Abstract
Background
While antiretroviral pre-exposure prophylaxis (PrEP) prevents HIV acquisition, it is not known if it alters HIV disease progression. This study assesses whether tenofovir gel impacted on disease progression among CAPRISA 004 microbicide trial seroconvertors.
Methods
Eighty-three seroconvertors from the tenofovir and placebo gel arms of the CAPRISA 004 trial were monitored prospectively for a minimum two years by CD4 count and viral load (VL). Linear mixed models were fitted to HIV VL, and log rank test was used to compare time to reach CD4<350.
Results
Median 2-week post-infection VL was 4.74 and 4.45 log copies/ml in women assigned to tenofovir gel (n=32) and placebo gel (n=51) (p=0.189). Corresponding 12-month post-infection VLs were 4.24 and 3.70 log copies/ml (p=0.016). After adjusting for clinical and behavioral characteristics and protective HLA alleles, mean VLs within the first two years were 4.51 and 4.02 log copies/ml in women from the tenofovir and placebo arms (p=0.013). Among women with vaginal tenofovir measurements, mean VL were 4.53 and 4.60 log copies/ml in those with detectable versus undetectable levels (p=0.840). Overall mean CD4 counts were 463 and 514 cells/μl in women assigned to tenofovir and placebo (p=0.290). Thirty-two (38.6%) women reached CD4<350 at median 9.4 months post-infection, 13 (40.6%) from the tenofovir and 19 (37.3%) from the placebo arms (p=0.786).
Conclusions
Tenofovir gel had no impact on post-infection CD4 counts or the rate of CD4 decline. While seroconvertors from the tenofovir arm experienced higher VLs, this did not result in a need for earlier antiretroviral therapy.
Keywords: Pre-exposure prophylaxis, HIV seroconversion, HIV disease progression, Viral load, Tenofovir gel
Introduction
Randomised controlled clinical trials have demonstrated that systemic pre-exposure prophylaxis (PrEP) prevents HIV acquisition in men who have sex with men (MSM)1, intravenous drug users2 and heterosexual men and women3,4. This has led to the licensing of oral tenofovir/emtricitabine (Truvada®) as PrEP in HIV uninfected individuals at risk of HIV infection. In addition, the use of antiretrovirals in topical formulations to prevent HIV acquisition has been actively pursued, but with variable results5,6, possibly attributable to differences in adherence between trials. Although concerns about drug side effects in healthy individuals7,8, selection for drug resistance and risk compensation have largely been allayed by recent studies9–11, the effect of failed PrEP on disease course remains an area of concern. Animal studies have suggested that macaques, which acquired SHIV after failed PrEP, may have lower acute viraemia and be at higher risk for the acquisition of drug-resistant virus12,13.
Few studies have assessed the effect of PrEP use on markers of HIV disease progression in individuals who acquire HIV despite PrEP14. In the CAPRISA 004 1% tenofovir gel microbicide trial5, women who acquired HIV in the tenofovir and placebo arms were offered enrolment into the CAPRISA 002 Acute Infection cohort study15. Research on these women from acute HIV infection has already established that the diversity of the transmitting virus was similar between seroconvertors from the two arms16 and tenofovir gel did not appear to select for viruses with enhanced fitness17. Interestingly, T cell responses were relatively preserved during early infection amongst women who were randomized to receive tenofovir gel but who seroconverted18. In addition, sensitive drug resistance screening of plasma and cervico-vaginal lavage specimen collected during acute HIV infection did not reveal any PrEP-related tenofovir drug resistance mutations 19.
Despite these reassuring findings, data from long-term follow-up of participants who seroconverted after tenofovir gel exposure is required to address the safety of topical PrEP, and to alleviate remaining concerns about its implementation. The CAPRISA 004 trial and the already established CAPRISA 002 Acute Infection study provided us with the opportunity to offer PrEP trial seroconvertors long-term follow-up. Therefore, the objective of this study was to compare markers of HIV disease progression among women who seroconverted during the CAPRISA 004 trial, and were exposed to either 1% vaginal tenofovir gel or placebo.
Methods
Design and study population
Women who acquired HIV infection during the CAPRISA 004 study, a randomized controlled, double-blinded trial to assess the safety and effectiveness of the vaginal microbicide 1% tenofovir gel, were offered enrolment into the CAPRISA 002 Acute Infection cohort study. Recruitment methods and eligibility criteria for both studies were previously described in detail5,15.
Briefly, 889 sexually active, HIV-uninfected women aged 18–40 years from urban and rural settings in South Africa participated in the CAPRISA 004 study between May 2007 and March 2010. At each monthly visit, women tested for HIV with two rapid point-of-care antibody tests. Suspected seroconvertors underwent HIV ribonucleic acid (RNA) polymerase chain reaction (PCR), laboratory enzyme-linked immunosorbent assay (ELISA) testing to confirm the HIV diagnosis. Product hold was instituted upon confirmation of HIV acquisition. The date of infection was estimated as the midpoint between the last negative and first positive antibody test, or if a woman had tested negative on rapid antibody tests, but positive on retrospective PCR testing, the infection date was estimated to be 14 days prior to the positive PCR result.
A total of 98 women acquired HIV during the trial, 38 in the tenofovir and 60 in the placebo arm. Of these, 83 women consented to enroll into the CAPRISA 002 study. Participants were assessed at enrolment, and were then followed-up fortnightly until 3 months, monthly during early infection until 12 months, and 3-monthly, or if medically indicated, thereafter, until antiretroviral therapy (ART) initiation. Participants had at least two years of follow-up. ART was commenced at a CD4 count of <350 cells/μl or an AIDS-defining diagnosis, as per South African Adult Treatment Guidelines [20].
Demographic and behavioral data were collected in both studies. Information on sexual behavior, condom and contraceptive use and adherence to the 1% tenofovir and placebo gels was collected in CAPRISA 004 and was previously reported [11].
Laboratory methods
During the CAPRISA 004 trial two HIV rapid tests, Determine HIV 1/2 (Abbott Laboratories, Illinois, USA) and Uni-Gold Recombigen® HIV test (Trinity Biotech, Wicklow, Ireland) were performed at each study visit. Participants with concordantly positive, discordant or indeterminate results were assessed for possible seroconversion by two separate RNA PCR assays (Roche Cobas Amplicor HIV-1 Monitor v1.5, Roche Diagnostics, Branchburg, New Jersey, USA), one week apart.
Once diagnosed and enrolled, participants had HIV viral loads (VL) and CD4+ T-cell counts using the TruCOUNT method (BD Biosciences, San Jose, US) performed at each visit. During the course of the study the VL PCR assay switched from Roche Ampliprep-Amplicor to Roche Taqman version 1.0 on 1 June 2010, and then to Roche Taqman version 2.0 on 9 Jan 2012. All analyses were therefore adjusted for assay type and all samples at the critical 12-month post-infection time point were re-tested on the Taqman version 2.0 assay, in order to eliminate potential measurement bias. The CAPRISA laboratory is accredited by the South African National Accreditation System (SANAS), ISO15189.
In addition, all women underwent a genital screen for sexually transmitted infections (STI) including Herpes Simplex Virus, Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, Trichomonas vaginalis PCR and hepatitis B core antibody testing at enrolment and 6-monthly during the study. HLA typing at four digit resolution was performed using sequence based typing (Atria AlleleSEQR kits, Abbott). Additional laboratory and tenofovir drug level data was available from the CAPRISA 004 trial.
Plasma samples were tested for antiretroviral drug levels in seroconvertors with a VL <400 copies/ml at 12-month post-infection. A liquid chromatography–mass spectrometry (LC MS/MS) semi-quantitative method was used for the detection of efavirenz, nevirapine, lopinavir and ritonavir developed in-house in the Division of Clinical Pharmacology, University of Cape Town, South Africa. Twenty microliters of plasma was extracted via protein precipitation and analyzed on an AB Sciex API 4000 mass spectrometer (AB Sciex). Control samples spiked to the cut-off limits were analyzed with each batch and used to determine presence or absence of the analytes. A deuterated internal standard was used to monitor the extraction efficiency of the method.
Outcome measures
We defined outcomes as follows; (i) a difference in overall VL measurements and, specifically, at 12-months post-infection, (ii) a difference in overall CD4 count measurements and at 12-months post-infection, and (iii) the rate of disease progression to CD4 count <350 cells/μl, i.e. the need for ART initiation. Decline of a CD4 count to <350 cells/μl was deemed an endpoint, if it occurred at least 6 months post-infection to allow for CD4+ T-cell lymphopaenia during primary infection that reverted by 6 months.
Statistical analysis
Basic descriptive statistics were used to summarize characteristics of seroconvertors at enrolment. Baseline differences between women from the tenofovir and placebo arms were compared using Wilcoxon rank sums and Fisher’s exact tests.
CD4 count and HIV VL measurements in the first two years of infection, at 3-monthly intervals, were described using medians and interquartile ranges. The Wilcoxon rank sum test was used to compare tenofovir and placebo arm seroconvertors at the specific time points. Linear mixed models, accounting for repeated measures, were fitted to all CD4 count and HIV VL measurements in the first two years of infection, in order to assess the impact of CAPRISA 004 arm. Unadjusted and adjusted analyses were performed controlling for possible confounders.
Time to CD4 count <350 cells/μl was calculated as the period from estimated date of infection to the date of the first measured CD4 count <350 cells/μl beyond 6 months post-infection. Those who did not reach this low CD4 count were censored at their last post-infection visit. A Kaplan-Meier survival curve and log rank test was used to compare the differences between the arms.
Linear mixed models were used to assess the association between tenofovir concentrations, as measured in the genital tract in the CAPRISA 004 study, and VL in the first two years of HIV infection.
Statistical analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary), while graphs were prepared using GraphPad Prism 5.
Ethical considerations
Ethical approval for the CAPRISA 002 study was obtained from the University of KwaZulu-Natal (E013/04), the University of Cape Town (025/2004), and the University of the Witwatersrand (M040202).
RESULTS
Cohort characteristics by trial arm
A total of 83 women of 98 CAPRISA 004 seroconvertors (84.7%) consented to participate in the CAPRISA 002 Acute Infection study and have so far contributed 296.1 women-years of ART-naïve follow-up with a retention rate of 94.0% and median follow up time of 48 months (IQR 31–57). Recruitment from the two trial arms was evenly balanced with 32 of 38 women (84.2%) from the tenofovir gel PrEP arm and 51 of 60 women (85.0%) from the placebo arm. Two thirds of women (67.5%) were diagnosed at the rural and one third (32.5%) at the urban CAPRISA research sites. The 15 women who did not enroll into the study had similar baseline characteristics to those who did enroll, apart from reporting a lower condom use at their last sex act (58.3% vs 84.2%, p=0.049), and showing lower rates of STI symptoms prior to seroconversion (0.0% vs 32.5%, p=0.005).
At enrolment into the study the median age was 22 years (IQR 21–25), most women (81.9%) had had one sexual partner in the previous month with a median of four sex-acts per month (IQR 2–7), and 61.5% were using depot medroxyprogesterone acetate (DMPA) injections as their main method of contraception (Table 1).
Table 1.
Baseline characteristics of CAPRISA 004 seroconvertors
| Characteristic | All (N=83) | Tenofovir arm (n=32) | Placebo arm (n=51) | p-value† |
|---|---|---|---|---|
| Median age at seroconversion in years (IQR) | 22 (21 – 25) | 23 (20 – 27) | 22 (21 – 25) | 0.460 |
| Median estimated days post infection at CAP002 enrolment (IQR) | 37 (24 – 65) | 40 (29 – 83) | 36 (22 – 59) | 0.133 |
| % Rural (n) | 67.5% (56) | 65.6% (21) | 68.6% (35) | 0.813 |
| % sexual partners prior to seroconversion | ||||
| More than one | 1.2% (1) | 0.0% (0) | 2.0% (1) | 0.690 |
| One only | 81.9% (68) | 78.1% (25) | 84.3% (43) | |
| None | 16.9% (14) | 21.9% (7) | 13.7% (7) | |
| Median number of sex acts in past 30 days at visit prior to seroconversion (IQR) | 4 (2 – 7) | 3 (2 – 5) | 5 (2 – 7) | 0.082 |
| Median number of returned used gels at visit prior to seroconversion (IQR) | 6 (3 – 10) | 5 (2 – 10) | 8 (4 – 10) | 0.287 |
| Median adherence in 004 (IQR) | 59.2% (50 – 100%) | 56.7% (50 – 100%) | 60.0% (50 – 100%) | 0.927 |
| % with condom use at last sex at reported at visit prior to seroconversion (n) | 84.2% (69) | 81.3% (26) | 86.0% (43) | 0.758 |
| % on Depo-Provera at end of 004 trial (n) | 61.5% (51) | 59.4% (19) | 62.8% (32) | 0.819 |
| % with any STI symptoms within 3 months prior to seroconversion (n) | 32.5% (27) | 34.4% (11) | 31.4% (16) | 0.813 |
| HSV-2 status during 004 trial | ||||
| % Baseline Positive (n) | 56.6% (47) | 53.1% (17) | 58.8% (30) | 0.866 |
| % Stayed Negative (n) | 24.1% (20) | 25.0% (8) | 23.5% (12) | |
| % Became Positive (n) | 19.3% (16) | 21.9% (7) | 17.7% (9) | |
| % BV at CAP002 enrolment* (n) | 55.6% (45/81) | 62.5% (20) | 51.0% (25/49) | 0.365 |
| % T. vaginalis at CAP002 enrolment* (n) | 4.9% (4/81) | 9.4% (3) | 2.0% (1/49) | 0.295 |
| % N. gonorrhoeae at CAP002 enrolment* (n) | 6.2% (5/81) | 3.1% (1) | 8.2% (4/49) | 0.643 |
| % C. trachomatis at CAP002 enrolment* (n) | 16.1% (13/81) | 18.8% (6) | 14.3% (7/49) | 0.758 |
| % M. genitalium at CAP002 enrolment* (n) | 8.6% (7/81) | 12.5% (4) | 6.1% (3/49) | 0.426 |
| % HSV-2 PCR at CAP002 enrolment* (n) | 11.1% (9/81) | 12.5% (4) | 10.2% (5/49) | 0.734 |
| % with any STI** at CAP002 enrolment excluding BV (n) | 40.7% (33/81) | 43.8% (14) | 38.8% (19/49) | 0.817 |
| Median first VL after HIV infection in 004 (log copies/ml) (IQR) | 4.60 (3.75–5.25) | 4.74 (3.97–5.37) | 4.45 (3.56–5.17) | 0.189 |
| Median weeks post infection at first VL in 004 (IQR) | 2 (2 – 2) | 2 (2 – 3) | 2 (2 – 2) | 0.224 |
| Median first CD4 count after HIV infection (IQR) | 498 (393 – 643) | 467 (380 – 530) | 519 (414 – 689) | 0.106 |
| Median weeks post infection at first CD4 count (IQR) | 5 (3 – 9) | 6 (4 – 12) | 5 (3 – 8) | 0.159 |
p-value calculated using Wilcoxon rank sums test (medians) or Fisher’s exact tests (proportions).
Bacterial vaginosis (BV). Missing STI data at CAPRISA 002 enrolment for 2 participants from the placebo arm.
Any STI defined as having either T. vaginalis, N. gonorrhoeae, C. trachomatis, M. genitalium, HSV-2 PCR or syphilis
The prevalence of STIs (40.7%) and bacterial vaginosis (55.6%) was high at enrolment. Specifically, 16.1% of women were diagnosed with Chlamydia trachomatis, 6.2% with Neisseria gonorrhoeae, 8.6% with Mycoplasma genitalium, and 4.9% with Trichomonas vaginalis. In addition, 11.1% of women tested positive for Herpes simplex virus (HSV) by genital fluid PCR.
The median first HIV VL at an estimated median 2 weeks post-infection was 4.60 log copies/ml (IQR 3.74–5.25) with median of 4.74 log copies/ml (IQR 3.97–5.37) in seroconvertors from the tenofovir arm versus 4.45 log copies/ml (IQR 3.56–5.17) from the placebo arm (p=0.189). The median first CD4 count at an estimated median 5 weeks (IQR 3–9) post-infection was 498 cells/μl (IQR 393–643) with 467 cells/μl (IQR 380–530) in the tenofovir arm versus 519 cells/μl (IQR 414–689) in the placebo arm (p=0.106).
Comparing seroconvertors from each trial arm, no statistically significant differences in baseline characteristics were found (Table 1).
Seroconvertors assigned to tenofovir gel have higher VLs, but similar CD4+ T-cell counts, compared to placebo recipients during the first two years of infection
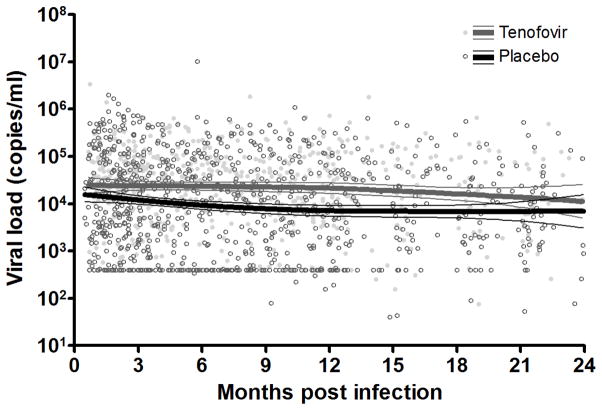
VL measurements remained higher from acute infection to 24 months post-infection in seroconvertors from the tenofovir compared to the placebo arm (Figure 1). The estimated overall mean VL for the tenofovir and placebo arms were 4.51 log copies/ml (SE=0.20) and 4.02 log copies/ml (SE=0.19), respectively, during the course of follow-up (p=0.013). The difference between arms was statistically significant by 12 months post-infection with a median VL of 4.24 log copies/ml (IQR 3.74 – 4.77) versus 3.70 log copies/ml (IQR 2.60 – 4.66) in women from the tenofovir and placebo arms respectively (p=0.016) (Table 1). Although, seroconvertors from the tenofovir arm generally had lower CD4 counts, this difference did not reach statistical significance at any point during the 24-month follow-up (Table 2). Even after adjusting for potential confounders including protective HLA types (B*1302, B*27, B*57, B*5801, B*8101), there was a strong association between assignment to the tenofovir gel arm and post-infection VL (Table 3). However, no association was found between trial arm and CD4 count with a mean CD4 count of 463 cells/μl (SE=46.87) in women from the tenofovir arm compared to 514 cells/μl (SE=45.44) in the placebo arm (p=0.290) during the follow-up period (Supplementary Figure).
Figure 1.
Scatterplot of VL measurements within the first two years of HIV infection, with a Loess smoothing line and 95% confidence intervals, stratified by CAPRISA 004 tenofovir gel and placebo arms
Table 2.
Comparison of HIV VL and CD4 count at 3-monthly intervals post-infection
| Month | 3 | 6 | 9 | 12 | 15 | 18 | 21 | 24 | |
|---|---|---|---|---|---|---|---|---|---|
| VL (log copies/ml) | |||||||||
| Tenofovir | n | 28 | 31 | 30 | 30 | 29 | 26 | 26 | 22 |
| Median (IQR) | 4.42 (3.74 – 4.91) | 4.69 (3.84– 4.99) | 4.46 (3.98 – 4.89) | 4.24 (3.75 – 4.77) | 4.23 (3.55 – 4.81) | 4.21 (3.49 – 4.88) | 3.96 (3.51 – 4.62) | 4.10 (3.46 – 4.86) | |
| Placebo | n | 45 | 47 | 46 | 46 | 43 | 37 | 41 | 33 |
| Median (IQR) | 4.03 (3.08 – 4.84) | 4.12 (3.13 – 4.95) | 4.08 (2.92 – 4.66) | 3.70 (2.60 – 4.66) | 3.88 (3.23 – 4.56) | 3.69 (3.19 – 4.55) | 3.76 (3.18 – 4.44) | 3.55 (3.12 – 4.58) | |
| p-value* | 0.154 | 0.065 | 0.055 | 0.016 | 0.189 | 0.122 | 0.351 | 0.189 | |
| CD4 count (cells/μl) | |||||||||
| Tenofovir | n | 28 | 30 | 30 | 30 | 29 | 26 | 26 | 22 |
| Median (IQR) | 492 (391 – 631) | 471 (365 – 602) | 443 (393 – 606) | 442 (301 – 578) | 434 (325 – 531) | 476 (367 – 679) | 477 (396 – 592) | 454 (412 – 640) | |
| Placebo | n | 45 | 47 | 46 | 46 | 43 | 39 | 42 | 34 |
| Median (IQR) | 537 (433 – 683) | 487 (374 – 737) | 461 (365 – 672) | 432 (368 – 655) | 480 (352 – 641) | 513 (409 – 653) | 536 (392 – 591) | 510 (374 – 626) | |
| p-value* | 0.475 | 0.487 | 0.629 | 0.323 | 0.181 | 0.512 | 0.331 | 1.000 | |
Wilcoxon rank sums test
Table 3.
Univariate and multivariate linear mixed model fitted to HIV VL within the first 24 months infection
| Variable | Univariate analysis* | Multivariate analysis | ||
|---|---|---|---|---|
| Effect estimate (SE) | p-value | Effect estimate (SE) | p-value | |
| Tenofovir vs Placebo | 0.38 (0.20) | 0.059 | 0.50 (0.20) | 0.013 |
| Age in years | −0.02 (0.02) | 0.373 | −0.02 (0.03) | 0.438 |
| Rural vs urban site | 0.25 (0.21) | 0.229 | 0.08 (0.26) | 0.745 |
| DMPA vs no DMPA** | 0.16 (0.20) | 0.423 | 0.40 (0.23) | 0.084 |
| Condom used at last sex act ** No vs Yes | 0.42 (0.27) | 0.127 | 0.49 (0.28) | 0.078 |
| Number of sexual partners** | 0.29 (0.25) | 0.244 | 0.39 (0.34) | 0.255 |
| Number of returned used applicators** | −0.01 (0.02) | 0.519 | −0.10 (0.05) | 0.058 |
| Number of sex acts in the last 30 days** | 0.01 (0.02) | 0.578 | 0.09 (0.05) | 0.079 |
| Median gel adherence in 004 | −0.003 (0.004) | 0.4436 | 0.003 (0.005) | 0.636 |
| STI symptoms within 3 months prior to seroconversion Yes vs No | −0.12 (0.21) | 0.562 | −0.22 (0.23) | 0.333 |
| HSV2 status in 004 | ||||
| Baseline positive vs remained negative | −0.04 (0.24) | 0.857 | −0.002 (0.26) | 0.995 |
| Became positive vs remained negative | 0.49 (0.30) | 0.106 | 0.40 (0.31) | 0.197 |
| Bacterial vaginosis at 002 enrolment positive vs negative | −0.04 (0.20) | 0.844 | −0.14 (0.22) | 0.511 |
| Any STIa at 002 enrolment Yes vs No | −0.11 (0.20) | 0.598 | −0.36 (0.22) | 0.094 |
| Protective HLA-B type# Yes vs No | −0.47 (0.22) | 0.036 | −0.35 (0.23) | 0.141 |
All univariate analyses were adjusted for time post infection and VL assay used (Taqman1 vs Amplicor)
As reported at last visit prior to HIV infection in 004
Any STI is defined as having either T. vaginalis, N. gonorrhoea, C. trachomatis, M. genitalium, HSV-2 PCR or syphilis.
Protective HLA-B types defined as B*1302, B*27, B*57, B*5801, B*8101
The multivariate model (Table 3) also revealed a weak negative association between the number of used gel applicators women returned and the post-infection VL [Effect estimate −0.10 (SE=0.05), p=0.058], and weak positive associations between the number of sex acts in the previous 30 days [0.09 (SE=0.05), p=0.079], not using a condom at the last sex act [0.49 (SE=0.28), p=0.078] and DMPA use [0.40 (SE=0.23), p=0.084].
Since the VL assay changed during the course of follow-up and, therefore, could have differentially affected VL estimates, we retested cryopreserved plasma using the same assay (Taqman version 2.0). The association between study arm and VLs remained significant: 4.40 vs 3.97 log copies/ml for tenofovir vs placebo women at 12 months post infection (p=0.034) (Supplementary Table).
An unusually high proportion of women (12/45, 26.7%) from the placebo arm had undetectable VLs (<400 copies/ml) compared with women from the tenofovir arm (1/30, 3.3%), (p=0.011). Assessment of stored samples from these women using the more sensitive Taqman version 2.0 assay, with a lower limit of detection of 20 copies/ml, demonstrated that 7/45 (15.6%) versus 1/30 (3.3%) women had undetectable VLs (p=0.134) (Supplementary Table). Further analysis of longitudinal data on the 13 women with VL <400 copies/ml at 12 months have revealed that two women have remained virologically suppressed and three are viraemic controllers (VL <2000 copies/ml). The remainder have lost virological control. Antiretroviral drugs (efavirenz, nevirapine, lopinavir and ritonavir) were undetectable in plasma samples from these 13 women with low VLs, therefore excluding undisclosed ART use.
Genital tract tenofovir levels at time of seroconversion are not associated with VL
Amongst women from the tenofovir gel arm of the CAPRISA 004 study, 32 participants had at least one tenofovir level measurement from a vaginal aspirate sample measured during pre-infection or at first visit post-infection. Mean VL measurements were 4.53 (SE=0.27) and 4.60 (SE=0.31) log copies/ml in women with detectable versus undetectable tenofovir levels (p=0.840). Participants with detectable and those with undetectable tenofovir levels had a mean CD4 count of 499 cells/μl (SE=87.84) and 570 cells/μl (SE=101.06), respectively (p=0.556).
Women who received tenofovir and placebo gel have similar rates of HIV disease progression
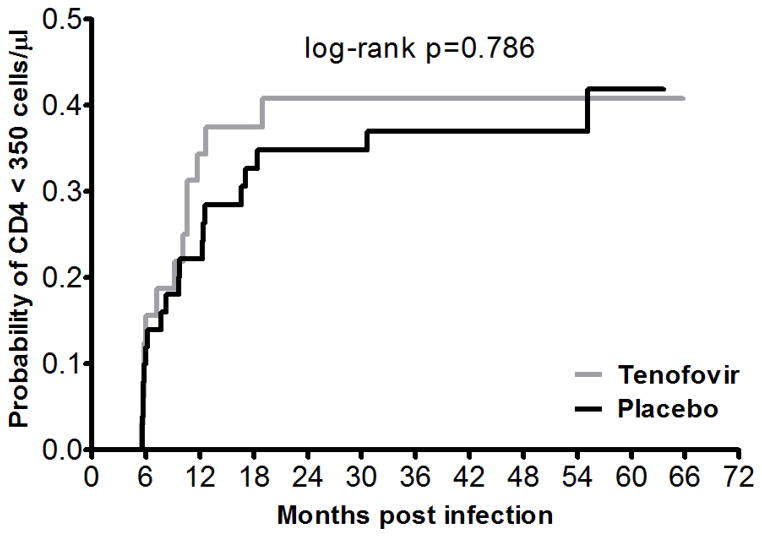
Of the 83 women, 32 (38.6%) reached the main endpoint of two consecutive CD4 counts <350 cells/μl, that is, 13 (40.6%) in the tenofovir arm and 19 (37.3%) in the placebo arm (Figure 2). This happened at a median of 9.4 months (IQR 5.8 – 12.5) post-infection. There was no association between trial arms and time of reaching CD4 count <350 cells/μl (p=0.786).
Figure 2.
Kaplan-Meier graph of time to CD4 count <350 cells/μl stratified by CAPRISA 004 arm
Discussion
Women assigned to tenofovir gel did not have altered HIV disease course, which is an important consideration for topical PrEP rollout. This prospective cohort study found higher HIV VLs among CAPRISA 004 seroconvertors from the tenofovir gel arm compared to the placebo arm. However, there was no corresponding difference in CD4 counts or earlier need for ART initiation comparing these two groups of seroconvertors. The difference in VL was only partially explained by a higher proportion of women with protective HLA alleles amongst women from the placebo arm. In addition, the study did not find a dose-response effect when comparing the presence of genital tract tenofovir with VL or CD4 counts. Therefore, although tenofovir gel prophylaxis may be associated with higher VLs, this does not translate into differences in HIV disease course.
This is one of the first human studies reporting on long-term follow-up of individuals who seroconverted after exposure to PrEP. Markers of disease progression and possible confounding characteristics were systematically and prospectively collected and retention rates in the study remain high.
The main limitation of this study is possible selection bias. The CAPRISA 004 trial showed a 39% effectiveness of the 1% tenofovir gel, thereby protecting some women participating in the tenofovir arm from HIV infection. Once HIV was diagnosed, women were no longer randomized and important population differences may have been selected, as evidenced, for example, by some differences in HLA alleles between women who seroconverted in the two arms. Despite this limitation, comparison of baseline demographic, behavioral and clinical characteristics revealed no obvious differences, thus, any differences must have been subtle or not studied. One of these differences may have been the level of genital tract inflammation at the time of transmission, which was measured to be significantly higher in tenofovir arm seroconvertors, and is most likely one of the main reasons for tenofovir gel failure in well-adherent women20.
A recent study by Chirwa et al14 reported on follow-up of 33 seroconvertors (9 assigned to tenofovir/emtricitabine and 24 to placebo) from the TDF2 oral PrEP trial. This smaller study found no significant differences in the CD4 count or VL profiles after two years of follow-up. While to our knowledge there are no other studies in humans, several macaque monkey studies have previously looked at the period immediately after SHIV acquisition in oral PrEP exposed animals. Zheng et al21 found that PrEP was limiting early virus evolution most likely because of the direct antiviral effect of PrEP and/or reduced target cell availability. Kersh et al12 reported a 100-fold lower peak viraemia and reduced systemic inflammation in their macaque model after oral TDF/FTC PrEP exposure. Furthermore, PrEP-treated macaques showed no significant CD4 cell count reduction during acute infection and developed more SHIV-specific central memory T cells relative to control macaques.
Similar to Kersh et al, a study by Curtis et al22 found a lower peak viraemia during acute infection in TDF/FTC-exposed macaques. Interestingly, although the timing of seroconversion, SHIV binding and neutralizing antibody levels were not impacted by treatment, lower maturation rates of antibody avidity for anti-p27, gp120, gp160 and gp41 were observed. Whether this could have led to higher VLs later on during the course of SHIV infection is not known. Furthermore, it is important to remember that these studies were all conducted with oral PrEP and in animals. Whether these findings also hold true in humans exposed to a 1% tenofovir gel microbicide is currently unknown.
Potential mechanisms for an increased VL after tenofovir gel failure could include a selection of fitter viruses at HIV acquisition, although preliminary studies do not support this, higher genital and systemic genital tract inflammation driving higher VLs, or a delay in VL decline after acute infection. In order to unravel whether the difference in VL in this study is a direct effect of PrEP, or due to the selection of two distinct cohorts after tenofovir gel exposure, further studies are underway. We have already established that the diversity, fitness and resistance pattern of the transmitted virus, as well as T cell responses16–19 were not adversely affected by 1% tenofovir gel exposure, and are currently investigating whether a delay in the antibody response of women who used the tenofovir gel could have been a contributing mechanism to explain the difference in VLs.
At the same time, long-term clinical follow-up of participants in this study will continue as part of the CAPRISA 002 study and in the CAPRISA 009 study, an open label randomized controlled trial (clinicaltrials.gov ref # NCT01387022) to assess the impact of prophylactic exposure to tenofovir gel on the efficacy of subsequent tenofovir-containing ART on viral suppression.
Supplementary Material
Supplementary Figure. Scatterplot of CD4 count measurements within the first two years of HIV infection, with a Loess smoothing line and 95% confidence intervals, stratified by CAPRISA 004 1% tenofovir gel and placebo arms
Supplementary Table. Comparison of the HIV VL of tenofovir and placebo arm seroconvertors according to VL assay used
Acknowledgments
We thank all the CARISA 002 Acute Infection Study participants who are continuing to make an important personal contribution to HIV research through their support and participation in our studies. The scientific and supportive role of the whole CAPRISA 004 and CAPRISA 002 study and protocol teams are gratefully acknowledged. We also thank Jennifer Norman for facilitating the antiretroviral drug level testing in the Division of Clinical Pharmacology, University of Cape Town, South Africa. NJG, LW, NN, VN, SS, NS, CG, CW, LM, QAK and SSAK contributed to study design, protocol writing, data collection, analysis and interpretation of data. NJG drafted the manuscript, while all other authors reviewed the final version of the manuscript.
Source of Funding: This research has been supported by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) (grant # AI51794), the National Research Foundation (grant # 67385), the Columbia University-Southern African Fogarty AIDS International Training and Research Programme (AITRP) funded by the Fogarty International Center, NIH (grant # D43TW00231) and a training grant from LifeLab, a biotechnology centre of the South African Government Department of Science and Technology. The parent trial (CAPRISA 004) was supported by the United States Agency for International Development (USAID), FHI360 [USAID co-operative agreement # GPO-A-00-05-00022-00, contract # 132119], and the Technology Innovation Agency (LIFElab). Tenofovir was provided by Gilead Sciences and the gel was manufactured and supplied for the CAPRISA 004 trial by CONRAD. The current studies are part of the CAPRISA TRAPS (Tenofovir gel Research for AIDS Prevention Science) Program, which is funded by CONRAD, Eastern Virginia Medical School [USAID co-operative grant #GP00-08-00005-00, subproject agreement # PPA-09-046]. The views expressed by the authors do not necessarily reflect the views of USAID, Gilead Sciences, Eastern Virginia Medical School or CONRAD.
Footnotes
Preliminary data was presented at CROI 2014, Boston, March 2014.
Conflicts of Interest
SSAK and QAK are co-inventors of two pending patents (61/354.050 and 61/357.892) of tenofovir gel against HSV-1 and HSV-2 with scientists from Gilead Sciences. The remaining authors have no conflict of interest to declare.
References
- 1.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Science translational medicine. 2012 Sep 12;4(151):151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013 Jun 15;381(9883):2083–2090. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. The New England journal of medicine. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. The New England journal of medicine. 2012 Aug 2;367(5):423–434. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 5.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010 Sep 3;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marrazzo JM, Ramjee G, Nair G, Palanee T, Mkhize B, Nakabiito C, Taljaard M, Piper J, Gomez K, Chirenje M for the VOICE Team. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir-emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). Conference for Retrovirusus and Opportunistic Infections; 2013; Atlanta. [Google Scholar]
- 7.McCormack S, Fidler S, Fisher M. The British HIV Association/British Association for Sexual Health and HIV Position Statement on pre-exposure prophylaxis in the UK. International journal of STD & AIDS. 2012 Jan;23(1):1–4. doi: 10.1258/ijsa.2011.051211. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MS, Muessig KE, Smith MK, Powers KA, Kashuba AD. Antiviral agents and HIV prevention: controversies, conflicts, and consensus. Aids. 2012 Aug 24;26(13):1585–1598. doi: 10.1097/QAD.0b013e3283543e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peterson L, Taylor D, Roddy R, et al. Tenofovir disoproxil fumarate for prevention of HIV infection in women: a phase 2, double-blind, randomized, placebo-controlled trial. PLoS clinical trials. 2007;2(5):e27. doi: 10.1371/journal.pctr.0020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grohskopf LA, Chillag KL, Gvetadze R, et al. Randomized Trial of Clinical Safety of Daily Oral Tenofovir Disoproxil Fumarate Among HIV-Uninfected Men Who Have Sex With Men in the United States. Journal of acquired immune deficiency syndromes. 2013 Sep 1;64(1):79–86. doi: 10.1097/QAI.0b013e31828ece33. [DOI] [PubMed] [Google Scholar]
- 11.Liu AY, Vittinghoff E, Chillag K, et al. Sexual Risk Behavior Among HIV-Uninfected Men Who Have Sex With Men Participating in a Tenofovir Preexposure Prophylaxis Randomized Trial in the United States. Journal of acquired immune deficiency syndromes. 2013 Sep 1;64(1):87–94. doi: 10.1097/QAI.0b013e31828f097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kersh EN, Luo W, Zheng Q, et al. Reduced inflammation and CD4 loss in acute SHIV infection during oral pre-exposure prophylaxis. The Journal of infectious diseases. 2012 Sep 1;206(5):770–779. doi: 10.1093/infdis/jis422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS medicine. 2008 Feb;5(2):e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chirwa LI, Johnson JA, Niska RW, et al. CD4+ cell count, viral load, and drug resistance patterns among heterosexual breakthrough HIV infections in a study of oral preexposure prophylaxis. Aids. 2014 Jan 14;28(2):223–226. doi: 10.1097/QAD.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 15.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PloS one. 2008;3(4):e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valley-Omar Z, Sibeko S, Anderson J, et al. CAPRISA 004 tenofovir microbicide trial: no impact of tenofovir gel on the HIV transmission bottleneck. The Journal of infectious diseases. 2012 Jul 1;206(1):35–40. doi: 10.1093/infdis/jis305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chopera DR, Mann JK, Mwimanzi P, et al. No evidence for selection of HIV-1 with enhanced gag-protease or Nef function among breakthrough infections in the CAPRISA 004 tenofovir microbicide trial. PloS one. 2013;8(8):e71758. doi: 10.1371/journal.pone.0071758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mureithi MW, Poole D, Naranbhai V, et al. Preservation HIV-1-specific IFNgamma+ CD4+ T-cell responses in breakthrough infections after exposure to tenofovir gel in the CAPRISA 004 microbicide trial. Journal of acquired immune deficiency syndromes. 2012 Jun 1;60(2):124–127. doi: 10.1097/QAI.0b013e31824f53a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei X, Hunt G, Abdool Karim SS, et al. Sensitive Tenofovir Resistance Screening of HIV-1 From the Genital and Blood Compartments of Women With Breakthrough Infections in the CAPRISA 004 Tenofovir Gel Trial. The Journal of infectious diseases. 2014 Feb 11; doi: 10.1093/infdis/jiu026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts LPJ, Williamson C, Little F, Naranbhai V, Sibeko S, Walzl G, Abdool Karim Q, Abdool Karim S. Genital Tract Inflammation in Women Participating in the CAP Tenofovir Microbicide Trial Who Became Infected with HIV: A Mechanism for Breakthrough Infection? CROI. 2011 [Google Scholar]
- 21.Zheng Q, Ruone S, Switzer WM, Heneine W, Garcia-Lerma JG. Limited SHIV env diversification in macaques failing oral antiretroviral pre-exposure prophylaxis. Retrovirology. 2012;9:40. doi: 10.1186/1742-4690-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtis KA, Kennedy MS, Luckay A, et al. Delayed maturation of antibody avidity but not seroconversion in rhesus macaques infected with simian HIV during oral pre-exposure prophylaxis. Journal of acquired immune deficiency syndromes. 2011 Aug 15;57(5):355–362. doi: 10.1097/QAI.0b013e3182234a51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Scatterplot of CD4 count measurements within the first two years of HIV infection, with a Loess smoothing line and 95% confidence intervals, stratified by CAPRISA 004 1% tenofovir gel and placebo arms
Supplementary Table. Comparison of the HIV VL of tenofovir and placebo arm seroconvertors according to VL assay used