Abstract
Purpose
To determine risk factors predictive of retinal detachment in patients with cytomegalovirus (CMV) retinitis in a setting with limited access to ophthalmic care.
Design
Case-control study.
Methods
Sixty-four patients with CMV retinitis and retinal detachment were identified from the Ocular Infectious Diseases and Retina Clinics at Chiang Mai University. Three control patients with CMV retinitis but no retinal detachment were selected for each case, matched by calendar date. The medical records of each patient were reviewed, with patient-level and eye-level features recorded for the clinic visit used to match cases and controls, and also for the initial clinic visit at which CMV retinitis was diagnosed. Risk factors for retinal detachment were assessed separately for each of these time points using multivariate conditional logistic regression models that included 1 eye from each patient.
Results
Patients with a retinal detachment were more likely than controls to have low visual acuity (OR, 1.24 per line of worse vision on the logMAR scale; 95%CI, 1.16-1.33) and bilateral disease (OR, 2.12; 95%CI, 0.92-4.90). Features present at the time of the initial diagnosis of CMV retinitis that predicted subsequent retinal detachment included bilateral disease (OR, 2.68; 95%CI, 1.18-6.08) and lesion size (OR, 2.64 per 10% increase in lesion size; 95%CI, 1.41-4.94).
Conclusion
Bilateral CMV retinitis and larger lesion sizes, each of which is a marker of advanced disease, were associated with subsequent retinal detachment. Earlier detection and treatment may reduce the likelihood that patients with CMV retinitis develop a retinal detachment.
Keywords: CMV Retinitis, HIV/AIDS, International Ophthalmology, Retinal Detachment
Introduction
Cytomegalovirus (CMV) retinitis is the most common ocular opportunistic infection and the leading cause of blindness among patients with the acquired immunodeficiency syndrome (AIDS).1, 2 Although it is now uncommon in the United States with the widespread use of highly active antiretroviral therapy (HAART),3-6 CMV retinitis continues to be the most common ocular opportunistic infections in AIDS patients and an important cause of blindness in many developing countries, especially in Asia.7-13 For example, at a tertiary ophthalmology center in Thailand, CMV retinitis affected 33% of AIDS patients and was the second leading cause of blindness among all patients seen at the clinic.9, 11 Of the major reasons for visual acuity loss associated with CMV retinitis, retinal detachments are perhaps the most devastating.14 Patients often have poor long-term visual outcomes even with surgical intervention, leading to permanent loss of vision.15-18 Because it can be a devastating complication of CMV retinitis, it is critical to understand the clinical characteristics that are associated with retinal detachment.
Risk factors for retinal detachment have been described for CMV retinitis patients in the United States.19-23 However, little information exists on CMV-related retinal detachment in the developing world, where the vast majority of CMV retinitis currently occurs and where retinal detachment is a common complication.13, 17 The circumstances in such resource-limited settings are very different from those seen in the United States. For example, patients at risk for CMV retinitis in Southeast Asia are not routinely screened, and the disease is often at an advanced stage at the time of initial diagnosis.10 Patients are often diagnosed several months after starting HAART, raising the possibility of preexisting CMV retinitis that becomes symptomatic due to intra-ocular inflammation from immune recovery. Furthermore, CMV retinitis in the developing world is treated almost exclusively with intravitreal ganciclovir injections, presenting additional risk for complications. Because the disease course and treatment regimen differ significantly in resource-limited settings, the risk factors for retinal detachment may be different than those found in the United States. In this study, we describe the results from a case-control study conducted in Thailand that investigated the risk factors for CMV retinitis-related retinal detachment.
Methods
Ethical approval was obtained from the Committee on Human Research at the University of California, San Francisco and the Chiang Mai University Faculty of Medicine Research Ethics Committee prior to conducting this case-control study. This study adhered to the tenets of the Declaration of Helsinki.
This study was conducted at the Ocular Infectious Diseases Clinic at Chiang Mai University, a tertiary referral ophthalmology clinic in northern Thailand. The clinic generally examines at-risk HIV patients with visual symptoms. We identified cases of CMV retinitis-related retinal detachment that were diagnosed between December 2004 and August 2010 by reviewing the medical records of all patients with CMV retinitis seen in the Ocular Infectious Diseases Clinic and all patients from the Retina Clinic logbook who had a procedure performed during this time. Cases were defined as any patient for whom retinal detachment was documented in the medical record. We selected 3 controls per case using incidence density sampling. Specifically, we reviewed the Ocular Infectious Diseases Clinic CMV retinitis logbook and enumerated all patients with a visit for CMV retinitis ± 3 months from the date the retinal detachment was initially diagnosed. We used a random number generator to select 3 patients without retinal detachment from this list to serve as controls. We sampled controls without replacement; each patient could serve as a control to only one case of retinal detachment. If a case had sequential bilateral retinal detachments, the date of the first retinal detachment was used to identify controls.
We collected eye-level and patient-level clinical and demographic information from the visit that the retinal detachment was first diagnosed for cases, and from the corresponding matched visit for controls. We also collected the same information from the initial clinic visit at which the diagnosis of CMV retinitis was initially made, in order to determine whether features that were present at baseline were predictive of future retinal detachment.
The medical record included detailed retinal drawings for all patients, drawn on a template that included the optic disc, fovea and vascular arcades, as well as the three zones traditionally used for CMV retinitis (zone 1 included the area within 3000 μm of the fovea or 1500 μm of the margin of the optic disc, zone 2 extended from zone 1 to the vortex veins, and zone 3 was the remaining retinal area that extended to the ora serrata).24 We noted the zone(s) with CMV retinitis and then grouped them based on the most anterior zone that was involved. In addition, we assessed 1) the area of retinitis as a proportion of the retinal surface area, 2) the most anterior extent of the retinitis, expressed as the proportion of the retinal radius as measured from the fovea to the ora serrata, and 3) the most posterior extent of the retinitis, also expressed as the proportion of the retinal radius. Measurements of the size and location of retinitis were performed by one of two graders using ImageJ software (Bethesda, MD),25 masked to whether the eye was a case or control. Lesion size measurements had good inter-rater reliability (intraclass correlation coefficient [ICC] between the two graders on a random set of 50 eyes was 0.88, 95%CI 0.81-0.94). Based on the charted retinal drawings it was often difficult to clearly distinguish detached retina from areas with CMV retinitis. Therefore, for this study we included lesion size and location only from eyes at the time of initial CMV retinitis diagnosis, before a retinal detachment had developed.
We constructed univariate and multivariate conditional logistic regression models to determine risk factors for retinal detachment, using the eye as the unit of analysis. Separate models were constructed for the matched visit (visit at which the retinal detachment was diagnosed or the time-matched visit for controls) and the visit at which CMV retinitis was first diagnosed. Because eyes from the same person are not independent, we selected only a single eye from each patient for analyses: for cases with sequential bilateral retinal detachments, we selected the first eye that developed a detachment; for cases with bilateral retinal detachments initially diagnosed at the same visit, one eye was randomly selected; and for controls with bilateral CMV retinitis, we randomly selected one of the eyes. Continuous predictors were tested for specification error (linktest in Stata) and transformations applied as needed. We explored the use of cubic splines for continuous variables but these did not significantly improve model fit and were therefore not used in the final model. All factors with P<0.05 in univariate models were included in the multivariate model, with a backwards stepwise selection algorithm employed until all covariates had a P<0.10 using the likelihood ratio test. All statistics were performed using Stata version 13 (Statacorp, College Station, Texas, USA).
Results
This study included 64 eyes from 64 patients with CMV retinitis-related retinal detachment and 192 matched control eyes from 192 patients with CMV retinitis but no retinal detachment. Greater than 90% of patients with a retinal detachment were on antiretroviral therapy, and the median CD4 count was 200 cells/μl (IQR 30 to 378) at the time of detachment (mean 226 cells/μl, 95%CI 172-279). Most retinal detachments occurred relatively soon after diagnosis of CMV retinitis (median 1.15 months, IQR 0 to 9.78; mean 8.2 months, 95%CI 4.5-11.9). Visual acuity was generally poor at the time of retinal detachment (median logMAR 1.8, IQR 1.0-1.8 [Snellen equivalent: hand motions, IQR 20/200 to Hand Motions]; mean logMAR 1.46, 95%CI 1.32 to 1.60).
Univariate analyses indicated that at the time the retinal detachment was diagnosed, cases with retinal detachment were more likely than controls to have CMV retinitis in the contralateral eye (67.2% vs. 52.6%; P=0.04), decreased visual acuity (mean logMAR 1.46 vs. 0.60 [Snellen equivalent 20/600 vs. 20/80]; P<0.001), and a shorter period of time since diagnosis of CMV retinitis (mean 8.2 months vs. 12.9 months; P=0.045; Table 1). Patients with retinal detachment were also more likely than controls to have received their initial CMV retinitis diagnosis at the time of the matched visit (35.9% vs. 14.1%; P<0.001), and therefore less likely to have started management for CMV retinitis before the time of detachment. In multivariate analysis, only decreased visual acuity was strongly associated with retinal detachment (OR 1.24 per line of logMAR acuity, 95%CI 1.16-1.33), although CMV retinitis in the contralateral eye was also included in the model (OR 2.12, 95%CI 0.92-4.90; Table 2).
Table 1.
Characteristics of patients with the acquired immunodeficiency syndrome (AIDS) at the time of diagnosis for cytomegalovirus retinitis and at the time of retinal detachment
| Initial CMV Retinitis Visita | Retinal Detachment Visitb | |||||
|---|---|---|---|---|---|---|
| Mean (95%CI) or N(%) |
Mean (95%CI) or N(%) |
|||||
| Characteristic | Cases (N=41) | Controls (N=123) | OR (95% CI) | Cases (N=64) | Controls (N=192) | OR (95% CI) |
| PATIENT CHARACTERISTICS | ||||||
| Age (years) | 37 (34-41) | 36 (31-40) | 1.02 (0.97-1.07) | 38 (34-42) | 38.5 (33-44) | 0.99 (0.96-1.03) |
| Female | 23 (56.1%) | 71 (57.7%) | 0.93 (0.46-1.92) | 30 (46.9%) | 109 (56.8%) | 0.68 (0.39-1.19) |
| CD4 count (cells/μm3)c | 83.0 (37-129) | 78.2 (56-111) | 1.00 (0.98-1.03) | 226 (172-279) | 189 (163-215) | 1.01 (0.99-1.02) |
| Taking antiretroviral therapyd | 26 (92.9%) | 90 (91.8%) | 1.08 (0.16-7.55) | 46 (95.8%) | 163 (95.3%) | 1.24 (0.24-6.33) |
| CMV retinitis in contralateral eye | 26 (63.4%) | 49 (39.8%) | 2.74 (1.25-6.03) | 43 (67.2%) | 101 (52.6%) | 1.91 (1.03-3.57) |
| No prior CMV retinitis diagnosis | 41 (100%) | 123 (100%) | - | 23 (35.9%) | 27 (14.1%) | 3.16 (1.67-6.01) |
| Months since CMV diagnosise | - | - | - | 8.2 (4.5-11.9) | 12.9 (10.3-15.6) | 0.98 (0.96-1.00) |
| EYE CHARACTERISTICS | ||||||
| Visual acuity (logMAR)f | 0.87 (0.68-1.06) | 0.72 (0.60-0.80) | 1.04 (0.98-1.10) | 1.46 (1.32-1.60) | 0.60 (0.50-0.70) | 1.24 (1.16-1.33) |
| Lesion active | 30 (73.2%) | 93 (75.6%) | 0.87 (0.38-2.00) | 18 (28.1%) | 52 (27.1%) | 1.06 (0.55-2.03) |
| Lesion size (% retinal surface)g | 12.8% (9.5-16.1%) | 8.2% (6.7-9.6%) | 2.67 (1.45-4.91) | - | - | - |
| Most posterior extent of retintiish | 28.3% (22.1-34.4%) | 27.9% (24.8-31.0%) | 1.01 (0.82-1.25) | - | - | - |
| Most anterior extent of retinitisi | 78.1% (72.9-83.3%) | 72.5% (69.4-75.5%) | 1.26 (0.99-1.60) | - | - | - |
| Number of lesions | 1.6 (1.3-1.9) | 1.3 (1.2-1.4) | 1.68 (1.03-2.74) | - | - | - |
| Vitreous haze present | 6 (14.6%) | 6 (4.9%) | 4.37 (1.06-18.11) | 4 (6.3%) | 3 (1.6%) | 4.00 (0.90-17.87) |
| Retinal hemorrhage present | 18 (43.9%) | 61 (49.6%) | 0.78 (0.37-1.64) | 13 (20.3%) | 43 (22.4%) | 0.89 (0.45-1.75) |
| Frosted branch angiitis present | 6 (14.6%) | 6 (4.9%) | 3.29 (0.99-10.92) | 2 (3.1%) | 1 (0.5%) | 6.00 (0.54-66.17) |
Bold values indicate a likelihood ratio p-value <0.05. CMV= cytomegalovirus; CD4= cluster of differentiation 4; logMAR= logarithm of the minimum angle of resolution
Longitudinal analysis of the risk factors at the time of initial CMV retinitis diagnosis that predict subsequent retinal detachment; excludes matched groups in which the case had a retinal detachment at the time of initial CMV retinitis diagnosis.
Cross-sectional analysis of the characteristics present at the time of retinal detachment diagnosis and their association with retinal detachment; includes all matched groups.
Data not available for all patients. 32 (78.0%) cases and 108 (87.8%) controls had data at the initial visit; 59 (92.2%) cases and 181 (94.3%) controls had data at the matched visit. Odds ratio indicates odds of retinal detachment per 10 cells/μm3.
Data not available for all patients. 28 (68.3%) cases and 98 (79.7%) controls had data at the initial visit; 48 (75.0%) cases and 171 (89.1%) controls had data at the matched visit.
Time since diagnosis of CMV retinitis. Numbers in table include all cases and controls; if the 23 cases with a retinal detachment at the time of CMV retinitis diagnosis and their 69 corresponding controls are excluded, the mean time since CMV retinitis diagnosis was 12.8 months (95%CI 7.5 to 18.1) for cases and 13.4 (95%CI 9.9 to 16.9) for controls.
Odds ratio indicates odds of retinal detachment per line of logMAR visual acuity.
Percent of the total retinal area occupied by the lesion by assessment of retinal drawings; odds ratio indicates odds of retinal detachment per 10% total retinal surface area
Distance from the fovea to the most proximal lesion border, calculated as a percent of the distance from the fovea to the ora serrata; odds ratio indicates odds of retinal detachment per 10% increase in the distance from the fovea to the most posterior border.
Distance from the fovea to the most distal lesion border, calculated as a percent of the distance from the fovea to the ora serrata; odds ratio indicates odds of retinal detachment per 10% increase in the distance from the fovea to the most anterior border.
Table 2.
Risk factors for retinal detachment in patients with the acquired immunodeficiency syndrome (AIDS) and cytomegalovirus retinitis: multivariate analyses
| Initial CMV Retinitis Visita |
Retinal Detachment Visitb |
|
|---|---|---|
| Characteristic | OR (95% CI)c | OR (95% CI)c |
| Lesion Size, per 10% total retinal surface area | 2.64 (1.41-4.94) | - |
| CMV retinitis in contralateral eye | 2.68 (1.18-6.08) | 2.12 (0.92-4.90) |
| Decreased visual acuity (per logMAR line) | - | 1.24 (1.16-1.33) |
CMV= cytomegalovirus; logMAR= logarithm of the minimum angle of resolution
Longitudinal analysis of the risk factors present at the time of initial CMV retinitis diagnosis that are predictive of subsequent retinal detachment; excludes matched groups in which the case had a retinal detachment at the time of initial CMV retinitis diagnosis.
Cross-sectional analysis of the characteristics present at the time of retinal detachment diagnosis and their association with retinal detachment; includes all matched groups.
Initial multivariate models included factors with P<0.05 in univariate analyses (bold items in Table 1); the final multivariate models shown above were selected with a backwards stepwise algorithm.
In order to assess for risk factors that might predict a subsequent retinal detachment, we performed similar analyses using data from the initial visit at which CMV retinitis was diagnosed. For this analysis, we excluded the 23 cases that had a retinal detachment at the time of initial CMV retinitis diagnosis and their corresponding 69 controls, leaving 41 patients with retinal detachment and 123 controls. In univariate analyses, cases were more likely than controls to have a larger lesion size (mean 12.8% of the total retinal surface area was involved with retinitis, vs. 8.2%, P<0.001), more peripheral retinitis (the most anterior border of the lesion was on average 78.1% of the total distance from the fovea to the ora serrata, vs. 72.5%; P=0.049), a higher number of retinal lesions (mean 1.6 vs. 1.3; P=0.04), CMV retinitis in the contralateral eye (63.4% vs. 39.8%, P=0.008), and vitreous haze (14.6% vs. 4.9%; P=0.04; Table 1). In the multivariate analysis, only lesion size (OR 2.64 per 10% increase in lesion size measured as a percentage of the total retinal surface area, 95%CI 1.41-4.94) and retinitis in the contralateral eye (OR 2.68, 95%CI 1.18-6.08) remained significant (Table 2).
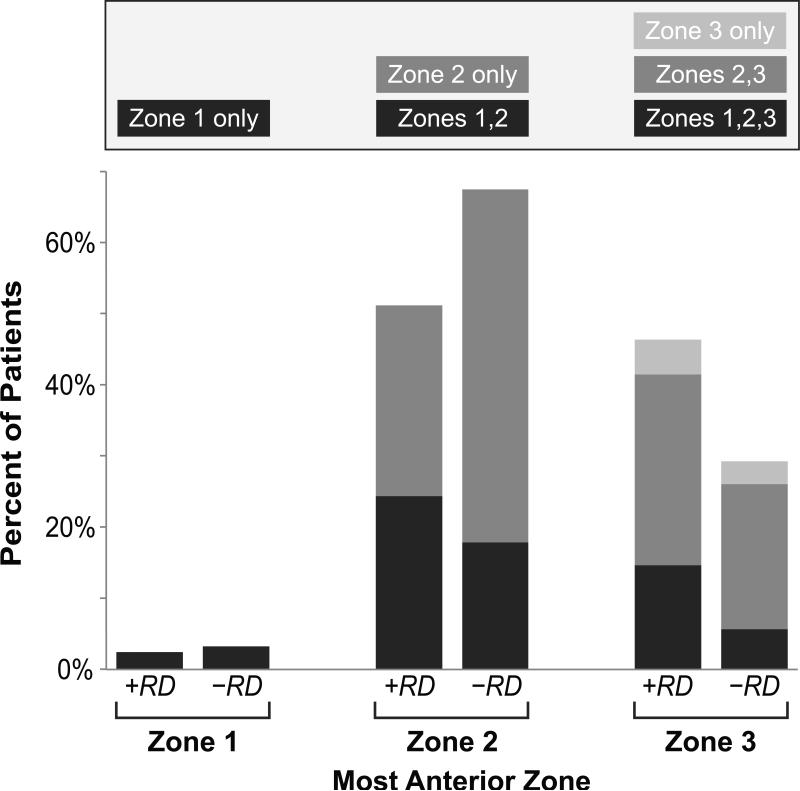
As noted in the Figure, cases with retinal detachment tended to have more anterior disease at the time of CMV retinitis diagnosis than did controls (46.3% of cases vs. 29.3% of controls had disease in zone 3; OR 2.01, 95%CI 0.99 to 4.09). However, we could not demonstrate an association between zone 3 disease and retinal detachment after adjusting for lesion size (OR 1.54, 95%CI 0.72 to 3.30).
Figure. Risk of retinal detachment in patients with the acquired immunodeficiency syndrome (AIDS) and cytomegalovirus retinitis, stratified by most anterior zone of retinal involvement.
Patients are grouped by the most anterior zone occupied by the lesion, with cases of retinal detachment (+RD) on the left and controls without retinal detachment (-RD) on the right. The zones represented by each pair of columns are shown in the key above the figure, with each colored box corresponding to the color of the bar.
Because a large number (35.9%) of cases presented with retinal detachment at the time of initial CMV retinitis diagnosis and were therefore ineligible for treatment before the retinal detachment occurred, we analyzed the effect of prior intravitreal injections only for the cases who did not have a retinal detachment at their initial visit (N=41) and their matched controls (N=123). At the time of retinal detachment or the matched control visit, there was no significant difference in the number of prior injections between cases (mean 6, 95%CI 4 to 9) and controls (mean 6, 95%CI 5 to 8; P=0.86). There was also no difference in the amount of time since the most recent injection (mean 30.4 weeks [95%CI 13.7 to 47.0] for cases, and 37.3 weeks [95%CI 23.7 to 50.9] for controls; P=0.57).
Discussion
Although most retinal detachments at this tertiary ophthalmology clinic in Thailand occurred approximately 1 month after diagnosis of CMV retinitis, 25% were diagnosed more than 9 months after the initial CMV retinitis diagnosis, suggesting that patients in this setting continued to be at risk for retinal detachments long after they had been diagnosed with and treated for CMV retinitis. CMV retinitis patients with a retinal detachment were more likely than controls to have bilateral disease and low visual acuity. At the time of initial CMV retinitis diagnosis, larger lesion size and bilateral disease were risk factors that significantly predicted a future retinal detachment. Neither the number of prior intravitreal injections nor the time since the most recent injection were associated with retinal detachment.
We found that development of a retinal detachment was strongly associated with large retinitis lesion size, a finding that has been described before.19-23 The lesion sizes reported here are somewhat smaller than those typically reported in previous studies. This discrepancy may be due to differences in the way lesion size was measured, with most prior studies using a categorical measurement from the clinical examination to estimate lesion size, whereas we used a continuous measure from tracings of retinal drawings. Our methodology is analogous to using retinal photographs to assess lesion size—a technique which has been shown to result in smaller lesion measurements compared with clinician assessment.26
Anterior lesions may predispose to retinal detachment because the anterior retina is thinner and more susceptible to tears, and also because it underlies the vitreous base, making it more vulnerable to the effects of vitreoretinal traction. Other studies have found zone 3 (i.e. more anterior) lesions to be significantly associated with retinal detachment.19, 20, 23 We expanded upon these findings by showing in univariate analyses that having a more anterior retinitis lesion at the time of initial CMV retinitis diagnosis was associated with subsequent retinal detachment. However, lesion location may be confounded by lesion size because large lesions are more likely to encompass more than one zone.27 The association between anterior lesion location and retinal detachment found in this study did not persist after adjusting for lesion size, suggesting that the size of a retinitis lesion is a stronger risk factor for retinal detachment than is anterior extent of the lesion, at least in this population.
In this study bilateral disease at the time of CMV retinitis diagnosis was a strong risk factor for future retinal detachment. Previous studies in the United States have not found retinitis in the contralateral eye to be an independent predictor of retinal detachment.19-23 In contrast, our study found that an eye was at increased risk for a retinal detachment if the contralateral eye also had CMV retinitis. In this setting with limited capacity for screening, bilateral disease is likely a marker of the duration of untreated CMV retinitis, as well as an indication of the amount of time that a patient has been severely immunocompromised.27 Although we did not find the time between diagnosis of CMV retinitis and retinal detachment to be predictive of retinal detachment in the multivariate analysis, this variable does not capture how long the patient had CMV retinitis prior to being diagnosed. In this Thai setting, patients likely develop CMV retinitis several months before they present to the ophthalmologist.10 Our finding that bilateral disease is a significant predictor of subsequent retinal detachment suggests that advanced disease due to delayed diagnosis plays a major role in the development of retinal detachment.
Intravitreal ganciclovir injections may prevent retinal detachments by limiting the progression of CMV retinitis.19 However, retinal detachment is also a known complication of intravitreal injections. 28, 29 It is difficult to determine whether intravitreal ganciclovir injections were a risk factor for retinal detachment in this population since this was the only treatment modality available to patients in the study. We did not find an association between the number of intravitreal injections and retinal detachment nor the time since the most recent injection, although this may be confounded by other factors such as the length of time in treatment. The overall effect is particularly difficult to determine given the retrospective design of this study, and could best be evaluated with a randomized controlled trial.
We found that patients had a higher CD4 count at the time of retinal detachment compared to the time of diagnosis of CMV retinitis, suggesting a possible role of inflammation due to immune reconstitution. However, when we compared cases to controls we did not find either CD4 count or HAART status to be a risk factor. Some studies carried out in the United States found that a low CD4 count was a risk factor for retinal detachment.21, 22 This discrepancy is likely due to differences in the patient population. Patients in the United States who developed CMV retinitis in the HAART era were often non-compliant, intolerant of, or failing HAART. 4, 30, 31 In contrast, almost all newly diagnosed CMV retinitis patients in our study clinic in Thailand started HAART within 2 months prior to the diagnosis of CMV retinitis.10 Thus, patients with CMV retinitis and a low CD4 count in our study population were likely in the early stages of immune reconstitution, in contrast to those in the studies carried out in the United States. Previous research has also identified HAART to be a protective factor against CMV retinitis-related retinal detachment.23 Because almost all cases and controls in our study were on three-drug antiretroviral therapy at the time of diagnosis, we were underpowered to detect a difference between those who were and were not receiving HAART. Nonetheless, HAART is clearly important for both the prevention and treatment of CMV retinitis and improving access to HAART in resource-poor settings should be a priority.5, 32-34
This study is novel in that it reports risk factors for CMV retinitis-related retinal detachment in a developing county setting in which almost all patients were treated with HAART and intravitreal ganciclovir injections. Previous studies have usually been limited to patients in the pre-HAART area treated systemically for CMV retinitis,19-21 although two studies did contain a small number of patients with retinal detachment who were treated with HAART.22, 23 In general, we found similar risk factors to those reported before, with the exception that anterior lesion location was less important than lesion size, and retinitis in the contralateral eye was found to be a strong risk factor for subsequent retinal detachment. Large retinitis lesion size and bilateral disease are likely signs of longstanding disease, suggesting that earlier detection of CMV retinitis could result in fewer retinal detachments and less blindness. Routine screening would result in earlier diagnosis and treatment, which could limit lesion growth and therefore reduce the chance of a retinal detachment.19, 29, 35 In addition, routine screening may detect patients who have signs that are concerning for a retinal detachment, such as a retinal tear or hole. Ophthalmologists could then pursue laser photocoagulation, which some hypothesize limits progression of a retinal detachment for lesions that are small and inactive.36-39 Adequately screening at-risk individuals can be challenging in resource-poor areas due to the relative lack of ophthalmologists.40, 41 Alternate methods could be explored to increase diagnostic capacity, including training primary care providers in indirect ophthalmoscopy42 and remote diagnosis via telemedicine.43-45
We acknowledge several limitations to this study. Besides the limitations inherent in all retrospective studies, this study was conducted at a tertiary center and is likely subject to referral bias. The case-control study design prevented us from determining the prevalence or rate of retinal detachments at this clinic. We used ophthalmologist drawings to determine the size and location of retinitis lesion sizes. Although drawings could introduce imprecision and misclassification error, their use should not introduce bias since the drawings were performed before the onset of retinal detachment.26 Moreover, in an attempt to reduce bias, all retinal lesion measurements were made by an individual masked to retinal detachment status. Finally, although this study included a much larger number of post-HAART era CMV retinitis-related retinal detachments than previous studies, the number was still relatively small. We improved the statistical power of the study by including 3 controls per case, but we may have nonetheless been underpowered to establish an association between certain risk factors and retinal detachment.
In conclusion, in this northern Thai population larger lesion size and bilateral disease at the time of diagnosis of CMV retinitis, both of which indicate late stage disease, were predictive of a retinal detachment. Efforts to expand the diagnostic capacity for CMV retinitis may lead to earlier detection and treatment, thereby reducing retinal detachments and blindness in these patients.
Acknowledgements and Disclosures
A. Funding/Support: This study was supported by the Gladstone Institute of Virology and Immunology Center for AIDS Research (San Francisco, California), the University of California, San Francisco Research Evaluation and Allocation Committee (San Francisco, California), That Man May See, (San Francisco, California), grant K23EY019071 from the National Eye Institute (National Institutes of Health, Bethesda, Maryland), the Littlefield Trust (San Francisco, CA), the Peierls Foundation (Austin, Texas), and the Doris Duke Charitable Foundation (New York, New York) through a grant supporting the Doris Duke International Clinical Research Fellows Program at the University of California, San Francisco. Michael Yen is a Doris Duke International Clinical Research Fellow.
D. Other Acknowledgements: None
Footnotes
C. Contributions of Authors:
Study Design and Conduct (JC, SoA, TPM, JDK)
Data Collection, Management, Analysis and Interpretation (MY, JC, SoA, PK, PV, SaA, CJ, JS, GNH, DH, TPM, JDK)
Preparation, Review or Approval of the Manuscript (MY, JC, SoA, PK, PV, SaA, CJ, JS, GNH, DH, TPM, JDK)
Disclosures
B. Financial Disclosures: Dr. Holland has served on Advisory Boards for the following companies: Novartis International AG (Basel, Switzerland); Santen, Incorporated (Emeryville, California); and Xoma Corporation (Berkeley, California).
References
- 1.Jabs DA, Van Natta ML, Holbrook JT, et al. Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment. Ophthalmology. 2007;114(4):780–6. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–83. [PMC free article] [PubMed] [Google Scholar]
- 3.Deayton JR, Wilson P, Sabin CA, et al. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS. 2000;14(9):1163–70. doi: 10.1097/00002030-200006160-00013. [DOI] [PubMed] [Google Scholar]
- 4.Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(1):231–3. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 5.Yust I, Fox Z, Burke M, et al. Retinal and extraocular cytomegalovirus end-organ disease in HIV-infected patients in Europe: a EuroSIDA study, 1994-2001. Eur J Clin Microbiol Infect Dis. 2004;23(7):550–9. doi: 10.1007/s10096-004-1160-2. [DOI] [PubMed] [Google Scholar]
- 6.Sugar EA, Jabs DA, Ahuja A, et al. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2012;153(6):1016–24. e5. doi: 10.1016/j.ajo.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiden D, Ford N, Wilson D, et al. Cytomegalovirus retinitis: the neglected disease of the AIDS pandemic. PLoS Med. 2007;4(12):e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford N, Shubber Z, Saranchuk P, et al. Burden of HIV-related cytomegalovirus retinitis in resource-limited settings: a systematic review. Clin Infect Dis. 2013;57(9):1351–61. doi: 10.1093/cid/cit494. [DOI] [PubMed] [Google Scholar]
- 9.Ausayakhun S, Watananikorn S, Ittipunkul N, Chaidaroon W, Patikulsila P, Patikulsila D. Epidemiology of the ocular complications of HIV infection in Chiang Mai. J Med Assoc Thai. 2003;86(5):399–406. [PubMed] [Google Scholar]
- 10.Ausayakhun S, Keenan JD, Ausayakhun S, et al. Clinical features of newly diagnosed cytomegalovirus retinitis in northern Thailand. Am J Ophthalmol. 2012;153(5):923–931. e1. doi: 10.1016/j.ajo.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pathanapitoon K, Ausayakhun S, Kunavisarut P, et al. Blindness and low vision in a tertiary ophthalmologic center in Thailand: the importance of cytomegalovirus retinitis. Retina. 2007;27(5):635–40. doi: 10.1097/01.iae.0000249575.38830.45. [DOI] [PubMed] [Google Scholar]
- 12.Sudharshan S, Kaleemunnisha S, Banu AA, et al. Ocular lesions in 1,000 consecutive HIV-positive patients in India: a long-term study. J Ophthalmic Inflamm Infect. 2013;3(1):2. doi: 10.1186/1869-5760-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gharai S, Venkatesh P, Garg S, Sharma SK, Vohra R. Ophthalmic manifestations of HIV infections in India in the era of HAART: analysis of 100 consecutive patients evaluated at a tertiary eye care center in India. Ophthalmic Epidemiol. 2008;15(4):264–71. doi: 10.1080/09286580802077716. [DOI] [PubMed] [Google Scholar]
- 14.Thorne JE, Jabs DA, Kempen JH, et al. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113(8):1441–5. doi: 10.1016/j.ophtha.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Raina J, Bainbridge JW, Shah SM. Decreased visual acuity in patients with cytomegalovirus retinitis and AIDS. Eye (Lond) 2000;14(Pt 1):8–12. doi: 10.1038/eye.2000.3. [DOI] [PubMed] [Google Scholar]
- 16.Irvine AR, Lonn L, Schwartz D, Zarbin M, Ballesteros F, Kroll S. Retinal detachment in AIDS: long-term results after repair with silicone oil. Br J Ophthalmol. 1997;81(3):180–3. doi: 10.1136/bjo.81.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tangmonkongvoragul C, Ausayakhun S. Causes of visual acuity loss among HIV-infected patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy in Chiang Mai University Hospital. J Med Assoc Thai. 2012;95(Suppl 4):S129–35. [PubMed] [Google Scholar]
- 18.Kunavisarut P, Bijlsma WR, Pathanapitoon K, Patikulsila D, Choovuthayakorn J, Rothova A. Proliferative vitreoretinopathy in human immunodeficiency virus-infected patients in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2010;150(2):218–22. doi: 10.1016/j.ajo.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Jabs DA, Enger C, Haller J, de Bustros S. Retinal detachments in patients with cytomegalovirus retinitis. Arch Ophthalmol. 1991;109(6):794–9. doi: 10.1001/archopht.1991.01080060058024. [DOI] [PubMed] [Google Scholar]
- 20.Freeman WR, Friedberg DN, Berry C, et al. Risk factors for development of rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis. Am J Ophthalmol. 1993;116(6):713–20. doi: 10.1016/s0002-9394(14)73471-3. [DOI] [PubMed] [Google Scholar]
- 21.Rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis: the Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial. The Studies of Ocular Complications of AIDS (SOCA) Research Group in Collaboration with the AIDS Clinical Trials Group (ACTG). Am J Ophthalmol. 1997;124(1):61–70. [PubMed] [Google Scholar]
- 22.Jabs DA, Van Natta ML, Thorne JE, et al. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. Second eye involvement and retinal detachment. Ophthalmology. 2004;111(12):2232–9. doi: 10.1016/j.ophtha.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 23.Kempen JH, Jabs DA, Dunn JP, West SK, Tonascia J. Retinal detachment risk in cytomegalovirus retinitis related to the acquired immunodeficiency syndrome. Arch Ophthalmol. 2001;119(1):33–40. [PubMed] [Google Scholar]
- 24.Holland GN, Buhles WC, Jr., Mastre B, Kaplan HJ. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. UCLA CMV Retinopathy. Study Group. Arch Ophthalmol. 1989;107(12):1759–66. doi: 10.1001/archopht.1989.01070020841024. [DOI] [PubMed] [Google Scholar]
- 25.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg DV, Holbrook JT, Hubbard LD, et al. Clinician versus reading center assessment of cytomegalovirus retinitis lesion size. Ophthalmology. 2005;112(4):559–66. doi: 10.1016/j.ophtha.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 27.Holland GN, Vaudaux JD, Jeng SM, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988 to 1994). Am J Ophthalmol. 2008;145(1):5–11. doi: 10.1016/j.ajo.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Ausayakhun S, Yuvaves P, Ngamtiphakom S, Prasitsilp J. Treatment of cytomegalovirus retinitis in AIDS patients with intravitreal ganciclovir. J Med Assoc Thai. 2005;88(Suppl 9):S15–20. [PubMed] [Google Scholar]
- 29.Engstrom RE, Jr., Holland GN. Local therapy for cytomegalovirus retinopathy. Am J Ophthalmol. 1995;120(3):376–85. doi: 10.1016/s0002-9394(14)72168-3. [DOI] [PubMed] [Google Scholar]
- 30.Holland GN, Vaudaux JD, Shiramizu KM, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in the era of highly active antiretroviral therapy (1997 to 2000). Am J Ophthalmol. 2008;145(1):12–22. doi: 10.1016/j.ajo.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 31.Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133(1):48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 32.Nolan W, Mitchell S. Do all patients with CMV retinitis require life long anti-CMV therapy? Br J Ophthalmol. 1998;82(7):844–5. doi: 10.1136/bjo.82.7.e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jouan M, Saves M, Tubiana R, et al. Discontinuation of maintenance therapy for cytomegalovirus retinitis in HIV-infected patients receiving highly active antiretroviral therapy. AIDS. 2001;15(1):23–31. doi: 10.1097/00002030-200101050-00006. [DOI] [PubMed] [Google Scholar]
- 34.Wohl DA, Kendall MA, Owens S, et al. The safety of discontinuation of maintenance therapy for cytomegalovirus (CMV) retinitis and incidence of immune recovery uveitis following potent antiretroviral therapy. HIV Clin Trials. 2005;6(3):136–46. doi: 10.1310/4J65-4YX1-4ET6-E5KR. [DOI] [PubMed] [Google Scholar]
- 35.Orellana J, Teich SA, Friedman AH, Lerebours F, Winterkorn J, Mildvan D. Combined short- and long-term therapy for the treatment of cytomegalovirus retinitis using ganciclovir (BW B759U). Ophthalmology. 1987;94(7):831–8. doi: 10.1016/s0161-6420(87)33536-5. [DOI] [PubMed] [Google Scholar]
- 36.McCluskey P, Grigg J, Playfair TJ. Retinal detachments in patients with AIDS and CMV retinopathy: a role for laser photocoagulation. Br J Ophthalmol. 1995;79(2):153–6. doi: 10.1136/bjo.79.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vrabec TR. Laser photocoagulation repair of macula-sparing cytomegalovirus-related retinal detachment. Ophthalmology. 1997;104(12):2062–7. doi: 10.1016/s0161-6420(97)30058-x. [DOI] [PubMed] [Google Scholar]
- 38.Davis JL, Hummer J, Feuer WJ. Laser photocoagulation for retinal detachments and retinal tears in cytomegalovirus retinitis. Ophthalmology. 1997;104(12):2053–60. doi: 10.1016/s0161-6420(97)30059-1. discussion 2060-1. [DOI] [PubMed] [Google Scholar]
- 39.Althaus C, Loeffler KU, Schimkat M, Hudde T, Sundmacher R. Prophylactic argon laser coagulation for rhegmatogenous retinal detachment in AIDS patients with cytomegalovirus retinitis. Graefes Arch Clin Exp Ophthalmol. 1998;236(5):359–64. doi: 10.1007/s004170050091. [DOI] [PubMed] [Google Scholar]
- 40.Resnikoff S, Felch W, Gauthier TM, Spivey B. The number of ophthalmologists in practice and training worldwide: a growing gap despite more than 200,000 practitioners. Br J Ophthalmol. 2012;96(6):783–7. doi: 10.1136/bjophthalmol-2011-301378. [DOI] [PubMed] [Google Scholar]
- 41.Estopinal CB, Ausayakhun S, Ausayakhun S, et al. Access to ophthalmologic care in Thailand: a regional analysis. Ophthalmic Epidemiol. 2013;20(5):267–73. doi: 10.3109/09286586.2013.821498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tun N, London N, Kyaw MK, et al. CMV retinitis screening and treatment in a resource-poor setting: three-year experience from a primary care HIV/AIDS programme in Myanmar. J Int AIDS Soc. 2011;14:41. doi: 10.1186/1758-2652-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ausayakhun S, Skalet AH, Jirawison C, et al. Accuracy and reliability of telemedicine for diagnosis of cytomegalovirus retinitis. Am J Ophthalmol. 2011;152(6):1053–1058. e1. doi: 10.1016/j.ajo.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah JM, Leo SW, Pan JC, et al. Telemedicine screening for cytomegalovirus retinitis using digital fundus photography. Telemed J E Health. 2013;19(8):627–31. doi: 10.1089/tmj.2012.0233. [DOI] [PubMed] [Google Scholar]
- 45.Yen M, Ausayakhun S, Chen J, et al. Telemedicine diagnosis of cytomegalovirus retinitis by nonophthalmologists. JAMA Ophthalmol. 2014;132(9):1052–8. doi: 10.1001/jamaophthalmol.2014.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]