Abstract
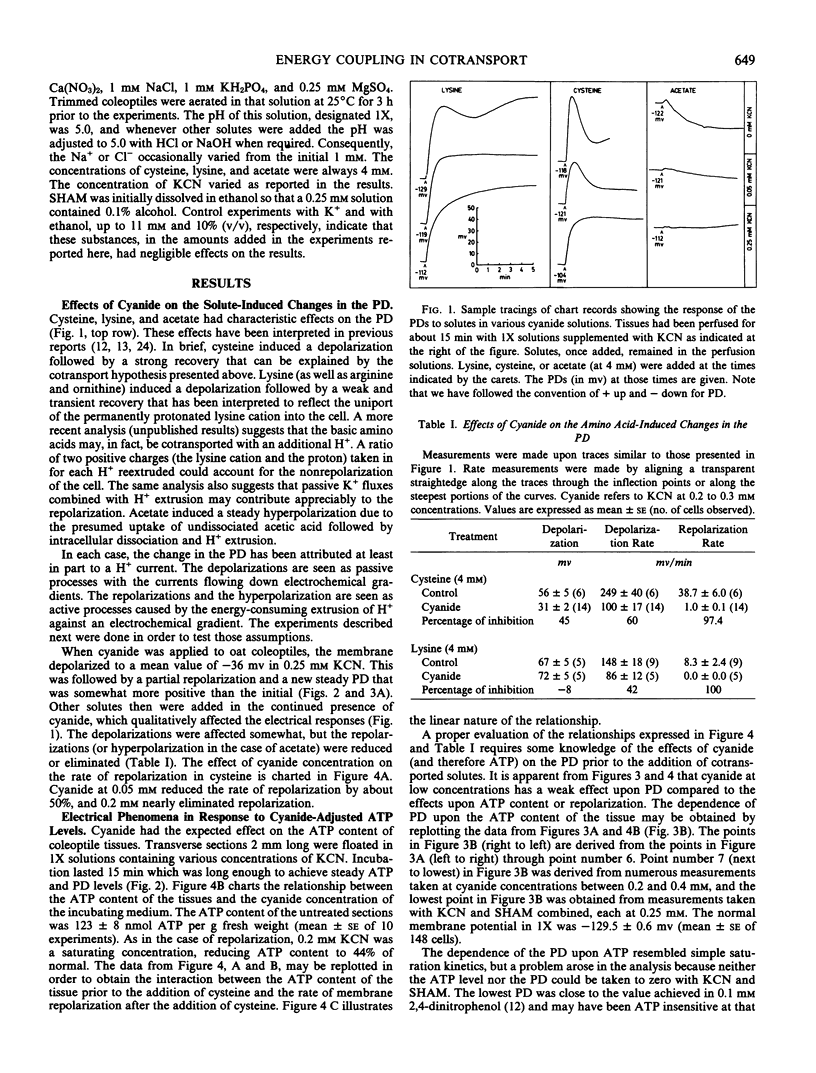
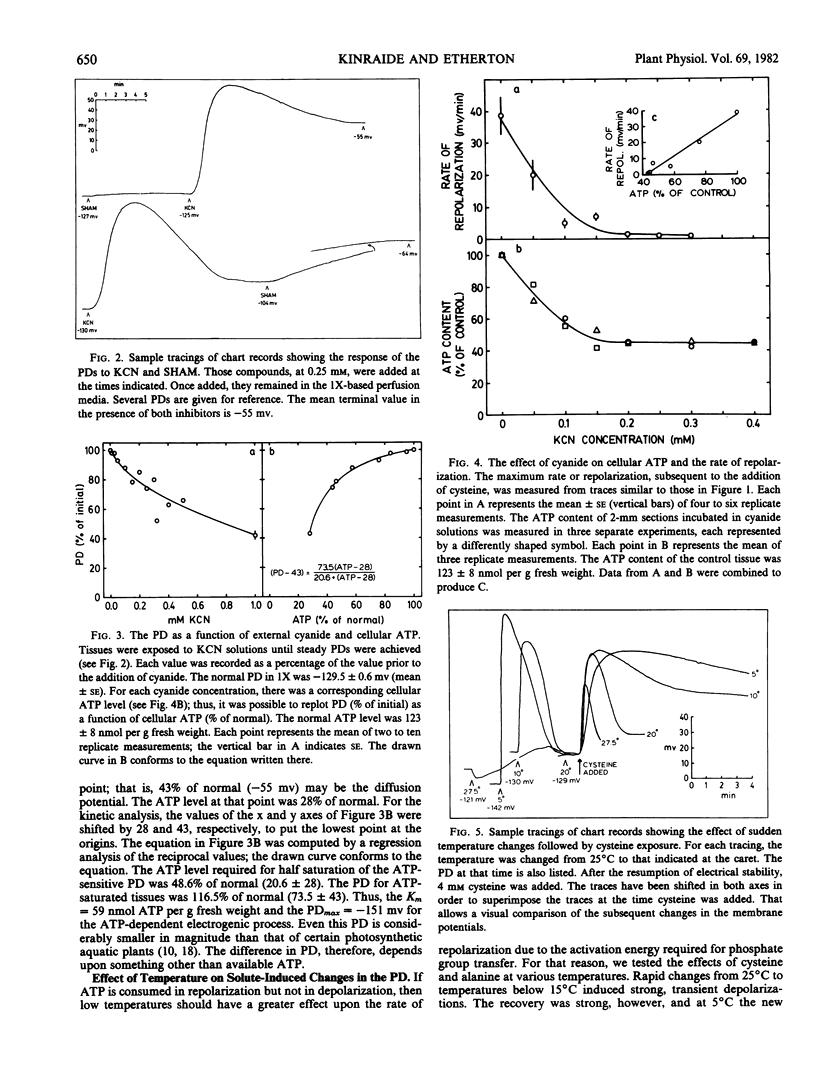
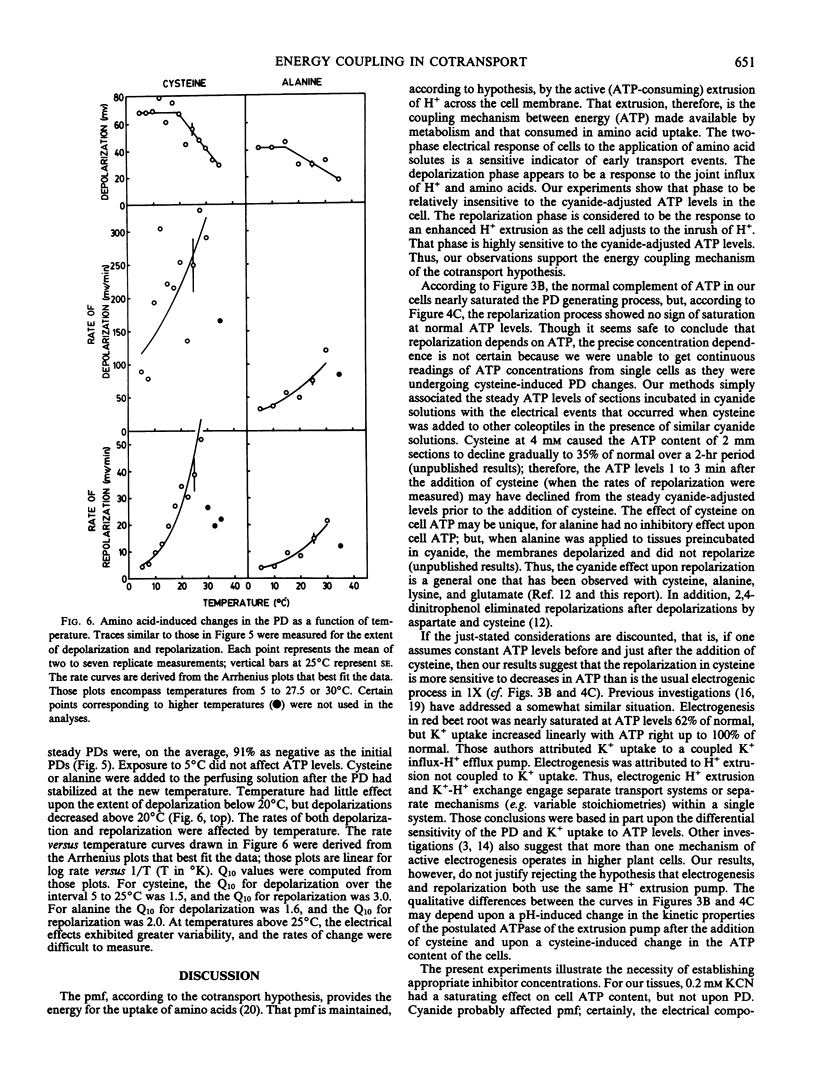
Experiments were undertaken in order to test the mechanism of energy coupling for amino acid uptake proposed in the cotransport hypothesis. According to the hypothesis an electrochemical potential difference in H+ is established by active H+ extrusion. That potential difference then drives the cotransport of H+ and amino acids into the cells. Application of amino acids to oat (Avena sativa var. Victory) coleoptiles induced transient depolarizations of the cell membrane electrical potentials considered to reflect the joint uptake of H+ and amino acids followed by an enhanced H+ extrusion. In the presence of KCN, cysteine induced strong depolarizations, but the rate of repolarization depended linearly upon the cyanide-adjusted ATP level of the tissue. At an ATP level 44% of normal, the membrane potential was 74% of normal, but the repolarization after cysteine-induced depolarization was practically nil. Sudden transitions from room temperature to temperatures below 15° C induced sharp depolarizations of the membrane which then repolarized within 3 min; the ATP content of the tissues was unaffected. Cysteine and alanine induced strong depolarizations at temperatures between 5 and 25°C, and the Q10 for the rate of depolarization was 1.5 for cysteine and 1.6 for alanine. The Q10 for the rate of repolarization was 3.0 for cysteine and 2.0 for alanine. These experiments support the prevailing view that the depolarizations are caused by the passive joint influx of H+ and amino acids and that the repolarizations depend upon the ATP-dependent extrusion of H+.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bravo-F P., Uribe E. G. An Analysis of the Relationship between Respiration and the Transmembrane Potential in Corn Roots. Plant Physiol. 1981 Apr;67(4):809–814. doi: 10.1104/pp.67.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman J. M., Lafayette P. R., Gronewald J. W., Hanson J. B. Effect of ATPase inhibitors on cell potential and k influx in corn roots. Plant Physiol. 1980 Jun;65(6):1139–1145. doi: 10.1104/pp.65.6.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Y. N., Nobel P. S. Amino Acid uptake by pea leaf fragments: specificity, energy sources, and mechanism. Plant Physiol. 1973 Dec;52(6):633–637. doi: 10.1104/pp.52.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont F. M., Burke L. L., Spanswick R. M. Characterization of a partially purified adenosine triphosphatase from a corn root plasma membrane fraction. Plant Physiol. 1981 Jan;67(1):59–63. doi: 10.1104/pp.67.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Evidence for amino Acid-h co-transport in oat coleoptiles. Plant Physiol. 1978 Jun;61(6):933–937. doi: 10.1104/pp.61.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington H. M., Smith I. K. Cysteine transport into cultured tobacco cells. Plant Physiol. 1977 Dec;60(6):807–811. doi: 10.1104/pp.60.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Spanswick R. M. Correlation of Adenosine Triphosphate Levels in Chara corallina with the Activity of the Electrogenic Pump. Plant Physiol. 1979 Aug;64(2):165–168. doi: 10.1104/pp.64.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Kinraide T. B., Etherton B. Electrical evidence for different mechanisms of uptake for basic, neutral, and acidic amino acids in oat coleoptiles. Plant Physiol. 1980 Jun;65(6):1085–1089. doi: 10.1104/pp.65.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed-Harel H., Reinhold L. Hysteresis in the responses of membrane potential, membrane resistance, and growth rate to cyclic temperature change. Plant Physiol. 1979 Jun;63(6):1089–1094. doi: 10.1104/pp.63.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraglia T., Poole R. J. ATP Levels and their Effects on Plasmalemma Influxes of Potassium Chloride in Red Beet. Plant Physiol. 1980 May;65(5):969–972. doi: 10.1104/pp.65.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]



