et al., 2013) | ||
| Inosine | Agonism | X | Chemical agonism in mice | (Kaster et al., 2004; Kaster et al., 2013) | ||
| Antagonism | X | a | Chemical antagonism in rats | (Wagner and Katz, 1983) | ||
| P2X4 receptor | Inhibition (allosteric modulation) | X | a | Chemical antagonism in mice and transgenic mice | (Bortolato et al., 2013) | |
| Antimanic Agents | ||||||
| Xanthine oxidase (XO) | Inhibition | X | Double-blinded placebo controlled studies | (Akhondzadeh et al., 2006; Machado-Vieira et al., 2008; Chan et al., 2010) | ||
| Adenosine non-specific | Agonism | X | Case reports | (Tondo and Rudas, 1991; Ogawa and Ueki, 2003; Hedges et al., 2009) | ||
a = anxiolytic
3. Human Studies
Various human studies implicate purinergic dysfunction in mood disorders. Regulation of the purinergic system has been associated with severe mood changes; specifically, altered genetic expression as well as peripheral and central biomarkers have been identified in individuals with mood disorders. On a molecular level, evidence of dysfunctional adenosine receptor downstream signaling in BD was demonstrated by studies showing altered cAMP, protein kinase C (PKC), and intracellular calcium levels in manic patients (reviewed in (Machado-Vieira et al., 2002)). To better understand the specific pathologies in this system, genetic analyses of purinergic gene expression and single nucleotide polymorphisms (SNPs) were conducted that indeed show an association with increased risk for mood disorders. Metabolites and markers of purinergic activity have also been identified in the periphery of these patients, and new pharmacological approaches with purinergic modulators have been attempted. Finally, imaging studies that allow for visualization of ATP metabolism and P2X7 receptor activity in the brain have also demonstrated unique findings when studying populations of depressed individuals.
3.1 Protein Expression and Genetic Studies
Diverse genetic studies have implicated purinergic targets in mood disorders. Zhang and colleagues found that low P2X7 transcription was associated with MDD, and lower mRNA expression was found in postmortem studies of victims of suicide (Zhang et al., 2011). Other studies found increased mRNA expression of P2 receptors, including the P2X7 subtype, in patients with treatment-resistant depression (Iacob et al., 2013) as well as in BD patients exposed to sleep deprivation (Backlund et al., 2012). Iacob and colleagues suggested a secondary compensatory mechanism of increased receptor expression to initially decreased purinergic activity (Iacob et al., 2013).
SNP variations in genes coding for purinergic system proteins play functional roles in individuals with mood disorders (summarized in Table 2). Twelve studies found an association between SNPs in the P2X7 gene and mood disorders: nine with the Gln460Arg allelic variation (rs2230912) (Barden et al., 2006; Lucae et al., 2006; Nagy et al., 2008; Hejjas et al., 2009; McQuillin et al., 2009; Soronen et al., 2011; Backlund et al., 2012; Mantere et al., 2012; Halmai et al., 2013), one with the His155Tyr (rs208294) allelic variation (Soronen et al., 2011), one with a SNP located in the 3′ untranslated region (rs1653625) (Halmai et al., 2013), and one with a trend toward significance with the Thr348Ala allele (rs1718119) (Erhardt et al., 2007). Interestingly, the study by Mantere and colleagues (2012) demonstrated that the P2X7 receptor subunit Gln460Arg polymorphism was specifically associated with neuroticism in the context of mood disorders. The Thr348Ala allele has been correlated with symptoms of mania including cognitive impairment, distractibility, and talkativeness (Backlund et al., 2011). Nevertheless, other studies found no correlation between P2X7 SNPs and mood disorders (Table 2).
Table 2.
Single nucleotide polymorphisms (SNPs) in purinergic genes implicated in mood disorders
| Genetic Variant and Poly- morphism |
Findings | Sample size |
Mood Disorders |
Correc- tions |
Gene coverage |
Subject criteria |
Study type & phenotype definition |
Fin- dings |
References |
|---|---|---|---|---|---|---|---|---|---|
| P2X7 | |||||||||
|
P2X7: rs2230912 Gln460Arg |
Not associated with diagnosis. BD subjects with at least one allele had higher symptom severity | 315 | BD and MDD | Bonferroni; adjusted for gender on HADS | GWAS (assessed multiple genotypes) | DSM-IV by psychiatrists | Case-control, symptom severity (MADRS and HDRS scores) | +/− | (Halmai et al., 2013) |
| Associated with length of current mood episode, which was moderated by neurotic traits | 424 | BD and MDD | Based on (Soronen et al., 2011) | Based on (Soronen et al., 2011); gene specific | Semi-structured interview | Length of episode moderated by neurotic traits | + | (Mantere et al., 2012) | |
| Associated with rapid cycling BD diagnosis | 690 (n=569 BD-I, n=121 rapid cycling) | BD only | Correction by permutation tests; gender-confounder adjusted | 3 SNPs | Lifetime assessment for specific symptoms, medical records, and interviews | Manic symptoms, then case-case | + | (Backlund et al., 2011) | |
|
450 (n=178 BD, n=272 MDD) | BD and MDD | Gender and age adjustment | Specific allelic association analyses | Semi-structured interview | Case-control, post-hoc by diagnosis, family history, duration of illness | + | (Soronen et al., 2011) | |
|
457 | Depression (including MDD, dysthymia, and mixed anxiety depression) | Adjusted for gender and age | Specific allelic association analysis | DSM-IV by MDI | Case-control; multiple scales (anxiety, obsessive- compulsive, depression, eating disorders, disability, alcohol abuse, well-being, environmental risks) | − | (Lavebratt et al., 2010) | |
| Associated with diagnosis of BD | 604 | BD only | Not corrected | Specific allelic association analysis | Structured interview | Case-control | + | (McQuillin et al., 2009) | |
| Associated with increased symptom severity (both depression and anxiety) in both BD and MDD | 171 | BD and MDD | Adjusted for gender and age | Specific allelic association analysis | DSM-IV | Case control, then symptom severity (HADS) | + | (Hejjas et al., 2009) | |
| Not associated with diagnosis | 1445 (European cohort) | BD and MDD | Corrected by permutations | Single marker association analysis | Structured interview | Case-control | − | (Grigoroiu-Serbanescu et al., 2009) | |
| Not associated with diagnosis | 1723 (n=687 BD, n=1036 MDD) | BD and MDD | Bonferroni; adjusted for gender and age | Specific allelic association analysis | DSM-IV and semi-structured interview | Case-control, familial study | − | (Green et al., 2009) | |
| Associated with increased symptom severity | 218 | Diabetics with mixed symptoms of anxiety and depression | False discovery rate correction | Specific allelic association analysis | Symptoms assessed by HADS | Case-case, symptom severity | + | (Nagy et al., 2008) | |
| Associated with diagnosis of depression | 1000 | MDD only | False discovery rate correction, then correction by permutation | Specific allelic association analysis | DSM-IV and semi-structured interview | Case-control | + | (Lucae et al., 2006) | |
| Associated with diagnosis of BD | 485 | BD only | Adjusted p-value in stats package vs. expected frequency of alleles | Specific allelic association analysis, linkage analysis | Structured interview | Case-control, familial study | + | (Barden et al., 2006) | |
|
P2X7: rs208294 His155Tyr |
|
450 | BD and MDD | Adjusted for gender and age | Specific allelic association analysis | Semi-structured interview | Case-control, post-hoc by diagnosis, family history, duration of illness | + | (Soronen et al., 2011) |
|
613 (n=218 with MDD) | MDD only | Adjusted for gender and age | Specific allelic association analyses | DSM-IV, MADRS | Case-control, Case-case, MADRS score change | − | (Viikki et al., 2011) | |
|
P2X7: rs171811 Thr348Ala |
Associated with symptoms of cognitive impairment, distractibility, talkativeness, and thought disorder | 690 | BD only | Correction by permutation tests, adjusted for gender | 3 SNPs | Lifetime assessment for specific symptoms, medical records, and interviews | Case-case by manic symptoms | + | (Backlund et al., 2011) |
| Trend towards an association with symptom severity for panic and agoraphobia | 179 pts, 462 controls | Anxiety disorders (including panic disorder and agoraphobia) | Correction by permutation tests, adjusted for gender | 25 SNPs | Structured interviews | Case-control, symptom severity by panic and agoraphobia scale | ~ | (Erhardt et al., 2007) | |
|
P2X7: rs1653625 AA (not AC or CC) in the 3′ un- translated region of the P2RX7 gene |
|
315 (n=195 MDD, n=120 BD) | BD and MDD | Bonferroni and adjusted for gender on HADS | GWAS (assessed multiple genotypes) | DSM-IV by psychiatrists | Case-control, symptom severity (MADRS and HDRS score) | −/~ | (Halmai et al., 2013) |
| Other Purinergic SNPs | |||||||||
|
A2aAR: 1976C/T A2A receptor |
Not associated with a mood disorder diagnosis | 192 patients (408 total) | BD and MDD | No | Single marker association analysis | DSM-IV criteria | Case-control | − | (Tsai et al., 2006) |
| Not associated with a diagnosis of panic disorder | 104 pts, 192 controls (Chinese cohort) | Panic disorder | No | Single marker association analysis | Semi- structured interview | Case-control | − | (Lam et al., 2005) | |
| Associated with symptoms of panic disorder and agorophobia | 70 subjects from pedigree, 83 familialtrios | Panic disorder | No | Haplotype combinations of 14 SNPs | Semi-structured interview | Familial pedigree study, panic disorder and agoraphobia scale | + | (Hamilton et al., 2004) | |
| Associated with greater anxiety after caffeine challenge (also with the 2592T/T A2A receptor SNP) | 100 | No mood disorder history | No | 4 loci of the A2A receptor | Telephone and in person screen | All healthy, double-blind study | + | (Alsene et al., 2003) | |
| Mito-chondrial DNA 5178 |
|
145 patients, 184 controls | BD only | No | Single marker association analysis | DSM-IV by two psychiatrists | Case-control | + | (Kato et al., 2000) |
|
SLC29A3, rs12256138 Variation in nucleoside (including adenosine) transporter gene transport |
Associated with a diagnosis of depression or sleep disturbance | 1423 (cohort of Finnish women) | MDD and symptoms of sleep disturbance | Bonferroni and adjusted for gender | 117 SNPs from 13 genes | Structured interview | Case-control | + | (Gass et al., 2010) |
|
GNB3, C825T poly-morphism G-protein B3 subunit |
Associated with antidepressant response | Meta-analysis | Depression (varied) | Varied | Single marker association analysis | Varied | Varied | + | (Klenke et al., 2011) |
indicates a positive association between the SNP and disease risk or a related phenotype
indicates no association between the SNP and disease risk or a related phenotype
Trend towards an association between the SNP and disease risk or a related phenotype
Abbreviations: BD: bipolar disorder; MDD: major depressive disorder; SNP: single nucleotide polymorphism; HDRS: Hamilton Depression Rating Scale, MADRS: Montgomery-Asberg Depression Rating Scale; MDI: Major Depressive Inventory; HADS: Hospital Anxiety and Depression Scale; GWAS: genome-wide association study; SSRI: selective serotonin reuptake inhibitor; ECT: electroconvulsive therapy.
By assessing the characteristics of the studies outlined in Table 2, some overall conclusions can be drawn. First, the Gln460Arg (rs2230912) polymorphism has been studied in nine case-control studies, two case-case studies, and one cohort study. Though sample sizes varied, the studies with the largest sample sizes (three studies n > 1000 subjects) were case-control studies (Lucae et al., 2006; Green et al., 2009; Grigoroiu-Serbanescu et al., 2009). All but three studies (two in BD and one in MDD) found no association with mood disorders (either BD or MDD) (Barden et al., 2006; Lucae et al., 2006; McQuillin et al., 2009). Five studies found an association with symptom profile (Hejjas et al., 2009; Backlund et al., 2011; Soronen et al., 2011; Mantere et al., 2012; Halmai et al., 2013). Notably, only two studies found no association with symptom profile, and in both cases this was in subjects with depression (Lavebratt et al., 2010; Halmai et al., 2013). Collectively, this demonstrates substantial evidence suggesting that the Gln460Arg SNP is associated with mood disorders and, specifically, with BD symptom severity or profile (such as length of mood episode). Given that only one study to date found an association between symptom profile and any other P2X7 gene polymorphism (His155Tyr SNP (rs208294)), it is likely that the Gln460Arg polymorphism is the most significant genetic predisposition to purinergic dysfunction and mood disorders. Overall, the findings suggest a likely association between the Gln460Arg P2X7 gene polymorphism and state (symptom severity or profile), but not trait (diagnosis). Similarly, though it has not been frequently studied, the 348Ala SNP has been associated with BD and anxiety disorders (panic and agoraphobia), though only the anxiety disorders study was of a case-control design (Erhardt et al., 2007; Backlund et al., 2011).
In addition, two case-control studies, one familial study, and one study of healthy subjects investigating an A2A receptor polymorphism (1976C/T) found a possible association with anxiety disorders but not depression (Alsene et al., 2003; Hamilton et al., 2004; Lam et al., 2005; Tsai et al., 2006).
Other purinergic system genes have also been associated with mood disorders. A SNP coding for mitochondrial DNA was found to be associated with BD as well as brain pH of BD subjects (Hamilton et al., 2004). A gene encoding SCL29A3, an adenosine transporter, was associated with depression and sleep disturbance (Gass et al., 2010). However, since each of these SNPs has only been identified in one study, more research on these potential novel purinergic targets is warranted.
These studies point to the involvement of chromosome 12q24 in mood disorders (Curtis et al., 2003; Ewald et al., 2003; Shink et al., 2005; Barden et al., 2006; Christiansen et al., 2009; Green et al., 2009; McQuillin et al., 2009). P2X7 genes have also been associated with inflammation, immune function, and neuronal survival (Harvey et al., 2007), all of which are involved in the pathophysiology of mood disorders. Although the findings of genetic studies vary, many indicate that the P2X7 receptor gene on this chromosome is the source of a susceptibility locus for mood disorders, but its functional role in mood disorders is still under investigation.
A variety of findings have linked the rs2230912 polymorphism with clinical outcomes (Halmai et al., 2013), however, it is possible that these polymorphisms may be associated with severity of depression rather than with risk for mood disorders (Halmai et al., 2013). It is also possible that inheritance is multifactorial and these polymorphisms only indicate increased risk to developing depressive illness through the rs208294 SNP (Soronen et al., 2011). Taken together, these findings suggest that the P2X7 receptor gene plays a potential key role in the pathophysiology of mood disorders.
3.2 Imaging Studies
Imaging studies have shown changes in brain compounds associated with purinergic system dysfunction in subjects with mood disorders. Because the high energy bonds to ATP are composed of phosphorous, quantification of its presence allows the identification of ATP activity in various brain regions. The magnetic properties of nuclei, such as that of phosphorous-31 (an isotope of phosphorous), are used in magnetic resonance spectroscopy (MRS) (Prichard et al., 1999). PET compounds with an affinity for adenosine receptors have also been developed (Bauer and Ishiwata, 2009) and could allow the evaluation of purinergic involvement in psychiatric disorders. The focus of ligand development is on molecules that can penetrate the blood brain barrier, such as those with xanthine-related structures. More than six chemicals fitting this structure have been identified as possible A1 receptor ligands, and 12 have been identified as possible A2A receptor ligands, many of them derived from similar chemicals as the A1 receptor ligands (Bauer and Ishiwata, 2009). For example, one recent study demonstrated the potential efficacy of [18]F-CPFPX, an A1 receptor ligand, by visualizing cerebral caffeine binding (Elmenhorst et al., 2012). No clinical studies in mood disorders have yet been completed using these ligands.
Initial studies using Phosphorous-31 (31P) MRS showed elevated 31P incorporation in manic patients treated with lithium (Kato et al., 1991; Kato et al., 1993) and diminished levels of phosphorous compounds during depressive episodes (Kato et al., 1992; Kato et al., 1995). Since then, other investigators have used 31P imaging to confirm abnormal phosphorus metabolism in subjects with BD (Deicken et al., 1995) and MDD (Moore et al., 1997), suggesting mitochondrial dysfunction related to low ATP levels. Furthermore, other studies have noted elevated levels of phosphate compounds in MDD patients after treatment of a major depressive episode (Forester et al., 2009), and a decrease after treatment with choline in BD (Lyoo et al., 2003). Notably, no differences in brain 31P PET were seen in sleep deprivation studies.
Subsequently, numerous studies identified abnormal ATP metabolism in mood disorders (Regenold et al., 2012). One MRS study found no difference when assessing purine levels in depressed and healthy individuals, but did note a difference in females who responded to treatment, indicating the potential involvement of adenosine in the efficacy of antidepressant treatment (Renshaw et al., 2001). Similar findings have been seen in adolescents and children (Kondo et al., 2011; Shi et al., 2012; Sikoglu et al., 2013; Weber et al., 2013). Most of these studies were small and require further investigation. Recently, PET has been used to study microglial and astrocyte metabolism and activity in the CNS through ligands to translocator protein (TSPO) (Brown et al., 2007; Zhang et al., 2007; Martin et al., 2011). Considering that microglia and astrocytes are a major source of purinergic receptor expression and related activity in the CNS, these studies may help us understand the interaction between purinergic and microglial dysfunction in the etiology of mood disorders.
3.3 Purinergic Biomarkers
Purinergic system biomarkers include enzymes of ATP metabolism (eg, XO, adenosine deaminase (ADA) and CD73), xanthines, uric acid, and products of their metabolism (eg, ATP levels). Early studies conducted in the 1960s and 1970s implicated purinergic system dysfunction in BD (Anumonye et al., 1968; Brown et al., 1972; Jenner et al., 1972; Hansen and Dimitrakoudi, 1974). Subsequently, CSF levels of the purine metabolites hypoxanthine and xanthine were linked with depressive symptoms in MDD. Lower CSF xanthine levels were associated with depressive symptoms, and higher levels were associated with poor appetite, which was associated with activation of the monoaminergic system (Agren et al., 1983; Niklasson et al., 1983). A more recent study identified significantly elevated levels of both serum ADA and XO levels in MDD subjects compared with healthy subjects (Herken et al., 2007). In that study, increased ADA levels were inversely correlated with duration of illness; levels increased with antidepressant treatment, suggesting that ADA levels may be an acute biomarker of disease. In addition, XO levels decreased with antidepressant treatment, suggesting that dysfunctional purinergic activity in depression is affected by pharmacologic intervention.
Uric acid has been linked to many physiological functions including sleep, locomotion, cognitive function, appetite, and socialization. It has also been implicated in the pathophysiology and therapeutics of mood disorders, particularly impulsivity and excitement-seeking behavior (Machado-Vieira et al., 2002; Sutin et al., 2014). Elevated uric acid levels—which suggest increased purinergic transformation—have been observed in acute mania. In addition, these levels were positively correlated with symptom severity and with the improvement of manic symptoms in diverse studies (De Berardis et al., 2008; Machado-Vieira et al., 2008; Salvadore et al., 2010). It should also be noted that peripheral uric acid levels correlate positively with central levels (Bowman et al., 2010); recently, plasma uric acid levels were found to be higher in BD patients than controls (Kesebir et al., 2014). As a result of these findings, testing for uric acid levels has been proposed as a screening test in acute mania (Machado-Vieira, 2012).
Uric acid levels have also been correlated with specific character traits including drive and disinhibition, both of which are common in mania. However, at least one study found no differences in uric acid levels between BD subjects and healthy controls (Wen et al., 2012). Uric acid, as one of a cluster of urinary metabolites, was correlated with first depressive episode in drug-naïve MDD subjects (Zheng et al., 2013). Another study compared healthy controls and MDD patients and found that lower levels of uric acid were associated with MDD (Chaudhari et al., 2010); indeed, the authors noted a strong inverse relation between Hamilton Depression Rating Scale (HDRS) scores and uric acid levels after 12 weeks of antidepressant treatment (Chaudhari et al., 2010). Interestingly, in another study, subjects with MDD showed lower plasma uric acid levels than healthy controls and BD patients (Kesebir et al., 2014). A smaller study also found that lower levels of uric acid were associated with MDD compared to other psychiatric conditions; these normalized after five weeks of antidepressant treatment, though no significant differences were observed between those with depressive and anxiety disorders (Wen et al., 2012). Moreover, selective serotonin reuptake inhibitors (SSRIs) modulated peripheral ADA and XO levels in individuals with MDD (Herken et al., 2007), and adenosine levels increased in individuals treated with citalopram (Blardi et al., 2005).
Finally, a large study found that prevalence of social phobia was inversely associated with moderately elevated levels of serum uric acid (Lyngdoh et al., 2013). The variability of these diverse findings may be due to the possibility that the relationship between peripheral uric acid levels and mood disorders is state- rather than trait-dependent (Kesebir et al., 2013). For instance, uric acid levels are higher in manic patients than in those with bipolar depression. In the study by Kesebir and colleagues, a positive correlation was identified between hyperthymic and irritable temperament scores and uric acid levels across the total sample of MDD and BD patients (Kesebir et al., 2014). Taken together, the evidence presented above suggests that uric acid, and perhaps other purinergic system biomarkers, may be used in the clinical assessment of patients with mood disorders.
4. Systemic Purinergic Dysfunction: Mood Disorders & Medical Comorbidities
The medical conditions most commonly comorbid with mood disorders may reveal important connections with their underlying biochemical imbalances. A nationwide study recently confirmed that patients with BD were at greater risk of developing gout than healthy matched controls, further linking uric acid imbalance with the etiology of BD (Chung et al., 2010). BD is also comorbid with many medical conditions of metabolic dysfunction including obesity, thyroid disease, cardiovascular disease, and diabetes (Hamdani et al., 2013), which implicates dysfunction of energy systems and metabolism in the etiology of BD. The links between BD and Lesch-Nyhan disease—a congenital deficiency of hypoxanthine-guanine phosphoribosyltransferase (HPST), an enzyme that regenerates purines in the purine salvage pathway (Jinnah, 2009)—lends further credence to the notion that purinergic systems play a role in mood, behavior, cognitive function, and motor activity in humans. Lesch-Nyhan disease is characterized by hyperurecemia leading to gout, neurodevelopmental delay, abnormal movements, self-injurious behavior, and mood instability (Kish et al., 1985; Stone et al., 2009), echoing some of the symptoms of mood disorders.
The purinergic system, and particularly the P2X7 receptor, has been implicated in numerous CNS conditions including neurodegenerative and neuroinflammation-related disorders such as multiple sclerosis, pain, seizures, and migraine (Burnstock and Kennedy, 2011) as well as numerous other systemic medical conditions (Vasileiou et al., 2010; Burnstock and Kennedy, 2011). Most of them can be comorbid with MDD or BD, including neurodegenerative disorders and multiple sclerosis (Stone et al., 2009; Hellmann-Regen et al., 2013), migraine (Jerrell et al., 2010; Ligthart et al., 2013), pain (Bair et al., 2003), seizure (Gilliam, 2005), obesity, type 2 diabetes mellitus, endocrine disorders, epilepsy, cardiovascular disorders, asthma, and other psychiatric conditions (Jerrell et al., 2010). The evidence that these conditions involve purinergic system dysfunction through central and peripheral pathological processes supports the hypothesis that mood disorders are systemic illnesses.
5. Treatment-related studies and clinical trials
The purinergic system offers an array of potential therapeutic targets for mood disorders, and clinical trials of pharmacologic agents that target the purinergic system have demonstrated promising results in BD. Potential targets drawn from clinical and preclinical studies are outlined in Table 1. However, most existing clinical trials of purinergic modulation have focused on XO inhibition. These are outlined in Table 3.
Table 3.
Clinical trials of purinergic modulators in bipolar disorder
| Drug | Target or class | State | Mood outcome measure(s) | Other outcomes measured | Design | Findings | Authors |
|---|---|---|---|---|---|---|---|
| Zotepine and haloperidol | Antipsychotics | Acute mania | YMRS | Uric acid levels | Single-blind, comparative |
|
(Chan et al., 2010) |
| Allopurinol with lithium | XO inhibitor as add-on to lithium | Acute mania | YMRS | Uric acid levels | Randomized, double-blind, placebo- controlled trial |
|
(Machado-Vieira et al., 2008) |
| Allopurinol with lithium and haloperidol | XO inhibitor as add-on to lithium plus haloperidol | Acute mania | YMRS | Uric acid levels | Randomized, double-blind, placebo- controlled trial |
|
(Akhondzadeh et al., 2006) |
| Allopurinol (plus complex medical regimens) | XO inhibitor with a mix of antipsychotics, anticonvulsants, antidepressants, lithium, benzodiazepines, and hypnotics | Acute mania | YMRS | None | Case-case study |
|
(Fan et al., 2012) |
| Allopurinol | XO inhibitor add-on to antipsychotics and/or mood stabilizers (polypharmacy, regimen unknown) | Acute mania | YMRS, CGI-BP | None | Multi-center randomized placebo controlled trial |
|
(Weiser et al., 2014) |
| Allopurinol and valproate | XO inhibitor and mood stabilizer | Acute mania | YMRS | Uric acid levels | Randomized, double-blind, placebo-controlled trial |
|
(Jahangard et al., 2014) |
Abbreviations: YMRS: Young Mania Rating Scale; CGI-BP: Clinical Global Impression for Bipolar Disorder; XO: xanthine oxidase.
One study that compared zotepine and haloperidol added to mood stabilizers in the treatment of acute mania found that zotepine significantly decreased uric acid levels; Young Mania Rating Scale (YMRS) ratings were equally improved with both treatments (Chan et al., 2010). This suggests that pharmacologic agents that are able to cross the blood brain barrier and inhibit adenosine metabolism, leading to decreased uric acid levels, may serve as effective treatments for mania and BD. However, not all mood stabilizers may work via this mechanism. Though there are no known studies of uric acid levels in patients with mood disorders taking valproic acid, one study of patients with epilepsy found that valproate did not lower uric acid levels (Ring et al., 1991). It should also be noted that pharmacological agents that modulate purinergic activity, such as caffeine, increase the risk of manic symptoms, severity of seasonal BD, and can induce schizophrenia in susceptible individuals (Tondo and Rudas, 1991; Ogawa and Ueki, 2003; Hedges et al., 2009).
Notably, the anti-gout agent allopurinol is an XO inhibitor that inhibits purine degradation, leading to increased adenosine levels (Pacher et al., 2006). The clinical potential of allopurinol for treating BD was recently emphasized (Bishnoi, 2014). Ten years ago, we delivered the first report on the efficacy of allopurinol in treatment-resistant mania comorbid with hyperuricemia; symptoms improved in two patients, and these improvements were associated with a consistent decrease in uric acid levels (Machado-Vieira et al., 2001). Three randomized, placebo-controlled trials in mania were subsequently conducted. A randomized, double-blind, placebo-controlled trial of allopurinol (600 mg/day) concomitantly with lithium in BD patients significantly alleviated manic symptoms compared to dipyridamole and placebo (Machado-Vieira et al., 2008); these antimanic treatment outcomes correlated with decreased uric acid levels (Machado-Vieira et al., 2008). A similar trial with allopurinol (300mg/day) as an add-on to lithium and haloperidol showed similar results (Akhondzadeh et al., 2006). In addition, a recent pilot study of allopurinol in BD subjects showed that those with restricted caffeine use compared to caffeine users had better outcomes (Machado-Vieira et al., 2008; Fan et al., 2012). It should be noted, however, that this study was small, and that participants were taking a complex medication regimen that included antipsychotics, anticonvulsants, antidepressants, lithium, benzodiazepines, and hypnotics; poor compliance and potential substance abuse were issues of additional concern (Fan et al., 2012). Another recent study found no difference between allopurinol (at a lower dose, 300mg/day) and placebo in BD patients experiencing an acute manic episode. However, these patients were treated with a variety of mood stabilizers and antipsychotics, potentially causing a ceiling effect (Weiser et al., 2014). Unfortunately, the authors provided no information on the number of concomitant medications, dose, or even the duration of treatment with mood stabilizers and antipsychotic medications during the follow-up period (Weiser et al., 2014), which prevents consistent conclusions from being drawn. More recently, a four-week, double-blind, placebo-controlled trial with allopurinol (300mg/day) as an add-on treatment to valproate showed significant antimanic effects compared to placebo (Jahangard et al., 2014). Echoing our previous findings, this study also found an association between lower uric acid levels and symptom improvement at endpoint (Jahangard et al., 2014). Finally, a recent systematic review and meta-analysis of adenosine modulators—with a special focus on allopurinol—supported the efficacy of these agents as adjuvant therapy in bipolar mania compared to placebo (Hirota and Kishi, 2013).
Taken together, the present findings support a role for allopurinol as add-on therapy in mania (see Table 3). To date, no trials of purinergic modulators have been conducted in MDD; however, some promising novel P2X receptor ligands are now in Phase I and II trials (Gunosewoyo and Kassiou, 2010).
6. Discussion
The evidence presented above underscores the manner in which purinergic signaling dysfunction appears to be directly involved in the pathophysiology and therapeutics of mood disorders. As reviewed above, recent findings from animal studies implicate purinergic signaling from the upstream receptor level to downstream behavioral effects. In addition, human genetic and clinical studies further support this hypothesis.
Though human studies have begun to assess clinical outcomes associated with purinergic modulators (for instance, through neuroimaging, genetic, and biomarker studies), future research is needed to explore their relationship to purinergic dysfunction in depth. As regards translational research, the application of new technologies—for instance, epigenetics, proteomics, and molecularly targeted PET imaging—should be included in future studies. In therapeutic applications, more selective purinergic modulators developed for (or tested in) non-psychiatric medical indications should be tested in mood disorders such as BD. For existing purinergic modulators discussed in this review, such as XO inhibitors, additional studies with larger sample sizes are needed, including multi-center clinical trials. Such studies should target a variety of other mood states beyond mania and include dimensions such as suicidality and anhedonia. Finally, such studies should take place in a variety of settings, including outpatient, inpatient, and emergency room environments.
The research conducted to date suggests that various components of purinergic signaling may contribute to the underlying etiology of mood disorders; targeting this system may help us identify therapeutic targets and develop new, improved purinergic modulators for mood disorders.
Research Highlights.
Purines and their metabolites regulate mood, cognition, and behavior
P1 and P2 receptor function and SNPs play a role in mood disorders
We review purinergic pathophysiology in mood disorders
We review the potential therapeutic role of purinergic agents in mood disorders
Acknowledgments
The authors gratefully acknowledge the support and funding of the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). Ioline Henter provided invaluable editorial assistance.
Role of Funding Source
This study was supported in part by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). The NIMH had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Author Contributions
RO: Conducted background research, drafted the review, and substantially edited and revised the manuscript.
RM-V: Conceptualized the study, conducted background research, drafted the review, and substantially edited and revised the manuscript.
HU: Conducted background research, and substantially edited and revised the manuscript.
CAZ: Conducted background research, and substantially edited and revised the manuscript.
Financial Disclosures/Conflict of Interest
The authors gratefully acknowledge the support and funding of the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH). Robin Ortiz was supported through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and by generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Alexandria Real Estate Equities, Inc., Mr. and Mrs. Joel S. Marcus, and the Howard Hughes Medical Institute, as well as other private donors.
Dr. Zarate is listed as a coinventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. The other authors have no conflict of interest to disclose, financial or otherwise.
All authors have seen and approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robin Ortiz, Email: robinortiz.mail@gmail.com.
Henning Ulrich, Email: henning@iq.usp.br.
Carlos A Zarate, Jr, Email: zaratec@mail.nih.gov.
Rodrigo Machado-Vieira, Email: machadovieirar@mail.nih.gov.
References
- Agren H, Niklasson F, Hallgren R. Brain purinergic activity linked with depressive symptomatology: hypoxanthine and xanthine in CSF of patients with major depressive disorders. Psychiatry Res. 1983;9:179–189. doi: 10.1016/0165-1781(83)90042-2. [DOI] [PubMed] [Google Scholar]
- Akhondzadeh S, Milajerdi MR, Amini H, Tehrani-Doost M. Allopurinol as an adjunct to lithium and haloperidol for treatment of patients with acute mania: a double-blind, randomized, placebo-controlled trial. Bipolar Disord. 2006;8:485–489. doi: 10.1111/j.1399-5618.2006.00363.x. [DOI] [PubMed] [Google Scholar]
- Almeida RF, Cereser VH, Jr, Faraco RB, Bohmer AE, Souza DO, Ganzella M. Systemic administration of GMP induces anxiolytic-like behavior in rats. Pharmacol Biochem Behav. 2010;96:306–311. doi: 10.1016/j.pbb.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Alsene K, Deckert J, Sand P, de Wit H. Association between A2a receptor gene polymorphisms and caffeine-induced anxiety. Neuropsychopharmacology. 2003;28:1694–1702. doi: 10.1038/sj.npp.1300232. [DOI] [PubMed] [Google Scholar]
- Anumonye A, Reading HW, Knight F, Ashcroft GW. Uric-acid metabolism in manic-depressive illness and during lithium therapy. Lancet. 1968;1:1290–1293. doi: 10.1016/s0140-6736(68)92300-3. [DOI] [PubMed] [Google Scholar]
- Backlund L, Nikamo P, Hukic DS, Ek IR, Transkman-Bendz L, Landen M, Edman G, Schalling M, Frisen L, Osby U. Cognitive manic symptoms associated with the P2RX7 gene in bipolar disorder. Bipolar Disord. 2011;13:500–508. doi: 10.1111/j.1399-5618.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- Backlund L, Lavebratt C, Frisen L, Nikamo P, Hukic Sudic D, Traskman-Bendz L, Landen M, Edman G, Vawter MP, Osby U, Schalling M. P2RX7: expression responds to sleep deprivation and associates with rapid cycling in bipolar disorder type 1. PLoS One. 2012;7:e43057. doi: 10.1371/journal.pone.0043057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- Baker JG, Hill SJ. A comparison of the antagonist affinities for the Gi- and Gs-coupled states of the human adenosine A1-receptor. J Pharmacol Exp Ther. 2007;320:218–228. doi: 10.1124/jpet.106.113589. [DOI] [PubMed] [Google Scholar]
- Barden N, Harvey M, Gagne B, Shink E, Tremblay M, Raymond C, Labbe M, Villeneuve A, Rochette D, Bordeleau L, Stadler H, Holsboer F, Muller-Myhsok B. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:374–382. doi: 10.1002/ajmg.b.30303. [DOI] [PubMed] [Google Scholar]
- Basso AM, Bratcher NA, Harris RR, Jarvis MF, Decker MW, Rueter LE. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav Brain Res. 2009;198:83–90. doi: 10.1016/j.bbr.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Bauer A, Ishiwata K. Adenosine receptor ligands and PET imaging of the CNS. Handb Exp Pharmacol. 2009:617–642. doi: 10.1007/978-3-540-89615-9_19. [DOI] [PubMed] [Google Scholar]
- Bennett MR. Synaptic P2X7 receptor regenerative-loop hypothesis for depression. Aust N Z J Psychiatry. 2007;41:563–571. doi: 10.1080/00048670701399994. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Wang Q, Ao H, Shoblock JR, Lord B, Aluisio L, Fraser I, Nepomuceno D, Neff RA, Welty N, Lovenberg TW, Bonaventure P, Wickenden AD, Letavic MA. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br J Pharmacol. 2013;170:624–640. doi: 10.1111/bph.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Fiebich BL, Gebicke-Härter P, van Calker D. Carbamazepine-Induced Upregulation of Adenosine A1-Receptors in Astrocyte Cultures Affects Coupling to the Phosphoinositol Signaling Pathway. Neuropsychopharmacology. 1999;20:271–278. doi: 10.1016/S0893-133X(98)00059-1. [DOI] [PubMed] [Google Scholar]
- Bishnoi RJ. Clinical potential of allopurinol in the treatment of bipolar disorder. Indian J Psychol Med. 2014;36:218–220. doi: 10.4103/0253-7176.131008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blardi P, de Lalla A, Urso R, Auteri A, Dell’Erba A, Bossini L, Castrogiovanni P. Activity of citalopram on adenosine and serotonin circulating levels in depressed patients. J Clin Psychopharmacol. 2005;25:262–266. doi: 10.1097/01.jcp.0000161500.58266.90. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Yardley MM, Khoja S, Godar SC, Asatryan L, Finn DA, Alkana RL, Louie SG, Davies DL. Pharmacological insights into the role of P2X4 receptors in behavioural regulation: lessons from ivermectin. Int J Neuropsychopharmacol. 2013;16:1059–1070. doi: 10.1017/S1461145712000909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher AA, Arnold JC, Hunt GE, Spiro A, Spencer J, Brown C, McGregor IS, Bennett MR, Kassiou M. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience. 2011;189:170–177. doi: 10.1016/j.neuroscience.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Shannon J, Frei B, Kaye JA, Quinn JF. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010;19:1331–1336. doi: 10.3233/JAD-2010-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SC, Linn JJ, Disney N. Serotonin, folic acid, and uric acid metabolism in the diagnosis of neuropsychiatric disorders. Biol Psychiatry. 1978;13:671–684. [PubMed] [Google Scholar]
- Brown AK, Fujita M, Fujimura Y, Liow JS, Stabin M, Ryu YH, Imaizumi M, Hong J, Pike VW, Innis RB. Radiation dosimetry and biodistribution in monkey and man of 11C-PBR28: a PET radioligand to image inflammation. J Nucl Med. 2007;48:2072–2079. doi: 10.2967/jnumed.107.044842. [DOI] [PubMed] [Google Scholar]
- Brown BL, Salway JG, Albano JD, Hullin RP, Ekins RP. Urinary excretion of cyclic AMP and manic-depressive psychosis. Br J Psychiatry. 1972;120:405–408. doi: 10.1192/bjp.120.557.405. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- Burnstock G. The purinergic nerve hypothesis. Ciba Found Symp. 1977;48:295–314. doi: 10.1002/9780470720301.ch17. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol. 2013;986:1–12. doi: 10.1007/978-94-007-4719-7_1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in endocrine organs. Purinergic Signal. 2014;10:189–231. doi: 10.1007/s11302-013-9396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. P2X receptors in health and disease. Adv Pharmacol. 2011;61:333–372. doi: 10.1016/B978-0-12-385526-8.00011-4. [DOI] [PubMed] [Google Scholar]
- Cao X, Li LP, Wang Q, Wu Q, Hu HH, Zhang M, Fang YY, Zhang J, Li SJ, Xiong WC, Yan HC, Gao YB, Liu JH, Li XW, Sun LR, Zeng YN, Zhu XH, Gao TM. Astrocyte-derived ATP modulates depressive-like behaviors. Nat Med. 2013;19:773–777. doi: 10.1038/nm.3162. [DOI] [PubMed] [Google Scholar]
- Caporali MG, Popoli P, de Carolis AS. Is the purinergic system involved in the control of pathological movements? Eur J Pharmacol. 1987;137:247–249. doi: 10.1016/0014-2999(87)90229-9. [DOI] [PubMed] [Google Scholar]
- Chan HY, Jou SH, Juang YY, Chang CJ, Chen JJ, Chen CH, Chiu NY. A single-blind, comparative study of zotepine versus haloperidol in combination with a mood stabilizer for patients with moderate-to-severe mania. Psychiatry Clin Neurosci. 2010;64:162–169. doi: 10.1111/j.1440-1819.2010.02066.x. [DOI] [PubMed] [Google Scholar]
- Chaudhari K, Khanzode S, Khanzode S, Dakhale G, Saoji A, Sarode S. Clinical correlation of alteration of endogenous antioxidant-uric acid level in major depressive disorder. Indian J Clin Biochem. 2010;25:77–81. doi: 10.1007/s12291-010-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, Aloyo VJ, Fink JS, Schwarzschild MA. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Christiansen L, Tan Q, Kruse TA, McGue M, Christensen K. Candidate region linkage analysis in twins discordant or concordant for depression symptomatology. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:581–584. doi: 10.1002/ajmg.b.30841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KH, Huang CC, Lin HC. Increased risk of gout among patients with bipolar disorder: a nationwide population-based study. Psychiatry Res. 2010;180:147–150. doi: 10.1016/j.psychres.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Cordeaux Y, Ijzerman AP, Hill SJ. Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br J Pharmacol. 2004;143:705–714. doi: 10.1038/sj.bjp.0705925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crema LM, Pettenuzzo LF, Schlabitz M, Diehl L, Hoppe J, Mestriner R, Laureano D, Salbego C, Dalmaz C, Vendite D. The effect of unpredictable chronic mild stress on depressive-like behavior and on hippocampal A1 and striatal A2A adenosine receptors. Physiol Behav. 2013;109:1–7. doi: 10.1016/j.physbeh.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Csolle C, Baranyi M, Zsilla G, Kittel A, Goloncser F, Illes P, Papp E, Vizi ES, Sperlagh B. Neurochemical Changes in the Mouse Hippocampus Underlying the Antidepressant Effect of Genetic Deletion of P2X7 Receptors. PLoS One. 2013;8:e66547. doi: 10.1371/journal.pone.0066547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Ferre S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D, Kalsi G, Brynjolfsson J, McInnis M, O’Neill J, Smyth C, Moloney E, Murphy P, McQuillin A, Petursson H, Gurling H. Genome scan of pedigrees multiply affected with bipolar disorder provides further support for the presence of a susceptibility locus on chromosome 12q23-q24, and suggests the presence of additional loci on 1p and 1q. Psychiatr Genet. 2003;13:77–84. doi: 10.1097/01.ypg.0000056684.89558.d2. [DOI] [PubMed] [Google Scholar]
- De Berardis D, Conti CM, Campanella D, Carano A, Di Giuseppe B, Valchera A, Tancredi L, Serroni N, Pizzorno AM, Fulcheri M, Gambi F, Sepede G, Moschetta FS, Salerno RM, Ferro FM. Evaluation of plasma antioxidant levels during different phases of illness in adult patients with bipolar disorder. J Biol Regul Homeost Agents. 2008;22:195–200. [PubMed] [Google Scholar]
- Deicken RF, Fein G, Weiner MW. Abnormal frontal lobe phosphorous metabolism in bipolar disorder. Am J Psychiatry. 1995;152:915–918. doi: 10.1176/ajp.152.6.915. [DOI] [PubMed] [Google Scholar]
- Dias RB, Rombo DM, Ribeiro JA, Henley JM, Sebastiao AM. Adenosine: setting the stage for plasticity. Trends Neurosci. 2013;36:248–257. doi: 10.1016/j.tins.2012.12.003. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology. 2003;45:977–985. doi: 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Bertorelli R, Ongini E, Costentin J, Vaugeois JM. Adenosine A2A receptor antagonists are potential antidepressants: evidence based on pharmacology and A2A receptor knockout mice. Br J Pharmacol. 2001;134:68–77. doi: 10.1038/sj.bjp.0704240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Basheer R, McCarley RW, Bauer A. Sleep deprivation increases A(1) adenosine receptor density in the rat brain. Brain Res. 2009;1258:53–58. doi: 10.1016/j.brainres.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmenhorst D, Meyer PT, Matusch A, Winz OH, Bauer A. Caffeine occupancy of human cerebral A1 adenosine receptors: in vivo quantification with 18F-CPFPX and PET. J Nucl Med. 2012;53:1723–1729. doi: 10.2967/jnumed.112.105114. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Lucae S, Unschuld PG, Ising M, Kern N, Salyakina D, Lieb R, Uhr M, Binder EB, Keck ME, Muller-Myhsok B, Holsboer F. Association of polymorphisms in P2RX7 and CaMKKb with anxiety disorders. J Affect Disord. 2007;101:159–168. doi: 10.1016/j.jad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Ewald H, Kruse TA, Mors O. Genome wide scan using homozygosity mapping and linkage analyses of a single pedigree with affective disorder suggests oligogenic inheritance. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:63–71. doi: 10.1002/ajmg.b.20039. [DOI] [PubMed] [Google Scholar]
- Fan A, Berg A, Bresee C, Glassman LH, Rapaport MH. Allopurinol augmentation in the outpatient treatment of bipolar mania: a pilot study. Bipolar Disord. 2012;14:206–210. doi: 10.1111/j.1399-5618.2012.01001.x. [DOI] [PubMed] [Google Scholar]
- Fedorova IM, Jacobson MA, Basile A, Jacobson KA. Behavioral characterization of mice lacking the A3 adenosine receptor: sensitivity to hypoxic neurodegeneration. Cell Mol Neurobiol. 2003;23:431–447. doi: 10.1023/A:1023601007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Wang Y, Simms JA, Yi HY, Li R, Bjeldanes L, Ye C, Bartlett SE. Locomotor activation by theacrine, a purine alkaloid structurally similar to caffeine: involvement of adenosine and dopamine receptors. Pharmacol Biochem Behav. 2012;102:241–248. doi: 10.1016/j.pbb.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Stroh C, Schulze-Osthoff K. P2X7/P2Z purinoreceptor-mediated activation of transcription factor NFAT in microglial cells. J Biol Chem. 1999;274:13205–13210. doi: 10.1074/jbc.274.19.13205. [DOI] [PubMed] [Google Scholar]
- Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–436. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester BP, Harper DG, Jensen JE, Ravichandran C, Jordan B, Renshaw PF, Cohen BM. 31Phosphorus magnetic resonance spectroscopy study of tissue specific changes in high energy phosphates before and after sertraline treatment of geriatric depression. Int J Geriatr Psychiatry. 2009;24:788–797. doi: 10.1002/gps.2230. [DOI] [PubMed] [Google Scholar]
- Franke H, Krugel U, Illes P. P2 receptors and neuronal injury. Pflugers Arch. 2006;452:622–644. doi: 10.1007/s00424-006-0071-8. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T. Roles of dopamine and inflammation-related molecules in behavioral alterations caused by repeated stress. J Pharmacol Sci. 2012;120:63–69. doi: 10.1254/jphs.12r09cp. [DOI] [PubMed] [Google Scholar]
- Gass N, Ollila HM, Utge S, Partonen T, Kronholm E, Pirkola S, Suhonen J, Silander K, Porkka-Heiskanen T, Paunio T. Contribution of adenosine related genes to the risk of depression with disturbed sleep. J Affect Disord. 2010;126:134–139. doi: 10.1016/j.jad.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Gilliam FG. Diagnosis and treatment of mood disorders in persons with epilepsy. Curr Opin Neurol. 2005;18:129–133. doi: 10.1097/01.wco.0000162853.29650.ec. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Fernandez-Teruel A, Escorihuela RM, Fredholm BB, Tobena A, Pekny M, Johansson B. Mice lacking the adenosine A1 receptor are anxious and aggressive, but are normal learners with reduced muscle strength and survival rate. Eur J Neurosci. 2002;16:547–550. doi: 10.1046/j.1460-9568.2002.02122.x. [DOI] [PubMed] [Google Scholar]
- Green EK, Grozeva D, Raybould R, Elvidge G, Macgregor S, Craig I, Farmer A, McGuffin P, Forty L, Jones L, Jones I, O’Donovan MC, Owen MJ, Kirov G, Craddock N. P2RX7: A bipolar and unipolar disorder candidate susceptibility gene? Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1063–1069. doi: 10.1002/ajmg.b.30931. [DOI] [PubMed] [Google Scholar]
- Grigoroiu-Serbanescu M, et al. Variation in P2RX7 candidate gene (rs2230912) is not associated with bipolar I disorder and unipolar major depression in four European samples. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1017–1021. doi: 10.1002/ajmg.b.30952. [DOI] [PubMed] [Google Scholar]
- Gunosewoyo H, Kassiou M. P2X purinergic receptor ligands: recently patented compounds. Expert Opin Ther Pat. 2010;20:625–646. doi: 10.1517/13543771003702424. [DOI] [PubMed] [Google Scholar]
- Halldner L, Aden U, Dahlberg V, Johansson B, Ledent C, Fredholm BB. The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: a study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology. 2004;46:1008–1017. doi: 10.1016/j.neuropharm.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Halmai Z, Dome P, Vereczkei A, Abdul-Rahman O, Szekely A, Gonda X, Faludi G, Sasvari-Szekely M, Nemoda Z. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J Affect Disord. 2013;150:104–109. doi: 10.1016/j.jad.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Hamdani N, Doukhan R, Kurtlucan O, Tamouza R, Leboyer M. Immunity, inflammation, and bipolar disorder: diagnostic and therapeutic implications. Curr Psychiatry Rep. 2013;15:387. doi: 10.1007/s11920-013-0387-y. [DOI] [PubMed] [Google Scholar]
- Hamilton N, Vayro S, Kirchhoff F, Verkhratsky A, Robbins J, Gorecki DC, Butt AM. Mechanisms of ATP- and glutamate-mediated calcium signaling in white matter astrocytes. Glia. 2008;56:734–749. doi: 10.1002/glia.20649. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, De Leon AB, Heiman GA, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA. Evidence for genetic linkage between a polymorphism in the adenosine 2A receptor and panic disorder. Neuropsychopharmacology. 2004;29:558–565. doi: 10.1038/sj.npp.1300311. [DOI] [PubMed] [Google Scholar]
- Hansen O, Dimitrakoudi M. The relation of blood adenosine triphosphate to changes of mood in affective disorders. Br J Psychiatry. 1974;125:268–274. doi: 10.1192/bjp.125.3.268. [DOI] [PubMed] [Google Scholar]
- Harvey M, Belleau P, Barden N. Gene interactions in depression: pathways out of darkness. Trends Genet. 2007;23:547–556. doi: 10.1016/j.tig.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Hedges DW, Woon FL, Hoopes SP. Caffeine-induced psychosis. CNS Spectr. 2009;14:127–129. doi: 10.1017/s1092852900020101. [DOI] [PubMed] [Google Scholar]
- Hejjas K, Szekely A, Domotor E, Halmai Z, Balogh G, Schilling B, Sarosi A, Faludi G, Sasvari-Szekely M, Nemoda Z. Association between depression and the Gln460Arg polymorphism of P2RX7 gene: a dimensional approach. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:295–299. doi: 10.1002/ajmg.b.30799. [DOI] [PubMed] [Google Scholar]
- Hellmann-Regen J, Piber D, Hinkelmann K, Gold SM, Heesen C, Spitzer C, Endres M, Otte C. Depressive syndromes in neurological disorders. Eur Arch Psychiatry Clin Neurosci. 2013;263(Suppl 2):S123–136. doi: 10.1007/s00406-013-0448-6. [DOI] [PubMed] [Google Scholar]
- Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, Kap O, Yumru M, Savas HA, Akyol O. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Herzog AG. Neuroactive properties of reproductive steroids. Headache. 2007;47 (Suppl 2):S68–S78. doi: 10.1111/j.1526-4610.2007.00817.x. [DOI] [PubMed] [Google Scholar]
- Hirota T, Kishi T. Adenosine hypothesis in schizophrenia and bipolar disorder: a systematic review and meta-analysis of randomized controlled trial of adjuvant purinergic modulators. Schizophr Res. 2013;149:88–95. doi: 10.1016/j.schres.2013.06.038. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S, Cohen-Williams ME, Higgins GA, Impagnatiello F, Nicolussi E, Parra LE, Foster C, Zhai Y, Neustadt BR, Stamford AW, Parker EM, Reggiani A, Hunter JC. Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression. J Pharmacol Exp Ther. 2009;330:294–303. doi: 10.1124/jpet.108.149617. [DOI] [PubMed] [Google Scholar]
- Huang ZL, Urade Y, Hayaishi O. The role of adenosine in the regulation of sleep. Curr Top Med Chem. 2011;11:1047–1057. doi: 10.2174/156802611795347654. [DOI] [PubMed] [Google Scholar]
- Iacob E, Light KC, Tadler SC, Weeks HR, White AT, Hughen RW, Vanhaitsma TA, Bushnell L, Light AR. Dysregulation of leukocyte gene expression in women with medication-refractory depression versus healthy non-depressed controls. BMC Psychiatry. 2013;13:273. doi: 10.1186/1471-244X-13-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K. The functions of ATP receptors in the hippocampus. Pharmacol Res. 1998;38:323–331. doi: 10.1006/phrs.1998.0382. [DOI] [PubMed] [Google Scholar]
- Jahangard L, Soroush S, Haghighi M, Ghaleiha A, Bajoghli H, Holsboer-Trachsler E, Brand S. In a double-blind, randomized and placebo-controlled trial, adjuvant allopurinol improved symptoms of mania in in-patients suffering from bipolar disorder. Eur Neuropsychopharmacol. 2014;24:1210–1221. doi: 10.1016/j.euroneuro.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Jenner FA, Sampson GA, Thompson EA, Somerville AR, Beard NA, Smith AA. Manic-depressive psychosis and urinary excretion of cyclic AMP. Br J Psychiatry. 1972;121:236–237. doi: 10.1192/bjp.121.2.236. [DOI] [PubMed] [Google Scholar]
- Jerrell JM, McIntyre RS, Tripathi A. A cohort study of the prevalence and impact of comorbid medical conditions in pediatric bipolar disorder. J Clin Psychiatry. 2010;71:1518–1525. doi: 10.4088/JCP.09m05585ora. [DOI] [PubMed] [Google Scholar]
- Jimenez-Pacheco A, Mesuret G, Sanz-Rodriguez A, Tanaka K, Mooney C, Conroy R, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC, Engel T. Increased neocortical expression of the P2X7 receptor after status epilepticus and anticonvulsant effect of P2X7 receptor antagonist A-438079. Epilepsia. 2013;54:1551–1561. doi: 10.1111/epi.12257. [DOI] [PubMed] [Google Scholar]
- Jinnah HA. Lesch-Nyhan disease: from mechanism to model and back again. Dis Model Mech. 2009;2:116–121. doi: 10.1242/dmm.002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, Escorihuela RM, Fernandez-Teruel A, Wiesenfeld-Hallin Z, Xu XJ, Hardemark A, Betsholtz C, Herlenius E, Fredholm BB. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karve AV, Jagtiani SS, Chitnis KA. Evaluation of effect of allopurinol and febuxostat in behavioral model of depression in mice. Indian J Pharmacol. 2013;45:244–247. doi: 10.4103/0253-7613.111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaster MP, Santos AR, Rodrigues AL. Involvement of 5-HT1A receptors in the antidepressant-like effect of adenosine in the mouse forced swimming test. Brain Res Bull. 2005a;67:53–61. doi: 10.1016/j.brainresbull.2005.05.025. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Ferreira PK, Santos AR, Rodrigues AL. Effects of potassium channel inhibitors in the forced swimming test: possible involvement of L-arginine-nitric oxide-soluble guanylate cyclase pathway. Behav Brain Res. 2005b;165:204–209. doi: 10.1016/j.bbr.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Rosa AO, Santos AR, Rodrigues AL. Involvement of nitric oxide-cGMP pathway in the antidepressant-like effects of adenosine in the forced swimming test. Int J Neuropsychopharmacol. 2005c;8:601–606. doi: 10.1017/S1461145705005316. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Budni J, Santos AR, Rodrigues AL. Pharmacological evidence for the involvement of the opioid system in the antidepressant-like effect of adenosine in the mouse forced swimming test. Eur J Pharmacol. 2007a;576:91–98. doi: 10.1016/j.ejphar.2007.08.026. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Budni J, Binfare RW, Santos AR, Rodrigues AL. The inhibition of different types of potassium channels underlies the antidepressant-like effect of adenosine in the mouse forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2007b;31:690–696. doi: 10.1016/j.pnpbp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett. 2004;355:21–24. doi: 10.1016/j.neulet.2003.10.040. [DOI] [PubMed] [Google Scholar]
- Kaster MP, Budni J, Gazal M, Cunha MP, Santos AR, Rodrigues AL. The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A 2A receptors. Purinergic Signal. 2013;9:481–486. doi: 10.1007/s11302-013-9361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Shioiri T, Takahashi S, Inubushi T. Measurement of brain phosphoinositide metabolism in bipolar patients using in vivo 31P-MRS. J Affect Disord. 1991;22:185–190. doi: 10.1016/0165-0327(91)90064-y. [DOI] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Inubushi T. Brain phosphorous metabolism in depressive disorders detected by phosphorus-31 magnetic resonance spectroscopy. J Affect Disord. 1992;26:223–230. doi: 10.1016/0165-0327(92)90099-r. [DOI] [PubMed] [Google Scholar]
- Kato T, Takahashi S, Shioiri T, Inubushi T. Alterations in brain phosphorous metabolism in bipolar disorder detected by in vivo 31P and 7Li magnetic resonance spectroscopy. J Affect Disord. 1993;27:53–59. doi: 10.1016/0165-0327(93)90097-4. [DOI] [PubMed] [Google Scholar]
- Kato T, Kunugi H, Nanko S, Kato N. Association of bipolar disorder with the 5178 polymorphism in mitochondrial DNA. Am J Med Genet. 2000;96:182–186. [PubMed] [Google Scholar]
- Kato T, Shioiri T, Murashita J, Hamakawa H, Takahashi Y, Inubushi T, Takahashi S. Lateralized abnormality of high energy phosphate metabolism in the frontal lobes of patients with bipolar disorder detected by phase-encoded 31P-MRS. Psychol Med. 1995;25:557–566. doi: 10.1017/s003329170003347x. [DOI] [PubMed] [Google Scholar]
- Kesebir SO, Tatlidil Yaylaci E, Suner O, Gultekin BK. Uric acid levels may be a biological marker for the differentiation of unipolar and bipolar disorder: the role of affective temperament. J Affect Disord. 2014;165:131–134. doi: 10.1016/j.jad.2014.04.053. [DOI] [PubMed] [Google Scholar]
- Kesebir SO, Suner O, Yaylaci ET, Bayrak A, Turan C. Increased uric acid levels in bipolar disorder: is it trait or state? J Biol Regul Homeost Agents. 2013;27:981–988. [PubMed] [Google Scholar]
- Kirshenbaum GS, Saltzman K, Rose B, Petersen J, Vilsen B, Roder JC. Decreased neuronal Na+, K+-ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes Brain Behav. 2011;10:542–550. doi: 10.1111/j.1601-183X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Fox IH, Kapur BM, Lloyd K, Hornykiewicz O. Brain benzodiazepine receptor binding and purine concentration in Lesch-Nyhan syndrome. Brain Res. 1985;336:117–123. doi: 10.1016/0006-8993(85)90422-6. [DOI] [PubMed] [Google Scholar]
- Kittner H, Franke H, Fischer W, Schultheis N, Krugel U, Illes P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology. 2003;28:435–444. doi: 10.1038/sj.npp.1300043. [DOI] [PubMed] [Google Scholar]
- Klenke S, Kussmann M, Siffert W. The GNB3 C825T polymorphism as a pharmacogenetic marker in the treatment of hypertension, obesity, and depression. Pharmacogenet Genomics. 2011;21:594–606. doi: 10.1097/FPC.0b013e3283491153. [DOI] [PubMed] [Google Scholar]
- Kondo DG, Hellem TL, Sung YH, Kim N, Jeong EK, Delmastro KK, Shi X, Renshaw PF. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat. 2011;2011:650450. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E. Manic-Depressive Insanity and Paranoia. Chicago, IL: Chicago Medical Book Company; 1921. [Google Scholar]
- Krugel U, Spies O, Regenthal R, Illes P, Kittner H. P2 receptors are involved in the mediation of motivation-related behavior. Purinergic Signal. 2004;1:21–29. doi: 10.1007/s11302-004-4745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaria A, Tolias CM, Burnstock G. ATP signalling in epilepsy. Purinergic Signal. 2008;4:339–346. doi: 10.1007/s11302-008-9115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P, Hong CJ, Tsai SJ. Association study of A2a adenosine receptor genetic polymorphism in panic disorder. Neurosci Lett. 2005;378:98–101. doi: 10.1016/j.neulet.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Lang UE, Lang F, Richter K, Vallon V, Hipp HP, Schnermann J, Wolfer DP. Emotional instability but intact spatial cognition in adenosine receptor 1 knock out mice. Behav Brain Res. 2003;145:179–188. doi: 10.1016/s0166-4328(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Aberg E, Sjoholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen HY, Cherasse Y, Qu WM, Huang ZL, Bass CE, Winsky-Sommerer R, Semba K, Fredholm BB, Boison D, Hayaishi O, Urade Y, Chen JF. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekic DM. Facilitation and antagonism of lidocaine-kindled seizures: the behavioral feature. Physiol Behav. 1988;42:83–85. doi: 10.1016/0031-9384(88)90264-8. [DOI] [PubMed] [Google Scholar]
- Lewin E, Bleck V. Electroshock seizures in mice: effect on brain adenosine and its metabolites. Epilepsia. 1981;22:577–581. doi: 10.1111/j.1528-1157.1981.tb04129.x. [DOI] [PubMed] [Google Scholar]
- Ligthart L, Hottenga JJ, Lewis CM, Farmer AE, Craig IW, Breen G, Willemsen G, Vink JM, Middeldorp CM, Byrne EM, Heath AC, Madden PA, Pergadia ML, Montgomery GW, Martin NG, Penninx BW, McGuffin P, Boomsma DI, Nyholt DR. Genetic risk score analysis indicates migraine with and without comorbid depression are genetically different disorders. Hum Genet. 2013;133:173–186. doi: 10.1007/s00439-013-1370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Tsai SJ, Hong CJ. Association analysis of a functional G protein beta3 subunit gene polymorphism (C825T) in mood disorders. Neuropsychobiology. 2001;44:118–121. doi: 10.1159/000054929. [DOI] [PubMed] [Google Scholar]
- Lucae S, Salyakina D, Barden N, Harvey M, Gagne B, Labbe M, Binder EB, Uhr M, Paez-Pereda M, Sillaber I, Ising M, Bruckl T, Lieb R, Holsboer F, Muller-Myhsok B. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum Mol Genet. 2006;15:2438–2445. doi: 10.1093/hmg/ddl166. [DOI] [PubMed] [Google Scholar]
- Lyngdoh T, Bochud M, Glaus J, Castelao E, Waeber G, Vollenweider P, Preisig M. Associations of Serum Uric Acid and SLC2A9 Variant with Depressive and Anxiety Disorders: A Population-Based Study. PLoS One. 2013;8:e76336. doi: 10.1371/journal.pone.0076336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Demopulos CM, Hirashima F, Ahn KH, Renshaw PF. Oral choline decreases brain purine levels in lithium-treated subjects with rapid-cycling bipolar disorder: a double-blind trial using proton and lithium magnetic resonance spectroscopy. Bipolar Disord. 2003;5:300–306. doi: 10.1034/j.1399-5618.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R. Purinergic system in the treatment of bipolar disorder: uric acid levels as a screening test in mania. J Clin Psychopharmacol. 2012;32:735–736. doi: 10.1097/JCP.0b013e318268391d. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Therapeutic efficacy of allopurinol in mania associated with hyperuricemia. J Clin Psychopharmacol. 2001;21:621–622. doi: 10.1097/00004714-200112000-00017. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Lara DR, Souza DO, Kapczinski F. Purinergic dysfunction in mania: an integrative model. Med Hypotheses. 2002;58:297–304. doi: 10.1054/mehy.2001.1543. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Soares JC, Lara DR, Luckenbaugh DA, Busnello JV, Marca G, Cunha A, Souza DO, Zarate CA, Jr, Kapczinski F. A double-blind, randomized, placebo-controlled 4-week study on the efficacy and safety of the purinergic agents allopurinol and dipyridamole adjunctive to lithium in acute bipolar mania. J Clin Psychiatry. 2008;69:1237–1245. doi: 10.4088/jcp.v69n0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard MC, Nikodijevic O, LaNoue KF, Berkich D, Ji XD, Bartus R, Jacobson KA. Adenosine receptor prodrugs: synthesis and biological activity of derivatives of potent, A1-selective agonists. J Pharm Sci. 1994;83:46–53. doi: 10.1002/jps.2600830112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder P, Trujillo CA, Lopes CG, Resende RR, Gomes KN, Yuahasi KK, Britto LR, Ulrich H. New insights into purinergic receptor signaling in neuronal differentiation, neuroprotection, and brain disorders. Purinergic Signal. 2007;3:317–331. doi: 10.1007/s11302-007-9074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantere O, Soronen P, Uher R, Ketokivi M, Jylha P, Melartin T, Paunio T, Isometsa E. Neuroticism mediates the effect of P2RX7 on outcomes of mood disorders. Depress Anxiety. 2012;29:816–823. doi: 10.1002/da.21945. [DOI] [PubMed] [Google Scholar]
- Martin A, Boisgard R, Kassiou M, Dolle F, Tavitian B. Reduced PBR/TSPO expression after minocycline treatment in a rat model of focal cerebral ischemia: a PET study using [(18)F]DPA-714. Mol Imaging Biol. 2011;13:10–15. doi: 10.1007/s11307-010-0324-y. [DOI] [PubMed] [Google Scholar]
- McQuillin A, Bass NJ, Choudhury K, Puri V, Kosmin M, Lawrence J, Curtis D, Gurling HM. Case-control studies show that a non-conservative amino-acid change from a glutamine to arginine in the P2RX7 purinergic receptor protein is associated with both bipolar- and unipolar-affective disorders. Mol Psychiatry. 2009;14:614–620. doi: 10.1038/mp.2008.6. [DOI] [PubMed] [Google Scholar]
- Minor TR, Winslow JL, Chang WC. Stress and adenosine: II. Adenosine analogs mimic the effect of inescapable shock on shuttle-escape performance in rats. Behav Neurosci. 1994;108:265–276. doi: 10.1037//0735-7044.108.2.265. [DOI] [PubMed] [Google Scholar]
- Moore CM, Christensen JD, Lafer B, Fava M, Renshaw PF. Lower levels of nucleoside triphosphate in the basal ganglia of depressed subjects: a phosphorous-31 magnetic resonance spectroscopy study. Am J Psychiatry. 1997;154:116–118. doi: 10.1176/ajp.154.1.116. [DOI] [PubMed] [Google Scholar]
- Nagy G, Ronai Z, Somogyi A, Sasvari-Szekely M, Rahman OA, Mate A, Varga T, Nemoda Z. P2RX7 Gln460Arg polymorphism is associated with depression among diabetic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1884–1888. doi: 10.1016/j.pnpbp.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Niklasson F, Agren H, Hallgren R. Purine and monoamine metabolites in cerebrospinal fluid: parallel purinergic and monoaminergic activation in depressive illness? J Neurol Neurosurg Psychiatry. 1983;46:255–260. doi: 10.1136/jnnp.46.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Gene, brains, and environment-genetic neuroimaging of depression. Curr Opin Neurobiol. 2013;23:133–142. doi: 10.1016/j.conb.2012.08.004. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of the adenosine A(2A) antagonist KW-6002 on motor and motivational processes in the rat. Psychopharmacology (Berl) 2006;184:46–55. doi: 10.1007/s00213-005-0240-z. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Ueki H. Secondary mania caused by caffeine. Gen Hosp Psychiatry. 2003;25:138–139. doi: 10.1016/s0163-8343(02)00273-6. [DOI] [PubMed] [Google Scholar]
- da Oliveira RL, Seibt KJ, Rico EP, Bogo MR, Bonan CD. Inhibitory effect of lithium on nucleotide hydrolysis and acetylcholinesterase activity in zebrafish (Danio rerio) brain. Neurotoxicol Teratol. 2011;33:651–657. doi: 10.1016/j.ntt.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev. 2006;58:87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Muller CE, Salamone JD, Correa M. Effect of subtype-selective adenosine receptor antagonists on basal or haloperidol-regulated striatal function: studies of exploratory locomotion and c-Fos immunoreactivity in outbred and A(2A)R KO mice. Behav Brain Res. 2013;247:217–226. doi: 10.1016/j.bbr.2013.03.035. [DOI] [PubMed] [Google Scholar]
- Paul S, Elsinga PH, Ishiwata K, Dierckx RA, van Waarde A. Adenosine A(1) receptors in the central nervous system: their functions in health and disease, and possible elucidation by PET imaging. Curr Med Chem. 2011;18:4820–4835. doi: 10.2174/092986711797535335. [DOI] [PubMed] [Google Scholar]
- Pereira VS, Casarotto PC, Hiroaki-Sato VA, Sartim AG, Guimaraes FS, Joca SR. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: Involvement of nitric oxide. Eur Neuropsychopharmacol. 2013;23:1769–1778. doi: 10.1016/j.euroneuro.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Phillis JW. Potentiation of the action of adenosine on cerebral cortical neurones by the tricyclic antidepressants. Br J Pharmacol. 1984;83:567–575. doi: 10.1111/j.1476-5381.1984.tb16521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Kostopoulos GK. Adenosine as a putative transmitter in the cerebral cortex. Studies with potentiators and antagonists. Life Sci. 1975;17:1085–1094. doi: 10.1016/0024-3205(75)90329-x. [DOI] [PubMed] [Google Scholar]
- Pinna A, Wardas J, Cozzolino A, Morelli M. Involvement of adenosine A2A receptors in the induction of c-fos expression by clozapine and haloperidol. Neuropsychopharmacology. 1999;20:44–51. doi: 10.1016/S0893-133X(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Popoli P, Benedetti M, Scotti de Carolis A. Anticonvulsant activity of carbamazepine and N6-L-phenylisopropyladenosine in rabbits. Relationship to adenosine receptors in central nervous system. Pharmacol Biochem Behav. 1988;29:533–539. doi: 10.1016/0091-3057(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Porciuncula LO, Vinade L, Wofchuk S, Souza DO. Guanine based purines inhibit [(3)H]glutamate and [(3)H]AMPA binding at postsynaptic densities from cerebral cortex of rats. Brain Res. 2002;928:106–112. doi: 10.1016/s0006-8993(01)03368-6. [DOI] [PubMed] [Google Scholar]
- Potucek YD, Crain JM, Watters JJ. Purinergic receptors modulate MAP kinases and transcription factors that control microglial inflammatory gene expression. Neurochem Int. 2006;49:204–214. doi: 10.1016/j.neuint.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Prediger RD, Batista LC, Takahashi RN. Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol. 2004;499:147–154. doi: 10.1016/j.ejphar.2004.07.106. [DOI] [PubMed] [Google Scholar]
- Prichard JW, Alger JR, Turner R. Brain imaging: the NMR revolution. Interview by Clare Thompson. BMJ. 1999;319:1302. doi: 10.1136/bmj.319.7220.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenold WT, Pratt M, Nekkalapu S, Shapiro PS, Kristian T, Fiskum G. Mitochondrial detachment of hexokinase 1 in mood and psychotic disorders: implications for brain energy metabolism and neurotrophic signaling. J Psychiatr Res. 2012;46:95–104. doi: 10.1016/j.jpsychires.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Renshaw PF, Parow AM, Hirashima F, Ke Y, Moore CM, de Frederick BB, Fava M, Hennen J, Cohen BM. Multinuclear magnetic resonance spectroscopy studies of brain purines in major depression. Am J Psychiatry. 2001;158:2048–2055. doi: 10.1176/appi.ajp.158.12.2048. [DOI] [PubMed] [Google Scholar]
- Ring HA, Heller AJ, Marshall WJ, Johnson AL, Reynolds EH. Plasma uric acid in patients receiving anticonvulsant monotherapy. Epilepsy Res. 1991;8:241–244. doi: 10.1016/0920-1211(91)90070-v. [DOI] [PubMed] [Google Scholar]
- Sadek AR, Knight GE, Burnstock G. Electroconvulsive therapy: a novel hypothesis for the involvement of purinergic signalling. Purinergic Signal. 2011;7:447–452. doi: 10.1007/s11302-011-9242-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M. Dopamine/adenosine interactions involved in effort-related aspects of food motivation. Appetite. 2009;53:422–425. doi: 10.1016/j.appet.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvadore G, Viale CI, Luckenbaugh DA, Zanatto VC, Portela LV, Souza DO, Zarate CA, Jr, Machado-Vieira R. Increased uric acid levels in drug-naive subjects with bipolar disorder during a first manic episode. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:819–821. doi: 10.1016/j.pnpbp.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos TG, Souza DO, Tasca CI. GTP uptake into rat brain synaptic vesicles. Brain Res. 2006;1070:71–76. doi: 10.1016/j.brainres.2005.10.099. [DOI] [PubMed] [Google Scholar]
- Savelyev SA, Rantamaki T, Rytkonen KM, Castren E, Porkka-Heiskanen T. Sleep homeostasis and depression: studies with the rat clomipramine model of depression. Neuroscience. 2012;212:149–158. doi: 10.1016/j.neuroscience.2012.03.029. [DOI] [PubMed] [Google Scholar]
- Schmidt AP, Lara DR, Souza DO. Proposal of a guanine-based purinergic system in the mammalian central nervous system. Pharmacol Ther. 2007;116:401–416. doi: 10.1016/j.pharmthera.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, Yanai K, Weinshenker D, Sumiyoshi T. Psychosis pathways converge via D2high dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Shen HY, Chen JF. Adenosine A(2A) receptors in psychopharmacology: modulators of behavior, mood and cognition. Curr Neuropharmacol. 2009;7:195–206. doi: 10.2174/157015909789152191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA, Linden J, Tsien JZ, Chen JF. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci. 2008;28:2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XF, Kondo DG, Sung YH, Hellem TL, Fiedler KK, Jeong EK, Huber RS, Renshaw PF. Frontal lobe bioenergetic metabolism in depressed adolescents with bipolar disorder: a phosphorus-31 magnetic resonance spectroscopy study. Bipolar Disord. 2012;14:607–617. doi: 10.1111/j.1399-5618.2012.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shink E, Harvey M, Tremblay M, Gagne B, Belleau P, Raymond C, Labbe M, Dube MP, Lafreniere RG, Barden N. Analysis of microsatellite markers and single nucleotide polymorphisms in candidate genes for susceptibility to bipolar affective disorder in the chromosome 12Q24.31 region. Am J Med Genet B Neuropsychiatr Genet. 2005;135B:50–58. doi: 10.1002/ajmg.b.30165. [DOI] [PubMed] [Google Scholar]
- Short JL, Ledent C, Borrelli E, Drago J, Lawrence AJ. Genetic interdependence of adenosine and dopamine receptors: evidence from receptor knockout mice. Neuroscience. 2006;139:661–670. doi: 10.1016/j.neuroscience.2005.12.052. [DOI] [PubMed] [Google Scholar]
- Sikoglu EM, Jensen JE, Vitaliano G, Liso Navarro AA, Renshaw PF, Frazier JA, Moore CM. Bioenergetic measurements in children with bipolar disorder: a pilot 31P magnetic resonance spectroscopy study. PLoS One. 2013;8:e54536. doi: 10.1371/journal.pone.0054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- Smolen TN, Smolen A. Purinergic modulation of ethanol-induced sleep time in long-sleep and short-sleep mice. Alcohol. 1991;8:123–130. doi: 10.1016/0741-8329(91)91320-2. [DOI] [PubMed] [Google Scholar]
- Soronen P, Mantere O, Melartin T, Suominen K, Vuorilehto M, Rytsala H, Arvilommi P, Holma I, Holma M, Jylha P, Valtonen HM, Haukka J, Isometsa E, Paunio T. P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:435–447. doi: 10.1002/ajmg.b.31179. [DOI] [PubMed] [Google Scholar]
- Sperlagh B, Csolle C, Ando RD, Goloncser F, Kittel A, Baranyi M. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol Hung. 2012;14:231–238. [PubMed] [Google Scholar]
- Spinelli MG. Neuroendocrine effects on mood. Rev Endocr Metab Disord. 2005;6:109–115. doi: 10.1007/s11154-005-6723-8. [DOI] [PubMed] [Google Scholar]
- Stone TW, Taylor DA. Antagonism by clonidine of neuronal depressant responses to adenosine, adenosine-5′-monophosphate and adenosine triphosphate. Br J Pharmacol. 1978;64:369–374. doi: 10.1111/j.1476-5381.1978.tb08659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Ceruti S, Abbracchio MP. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb Exp Pharmacol. 2009:535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, Zonderman AB, Mattson MP, Ferrucci L, Schlessinger D, Terracciano A. Impulsivity is associated with uric acid: evidence from humans and mice. Biol Psychiatry. 2014;75:31–37. doi: 10.1016/j.biopsych.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taayedi R, Kohler C, Drendel V, Seidel B, Illes P, Krugel U. Purinergic mechanisms mediate depression-like responses to chronic stress. J Neurochem. 2007;102:288. [Google Scholar]
- Tondo L, Rudas N. The course of a seasonal bipolar disorder influenced by caffeine. J Affect Disord. 1991;22:249–251. doi: 10.1016/0165-0327(91)90071-y. [DOI] [PubMed] [Google Scholar]
- Tosh DK, Paoletta S, Deflorian F, Phan K, Moss SM, Gao ZG, Jiang X, Jacobson KA. Structural sweet spot for A1 adenosine receptor activation by truncated (N)-methanocarba nucleosides: receptor docking and potent anticonvulsant activity. J Med Chem. 2012;55:8075–8090. doi: 10.1021/jm300965a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SJ, Hong CJ, Hou SJ, Yen FC. Association study of adenosine A2a receptor (1976C>T) genetic polymorphism and mood disorders and age of onset. Psychiatr Genet. 2006;16:185. doi: 10.1097/01.ypg.0000218627.26622.eb. [DOI] [PubMed] [Google Scholar]
- Ulrich H, Abbracchio MP, Burnstock G. Extrinsic purinergic regulation of neural stem/progenitor cells: implications for CNS development and repair. Stem Cell Rev. 2012;8:755–767. doi: 10.1007/s12015-012-9372-9. [DOI] [PubMed] [Google Scholar]
- Ushijima I, Mizuki Y, Hara T, Kaneyuki H, Mashimoto S, Kajimura N, Yamada M. Effects of dilazep (Comelian) on the central purinergic system: inhibitory effects on clonidine-induced aggressive behavior. Eur J Pharmacol. 1989;161:245–248. doi: 10.1016/0014-2999(89)90853-4. [DOI] [PubMed] [Google Scholar]
- Vasileiou E, Montero RM, Turner CM, Vergoulas G. P2X(7) receptor at the heart of disease. Hippokratia. 2010;14:155–163. [PMC free article] [PubMed] [Google Scholar]
- Vavra V, Bhattacharya A, Jindrichova M, Zemkova H. Facilitation of neurotransmitter and hormone release by P2X purinergic receptors. In: Contreras CM, editor. Neuroscience--Dealing with Frontiers. InTech; 2012. Available from: http://www.intechopen.com/books/neuroscience-dealing-with-frontiers/facilitation-of-neurotransmitter-and-hormone-release-by-p2x-purinergic-receptors- [DOI] [Google Scholar]
- Viikki M, Kampman O, Anttila S, Illi A, Setala-Soikkeli E, Huuhka M, Mononen N, Lehtimaki T, Leinonen E. P2RX7 polymorphisms Gln460Arg and His155Tyr are not associated with major depressive disorder or remission after SSRI or ECT. Neurosci Lett. 2011;493:127–130. doi: 10.1016/j.neulet.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Vinade ER, Izquierdo I, Lara DR, Schmidt AP, Souza DO. Oral administration of guanosine impairs inhibitory avoidance performance in rats and mice. Neurobiol Learn Mem. 2004;81:137–143. doi: 10.1016/j.nlm.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DK, Beenhakker M, Lin RC, Carter MF, Paul IA, Bischofberger N, Jacobson KA. Reduction of postischemic brain damage and memory deficits following treatment with the selective adenosine A1 receptor agonist. Eur J Pharmacol. 1996;302:43–48. doi: 10.1016/0014-2999(96)00101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JA, Katz RJ. Purinergic control of anxiety: direct behavioral evidence in the rat. Neurosci Lett. 1983;43:333–337. doi: 10.1016/0304-3940(83)90210-0. [DOI] [PubMed] [Google Scholar]
- Weber WA, Dudley J, Lee JH, Strakowski SM, Adler CM, DelBello MP. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. J Affect Disord. 2013;150:1109–1113. doi: 10.1016/j.jad.2013.04.047. [DOI] [PubMed] [Google Scholar]
- Wei CJ, Li W, Chen JF. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta. 2011;1808:1358–1379. doi: 10.1016/j.bbamem.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Weiser M, Burshtein S, Gershon AA, Marian G, Vlad N, Grecu IG, Tocari E, Tiugan A, Hotineanu M, Davis JM. Allopurinol for mania: a randomized trial of allopurinol versus placebo as add-on treatment to mood stabilizers and/or antipsychotic agents in manic patients with bipolar disorder. Bipolar Disord. 2014;16:441–447. doi: 10.1111/bdi.12202. [DOI] [PubMed] [Google Scholar]
- Weisman GA, Woods LT, Erb L, Seye CI. P2Y receptors in the mammalian nervous system: pharmacology, ligands and therapeutic potential. CNS Neurol Disord Drug Targets. 2012;11:722–738. doi: 10.2174/187152712803581047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen S, Cheng M, Wang H, Yue J, Wang H, Li G, Zheng L, Zhong Z, Peng F. Serum uric acid levels and the clinical characteristics of depression. Clin Biochem. 2012;45:49–53. doi: 10.1016/j.clinbiochem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Invest. 2013;123:3552–3563. doi: 10.1172/JCI65636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson JC, Minor TR, Job RF. Inhibition of adenosine deaminase by erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) mimics the effect of inescapable shock on escape learning in rats. Behav Neurosci. 1998;112:399–409. doi: 10.1037//0735-7044.112.2.399. [DOI] [PubMed] [Google Scholar]
- Wu PH, Phillis JW. Distribution and release of adenosine triphosphate in rat brain. Neurochem Res. 1978;3:563–571. doi: 10.1007/BF00963759. [DOI] [PubMed] [Google Scholar]
- Yu C, Gupta J, Chen JF, Yin HH. Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci. 2009;29:15100–15103. doi: 10.1523/JNEUROSCI.4215-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zezula J, Freissmuth M. The A(2A)-adenosine receptor: a GPCR with unique features? Br J Pharmacol. 2008;153(Suppl 1):S184–190. doi: 10.1038/sj.bjp.0707674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Su TP, Choi K, Maree W, Li CT, Chung MY, Chen YS, Bai YM, Chou YH, Barker JL, Barrett JE, Li XX, Li H, Benedek DM, Ursano R. P11 (S100A10) as a potential biomarker of psychiatric patients at risk of suicide. J Psychiatr Res. 2011;45:435–441. doi: 10.1016/j.jpsychires.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Zhang MR, Kumata K, Maeda J, Yanamoto K, Hatori A, Okada M, Higuchi M, Obayashi S, Suhara T, Suzuki K. 11C-AC-5216: a novel PET ligand for peripheral benzodiazepine receptors in the primate brain. J Nucl Med. 2007;48:1853–1861. doi: 10.2967/jnumed.107.043505. [DOI] [PubMed] [Google Scholar]
- Zheng P, Chen JJ, Huang T, Wang MJ, Wang Y, Dong MX, Huang YJ, Zhou LK, Xie P. A novel urinary metabolite signature for diagnosing major depressive disorder. J Proteome Res. 2013;12:5904–5911. doi: 10.1021/pr400939q. [DOI] [PubMed] [Google Scholar]
- Zlomuzica A, Burghoff S, Schrader J, Dere E. Superior working memory and behavioural habituation but diminished psychomotor coordination in mice lacking the ecto-5′-nucleotidase (CD73) gene. Purinergic Signal. 2013;9:175–182. doi: 10.1007/s11302-012-9344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]