Abstract
Gonadectomy (GDX) induces sex steroid-producing adrenocortical tumors in certain mouse strains and in the domestic ferret. Transcriptome analysis and DNA methylation mapping were used to identify novel genetic and epigenetic markers of GDX-induced adrenocortical neoplasia in female DBA/2J mice. Markers were validated using a combination of laser capture microdissection, quantitative RT-PCR, in situ hybridization, and immunohistochemistry. Microarray expression profiling of whole adrenal mRNA from ovariectomized vs. intact mice demonstrated selective upregulation of gonadal-like genes including Spinlw1 and Insl3 in GDX-induced adrenocortical tumors of the mouse. A complementary candidate gene approach identified Foxl2 as another gonadal-like marker expressed in GDX-induced neoplasms of the mouse and ferret. That both “male-specific” (Spinlw1) and “female-specific” (Foxl2) markers were identified is noteworthy and implies that the neoplasms exhibit mixed characteristics of male and female gonadal somatic cells. Genome-wide methylation analysis showed that two genes with hypomethylated promoters, Igfbp6 and Foxs1, are upregulated in GDX-induced adrenocortical neoplasms. These new genetic and epigenetic markers may prove useful for studies of steroidogenic cell development and for diagnostic testing.
Keywords: adrenal cortex, endocrine tumor, gonadotropin, ovariectomy, steroidogenesis
1. Introduction
Steroidogenic cells in the adrenal cortex and gonads arise from a common pool of progenitors in the adrenogonadal primordia, but the mechanisms that determine whether a given precursor cell adopts an adrenocortical or gonadal phenotype are not fully understood (Val et al., 2006; Hu et al., 2007; Morohashi and Zubair, 2011; Wood and Hammer, 2011; Laufer et al., 2012; Shima et al., 2012; Simon and Hammer, 2012; Bandiera et al., 2013; Pihlajoki et al., 2013b; Wood et al., 2013). One experimentally tractable model for the study of steroidogenic cell fate determination is gonadectomy (GDX)-induced adrenocortical neoplasia. In response to GDX and the ensuing changes in serum hormone levels [↑ luteinizing hormone (LH), ↓ inhibins, etc.], sex steroid-producing tumors arise in the adrenal glands of certain mouse strains and ferrets (Bielinska et al., 2005; Bielinska et al., 2006; Johnsen et al., 2006; Bernichtein et al., 2007; Bernichtein et al., 2008; Bernichtein et al., 2009; Doghman and Lalli, 2009; Beuschlein et al., 2012; Miller et al., 2013). This phenomenon is thought to reflect gonadotropin-induced metaplasia of stem/progenitor cells in the adrenal capsule or cortex, although the term “neoplasia” is used more often than “metaplasia” to describe the process (Bielinska et al., 2006). The neoplastic tissue resembles luteinized ovarian stroma and expresses gonadal-like differentiation markers, including LH receptor (Lhcgr), anti-Müllerian hormone (Amh) and its receptor (Amhr2), inhibin-α (Inha), transcription factors Gata4 and Wt1, and enzymes required for sex steroid biosynthesis (Cyp17a1, Cyp19a1) (Bielinska et al., 2003; Bielinska et al., 2005; Bielinska et al., 2006; Johnsen et al., 2006; Krachulec et al., 2012; Bandiera et al., 2013). Prototypical markers of adrenocortical cell differentiation, such as the ACTH receptor (Mc2r) or corticoid biosynthetic enzymes (Cyp21a1, Cyp11b1, Cyp11b2), are not expressed in the neoplastic tissue (Bielinska et al., 2003; Bielinska et al., 2005; Bielinska et al., 2006; Johnsen et al., 2006).
The genetic basis of GDX-induced adrenocortical neoplasia has been investigated in the mouse. Hypophysectomy, parabiosis, and transplantation experiments have shown that the adrenal glands of susceptible strains of mice exhibit an inherent predisposition to develop tumors in response to gonadotropin stimulation [reviewed in (Bielinska et al., 2005; Bielinska et al., 2006)]. Linkage analysis of crosses between susceptible (DBA/2J) and non-susceptible (C57Bl/6) inbred strains has established that post-GDX adrenocortical neoplasia is a complex trait influenced by multiple genetic loci (Bernichtein et al., 2007; Bernichtein et al., 2008; Bernichtein et al., 2009). Targeted mutagenesis of Gata4, a gene normally expressed in gonadal but not adrenal steroidogenic cells of the adult mouse, attenuates post-GDX adrenocortical tumor formation in susceptible strains (Krachulec et al., 2012), and transgenic expression of Gata4 induces adrenocortical neoplasia in a non-susceptible strain (Chrusciel et al., 2013).
In addition to genetic factors, epigenetic changes such as DNA methylation may contribute to the pathogenesis of GDX-induced adrenocortical neoplasia. Altered methylation of cytosine residues in CpG dinucleotides has been shown to modulate gene expression and progenitor cell fate in various tissues, including endocrine organs (Aranda et al., 2009; Hoivik et al., 2011). For example, conditional mutagenesis of the mouse Dnmt1 gene, which encodes the maintenance DNA methyl-transferase, causes reprogramming of pancreatic β-cells into α-cells (Akerman et al., 2011; Dhawan et al., 2011). It has been suggested that GDX-induced adrenocortical neoplasia may be another example of DNA methylation-regulated cell fate interconversion in an endocrine tissue (Bielinska et al., 2009; Schillebeeckx et al., 2013). According to this hypothesis, epigenetic alterations affect the phenotypic plasticity of adrenocortical stem/progenitor cells, allowing them to respond to the hormonal changes associated with GDX (Feinberg et al., 2006; Bielinska et al., 2009).
The current study was undertaken to identify novel genetic and epigenetic markers of GDX-induced adrenocortical neoplasia, so as to gain a better foothold for investigations into the mechanistic basis of tumorigenesis. Complementary approaches, including genome-wide DNA methylation mapping and microarray expression profiling, were used to screen for genes that are hypomethylated and/or overexpressed in post-GDX adrenocortical neoplasms of the mouse. Candidate genes were validated using a combination of laser capture microdissection (LCM), quantitative RT-PCR (qRT-PCR), and in situ hybridization or immunohistochemistry. One of the validated genes was found to be a marker of post-GDX adrenocortical neoplasia in not only mice but also ferrets.
2. Materials and Methods
2.1. Experimental animals
Procedures involving mice were approved by an institutional committee for laboratory animal care and were conducted in accordance with NIH guidelines for the care and use of experimental animals. DBA/2J mice were purchased from Jackson Laboratories (Bar Harbor, ME). Female mice were anesthetized and gonadectomized at 3-4 weeks of age (Bielinska et al., 2005). We limited our analysis to females because they develop post-GDX adrenocortical neoplasms more readily than their male counterparts (Bielinska et al., 2006; Beuschlein et al., 2012). Adrenal tissue was harvested for analysis 3 months later.
2.2. Isolation of neoplastic and normal tissue using LCM
Cryosections (10 μm) of adrenal glands from ovariectomized or intact mice were collected on membrane slides (PEN-Membrane 2.0 μm; Leica), fixed in acetone (for collecting DNA) or ethanol (for collecting RNA) at -20° C, stained with hematoxylin and eosin (H&E) or crystal violet, and then dehydrated by passage through successively higher concentrations of ethanol followed by xylene (Pihlajoki et al., 2013a; Schillebeeckx et al., 2013). LCM was used to isolate samples from GDX-induced adrenocortical neoplasms, adjacent normal adrenocortical tissue [zona glomerulosa (zG) + zona fasciculata (zF) cells], and the adrenal X-zone. Dissectates were collected in SDS/proteinase K for genomic DNA isolation or in RNA extraction buffer (RNeasy Mini Kit, Qiagen, Valencia, CA).
2.3. DNA methylation analyses
DNA from neoplastic or normal adrenocortical tissue was subjected to genome-wide methylation analysis using LCM-reduced representation bisulfite sequencing (LCM-RRBS) (Schillebeeckx et al., 2013).
2.4. Microarray expression profiling
RNA was isolated from whole adrenal glands of intact, virgin (n = 3) or ovariectomized (n = 3) female DBA/2J mice using RNeasy® Mini Kit (Qiagen, Valencia, CA), amplified using the TotalPrep RNA amplification kit (Illumina, San Diego, CA), and hybridized on an Illumina Mouse6v2 oligonucleotide array. Array hybridization was performed by the GTAC Microarray Core facility at Washington University according to standard protocols.
Expression values were normalized using Partek Genomics Suite (Partek Inc.). More specifically, gene expression intensities were treated by background adjustment, quintile normalization, and log-transformation before statistical analyses. There were 45281 probes and 6 samples. Features were filtered first by “Detection Pvalue”; if all 6 samples had detection pvalue > 0.05, the probe was rejected. A total of 19620 probes were retained after the filtering. The R package genefilter was used to further filter on the basis of interquartile range (IQR), a measure of statistical dispersion. Applying the default value of 50% suggested by genefilter reduced the number of probes to 9810. Permutation-based analysis was performed using the significance analysis of microarrays (SAM) test with the R statistical package siggenes. After adjusting for multiple testing, there were 120 significant probes (corresponding to 95 genes) with permutation FDR < 0.1 (Supplemental Table 1). Unsupervised hierarchical clustering was performed with R function hclust, using a Euclidean distance metric and complete linkage.
2.5. Enrichment analysis
Genes that were differentially expressed in adrenal glands from gonadectomized vs. intact mice were compared to pooled Gene Expression Omnibus (GEO) data for different mouse tissues, using a recently developed method (Chen et al., 2013). Genes with a statistically significant expression specificity (p value < 0.01; Bonferroni corrected) for adrenal, brain, ovary, or testis tissues were considered to be tissue-specific (Supplemental Table 2). Enrichment for upregulated or downregulated genes within the tissue-specific gene dataset was determined using the Fisher’s Exact Test.
2.6. qRT-PCR
Total RNA was isolated and subjected to qRT-PCR analysis as described (Slott et al., 1993). Expression was normalized to the housekeeping genes Actb and Gapdh. Primer pairs are listed in Supplemental Table 3.
2.7. In situ hybridization
Nonradioactive in situ hybridization was performed (Val et al., 2006) using paraformaldehyde-fixed, paraffin-embedded adrenal sections (5 μm). To prepare riboprobes, cDNA fragments of Igfbp6, Foxs1, Spinlw1, and Foxl2 were amplified by RT-PCR (annealing temperature = 52° C) and cloned into the vector pCRII-TOPO (Invitrogen, Carlsbad, CA) using the manufacturer’s guidelines. RT-PCR primers and cDNA fragment sizes are specified in Supplemental Table 4. Digoxygenin-labeled antisense riboprobes were synthesized from EcoRV-linearized plasmids using Sp6 RNA polymerase (Heikinheimo et al., 1994). Bound riboprobe was detected using an alkaline phosphatase conjugated anti-digoxigenin antibody, as described (Val et al., 2006). Sections were subsequently counterstained with nuclear fast red.
2.8. Immunohistochemistry
Mouse tissues were fixed overnight in 4% paraformaldehyde in PBS, embedded in paraffin, sectioned (5 μm), and subjected to immunoperoxidase staining (Anttonen et al., 2003). Formalin-fixed, paraffin-embedded, adrenocortical neoplasms from gonadectomized ferrets were obtained from the archives of a veterinary diagnostic laboratory, as reported previously (Peterson et al., 2003; Peterson et al., 2004; Wagner et al., 2008); included were cases of adrenocortical carcinoma, adenoma, and nodular hyperplasia. Criteria for classification of these tumors are listed elsewhere (Peterson et al., 2003). The primary antibodies were: a) rabbit anti-INSL3 (sc-134587; Santa Cruz Biotechnology, Santa Cruz, CA; 1:200 dilution), b) goat anti-GATA4 (sc-1237, Santa Cruz Biotechnology; 1:200 dilution), and c) goat anti-FOXL2 (IMG-3228; Imgenex, San Diego, CA; 1:400 dilution). Secondary antibodies were: a) goat anti-rabbit biotinylated IgG (NEF-813, NEN Life Science, Boston, MA; 1:2000 dilution) and b) donkey anti-goat biotinylated IgG (Jackson Immunoresearch, West Grove, PA; 1:1000 dilution). The avidin-biotin immunoperoxidase system (Vectastain Elite ABC Kit, Vector Laboratories, Inc., Burlingame, CA) and diaminobenzidine were used to visualize the bound antibody. The analysis included negative control studies in which the primary antibodies were omitted.
2.9. Data Release
The DNA methylation data generated for the study can be found under GEO accession number GSE45361. DNA methylation and raw sequence data are also publically available at the Center for Genome Sciences (www.cgs.wustl.edu/~maxim/). The microarray hybridization data has been deposited under GEO accession number GSE54393.
3. Results and Discussion
3.1. RNA expression profiling identifies Spinlw1 and Insl3 as novel markers of GDX-induced adrenocortical neoplasia in the mouse
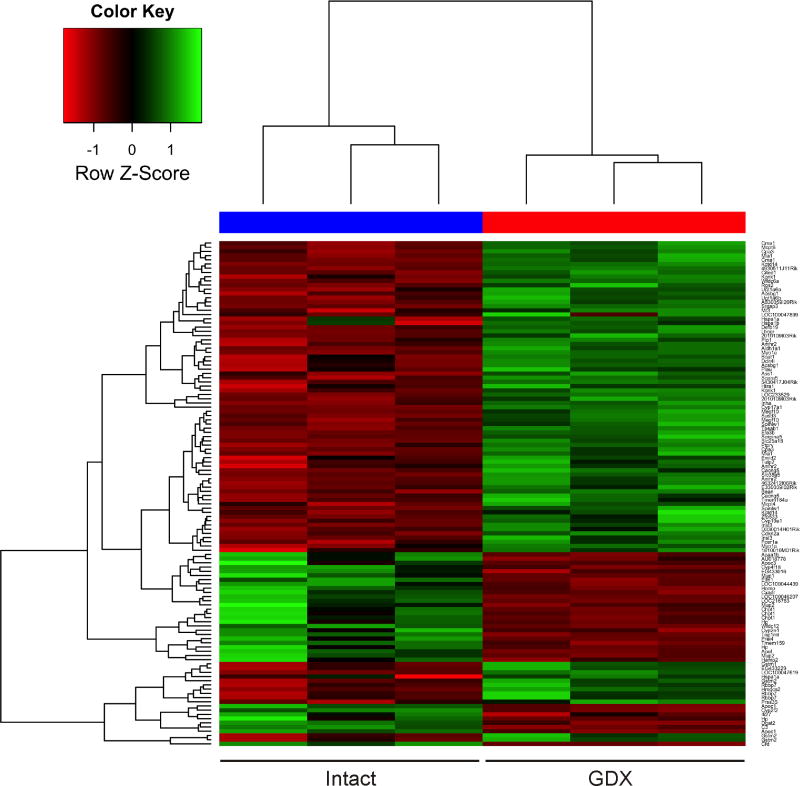
To systematically screen for markers of GDX-induced adrenocortical tumorigenesis, mRNA from whole adrenal glands of gonadectomized vs. intact female DBA/2J mice was subjected to microarray hybridization analysis. A total of 95 genes were found to be differentially regulated, of which 67 were upregulated and 28 were downregulated (FDR < 0.1) (Supplemental Table 1). Unsupervised hierarchical clustering the differentially expressed genes is shown in Fig. 1.
Figure 1. Hierarchical clustering of microarray hybridization results for mRNA isolated from whole adrenal glands of gonadectomized vs. intact female DBA2/J mice (n = 3 each).
Each row denotes the results for a distinct microarray probe. Some genes are represented by multiple probes. Each column signifies a different mouse. Colors vary from red to green for the lowest to highest gene expression levels, respectively. The distance corresponds to a Manhattan distance.
We hypothesized that genes upregulated in the adrenal glands of gonadectomized mice were enriched for markers of gonadal tissue. To test this, we utilized a recently developed method of enrichment analysis (Chen et al., 2013) wherein differentially expressed are compared to pooled GEO microarray data for various mouse tissues (adrenal gland, brown adipose tissue, brain, heart, intestine, kidney, liver, lung, muscle, ovary, skin, testis, thymus, and white adipose tissue). Enrichment analysis confirmed that genes upregulated in the adrenal glands of gonadectomized mice were more likely to be highly expressed in ovary (Fig. 2A; P = 3.0 × 10-9) or testis (Fig. 2B; P = 1.5 × 10-6) than in extragonadal tissues such as brain (Fig. 2D; P = 0.069). Genes downregulated in the adrenal glands of gonadectomized mice were more likely to be highly expressed in normal adrenal tissue (Fig. 2C; P = 4.1 × 10-4) than in brain (Fig. 2E; P = 1.0). This systematic, transcriptomic comparison reinforces the longstanding tenet that GDX induces the selective accumulation of gonadal-like cells in the adrenal glands of DBA/2J mice.
Figure 2. Systematic transcriptome analysis shows that genes upregulated in the adrenal glands of gonadectomized mice are more likely to be highly expressed in ovary or testis than in other tissues.
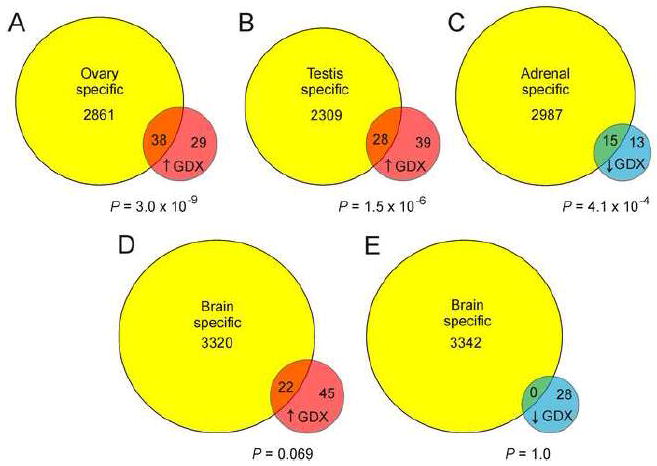
Genes that were differentially expressed in microarray of whole adrenal glands from gonadectomized vs. intact female DBA/2J mice were subjected to enrichment analysis using Fisher’s exact test. Genes defined as “specific” for ovary, testis, adrenal or brain, based on analysis of pooled GEO microarray data (Chen et al., 2013), are shown as yellow circles. Genes that were upregulated in adrenal glands from gonadectomized mice are shown as red circles (↑ GDX). Genes that were downregulated in adrenal glands from gonadectomized mice are shown as blue circles (↓ GDX). Enrichment analysis demonstrated that genes upregulated in the adrenal glands of gonadectomized mice were more likely to be highly expressed in ovary (A) or testis (B) than in brain (D). Genes that were downregulated in the adrenal glands of gonadectomized mice were more likely to be highly expressed in the normal adrenal tissue (C) than in brain (E).
Among the genes upregulated in the microarray analysis were gonadal-like markers previously shown to be expressed in GDX-induced adrenocortical neoplasms, such as Cyp17a1 (460-fold), Lhcgr (43-fold), Cyp19a1 (28-fold), Inha (17-fold), and Amhr2 (6.2- to 9.1-fold on different microarray probes) (Bielinska et al., 2006). In addition to gonadal differentiation markers, 5 mast cell protease genes (Cma1, Mcpt4, Mcpt6, Tpsab1, and Cpa3) were significantly upregulated (5.6- to 12-fold) in the adrenal glands of gonadectomized mice (Supplemental Table 1), consistent with the well-documented phenomenon of mast cell infiltration of post-GDX adrenocortical neoplasms (Kim et al., 1997; Bielinska et al., 2005).
In an effort to identify novel markers of tumorigenesis, genes that were upregulated in the adrenal glands of ovariectomized mice were subjected to further validation studies. For full validation, we stipulated that three criteria had to be met: 1) qRT-PCR analysis of mRNA isolated from whole adrenal glands had to show a significant increase in expression of the gene in gonadectomized vs. intact mice, 2) qRT-PCR analysis of mRNA isolated by LCM had to show a significant increase in expression of the gene in neoplastic vs. adjacent normal adrenocortical tissue, and 3) in situ hybridization or immunohistochemistry of adrenal tissue from gonadectomized mice had to demonstrate expression in the neoplastic cells. Two genes identified in the microarray analysis, Spinlw1 and Insl3, fulfilled all the criteria (Fig. 3A-E).
Figure 3. Two genes identified by microarray expression profiling, Spinlw1 and Insl3, are upregulated in GDX-induced adrenocortical neoplasms of the mouse.
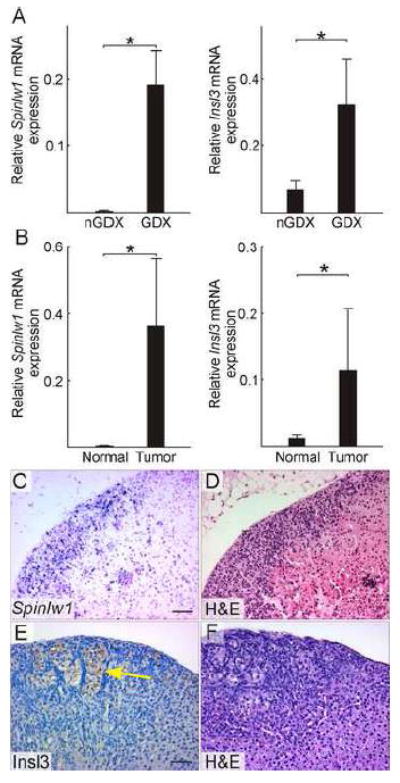
(A) qRT-PCR analysis of Spinlw1 and Insl3 in whole adrenal mRNA from non-gonadectomized (nGDX) or gonadectomized (GDX) female DBA/2J mice (n = 4 per group). (B) qRT-PCR analysis of Spinlw1 and Insl3 in normal or tumor tissue isolated by LCM from the adrenal cortex of ovariectomized DBA/2J mice (n = 4 per group). qRT-PCR results were normalized to expression of the housekeeping gene Actb (*, P < 0.05); normalization to Gapdh yielded similar results. Statistical significance was determined using ANOVA with Dunnett’s multiple comparison test. (C) In situ hybridization of adrenal tissue from an ovariectomized DBA/2J mouse using a Spinlw1 antisense riboprobe. (D) H&E staining of an adjacent tissue section. Spinlw1 mRNA localized to neoplastic cells in the subcapsular region. (C) Immunoperoxidase staining of adrenal tissue from an ovariectomized DBA/2J mouse using anti-INSL3. (D) H&E staining of an adjacent tissue section. INSL3 immunoreactivity was evident in lipid-laden type B neoplastic cells [see (Bielinska et al., 2006) for a description of this cell type]. Control experiments demonstrated INSL3 immunoreactivity in Leydig cells of the adult testis (data not shown). Bars = 50 μm.
Spinlw1, which was upregulated 16- to 51-fold on different microarray probes, encodes EPPIN, a serine protease inhibitor secreted by Sertoli cells and epididymal epithelial cells (O’Rand et al., 2011) but not by cells of the ovary or uterus (Sivashanmugam et al., 2003; Silva et al., 2012). Thus, EPPIN is generally considered to be a “male-specific” marker. Consistent with this notion, expression of Spinlw1 has been shown to be androgen-dependent (Denolet et al., 2006; Schauwaers et al., 2007; Willems et al., 2010; Silva et al., 2012).
Insl3, which was upregulated 50-fold on the microarray, encodes insulin-like 3, a hormone that is secreted by fetal Leydig cells. INSL3 mediates the trans-abdominal phase of testicular descent (Ivell et al., 2013)(Ivell and Anand-Ivell, 2011). Additionally, INSL3 is constitutively secreted by adult Leydig cells and serves as a serum biomarker of this cell type (Anand-Ivell et al., 2009), although the function of INSL3 in the adult testis remains unclear (Ivell et al., 2013). Insl3 is also expressed in the ovary, particularly in theca interna cells of the maturing follicle (Satchell et al., 2013), where it induces androgen production (Glister et al., 2013). It is conceivable that serum levels of INSL3 could be a biomarker of post-GDX adrenocortical neoplasms, and future experiments will explore this possibility.
3.2. A candidate gene approach identifies Foxl2 as a novel marker of GDX-induced adrenocortical neoplasia in the mouse
Due to limits in sensitivity, microarray hybridization analysis often fails to detect changes in expression of transcription factor genes. Indeed, one undisputed marker of GDX-induced adrenocortical neoplasia, Gata4 (Bielinska et al., 2006), showed a non-significant expression change of ~0.5-fold on the microarray, underscoring the inherent limitations of this technology. Therefore, we supplemented the microarray analysis with a candidate gene approach. Foxl2, a transcription factor gene that was marginally upregulated in the microarray analysis (1.3-fold, FDR > 0.1), was found to be another bona fide marker of GDX-induced adrenocortical neoplasia in the mouse, using the same rigorous criteria outlined above (Fig. 4A-F). Foxl2 encodes a forkhead transcription factor that is expressed in granulosa and interstitial cells of the ovary (Schmidt et al., 2004; Mork et al., 2012). Consequently, FOXL2 is generally considered to be a “female-specific” marker (Georges et al., 2013). Mice harboring null mutations in Foxl2 develop ovaries that express testicular differentiation markers (Schmidt et al., 2004; Uda et al., 2004; Ottolenghi et al., 2005; Garcia-Ortiz et al., 2009; Uhlenhaut et al., 2009). Of note, extracts of adrenal glands from non-gonadectomized mice have been shown to contain FOXL2 mRNA and protein at levels 40-50 times lower than extracts of ovary (Yang et al., 2010). The significance of this low level expression of Foxl2 in non-neoplastic adrenal glands is unclear.
Figure 4. Foxl2, a marker identified through a candidate gene approach, is upregulated in GDX-induced adrenocortical neoplasms of the mouse.
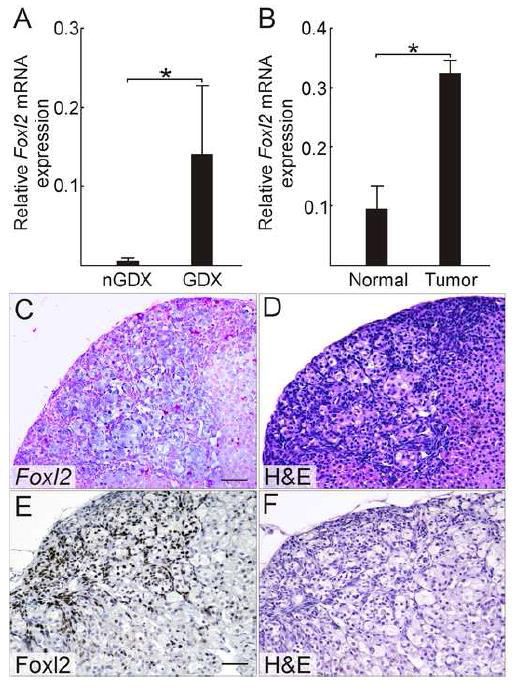
(A) qRT-PCR analysis of Foxl2 in whole adrenal mRNA from non-gonadectomized (nGDX) or gonadectomized (GDX) female DBA/2J mice (n = 4 per group). (B) qRT-PCR analysis of Foxl2 in normal or tumor tissue isolated by LCM from the adrenal cortex of ovariectomized DBA/2J mice (n = 4 per group). qRT-PCR results were normalized to expression of the housekeeping gene Actb (*, P < 0.05); normalization to Gapdh yielded similar results. Statistical significance was determined using ANOVA with Dunnett’s multiple comparison test. (C) In situ hybridization of adrenal tissue from an ovariectomized DBA/2J mouse using a Foxl2 antisense riboprobe. (D) H&E staining of an adjacent tissue section. Bar = 50 μm. (E) Immunoperoxidase staining of adrenal tissue from an ovariectomized DBA/2J mouse using anti-FOXL2. (F) H&E staining of an adjacent tissue section. Nuclear FOXL2 immunoreactivity was evident in both small, basophilic type A neoplastic cells and large, lipid-laden type B neoplastic cells [see (Bielinska et al., 2006) for a description of these cell types]. Control stainings demonstrated nuclear FOXL2 immunoreactivity in granulosa cells of the adult ovary (data not shown). Bar = 50 μm.
3.3. FOXL2 is a novel marker of adrenocortical neoplasia in gonadectomized ferrets
Sex steroid-producing neoplasms arise in up to 20% of gonadectomized ferrets and are a major cause of morbidity and mortality in this species (Bielinska et al., 2006; Beuschlein et al., 2012). Immunostaining of archival veterinary pathology specimens showed that FOXL2 is a marker of post-GDX adrenocortical neoplasia in the ferret (Fig. 5A-F; Supplemental Fig. 1). The specimens were obtained from gonadectomized ferrets with signs of ectopic sex steroid production (e.g., alopecia, vulvar hyperplasia, or stranguria) and included examples of adrenocortical carcinoma, adenoma, and nodular hyperplasia. Several of the specimens contained residual normal cortex, which served as a negative control (Fig. 5A, C, E). Nuclear FOXL2 immunoreactivity was detected in 50% of the specimens examined [4 of 10 cases of adrenocortical carcinoma, 4 of 6 cases of adrenocortical adenoma, and 1 of 2 cases of nodular hyperplasia]. FOXL2-positive tumors were seen in both female (Fig. 5) and male (Supplemental Fig. 1) ferrets. Only a minority of the cells within a given tumor reacted with FOXL2 antibody (Fig. 5D; Supplemental Fig. 1). By comparison, nuclear GATA4 immunoreactivity has been observed in >90% of ferret adrenocortical tumors (Peterson et al., 2004), and GATA4 antibody typically stains a higher percentage of cells within a given tumor (Fig. 5F).
Figure 5. FOXL2 immunoreactivity in neoplastic adrenocortical cells from a gonadectomized female ferret with clinical evidence of ectopic sex steroid production.
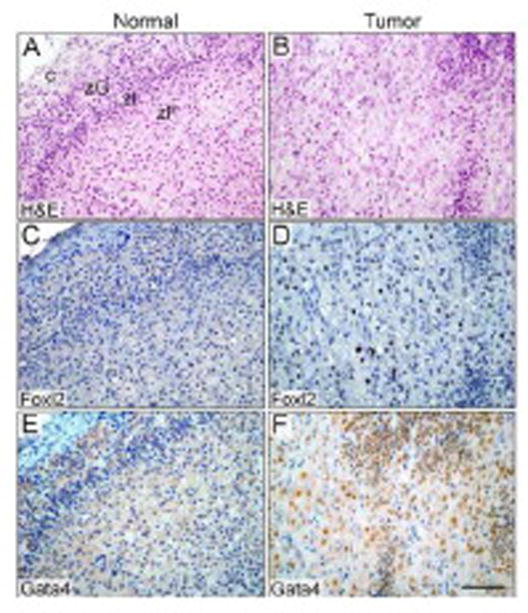
Sections of normal (A,C,E) or neoplastic (B,D,F) adrenal cortex were subjected to H&E staining (A,B) or to immunoperoxidase staining with anti-FOXL2 (C,D) or anti-GATA4 (E,F). The neoplastic tissue contained a mixture of small, basophilic cells and large, polyhedral cells. Nuclear GATA4 immunoreactivity was evident in both the small and large neoplastic cells, whereas nuclear FOXL2 immunoreactivity was limited to the large neoplastic cells in this specimen. Bar = 50 μm. Abbreviations: c, capsule; zF, zona fasciculata; zG, zona glomerulosa; zI, zona intermedia [a zone characteristic of ferrets and other carnivores (Holmes, 1961)].
We conclude that FOXL2 is a marker of GDX-induced adrenocortical tumors in not only the mouse but also the ferret. This observation expands the list of genes known to be expressed in sex steroidogenic adrenocortical neoplasms of the ferret (Schoemaker et al., 2002; Peterson et al., 2003; Peterson et al., 2004; Bielinska et al., 2006; Wagner et al., 2008; de Jong et al., 2013; de Jong et al., 2014). The relatively low percentage of FOXL2 immunoreactive cells within ferret adrenocortical tumors may limit the diagnostic utility of this marker. It remains to be determined whether the other markers of adrenocortical neoplasia that emerged from the microarray hybridization analysis of gonadectomized mice are also markers of tumorigenesis in ferrets.
3.4. Two hypomethylated genes, Igfbp6 and Foxs1, are upregulated in GDX-induced adrenocortical neoplasms of the mouse
In a prior report we used a highly sensitive method of global DNA methylation analysis, termed LCM-RRBS, to identify gene promoters that are differentially methylated in neoplastic vs. normal adrenocortical tissue from ovariectomized DBA/2J mice (Schillebeeckx et al., 2013). We reasoned that promoters that are hypomethylated in the neoplastic tissue could be novel markers of GDX-induced adrenocortical tumorigenesis. In the current study we subjected these hypomethylated candidate genes to validation studies, including qRT-PCR analysis of mRNA isolated by LCM and in situ hybridization. Two genes, Igfbp6 and Foxs1, were confirmed to be upregulated GDX-induced tumors (Fig. 6)
Figure 6. Two genes identified as hypomethylated by genome-wide mapping, Igfbp6 and Foxs1, are upregulated in GDX-induced adrenocortical neoplasms of the mouse.
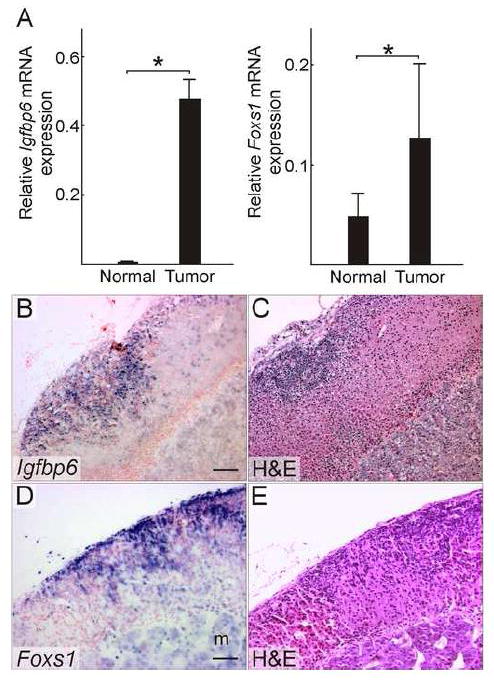
(A) qRT-PCR analysis of Igfbp6 and Foxs1 in normal or tumor tissue isolated by LCM from the adrenal cortex of ovariectomized DBA/2J mice (n = 4 per group). qRT-PCR results were normalized to expression of Actb (*, P < 0.05); normalization to Gapdh yielded similar results. Statistical significance was determined using ANOVA with Dunnett’s multiple comparison test. (B) In situ hybridization of adrenal tissue from an ovariectomized DBA/2J mouse using an Igfbp6 antisense riboprobe. (C) H&E staining of an adjacent tissue section. Igfbp6 mRNA localized to neoplastic cells in the subcapsular region. (D) In situ hybridization of adrenal tissue from an ovariectomized DBA/2J mouse using a Foxs1 antisense riboprobe. (E) H&E staining of an adjacent tissue section. Foxs1 mRNA localized to neoplastic tissue in the subcapsular region and to cells in the medulla (m). Bars = 50 μm.
Igfbp6 encodes a member of a family of insulin-related growth factor binding proteins (IGFBPs), which modulate the interaction of insulin-related growth factors (IGFs) with their cell surface receptors. IGFBP6 differs from the other 5 members of the IGFBP family in that it has a 30- to 100-fold preferential binding affinity for IGF2 over IGF1 (Bach et al., 1993). Both IGF2 and IGF1 impact the growth, differentiation, and function of adrenocortical cells (Weber et al., 1999), and IGFBP6 has been shown to inhibit the actions of IGF2 in experimental systems (Bach et al., 1994). Hormone-dependent Igfbp6 expression has been documented in somatic cells of the rodent ovary (Rohan et al., 1993). It is conceivable that IGFBP6 produced in post-GDX adrenocortical neoplasms serves an insulator function by blocking the activity of IGF2, thereby favoring gonadal-like differentiation over adrenocortical differentiation.
Foxs1 (Fkhl18) encodes a forkhead-domain transcription factor that is expressed in Sertoli cells and periendothelial cells of the developing mouse fetal testis (Sato et al., 2008). Male and female Foxs1 knockout mice are viable and fertile, but the mutant males accumulate blood in the fetal testis, presumably due to apoptosis of periendothelial cells (Sato et al., 2008). Foxs1 is also expressed in gonadal-like cells that accumulate in the adrenal subcapsule of Gata6flox/flox; Sf1-cre mice (Pihlajoki et al., 2013a). In addition to gonadal(-like) cells, Foxs1 is expressed in neural crest derivatives (Heglind et al., 2005; Montelius et al., 2007); consistent with this notion, in situ hybridization showed strong Foxs1 expression in the neoplastic adrenocortical cells and weak expression in chromaffin cells of the adrenal medulla (Fig. 6E).
That only 2 of the 37 hypomethylated genes identified in the original differential methylation screen (Schillebeeckx et al., 2013) were validated as upregulated markers is not surprising. Hypomethylation is correlated with increased gene expression, but the relationship is not absolute. Moreover, the small amounts of RNA isolated by LCM precluded qRT-PCR validation of a large number of genes.
None of the novel markers identified on the basis of transcriptome analysis showed evidence of differential methylation in the LCM-RRBS analysis (Schillebeeckx et al., 2013). The degree of methylation of the Foxl2 promoter was <1% in both neoplastic and normal tissue, and the degree of methylation of the Insl3 promoter was comparable in neoplastic and normal tissue (13% vs. 16%; σ = 4.1 and σ = 5.3, respectively). Coverage of the Spinlw1 promoter was inadequate for quantification of its methylation status.
4. Summary
Using complementary approaches, including microarray expression profiling, candidate gene analysis, and DNA methylation mapping, we have identified novel genetic (Spinlw1, Insl3, Foxl2) and epigenetic (Igfbp6, Foxs1) markers of GDX-induced adrenocortical neoplasia in the mouse. That both “male-specific” (Spinlw1) and “female-specific” (Foxl2) markers were detected is noteworthy and implies that the neoplasms exhibit mixed characteristics of male and female gonadal somatic cells. Such indeterminate steroidogenic cell phenotypes have been reported in other experimental models (Heikkila et al., 2002; Val et al., 2006). One of the markers, FOXL2, was observed in adrenocortical tumor specimens from gonadectomized ferrets. These new markers may prove useful for studies of steroidogenic cell development and for tumor classification.
Supplementary Material
Nuclear FOXL2 staining is evident a subset of the neoplastic cells (arrowheads) but not in normal adrenocortical cells (arrow). Bar = 50 μm.
Differentially expressed genes were selected on the basis of permutation FDR < 0.1. The q values in this table are adjusted p values derived using the Benjamini-Hochberg method.
The 4 lists, which were compiled using the method of Chen et al. (2013), are in shown in separate tabs of the spreadsheet. For convenience, microarray hybridization results, denoted as the ratio of expression in whole adrenal glands of gonadectomized (GDX) vs. non-gonadectomized (nGDX) mice, are included in the tables.
Highlights.
Spinlw1, Insl3, & Foxl2 are upregulated in murine post-GDX adrenocortical tumors.
FOXL2 immunoreactivity is evident in adrenocortical tumors from gonadectomized ferrets.
Igfbp6 & Foxs1 are hypomethylated and upregulated in murine post-GDX adrenocortical tumors.
Post-GDX adrenocortical tumors exhibit properties of female and male gonadal cells.
Acknowledgments
Grant support: NIH grants DK52574, DK075618, and DA025744, American Heart Association grant 13GRNT16850031, Children’s Discovery Institute, the Sigrid Jusélius Foundation, the Academy of Finland, and CIMO (Centre for International Mobility Finland) fellowship TM-13-8769.
We thank members of the histology, laser microscopy, and microarray cores for technical assistance. We thank Michael Brooks for providing assistance with the microarray analysis, Mikko Anttonen for helpful suggestions, and Matti Kiupel for providing ferret tissue specimens.
Footnotes
Disclosure summary: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akerman I, van Arensbergen J, Ferrer J. Removing the brakes on cell identity. Dev Cell. 2011;20:411–412. doi: 10.1016/j.devcel.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Anand-Ivell R, Heng K, Hafen B, Setchell B, Ivell R. Dynamics of INSL3 peptide expression in the rodent testis. Biol Reprod. 2009;81:480–487. doi: 10.1095/biolreprod.109.077552. [DOI] [PubMed] [Google Scholar]
- Anttonen M, Ketola I, Parviainen H, Pusa AK, Heikinheimo M. FOG-2 and GATA-4 are coexpressed in the mouse ovary and can modulate Mullerian-inhibiting substance expression. BiolReprod. 2003;68:1333–1340. doi: 10.1095/biolreprod.102.008599. [DOI] [PubMed] [Google Scholar]
- Aranda P, Agirre X, Ballestar E, Andreu EJ, Roman-Gomez J, Prieto I, Martin-Subero JI, Cigudosa JC, Siebert R, Esteller M, et al. Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS One. 2009;4:e7809. doi: 10.1371/journal.pone.0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach LA, Hsieh S, Brown AL, Rechler MM. Recombinant human insulin-like growth factor (IGF)-binding protein-6 inhibits IGF-II-induced differentiation of L6A1 myoblasts. Endocrinology. 1994;135:2168–2176. doi: 10.1210/endo.135.5.7525263. [DOI] [PubMed] [Google Scholar]
- Bach LA, Hsieh S, Sakano K, Fujiwara H, Perdue JF, Rechler MM. Towards identification of a binding site on insulin-like growth factor-II for IGF-binding proteins. Adv Exp Med Biol. 1993;343:55–61. doi: 10.1007/978-1-4615-2988-0_6. [DOI] [PubMed] [Google Scholar]
- Bandiera R, Vidal VP, Motamedi FJ, Clarkson M, Sahut-Barnola I, von Gise A, Pu WT, Hohenstein P, Martinez A, Schedl A. WT1 Maintains Adrenal-Gonadal Primordium Identity and Marks a Population of AGP-like Progenitors within the Adrenal Gland. Dev Cell. 2013;27:5–18. doi: 10.1016/j.devcel.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernichtein S, Alevizaki M, Huhtaniemi I. Is the adrenal cortex a target for gonadotropins? Trends EndocrinolMetab. 2008;19:231–238. doi: 10.1016/j.tem.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Peltoketo H, Huhtaniemi I. Adrenal hyperplasia and tumours in mice in connection with aberrant pituitary-gonadal function. Mol Cell Endocrinol. 2009;300:164–168. doi: 10.1016/j.mce.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Petretto E, Jamieson S, Goel A, Aitman TJ, Mangion JM, Huhtaniemi IT. Adrenal gland tumorigenesis after gonadectomy in mice is a complex genetic trait driven by epistatic loci. Endocrinology. 2007;149:651–661. doi: 10.1210/en.2007-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuschlein F, Galac S, Wilson DB. Animal models of adrenocortical tumorigenesis. Mol Cell Endocrinol. 2012;351:78–86. doi: 10.1016/j.mce.2011.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppaluoto J, Rahman N, Heikinheimo M, Wilson DB. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology. 2005;146:3975–3984. doi: 10.1210/en.2004-1643. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Kiiveri S, Parviainen H, Mannisto S, Heikinheimo M, Wilson DB. Gonadectomy-induced adrenocortical neoplasia in the domestic ferret (Mustela putorius furo) and laboratory mouse. VetPathol. 2006;43:97–117. doi: 10.1354/vp.43-2-97. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Parviainen H, Kiiveri S, Heikinheimo M, Wilson DB. Review paper: origin and molecular pathology of adrenocortical neoplasms. Vet Pathol. 2009;46:194–210. doi: 10.1354/vp.46-2-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M, Parviainen H, Porter-Tinge SB, Kiiveri S, Genova E, Rahman N, Huhtaniemi IT, Muglia LJ, Heikinheimo M, Wilson DB. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology. 2003;144:4123–4133. doi: 10.1210/en.2003-0126. [DOI] [PubMed] [Google Scholar]
- Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012;139:4661–4570. doi: 10.1242/dev.087247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen IB, Rathi VK, DeAndrade DS, Jay PY. Association of genes with physiological functions by comparative analysis of pooled expression microarray data. Physiol Genomics. 2013;45:69–78. doi: 10.1152/physiolgenomics.00116.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrusciel M, Vuorenoja S, Mohanty B, Rivero-Muller A, Li X, Toppari J, Huhtaniemi I, Rahman NA. Transgenic GATA-4 expression induces adrenocortical tumorigenesis in C57Bl/6 mice. J Cell Sci. 2013;126:1845–1857. doi: 10.1242/jcs.119347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MK, Schoemaker NJ, Mol JA. Expression of sfrp1 and activation of the Wnt pathway in the adrenal glands of healthy ferrets and neutered ferrets with hyperadrenocorticism. Vet J. 2013;196:176–180. doi: 10.1016/j.tvjl.2012.09.022. [DOI] [PubMed] [Google Scholar]
- de Jong MK, ten Asbroek EEM, Sleiderink AJ, Conley AJ, Mol JA, Schoemaker NJ. Gonadectomy-related adrenocortical tumors in ferrets demonstrate increased expression of androgen and estrogen synthesizing enzymes together with high inhibin expression. Domestic Animal Endocrinology. 2014;48:42–47. doi: 10.1016/j.domaniend.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Denolet E, De Gendt K, Allemeersch J, Engelen K, Marchal K, Van Hummelen P, Tan KA, Sharpe RM, Saunders PT, Swinnen JV, et al. The effect of a sertoli cell-selective knockout of the androgen receptor on testicular gene expression in prepubertal mice. Mol Endocrinol. 2006;20:321–334. doi: 10.1210/me.2005-0113. [DOI] [PubMed] [Google Scholar]
- Dhawan S, Georgia S, Tschen SI, Fan G, Bhushan A. Pancreatic beta cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell. 2011;20:419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doghman M, Lalli E. A matter of dosage: SF-1 in adrenocortical development and cancer. Ann Endocrinol (Paris) 2009;70:148–152. doi: 10.1016/j.ando.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. NatRevGenet. 2006;7:21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- Garcia-Ortiz JE, Pelosi E, Omari S, Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco A, et al. Foxl2 functions in sex determination and histogenesis throughout mouse ovary development. BMC Dev Biol. 2009;9:36. doi: 10.1186/1471-213X-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges A, Auguste A, Bessiere L, Vanet A, Todeschini AL, Veitia RA. FOXL2: a central transcription factor of the ovary. J Mol Endocrinol. 2013 doi: 10.1530/JME-13-0159. [DOI] [PubMed] [Google Scholar]
- Glister C, Satchell L, Bathgate RA, Wade JD, Dai Y, Ivell R, Anand-Ivell R, Rodgers RJ, Knight PG. Functional link between bone morphogenetic proteins and insulin-like peptide 3 signaling in modulating ovarian androgen production. Proc Natl Acad Sci U S A. 2013;110:E1426–1435. doi: 10.1073/pnas.1222216110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heglind M, Cederberg A, Aquino J, Lucas G, Ernfors P, Enerback S. Lack of the central nervous system- and neural crest-expressed forkhead gene Foxs1 affects motor function and body weight. Mol Cell Biol. 2005;25:5616–5625. doi: 10.1128/MCB.25.13.5616-5625.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikinheimo M, Scandrett JM, Wilson DB. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol. 1994;164:361–373. doi: 10.1006/dbio.1994.1206. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Hoivik EA, Bjanesoy TE, Bakke M. Epigenetic regulation of the gene encoding steroidogenic factor-1. Mol Cell Endocrinol. 2013;371:133–139. doi: 10.1016/j.mce.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Hoivik EA, Bjanesoy TE, Mai O, Okamoto S, Minokoshi Y, Shima Y, Morohashi K, Boehm U, Bakke M. DNA methylation of intronic enhancers directs tissue-specific expression of steroidogenic factor 1/adrenal 4 binding protein (SF-1/Ad4BP) Endocrinology. 2011;152:2100–2112. doi: 10.1210/en.2010-1305. [DOI] [PubMed] [Google Scholar]
- Holmes RL. The adrenal glands of the ferret. JAnat. 1961;95:325–336. [PMC free article] [PubMed] [Google Scholar]
- Hu L, Monteiro A, Johnston H, King P, O’Shaughnessy PJ. Expression of Cyp21a1 and Cyp11b1 in the fetal mouse testis. Reproduction. 2007;134:585–591. doi: 10.1530/REP-07-0133. [DOI] [PubMed] [Google Scholar]
- Ivell R, Anand-Ivell R. Biological role and clinical significance of insulin-like peptide 3. Curr Opin Endocrinol Diabetes Obes. 2011;18:210–216. doi: 10.1097/MED.0b013e3283453fe6. [DOI] [PubMed] [Google Scholar]
- Ivell R, Wade JD, Anand-Ivell R. INSL3 as a Biomarker of Leydig Cell Functionality. Biol Reprod. 2013;88:147. doi: 10.1095/biolreprod.113.108969. [DOI] [PubMed] [Google Scholar]
- Johnsen IK, Slawik M, Shapiro I, Hartmann MF, Wudy SA, Looyenga BD, Hammer GD, Reincke M, Beuschlein F. Gonadectomy in mice of the inbred strain CE/J induces proliferation of sub-capsular adrenal cells expressing gonadal marker genes. JEndocrinol. 2006;190:47–57. doi: 10.1677/joe.1.06750. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kubota H, Kiuchi Y, Doi K, Saegusa J. Subcapsular cell hyperplasia and mast cell infiltration in the adrenal cortex of mice: comparative study in 7 inbred strains. Exp Anim. 1997;46:303–306. doi: 10.1538/expanim.46.303. [DOI] [PubMed] [Google Scholar]
- Krachulec J, Vetter M, Schrade A, Lobs AK, Bielinska M, Cochran R, Kyronlahti A, Pihlajoki M, Parviainen H, Jay PY, et al. GATA4 Is a critical regulator of gonadectomy-induced adrenocortical tumorigenesis in mice. Endocrinology. 2012;153:2599–2611. doi: 10.1210/en.2011-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer E, Kesper D, Vortkamp A, King P. Sonic hedgehog signaling during adrenal development. Mol Cell Endocrinol. 2012;351:19–27. doi: 10.1016/j.mce.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LA, Fagerstone KA, Wagner RA, Finkler M. Use of a GnRH vaccine, GonaCon, for prevention and treatment of adrenocortical disease (ACD) in domestic ferrets. Vaccine. 2013;31:4619–4623. doi: 10.1016/j.vaccine.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Montelius A, Marmigere F, Baudet C, Aquino JB, Enerback S, Ernfors P. Emergence of the sensory nervous system as defined by Foxs1 expression. Differentiation. 2007;75:404–417. doi: 10.1111/j.1432-0436.2006.00154.x. [DOI] [PubMed] [Google Scholar]
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, Capel B. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod. 2012;86:37. doi: 10.1095/biolreprod.111.095208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K, Zubair M. The fetal and adult adrenal cortex. Mol Cell Endocrinol. 2011;336:193–197. doi: 10.1016/j.mce.2010.11.026. [DOI] [PubMed] [Google Scholar]
- O’Rand MG, Widgren EE, Hamil KG, Silva EJ, Richardson RT. Functional studies of eppin. Biochem Soc Trans. 2011;39:1447–1449. doi: 10.1042/BST0391447. [DOI] [PubMed] [Google Scholar]
- Ottolenghi C, Omari S, Garcia-Ortiz JE, Uda M, Crisponi L, Forabosco A, Pilia G, Schlessinger D. Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet. 2005;14:2053–2062. doi: 10.1093/hmg/ddi210. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Kiupel M, Bielinska M, Kiiveri S, Heikinheimo M, Capen CC, Wilson DB. Transcription factor GATA-4 is a marker of anaplasia in adrenocortical neoplasms of the domestic ferret (Mustela putorius furo) VetPathol. 2004;41:446–449. doi: 10.1354/vp.41-4-446. [DOI] [PubMed] [Google Scholar]
- Peterson RA, Kiupel M, Capen CC. Adrenal cortical carcinomas with myxoid differentiation in the domestic ferret (Mustela putorius furo) VetPathol. 2003;40:136–142. doi: 10.1354/vp.40-2-136. [DOI] [PubMed] [Google Scholar]
- Pihlajoki M, Gretzinger E, Cochran R, Kyronlahti A, Schrade A, Hiller T, Sullivan L, Shoykhet M, Schoeller EL, Brooks MD, et al. Conditional Mutagenesis of Gata6 in SF1-Positive Cells Causes Gonadal-Like Differentiation in the Adrenal Cortex of Mice. Endocrinology. 2013a;154:1754–1767. doi: 10.1210/en.2012-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajoki M, Heikinheimo M, Wilson DB. Never underestimate the complexity of remodeling. Endocrinology. 2013b;154:4446–4449. doi: 10.1210/en.2013-1982. [DOI] [PubMed] [Google Scholar]
- Rohan RM, Ricciarelli E, Kiefer MC, Resnick CE, Adashi EY. Rat ovarian insulin-like growth factor-binding protein-6: a hormonally regulated theca-interstitial-selective species with limited antigonadotropic activity. Endocrinology. 1993;132:2507–2512. doi: 10.1210/endo.132.6.7684977. [DOI] [PubMed] [Google Scholar]
- Satchell L, Glister C, Bleach EC, Glencross RG, Bicknell AB, Dai Y, Anand-Ivell R, Ivell R, Knight PG. Ovarian Expression of Insulin-Like Peptide 3 (INSL3) and Its Receptor (RXFP2) During Development of Bovine Antral Follicles and Corpora Lutea and Measurement of Circulating INSL3 Levels During Synchronized Estrous Cycles. Endocrinology. 2013;154:1897–1906. doi: 10.1210/en.2012-2232. [DOI] [PubMed] [Google Scholar]
- Sato Y, Baba T, Zubair M, Miyabayashi K, Toyama Y, Maekawa M, Owaki A, Mizusaki H, Sawamura T, Toshimori K, et al. Importance of forkhead transcription factor Fkhl18 for development of testicular vasculature. Mol Reprod Dev. 2008;75:1361–1371. doi: 10.1002/mrd.20888. [DOI] [PubMed] [Google Scholar]
- Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, et al. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A. 2007;104:4961–4966. doi: 10.1073/pnas.0610814104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillebeeckx M, Schrade A, Lobs AK, Pihlajoki M, Wilson DB, Mitra RD. Laser capture microdissection-reduced representation bisulfite sequencing (LCM-RRBS) maps changes in DNA methylation associated with gonadectomy-induced adrenocortical neoplasia in the mouse. Nucleic Acids Res. 2013;41:e116. doi: 10.1093/nar/gkt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development. 2004;131:933–942. doi: 10.1242/dev.00969. [DOI] [PubMed] [Google Scholar]
- Schoemaker NJ, Teerds KJ, Mol JA, Lumeij JT, Thijssen JH, Rijnberk A. The role of luteinizing hormone in the pathogenesis of hyperadrenocorticism in neutered ferrets. MolCell Endocrinol. 2002;197:117–125. doi: 10.1016/s0303-7207(02)00285-x. [DOI] [PubMed] [Google Scholar]
- Shima Y, Miyabayashi K, Baba T, Otake H, Oka S, Zubair M, Morohashi K. Identification of an enhancer in the Ad4BP/SF-1 gene specific for fetal Leydig cells. Endocrinology. 2012;153:417–425. doi: 10.1210/en.2011-1407. [DOI] [PubMed] [Google Scholar]
- Silva EJ, Patrao MT, Tsuruta JK, O’Rand MG, Avellar MC. Epididymal protease inhibitor (EPPIN) is differentially expressed in the male rat reproductive tract and immunolocalized in maturing spermatozoa. Mol Reprod Dev. 2012;79:832–842. doi: 10.1002/mrd.22119. [DOI] [PubMed] [Google Scholar]
- Simon DP, Hammer GD. Adrenocortical stem and progenitor cells: implications for adrenocortical carcinoma. Mol Cell Endocrinol. 2012;351:2–11. doi: 10.1016/j.mce.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivashanmugam P, Hall SH, Hamil KG, French FS, O’Rand MG, Richardson RT. Characterization of mouse Eppin and a gene cluster of similar protease inhibitors on mouse chromosome 2. Gene. 2003;312:125–134. doi: 10.1016/s0378-1119(03)00608-5. [DOI] [PubMed] [Google Scholar]
- Slott VL, Suarez JD, Poss PM, Linder RE, Strader LF, Perreault SD. Optimization of the Hamilton-Thorn computerized sperm motility analysis system for use with rat spermatozoa in toxicological studies. Fundam Appl Toxicol. 1993;21:298–307. doi: 10.1006/faat.1993.1102. [DOI] [PubMed] [Google Scholar]
- Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet. 2004;13:1171–1181. doi: 10.1093/hmg/ddh124. [DOI] [PubMed] [Google Scholar]
- Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Val P, Jeays-Ward K, Swain A. Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol. 2006;299:250–256. doi: 10.1016/j.ydbio.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Wagner S, Kiupel M, Peterson RA, Heikinheimo M, Wilson DB. Cytochrome b5 expression in gonadectomy-induced adrenocortical neoplasms of the domestic ferret (Mustela putorius furo) VetPathol. 2008;45:439–442. doi: 10.1354/vp.45-4-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber MM, Fottner C, Schmidt P, Brodowski KM, Gittner K, Lahm H, Engelhardt D, Wolf E. Postnatal overexpression of insulin-like growth factor II in transgenic mice is associated with adrenocortical hyperplasia and enhanced steroidogenesis. Endocrinology. 1999;140:1537–1543. doi: 10.1210/endo.140.4.6660. [DOI] [PubMed] [Google Scholar]
- Willems A, De Gendt K, Allemeersch J, Smith LB, Welsh M, Swinnen JV, Verhoeven G. Early effects of Sertoli cell-selective androgen receptor ablation on testicular gene expression. Int J Androl. 2010;33:507–517. doi: 10.1111/j.1365-2605.2009.00964.x. [DOI] [PubMed] [Google Scholar]
- Wood MA, Acharya A, Finco I, Swonger JM, Elston MJ, Tallquist MD, Hammer GD. Fetal adrenal capsular cells serve as progenitor cells for steroidogenic and stromal adrenocortical cell lineages in M. musculus. Development. 2013;140:4522–4532. doi: 10.1242/dev.092775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood MA, Hammer GD. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336:206–212. doi: 10.1016/j.mce.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Gutierrez NM, Wang L, Ellsworth BS, Wang CM. Synergistic activation of the Mc2r promoter by FOXL2 and NR5A1 in mice. Biol Reprod. 2010;83:842–851. doi: 10.1095/biolreprod.110.085621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Ishihara S, Oka S, Okumura K, Morohashi K. Two-step regulation of Ad4BP/SF-1 gene transcription during fetal adrenal development: initiation by a Hox-Pbx1-Prep1 complex and maintenance via autoregulation by Ad4BP/SF-1. Mol Cell Biol. 2006;26:4111–4121. doi: 10.1128/MCB.00222-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Parker KL, Morohashi K. Developmental links between the fetal and adult zones of the adrenal cortex revealed by lineage tracing. Mol Cell Biol. 2008;28:7030–7040. doi: 10.1128/MCB.00900-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nuclear FOXL2 staining is evident a subset of the neoplastic cells (arrowheads) but not in normal adrenocortical cells (arrow). Bar = 50 μm.
Differentially expressed genes were selected on the basis of permutation FDR < 0.1. The q values in this table are adjusted p values derived using the Benjamini-Hochberg method.
The 4 lists, which were compiled using the method of Chen et al. (2013), are in shown in separate tabs of the spreadsheet. For convenience, microarray hybridization results, denoted as the ratio of expression in whole adrenal glands of gonadectomized (GDX) vs. non-gonadectomized (nGDX) mice, are included in the tables.