Abstract
Introduction
Myrosinase (thioglucoside glucohydrolase; E.C. 3.2.1.147), is a plant enzyme of increasing interest and importance to the biomedical community. Myrosinase catalyses the formation of isothiocyanates such as sulforaphane (frombroccoli) and 4-(α-l-rhamnopyranosyloxy)benzyl isothiocyanate (from moringa), which are potent inducers of the cytoprotective phase-2 response in humans, by hydrolysis of their abundant glucosinolate (β-thioglucoside N-hydroxysulphate) precursors.
Objective
To develop an aqueous two-phase counter-current chromatography (CCC) system for the rapid, three-step purification of catalytically active myrosinase.
Methods
A high-concentration potassium phosphate and polyethylene glycol biphasic aqueous two-phase system (ATPS) is used with a newly developed CCC configuration that utilises spiral-wound, flat-twisted tubing (with an ovoid cross-section).
Results
Making the initial crude plant extract directly in the ATPS and injecting only the lower phase permitted highly selective partitioning of the myrosinase complex before a short chromatography on a spiral disk CCC. Optimum phase retention and separation of myrosinase from other plant proteins afforded a 60-fold purification.
Conclusion
Catalytically active myrosinase is purified from 3-day broccoli sprouts, 7-day daikon sprouts, mustard seeds and the leaves of field-grown moringa trees, in a CCC system that is predictably scalable.
Keywords: Counter-current chromatography, broccoli, daikon, glucosinolate, isothiocyanate, moringa, mustard
Introduction
Myrosinase is a plant enzyme of growing interest to the biomedical and nutrition communities because of its central role in the bioavailability of a large group of chemoprotective natural products. Myrosinase converts glucosinolates (β-thioglucoside N-hydroxysulphates) in plant cells to their cognate bioactive isothiocyanates in a set of reactions widely referred to as the ‘mustard-oil bomb’ (Matile, 1980). This enzyme is present as part of the defensive mechanism against insect, fungal and bacterial pathogens that certain dietary plants possess, primarily the Brassica vegetables such as broccoli (Brassica oleracea var. italica), daikon (Raphanus sativus), mustard (Sinapis alba) and moringa (Moringa oleifera and all other known species in this edible tropical genus) (Fahey et al., 2001; Fahey, 2005; Waterman et al., 2014; J. W. Fahey, unpublished data). It is also present in the gastrointestinal microflora of humans (Fahey et al., 2012).
The isothiocyanate products of the myrosinase-catalysed reaction induce the cytoprotective (phase 2) response in mammals and they also have selective antibiotic, anti-inflammatory and even radioprotective activity (Fan et al., 2013). They are of special importance in the prevention of cancer and chronic diseases such as type 2 diabetes, asthma and air-pollution-related injury (Dinkova-Kostova and Kostov, 2012; Egner et al., 2014). The rates of conversion of the over 120 naturally occurring glucosinolates to isothiocyanates (Fahey et al., 2012) and the abundance and localisation of microbial myrosinases within the gastrointestinal tract (Li et al., 2011) are key factors in modulating the bioavailability of these compounds from cooked and, to a lesser extent, raw dietary crucifers (Brassica vegetables) and, ultimately, to the disease preventive activity of these vegetables (Tang et al., 2010; Li et al., 2013). The importance of myrosinase to food scientists and nutritionists developing foods, functional foods and dietary supplements directed towards improving health from the perspectives of food quality, processing, stability and preservation has escalated over the past 10 years (Fahey and Kensler, 2013; Waterman et al., 2014).
Compounds are separated by counter-current chromatography (CCC) based on their partition coefficients between two immiscible solvent phases that are repeatedly mixed and separated as they flow past each other in opposite directions. Thus, CCC is a form of partition chromatography without the solid support system that is typically found in HPLC or fast protein liquid chromatography (FPLC) columns and, thus, without the potential irreversible adsorption onto the solid support phases and the problems that may hinder scaling up such separations (Ito, 2005). Early (and ongoing) applications of CCC focused upon two-phase systems containing ratios of four or five solvents that typically included hexane, ethyl acetate, acetonitrile, butanol, methanol and water, which produce a biphasic system that can be adjusted to favour the separation of compounds of a wide range of polarity, based upon their partition coefficients (Ito, 2005; Friesen and Pauli, 2007). The development of aqueous–aqueous polymer phase systems (otherwise known as aqueous two-phase systems or ATPS) for partitioning macromolecules and cell particles required further modification of CCC design in order to maximise retention of both phases (Albertsson, 1986). The two aqueous phases are kept immiscible by judicious addition of a polymer and high concentrations of salts. Without organic solvents present, tertiary protein structure is well preserved, provided that the pH of the ATPS is appropriately controlled. Specifically, polyethylene glycol (PEG)-potassium phosphate ATPS unilaterally partitions low molecular weight (MW) compounds to either the upper or lower phase, whereas macromolecules such as proteins are usually much more evenly distributed between phases. This provides great advantages for the isolation and purification of certain proteins by CCC, as initially demonstrated by Shibusawa et al. (2006, 2007).
The initial report of CCC isolation of an active enzyme from biological material utilised a lysate of Escherichia coli, from which 80% of the initial ketosteroid isomerase (3-oxo-Δ5-steroid isomerase) was recovered at 98% purity. This first recovery of active enzyme from CCC (cross-axis coil planet and toroidal coil designs) still required dialysis, ion exchange and concentration to produce a small quantity of enzyme (Qi et al., 1998). Cholinesterase was subsequently purified directly from human serum by CCC (Shibusawa et al., 2001), as was horseradish peroxidase from horseradish, but with very low enrichment and low yields (Magri et al., 2003). No other plant enzymes have been purified by CCC utilising an ATPS, but others have exploited the properties of the ATPS to purify glucosyltransferase from Streptococcus mutans (Yanagida et al., 2004), lactic acid dehydrogenase from bovine heart, alcohol dehydrogenase from bovine liver and histone deacetylase from E. coli (reviewed in Shibusawa et al., 2006).
The purifications reported herein, achieve good initial purification of the enzyme by optimising ATPS selection based on differential solubility of myrosinase, and loading only the phase in which myrosinase (but not most other plant enzymes) is most soluble. Furthermore, a spiral tubing assembly (STA) CCC configuration was utilised which resolved some of the difficulties in mixing and settling required by CCC separation that depend upon viscosity, but that with slow settling speed cannot be fully exploited owing to unacceptable loss of stationary phase. These procedures address retention of full catalytic activity, potential scalability, freedom from toxic solvents and minimising the number of processing steps.
Experimental
Apparatus
Two mixer–settler barricaded disk (MSBD) CCC assemblies were used (Ito et al., 2013). The first unit (90 mL capacity, constructed by CC Biotech, Rockville, MD, USA) contained five stacked disks, each with four interwoven spiral channels that were barricaded every 5 mm with glass beads (1 mm) (3 M Reflective Products Division, Minnesota Mining & Manufacturing Co., St Paul, MN, USA) in every fourth section. Disks were sealed with Viton septa and sandwiched with a pair of aluminum flanges. The second unit (160 mL capacity, constructed by R. Clary, NIH, Bethesda, MD, USA) contained eight stacked disks, each with 300 barricaded chambers.
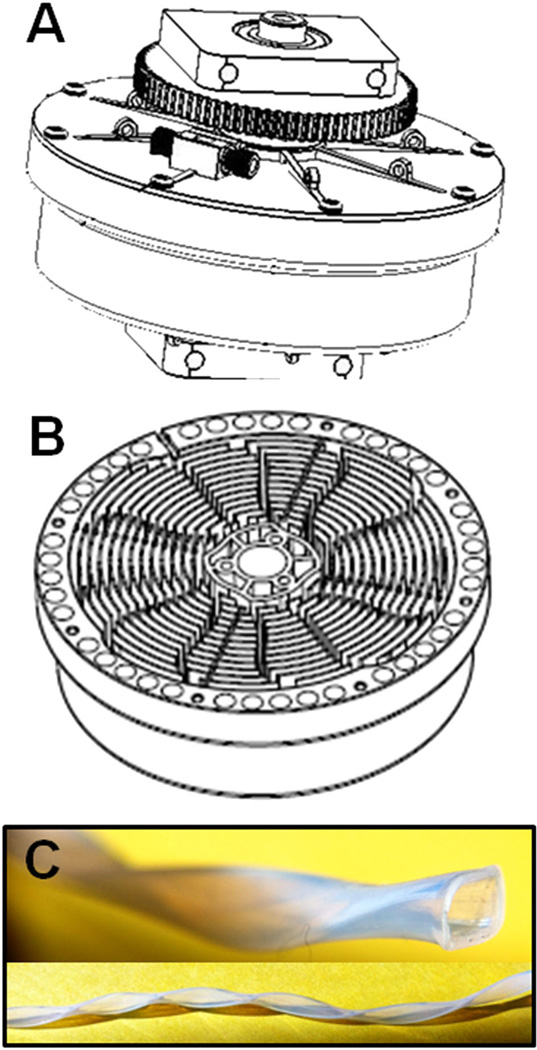
A single STA CCC unit was manufactured as described (Ito et al., 2013). Briefly, it had a 10 cm revolution radius (for the spiral tube support plus counterweight; Fig. 1A) and a 90 mL capacity spiral tube assembly (provided by CC BioTech, Rockville, MD, USA) with four interwoven spiral channels and 12 radial grooves (Fig. 1B) to accommodate 1.6 mm ID flat-twisted polytetrafluoroethylene (PTFE) tubing (Fig. 1C).
Figure 1.
Schematic view of spiral tube assembly (STA) counter-current chromatography (CCC) configuration: (A) external view of assembled spiral tubing support rotor in the 12-radial groove configuration shown in the inside view (B) and wound with flat-twisted PTFE tubing (C).
Reagents
Polyethylene glycol 1000 (PEG-1000), K2HPO4, KH2PO4, gel staining and destaining supplies, and other reagents were from Sigma (St Louis, MO, USA) unless otherwise specified. Water was distilled. All sodium dodecyl sulphate (SDS) polyacrylamide gel-electrophoresis (PAGE) materials and Coomassie Brilliant Blue R-250 were from BioRad (Hercules, CA, USA).
Procedures
Preparation of aqueous two-phase systems
A 1:1 (v/v)) mixture was made in a separatory funnel from stock solutions of (A) 32% w/w PEG-1000 in water and (B) 25% (w/w) potassium phosphate buffer (made by adding 62.5 g K2HPO4 and 62.5 g KH2PO4 to 375 g water). Both salt and PEG solutions were degassed prior to mixing and subsequent phasing out at room temperature. The two phases were separated for use as CCC mobile and stationary phase, as well as for making the crude plant extract preparations. Final pH for each phase was 6.6 (Shinomiya et al., 1996).
Preparation of crude myrosinase extracts
Seven-day-old daikon sprouts (Raphanus sativus) and 3-day-old broccoli sprouts (Brassica oleracea var. italica) were grown commercially by Hanover Foods (Ridgely, MD, USA), freeze-dried and powdered. Fresh mustard seed (Sinapis alba) powder was obtained commercially (Ground Mustard, Gourmet Collection, McCormick & Co., Inc., Sparks, MD, USA). Fresh leaves of the moringa, or horseradish tree (Moringa oleifera), were collected in California (Moringa Farms, Sherman Oaks, CA, USA), shipped at 4 °C to Baltimore, and freeze-dried immediately and powdered. Just before use, powdered sprouts (250 mg), ground mustard seed (250 mg) or powdered moringa leaves (400 mg), were added to a mixture containing 5 mL of the pre-equilibrated ATPS lower phase and 5 mL of the pre-equilibrated ATPS upper phase (7 mL of each were used for the moringa). The extract was vortexed for 2 min then centrifuged for 10 min using a Centrific Model (Fisher Scientific, Pittsburgh, PA, USA) at 900 × g. After centrifugation, the clear lower phase containing the myrosinase activity was collected and used for injection onto the CCC. Injection volumes were 2 mL for the daikon, broccoli and mustard samples, and 3 mL for the moringa samples. An aliquot of the yellowish to greenish upper phase was examined for myrosinase activity and run on SDS-PAGE for comparison purposes. The pelleted plant material was discarded.
Measurement of myrosinase activity
Disappearance of sinigrin, as a measure of myrosinase activity, was monitored spectrophotometrically (Shikita et al., 1999). Briefly, an aliquot of the myrosinase-containing desalted fraction was added to a 1 mL quartz cuvette containing 10 mm sodium phosphate buffer, pH 6.0, 500 µm ascorbic acid, and, finally, 50 µm sinigrin, added last, after blanking (final concentrations). Other samples, that is, upper and lower ATPS phases of the crude plant extracts and the CCC column contents following fractionation, already contained sufficient concentrations of potassium phosphate and needed no additional buffer in the assay reaction mixture. Absorbance at 227 nm was measured for 3 min. Myrosinase activity was based on a molar extinction coefficient for sinigrin of 7000 L/mol/cm. Total protein was measured by A280 and by the bicinchoninic acid (BCA) method (Smith et al., 1985) as adapted for microtitre plates (Fahey et al., 2004).
Determination of partition coefficient for myrosinase from crude daikon extract
Phase development was guided by determination of partition coefficients (Kup/lp; partition coefficient of upper phase to lower phase) of daikon proteins as described by Ito et al. (2007). Freeze-dried, powdered daikon sprouts (4 mg) were vortex-mixed in a test tube containing 1 mL pre-equilibrated ATPS. Following phase separation, 50 µL aliquots of each phase were added to separate cuvettes with appropriate volumes of buffer, and myrosinase activity was measured as described in the section entitled ‘Measurement of myrosinase activity’, in order to determine its partition coefficient.
Counter-current chromatography
Briefly, the multilayer spiral CCC coils were loaded with PEG-rich stationary phase. The lower phase of the crude plant extract was injected onto the column, and then eluted with the (lower) salt-rich mobile phase, at 800 rpm with counterclockwise, tail-to-head elution at 0.3–0.5 mL/min with a Shimadzu pump (Shimadzu Scientific Co., Columbia, MD, USA). Thus, the stationary phase is essentially being used to remove solubles from myrosinase, which then elutes rapidly in the mobile phase. A MicroKros hollow fibre (Spectrum Laboratory Products, New Brunswick, NJ, USA) was placed on-line, directly before the inlet of the UV detector monitoring at 280 nm (LKB Uvicord IIS, Pharmacia Biotech, Uppsala, Sweden) in order to continuously de-salt the effluent by tangential flow ultrafiltration at 3.0 mL/min. Enzyme activity was measured for each fraction (50–60 fractions at 4 min/fraction.)
Analysis of CCC fractions by SDS-PAGE
The SDS-PAGE was performed using a BioRad Mini-Protean II Apparatus (Bio-Rad, Hercules, CA, USA) and BioRad 10-well ReadyGels (15% Tris-HCl) or BioRad Mini-Protean TGX Precast 4–15% Gels. Tank buffer contained 25 mm Tris, 192 mm glycine and 0.1% w/w SDS. Loading buffer was 250 mm Tris, pH 7 with 40% glycerol and 2% SDS; dithiothreitol and bromophenol blue were added just before running (ReadyGels) or 31.5 mm Tris-HCl, pH 6.8, 10% glycerol, 1% SDS, 0.005% bromophenol blue, 355 mm 2-mercaptoethanol (TGX Gels). Crude plant extracts (upper and lower phases) were desalted with Zeba Spin 7 K MWCO desalting columns (Thermo-Fisher Scientific, Pittsburgh, PA, USA) and solutes in the CCC fractions were concentrated about fivefold with Amicon Ultra 3 K MWCO centrifugal filters (EMD Millipore, Billerica, MA, USA) and run at 125 V (ReadyGels) or 200 V (TGX gels) for 30–35 min. Preparation for loading in the 50 µL wells was as follows: plant extracts – about 10 µL of the upper and lower phases were mixed with 20 µL sample buffer; concentrated CCC fractions – a quantity approximately equivalent to 75 µL of the undiluted fractions was mixed with 20 µL sample buffer; myrosinase standard – 10 µL was mixed with 20 µL sample buffer. Prior to gel loading, samples were incubated with the sample buffer in a boiling water bath for 5 min. BioRad Precision Plus All Blue MW standards (10 µL loaded) were used with TGX Gels, and a mixture of bovine serum albumin, ovalbumin, carbonic anhydrase, soybean trypsin inhibitor and RNase was used with ReadyGels. Proteins were visualised with 0.03% Coomassie Brilliant Blue R-250 in acetic acid:isopropanol:water (2:5:13), and gels were destained in 4:4:2 acetic acid:isopropanol:water. Native gels were also run with BioRad Mini-Protean TGX Precast 4–15% Gels. Tank buffer contained 25 mm Tris and 192 mm glycine (no SDS), and samples were loaded with BioRad Native Sample Buffer. Gels were run at 200 V for 30–35 min and stained as above.
Further purification of myrosinase by affinity chromatography
The CCC fractions with myrosinase activity were maintained at 4 °C, and the proteins were concentrated in 15 mL CentriPrep-10 concentrators (Amicon, Beverly, MA, USA), prior to loading on an open 1 × 2 cm column of concanavalinA-Sepharose (Pharmacia, Piscataway, NJ, USA) that had been washed and pre-equilibrated at 0.2 mL/min with a buffer (10 mL) containing 0.5 m NaCl, 30% glycerol and 2 mm dithiothreitol (DTT). Fractions (1 mL) were then eluted from the column with the same buffer but also containing 250 mm methyl-α-d-mannopyranoside (Sigma, St Louis, MO, USA), at a flow rate of 0.1 mL/min. Active fractions were pooled and concentrated in a CentriPrep-10 concentrator.
Results and discussion
This paper describes the rapid isolation of catalytically active plant myrosinase (thioglucoside glucohydrolase; E.C. 3.2.1.147), at high yield and purity, involving three main steps: (i) selective solvation; (ii) counter-current chromatography (CCC) using an aqueous two-phase system (ATPS) mixture of potassium phosphate and polyethylene glycol (PEG); and (iii) in-line desalting. The CCC benefited from modifications of existing designs of both the mixer–settler barricaded disk (MSBD) CCC and the spiral tube assembly (STA) CCC. Using affinity chromatography and gel electrophoresis, we verify that the recovered enzymatic activity co-migrates with highly purified myrosinase standards.
The CCC configuration and design
A variety of CCC configurations were evaluated in an effort to maximise phase retention and resolution, thus enhancing the separation of myrosinase from other plant proteins and co-extractives. Initial experiments not reported here included the use of a hydrodynamic CCC (J-type) and a hydrostatic-type CCC (fast centrifugal partition chromatography). Active daikon myrosinase was isolated using the 160 mL mixer–settler CCC with a barricaded spiral disk assembly (Ito et al., 2007, 2013) with the ATPS described herein. High-yield purifications, however, were achieved using a STA CCC with flat-twisted PTFE tubing (Fig. 1), previously designed for purification of proteins in ATPS systems, which greatly facilitates the mixing, settling and retention of stationary phase (Ito, 2005). Stationary phase retention is generally similar to that of the 90 mL CCC (MSBD). Dipeptides and proteins can be separated on this system, whereas usually they cannot be separated on traditional multilayer coil CCC columns due to inadequate mixing of ATPS phases. The system was further modified by winding the spiral coils with flattened and then helically twisted PTFE tubing that presents an ovoidal cross-section (Fig. 1C), thus providing even greater phase mixing and peak resolution. This design is much more amenable to scaling than the MSBD CCC.
Choice of a suitable ATPS
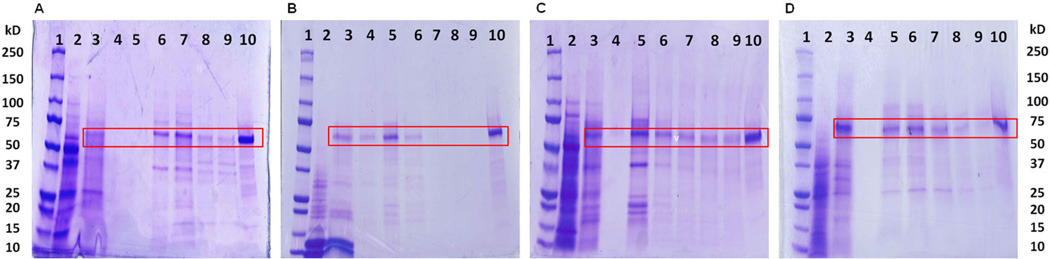
Initial partitioning and myrosinase activity assessments, the latter to avoid denaturing or inhibiting ATPSs, were carried out in 7 mL glass vials, with the ATPS PEG-1000 potassium phosphate system developed by Shinomiya et al. (1996). With this system, PEG-1000 is combined with mono- and di-basic potassium salt at various ratios to achieve ATPSs spanning a pH range from 9.2 to 6.6. Commercially available myrosinase (Sigma, St Louis, MO, USA) was used to evaluate the candidate ATPSs. The greatest enzymatic activity was maintained with the most acidic (pH = 6.6) ATPSs, with higher pH causing loss of activity. The partition coefficient (Kup/lp) for myrosinase was 0.08, determined by measuring activity in diluted aliquots of both upper and lower phases. Thus, interestingly, and fortunately, essentially all of the myrosinase activity was partitioned to the lower salt-rich phase, while many of the plant proteins and pigments migrated to the upper PEG-rich phase. The presence of myrosinase in the lower, but not in the upper phase, was confirmed using SDS-PAGE (Fig. 2A–D). Within the CCC, using this ATPS, myrosinase has a short retention time (tR), eluting just after the solvent front.
Figure 2.
The SDS-PAGE gels for myrosinase-rich fractions obtained by CCC (STA) from (A) daikon, (B) mustard, (C) broccoli and (D) moringa. Fractions were concentrated about fivefold with centrifugal filters (3 K MWCO) then prepared as described in the ‘Experimental’ section. Lanes: (1) MW standards; (2) crude tissue extract in upper phase of solvent system (not utilised for chromatography); (3) crude tissue extract in lower phase of solvent system; (4–9) peak myrosinase-containing fractions; (10) myrosinase standard. Red rectangles highlight myrosinase bands.
Further benchtop tests of ATPSs with mixtures of PEG-400 and PEG-1000, and alteration of the ratios of upper and lower phases, resulted in much better retention of myrosinase in the stationary phase and a higher Kup/lp (Table 1) but, in all cases, it eluted in a broad, low peak (data not shown) and this approach was abandoned in favour of the better resolution obtained with the original system, even though the tR of myrosinase was quite low. In contrast to the bulk of other proteins, highly preferential partitioning of myrosinase into the lower phase has permitted the use of a phase system that might otherwise be regarded as unconventional due to the fact that the partition coefficient (Kup/lp) for our target enzyme is extremely low in this system.
Table 1.
Determination of partition coefficients for daikon myrosinase
| Solvent systema | 32% PEG-1000 | 100% PEG-400 | 25% potassium phosphate (12.5% each, K2HPO4 and KH2PO4) |
Myrosinase partition coefficient (Kup/lp) |
|---|---|---|---|---|
| 1b | 1 | 0 | 1 | 0.08 |
| 2 | 4 | 1 | 15 | 0.03 |
| 3 | 3 | 2 | 10 | 0.33 |
| 4 | 2 | 3 | 10 | 1.27 |
| 5 | 1 | 4 | 15 | 5.27 |
| 6 | 0 | 5 | 30 | 6.57 |
Kup/lp, partition coefficient of upper phase to lower phase.
Stock solutions prepared by weight (w/w).
System that we ultimately chose for myrosinase purification.
Myrosinase stability
We determined in preliminary experiments that catalytic activity of purified myrosinase is best preserved with a low salt concentration and cold, but not freezing, temperatures (data not shown). This would prove problematic for myrosinase that elutes in the lower salt phase, which contains potassium phosphate concentrations as high as 1.64 m. The standard buffer used to assay myrosinase activity contains 20 mm sodium phosphate, buffer pH 6.0. Raising the potassium phosphate salt concentration to ca. 80 mm in a myrosinase enzyme assay (to simulate 1:20 dilution of an untreated column fraction) reduced activity by about one-third. The absence of any buffering salt reduces the activity by about 75%. Consequently, continuous on-line de-salting of the effluent from our CCC runs was performed in order to maintain a low, but not zero, phosphate concentration in the assay mixture. Thus, some ‘apparent reduction’ may occur as an unavoidable artefact of assay conditions and results may somewhat underestimate the activity of the eluted enzyme.
Applicability to range of plant sources of myrosinase
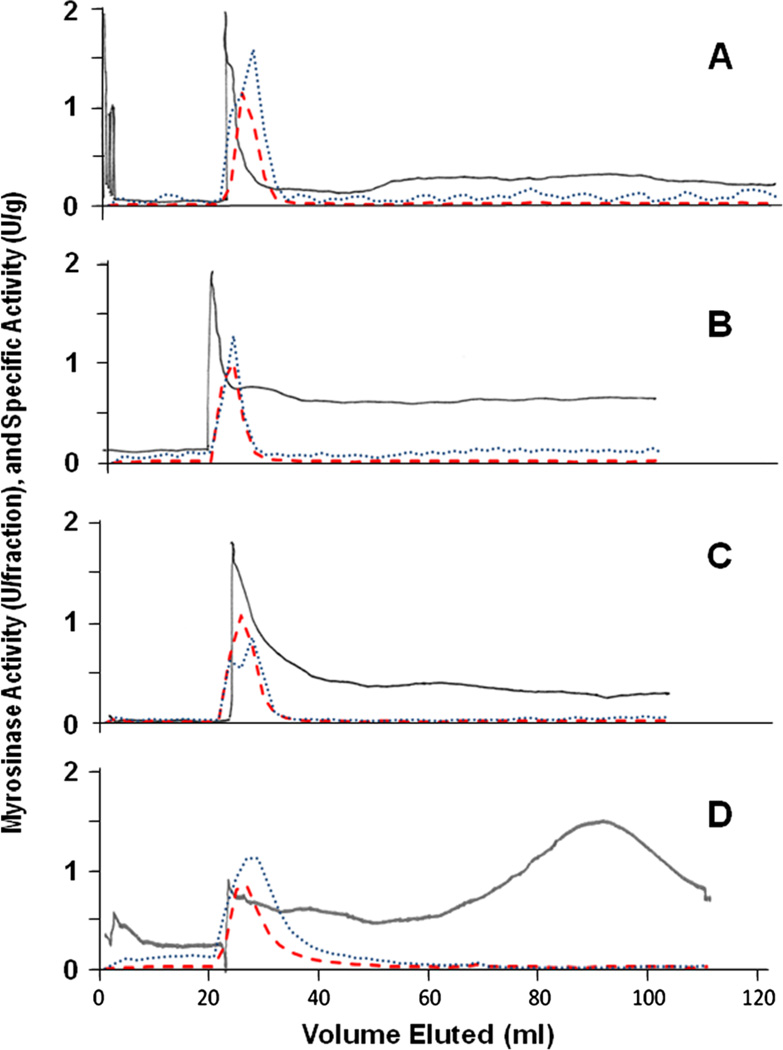
Separation of active myrosinase from crude extracts of daikon sprouts (freeze-dried sprouts resuspended in ATPS) was first evaluated on multiple CCC configurations by using the same ATPS solvent system. Enrichment was generally comparable in the MSBD and STA configurations (Table 2), and was as high as 45-fold. Other plant sources of crude myrosinase were then evaluated, each of which is known to differ from the daikon myrosinase, using the STA configuration. Purification of myrosinase from mustard seed, broccoli and moringa essentially worked as well as that from daikon, with all of the myrosinase activity partitioning to the lower ATPS phase before chromatography (Table 2) and sharp peaks of eluted activity (Fig. 3) with all four plant sources. The CCC stationary phase retention was about 75% in the chromatography of myrosinase from each plant source, and myrosinase purification was as high as 62-fold (from moringa), and between 58 and 79% of myrosinase activity was recovered (from broccoli and moringa respectively).
Table 2.
Counter-current chromatography purification of myrosinase from daikon sprouts, mustard seed, broccoli sprouts and moringa. Myrosinase yield and activity is shown for the initial aqueous plant extract, the lower (mobile) phase (prior to CCC loading) and the CCC fractions. All extracts were chromatographed using the spiral tube assembly (STA) CCC configuration, and daikon was also chromatographed using a 90 mL mixer–settler barricaded disk (MSBD) CCC configuration
| Source of myrosinase | Myrosinase Units (U) |
Recovery/purification | ||
|---|---|---|---|---|
| Specific activity (U/mg protein) |
Purification (-fold) |
Overall recovery (% of initial) |
||
| Initial aqueous plant extract | ||||
| Daikon | 5.48 | 0.069 | ||
| Mustard | 3.03 | 0.058 | ||
| Broccoli | 4.95 | 0.059 | ||
| Moringa | 4.73 | 0.018 | ||
| Lower (mobile) phase [CCC (STA)]a | ||||
| Daikon [for CCC (MSBD)] | 2.92 | 0.237 | 3.43 | |
| Daikon | 2.57 | 0.238 | 3.45 | |
| Mustard | 2.47 | 0.263 | 4.54 | |
| Broccoli | 4.39 | 0.322 | 5.51 | |
| Moringa | 2.66 | 0.040 | 2.20 | |
| CCC fractionsb | ||||
| Daikon, by CCC (MSBD) | 2.02 | 1.27 | 18.5 | 36.8 |
| Daikon, by CCC (STA) | 2.49 | 1.58 | 22.9 | 45.4 |
| Mustard, by CCC (STA) | 2.38 | 2.42 | 41.8 | 78.7 |
| Broccoli, by CCC (STA) | 2.89 | 0.85 | 14.6 | 58.5 |
| Moringa, by CCC (STA) | 3.74 | 1.12 | 61.5 | 79.2 |
The solubility of myrosinase in the CCC lower (mobile) phase is about the same as its solubility in the initial aqueous plant extract, however, substantial activity is lost if fractions are not de-salted immediately. This is performed in-line after collection from the CCC (see footnote b), but for practical reasons was not done pre-injection for these samples, thus these values are depressed as an experimental artefact.
‘Specific activity’ and ‘purification’ values provided for the fractions containing peak activity.
Figure 3.
The CCC (STA) chromatograms and myrosinase activity purified from four different sources: (A) daikon – freeze-dried 7-day-old daikon sprouts (Raphanus sativus); (B) mustard – commercial ground mustard seed (Sinapis alba); (C) broccoli – freeze-dried 3-day-old broccoli sprouts (Brassica oleracea var. italica); and (D) moringa – freeze-dried leaves of the horseradish tree (Moringa oleifera). Dry plant material was comminuted into equal portions of the solvent system upper and lower phases, which were vigorously mixed, then centrifuged, and only the lower phase was used for CCC with inline de-salting of the eluent prior to detection by absorbance at 280nm as described in the ‘Experimental’ section (solid line; relative scaling). Myrosinase activity (dashed red line) and specific activity (dotted blue line) are plotted against a common axis.
Purification of the myrosinase active complex
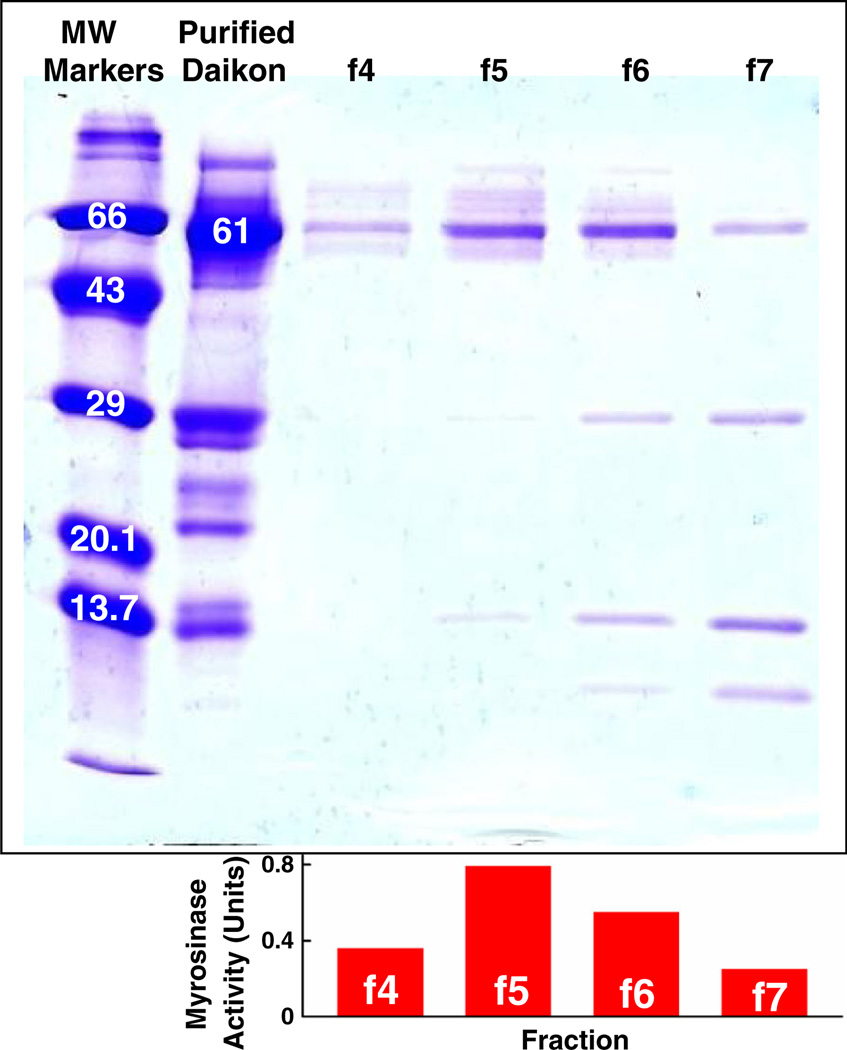
The SDS-PAGE gels (Fig. 2) demonstrated that the major band in CCC fractions containing myrosinase activity runs at approximately 63 kDa for each of the extracts, consistent with published values for the monomer MW for myrosinase from daikon (61 kDa) (Shikita et al., 1999), mustard seed (67 kDa) (Van Eylen et al., 2006) and broccoli (62 kDa) (Lianqing, 2009). Moringa myrosinase has not been characterised. A number of minor, lower MW bands are visible in each of the myrosinase fractions. These are presumed to be the myrosinase binding proteins and/or myrosinase-associated proteins that have been well documented (Bellostas et al., 2008). Myrosinase complexes range in size from 140 to 600 kDa and they contain proteins ranging from 10 to 110 kDa (Bellostas et al., 2008). A final concanavalin A purification step on representative fractions from CCC-purified daikon (Fig. 4) confirms the continued retention of these proteins that are critical parts of the myrosinase complex, and are required for full enzyme activity (Geshi et al., 1998; Bellostas et al., 2008).
Figure 4.
The SDS-PAGE gels of daikon myrosinase (0.038 U/mg protein) purified by a 160 mL mixer–settler barricaded disk (MSBD) CCC configuration (1.3 U/mg protein), then further purified by concanavalin A-Sepharose chromatography as described in the ‘Experimental’ section. Only the active fractions (f4–f7) eluted with methyl-α-d-mannopyranoside are shown (which yielded 1.9, 5.6, 12 and 10 U/mg protein, respectively).
The potential for scaling up isolation
The flat-twisted tubing configuration presented here appears to be most amenable to scale-up by adaptation to current J-type commercial machines. It permits enhanced mixing of the ATPS in a manner similar to the much larger multilayered toroidal coils that have been pioneered for use with ATPS on commercially available and highly scalable J-type CCC configurations (Guan et al., 2007; Sutherland, 2007; Sutherland et al., 2011). This work and that of others suggests that scale-up of this method should be facile and predictable (Fisher et al., 2005; Guan et al., 2007; Ito et al., 2007; Wood et al., 2007; Sutherland et al., 2013), and that the use of CCCs with greater column capacity will result in a concomitant increase in the yield of catalytically active myrosinase, if they can be adapted to this or other tubing design that permits greater retention of ATPS phases. Thus, based only upon first principles, 80 g of dried moringa leaves might be used to charge one of the largest available CCCs today (18 L column capacity), for a per-run yield of about 740 Units in a gram.
Acknowledgements
This work was supported by the Lewis and Dorothy Cullman Foundation, the American Institute for Cancer Research, and the NIH (CA 093780). We are most grateful to: Paul Talalay (Baltimore, MD, USA) for discussions; Janet Gutierrez (Monterrey, MX) for preliminary evaluation of alternative CCC configurations; Rodney Perdew (Sherman Oaks, CA) for providing fresh moringa leaves; and Katherine Stephenson (Baltimore, MD, USA) for technical assistance.
References
- Albertsson P-Å. Partition of Cell and Macromolecules. 3rd edn. Chichester: J. Wiley & Sons Ltd; 1986. pp. 265–327. [Google Scholar]
- Bellostas N, Petersen IL, Sørensen JC, Sørensen H. A fast and gentle method for the isolation of myrosinase complexes from Brassicaceous seeds. J Biochem Biophys Meth. 2008;70:918–925. doi: 10.1016/j.jprot.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Molec Med. 2012;18:337–347. doi: 10.1016/j.molmed.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Egner PA, Chen J-G, Zarth AT, Ng DK, Wang J-B, Kensler KH, Jacobson LP, Muñoz A, Johnson JL, Groopman JD, Fahey JW, Talalay P, Zhu J, Chen T-Y, Qian G-S, Carmella SG, Hecht SS, Kensler TW. Rapid and sustainable detoxication of airborne pollutants by broccoli sprout beverage: results of a randomized clinical trial in China. Cancer Prev Res. 2014 doi: 10.1158/1940-6207.CAPR-14-0103. (June 9. pii: canprevres.0103.2014 Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. [accessed 10 June 2014];Trees for Life Journal. 2005 1:5. http://www.tfljournal.org/article.php/20051201124931586. [Google Scholar]
- Fahey JW, Kensler TW. Health span extension through green chemoprevention. Virtual Mentor: Am Med Assoc J Ethics. 2013;15(4):311–318. doi: 10.1001/virtualmentor.2013.15.4.stas1-1304. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [corrigendum: Phytochemistry 59: 237.] [DOI] [PubMed] [Google Scholar]
- Fahey JW, Dinkova-Kostova AT, Talalay P. The ‘Prochaska’ microtiter plate bioassay for inducers of NQO1. Methods Enzymol. 2004;382(B):243–258. doi: 10.1016/S0076-6879(04)82014-7. [DOI] [PubMed] [Google Scholar]
- Fahey JW, Wehage SL, Holtzclaw WD, Kensler TW, Egner PA, Shapiro TA, Talalay P. Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora. Cancer Prev Res. 2012;5:603–611. doi: 10.1158/1940-6207.CAPR-11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan S, Meng Q, Xu J, Yang J, Zhao L, Zhang X, Sarkar FH, Brown ML, Dritschilo A, Rosen EM. DIM (3,3′-diindolylmethane) confers protection against ionizing radiation by a unique mechanism. Proc Natl Acad Sci U S A. 2013;46:18650–18655. doi: 10.1073/pnas.1308206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D, Garrard IJ, Van den Heuvel R, Sutherland IA, Chou FE, Fahey JW. Technology transfer and scale-up of a potential cancer-preventive plant secondary product: dynamic extraction of glucoraphanin. J Liq Chromatogr Rel Technol. 2005;28:1913–1922. [Google Scholar]
- Friesen JB, Pauli GF. Rational development of solvent system families in counter-current chromatography. J Chromatogr A. 2007;1151:51–59. doi: 10.1016/j.chroma.2007.01.126. [DOI] [PubMed] [Google Scholar]
- Geshi N, Andreasson E, Meijer J, Rask L, Brandt A. Co-localization of myrosinase and myrosinase-binding proteins in grains of myrosin cells in cotyledon of Brassica napus seedlings. Plant Physiol Biochem. 1998;36:583–590. [Google Scholar]
- Guan YH, Smulders J, Fisher D, Sutherland IA. Spiral coils for counter-current chromatography using aqueous polymer two-phase systems. J Chromatogr A. 2007;1151:115–120. doi: 10.1016/j.chroma.2006.12.097. [DOI] [PubMed] [Google Scholar]
- Ito Y. Origin and evolution of the coil planet centrifuge: A personal reflection of my 40 years of CCC research and development. Sep Purif Rev. 2005;34:131–154. [Google Scholar]
- Ito Y, Clary R, Sharpnak F, Metger H, Powell J. Mixer–settler counter-current chromatography with multiple spiral disk assembly. J Chromatogr A. 2007;1172:151–159. doi: 10.1016/j.chroma.2007.09.078. [DOI] [PubMed] [Google Scholar]
- Ito Y, Knight M, Finn TM. Spiral countercurrent chromatography. J Chromat Sci. 2013;51:726–738. doi: 10.1093/chromsci/bmt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Hullar MAJ, Beresford SAA, Lampe JW. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br J Nutr. 2011;106:408–416. doi: 10.1017/S0007114511000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang T, Li X, Zou P, Schwartz SJ, Sun D. Kinetics of sulforaphane in mice after consumption of sulforaphane-enriched broccoli sprout preparation. Mol Nutr Food Res. 2013;57(12):2128–2136. doi: 10.1002/mnfr.201300210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lianqing LJS. Molecular cloning and characteristic analysis of the cDNA encoding myrosinase from broccoli. [accessed 10 June 2014];J Chin Inst Food Sci Technol. 2009 http://en.cnki.com.cn/Article_en/CJFDTotal-ZGSP200901005.htm. [Google Scholar]
- Magri ML, Cabrera RB, Miranda MV, Fernández-Lahore HM, Cascone O. Performance of an aqueous two-phase-based countercurrent chromatographic system for horseradish peroxidase purification. J Sep Sci. 2003;26:1701–1706. [Google Scholar]
- Matile P. The mustard oil bomb. Compartmentation of the myrosinase system. Biochem Physiol Pflanzen. 1980;175:722–731. [Google Scholar]
- Qi L, Ma Y, Ito Y, Fales HM. Isolation and purification of 3-oxo-Δ5-steroid isomerase from crude E. coli lysate by countercurrent chromatography. J Liq Chromatogr Rel Technol. 1998;21:83–92. [Google Scholar]
- Shibusawa Y, Hosojima T, Nakata M, Shindo H, Ito Y. One-step purification of cholinesterase from human serum by CCC. J Liq Chromatogr Rel Technol. 2001;24:1733–1744. [Google Scholar]
- Shibusawa Y, Takeuchi N, Sugawara K, Yanagida A, Shindo H, Ito Y. Aqueous–aqueous two-phase systems composed of low molecular weight of polyethylene glycols and dextrans for counter-current chromatographic purification of proteins. J Chromatogr B. 2006;844:217–222. doi: 10.1016/j.jchromb.2006.07.028. [DOI] [PubMed] [Google Scholar]
- Shibusawa Y, Takeuchi N, Tsutsumi K, Nakano S, Yanagida A, Shindo H, Ito Y. One-step purification of histone deacetylase from Escherichia coli cell-lysate by counter-current chromatography using aqueous two-phase system. J Chromatogr A. 2007;1151:158–163. doi: 10.1016/j.chroma.2007.01.111. [DOI] [PubMed] [Google Scholar]
- Shikita M, Fahey JW, Golden TR, Holtzclaw WD, Talalay P. An unusual case of ‘uncompetitive activation’ by ascorbic acid: purification and kinetic properties of a myrosinase from Raphanus sativus seedlings. Biochem J. 1999;341:725–732. doi: 10.1042/0264-6021:3410725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya K, Muto M, Kabasawa Y, Fales HM, Ito Y. Protein separation by improved cross-axis coil planet centrifuge with eccentric coil assemblies. J Liq Chromatogr Rel Technol. 1996;19:415–425. [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sutherland IA. Review of centrifugal liquid-liquid chromatography using aqueous two-phase solvent (ATPS) systems: its scale-up and prospects for the future production of high-value biologics. Curr Opin Drug Discov Devel. 2007;10(6):540–549. [PubMed] [Google Scholar]
- Sutherland I, Hewitson P, Siebers R, van den Heuvel R, Arbenz L, Kinkel J, Fisher D. Scale-up of protein purifications using aqueous two-phase systems: Comparing multilayer toroidal coil chromatography with centrifugal partition chromatography. J Chromatogr A. 2011;1218(32):5527–5530. doi: 10.1016/j.chroma.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Sutherland I, Thickitt C, Douillet N, Freebairn K, Johns D, Mountain C, Wood P, Edwards N, Rooke D, Harris G, Keay D, Mathews B, Brown R, Garrard I, Hewitson P, Ignatova S. Scalable technology for the extraction of pharmaceutics: Outcomes from a 3 year collaborative industry/academia research programme. J Chromatogr A. 2013;1282:84–94. doi: 10.1016/j.chroma.2013.01.049. [DOI] [PubMed] [Google Scholar]
- Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, McCann SE. Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1806–1811. doi: 10.1158/1055-9965.EPI-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eylen D, Indrawati I, Hendrickx M, Van Loey A. Temperature and pressure stability of mustard seed (Sinapis alba L.) myrosinase. Food Chem. 2006;97:263–271. [Google Scholar]
- Waterman C, Cheng DM, Rojas-Silva P, Poulev A, Dreifus J, Lila MA, Raskin I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry. 2014;103:114–122. doi: 10.1016/j.phytochem.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P, Ignatova S, Janaway L, Keay D, Hawes D, Garrard I, Sutherland IA. Counter-current chromatography separation scaled up from an analytical column to a production column. J Chromatogr A. 2007;1151:25–30. doi: 10.1016/j.chroma.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Yanagida A, Isozaki M, Shibusawa Y, Shindo H, Ito Y. Purification of glucosyltransferase from cell-lysate of Streptococcus mutans by counter-current chromatography using aqueous polymer two-phase system. J Chromatogr B. 2004;805:155–160. doi: 10.1016/j.jchromb.2004.02.039. [DOI] [PubMed] [Google Scholar]