Abstract
Background
Some cells, tissues and organs release 2’,3’-cAMP (a positional isomer of 3’,5’-cAMP) and convert extracellular 2’,3’-cAMP to 2’-AMP plus 3’-AMP and convert these AMPs to adenosine (called the extracellular 2’,3’-cAMP-adenosine pathway). Recent studies show that microglia have an extracellular 2’,3’-cAMP-adenosine pathway. The goal of the present study was to investigate whether the extracellular 2’,3’-cAMP-adenosine pathway could have functional consequences on the production of cytokines/chemokines by activated microglia.
Methods
Experiments were conducted in cultures of primary murine microglia. In the first experiment, the effect of 2’,3’-cAMP, 3’-AMP, 2’-AMP and adenosine on LPS-induced TNF-α and CXCL10 production was determined. In the next experiment, the first protocol was replicated but with the addition of 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX) (0.1 µM; antagonist of adenosine receptors). The last experiment compared the ability of 2-chloro-N6-cyclopentyladenosine (CCPA) (10 µM; selective A1 agonist), 5’-N-ethylcarboxamide adenosine (NECA) (10 µM; agonist for all adenosine receptor subtypes) and CGS21680 (10 µM; selective A2A agonist) to inhibit LPS-induced TNF-α and CXCL10 production.
Results
1) 2’,3’-cAMP, 3’-AMP, 2’-AMP and adenosine similarly inhibited LPS-induced TNF-α and CXCL10 production; 2) DPSPX nearly eliminated the inhibitory effects of 2’,3’-cAMP, 3’-AMP, 2’-AMP and adenosine on LPS-induced TNF-α and CXCL10 production; 3) CCPA did not affect LPS-induced TNF-α and CXCL10; 4) NECA and CGS21680 similarly inhibited LPS-induced TNF-α and CXCL10 production.
Conclusions
2’,3’-cAMP and its metabolites (3’-AMP, 2’-AMP and adenosine) inhibit LPS-induced TNF-α and CXCL10 production via A2A-receptor activation. Adenosine and its precursors, via A2A receptors, likely suppress TNF-α and CXCL10 production by activated microglia in brain diseases.
Keywords: Primary Microglia; CXCL10; TNF; 2’,3’-cAMP; 2’-AMP; 3’-AMP; Adenosine; Adenosine Receptors; A2A Receptors
1. INTRODUCTION
As the primary innate immune cell in the central nervous system, microglia continuously monitor neural parenchyma for pathologic changes and subsequently may assume a variety of activation states [1, 2]. While microglia are recognized to have neuroprotective potential with the ability to limit injury and contribute to neurogenesis [3, 4], they also may develop a neurotoxic phenotype marked by the secretion of high levels of pro-inflammatory cytokines and chemokines, reactive oxygen species, and nitric oxide. In this study we have focused on two cytokine/chemokines released by activated microglia, TNF-α and CXCL10. Both mediators have been shown to be increased in the human brain following TBI and both have a growing body of evidence supporting their importance in neurologic disease [5].
Because of the limited restorative potential of the central nervous system, microglial activation, including inflammatory mediator release, must be a tightly controlled process. As such, the exact signals that dictate the development of various microglia activation phenotypes remain under study. One metabolite that may be involved in modulating the activation phenotype of microglia is adenosine. Following brain injury, adenosine is elevated extracellulary and is widely recognized to have neuroprotective effects including inhibition of excitatory neurotransmission, inhibition of neuroinflammation, and improved cerebral blood flow [6–8]. Extracellular adenosine exerts it actions via four G-protein coupled cell surface receptors (A1, A2A, A2B, A3) with different effects attributed to activation of different receptor subtypes [9]. While adenosine has been shown to inhibit secretion of certain pro-inflammatory cytokines, controversy exists regarding the adenosine receptor subtype responsible for this effect. Additionally, there is literature to support deleterious effects of certain receptor subtypes, in particular A2A receptors, following neurologic injury.
Extracellular adenosine may become elevated via a variety of mechanisms including efflux of intracellular adenosine through transporters or the extracellular generation of adenosine from ATP [10, 11]. In addition, some cells, tissues and organs express the extracellular 3’,5’-cAMP-adenosine pathway (conversion of extracellular 3’,5’-cAMP to 5’-AMP and 5’-AMP to adenosine) [12–17]. Also some cells, tissues and organs release 2’,3’-cAMP (positional isomer of 3’,5’-cAMP) and convert extracellular 2’,3’-cAMP to 2’-AMP and 3’-AMP and convert these AMPs to adenosine (extracellular 2’,3’-cAMP-adenosine pathway) [18–24]. Because microglia are important participants in the response to brain injury and adenosine is an endogenous neuroprotectant, recently we investigated whether these extracellular cAMP-adenosine pathways exist in primary murine microglia in culture [23]. Although there was little evidence of a 3’,5’-cAMP-adenosine pathway in microglia, primary murine microglia readily converted 2’,3’-cAMP to 2’-AMP and 3’-AMP and 3’-AMP and 2’-AMP to adenosine. Moreover, subsequent in vivo studies in murine brains [25] demonstrated: 1) conversion of exogenous 2’,3’-cAMP to 2’-AMP and 3’-AMP; 2) conversion of exogenous 3’-AMP and 2’-AMP to adenosine; 3) attenuation of exogenous 2’,3’-cAMP metabolism in mice null for 2’,3’-cyclic nucleotide-3’-phosphodiesterase (CNPase −/−); 4) activation of the 2’,3’-cAMP-adenosine pathway by traumatic brain injury (TBI); 5) attenuated activation of the 2’,3’-cAMP-adenosine pathway by TBI in CNPase −/− mice; and 6) worsened histopathology in CNPase −/− mice following TBI. Also, we observed 2’,3’-cAMP, 3’-AMP and 2’-AMP in the cerebrospinal fluid of TBI patients. Thus, it appears that the 2’,3’-cAMP-adenosine pathway may be an important source of adenosine in TBI and in microglia. However, whether the 2’,3’-cAMP-adenosine pathway modulates microglial activation is unknown. Therefore, this study was designed to investigate the ability of 2’,3’-cAMP, 3’-AMP, 2’-AMP and adenosine to alter the inflammatory response of activated microglia via adenosine receptor subtypes.
2. RESULTS
2.1. Adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP Inhibit LPS-Induced TNF-α and CXCL10 Production
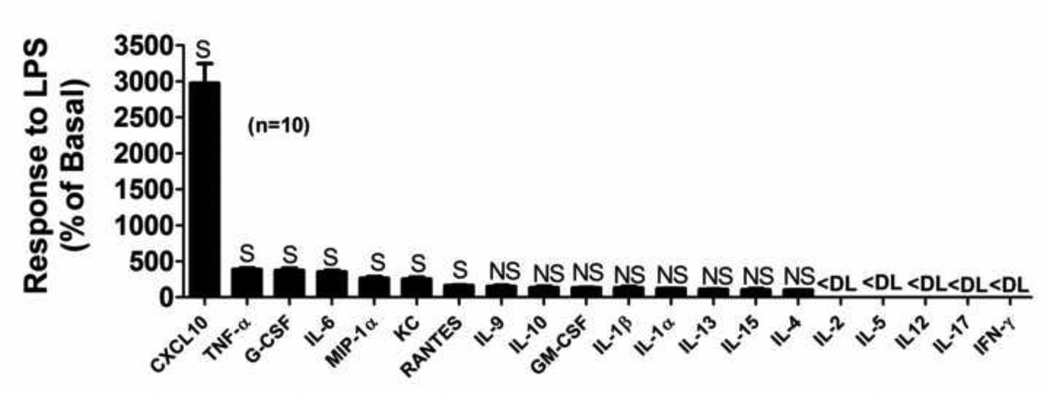
In a preliminary experiment, primary microglia cultures were treated for 24 hours with LPS (100 ng/ml), and cytokines/chemokines in the medium were assessed using a semiquantitative screening assay [LabMAP™ system (Luminex Corporation, Austin, TX) with a Milliplex® kit (EMD Millipore, Billerica, MA)]. As summarized in Figure 1, IL-2, IL-5, IL-12, IL-17 and INF-γ were below detection limit, and IL-9, IL-10, GM-CSF, IL-1β, IL-1α, IL-13, IL-15 and IL-4 although detectable did not change significantly with LPS treatment. LPS did increase medium levels of CXCL-10, TNF-α, G-CSF, IL-6, G-CSF, MIP-1α, KC and RANTES; however, the LPS-induced changes in CXCL10 and TNF-α were the largest. As mentioned above, there is a growing body of evidence supporting the importance of TNF-α and CXCL10 production by microglia in neurologic disease [5]. Also, while previous studies have explored the role of adenosine in inhibiting microglia production of TNF-α [26], the effects of adenosine on microglial CXCL10 production are unknown. Furthermore, the effects of 2’,3’-cAMP and its downstream metabolites on microglia TNF-α and CXCL10 production are entirely unknown as this has never been studied. Thus in the present experiments, we focussed on the ability of the 2’,3’-cAMP-adenosine pathway to alter LPS-induced TNF-α and CXCL10 production by microglia.
Figure 1. Profile of the effects of LPS on release of cytokines and chemokines in primary murine microglia with LPS.
Primary microglia were treated with LPS (100 ng/mL) or phosphate-buffered saline (PBS) for 24 hours. Released cytokines and chemokines were measured using multianalyte profiling (LabMAP™ system; Luminex Corporation, Austin, TX) with a Milliplex® kit (EMD Millipore, Billerica, MA). Production of cytokines and chemokines are expressed as percentage of basal levels (PBS-treated controls). <DL, NS and S indicate less than assay detection limit, non-significant change and significant change, respectively.
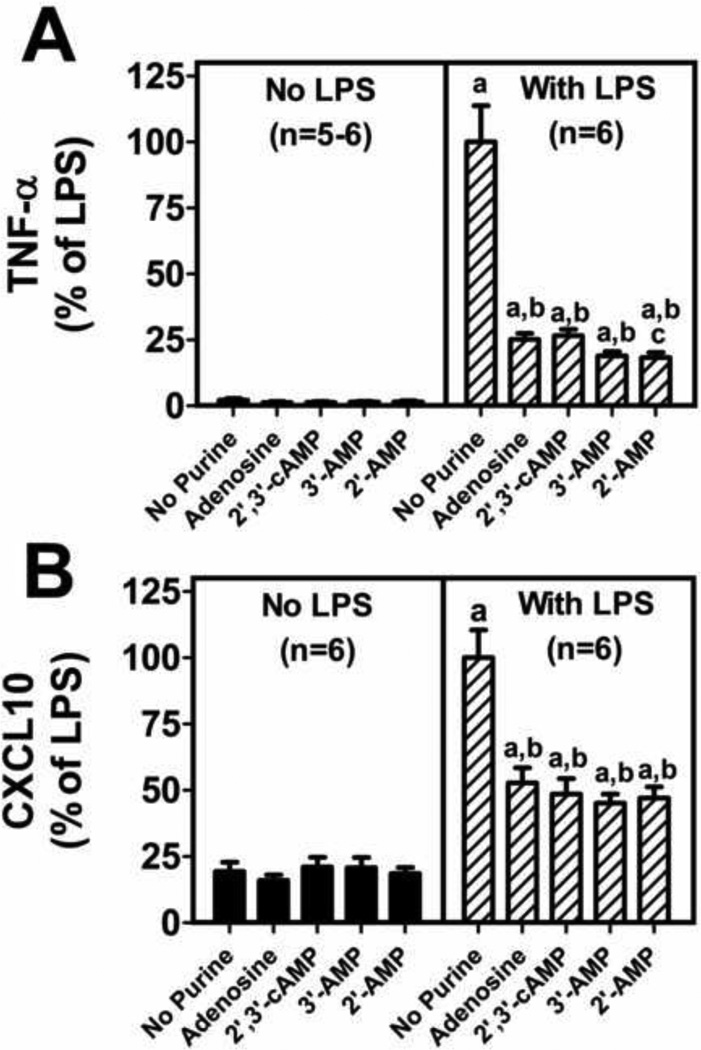
To determine if adenosine, 2’,3’-cAMP, 3’-AMP or 2’-AMP inhibit LPS-induced TNF-α and CXCL10 production, microglia were stimulated with LPS (100 ng/ml for 24 hours) and also treated with adenosine, 2,’3’-cAMP, 3’-AMP or 2’-AMP, and levels of TNF-α and CXCL10 were measured in the culture medium using murine TNF-α and CXCL10 ELISA kits from R&D Systems (Minneapolis, MN). In the absence of LPS, adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP did not affect TNF-α or CXCL10 production by microglia (Figure 2). LPS per se significantly stimulated TNF-α and CXCL10 production in the absence or presence of purines; however, the stimulation of the production of TNF-α and CXCL10 by LPS was significantly attenuated by adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP (Figure 2). Moreover, 2’,3’-cAMP, 3’-AMP and 2’-AMP suppressed LPS-induced TNF-α and CXCL10 production at least as much as did adenosine, and in fact 2’-AMP suppressed TNF-α slightly more than adenosine (Figure 2). These experiments suggest that adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP inhibit LPS-induced activation of primary microglia.
Figure 2. Adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP inhibit lipopolysaccharide (LPS) induced TNF-α and CXCL10 production in primary murine microglia.
Primary microglia were treated without and with LPS (100 ng/mL) and without and with adenosine (30 µM), 2’,3’-cAMP (30 µM), 3’-AMP (30 µM), or 2’-AMP (30 µM) for 24 hours. TNF-α and CXCL10 production were measured by ELISA. Data represent means and SEMs and are expressed as % of TNF-α and CXCL10 levels in LPS-treated cells in the absence of purines. LPS treatment alone resulted in TNF-α levels of 2692±371 pg/ml and CXCL10 levels of 9828±1033 pg/ml. “a” indicates significantly different from corresponding group without LPS, “b” indicates significantly different from “No Purine” LPS-treated group, and “c” indicates significantly different from “Adenosine” LPS-treated group.
2.2 Blockade of Adenosine Receptors Diminishes the Ability of Adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP to Inhibit LPS-Induced TNF-α and CXCL10
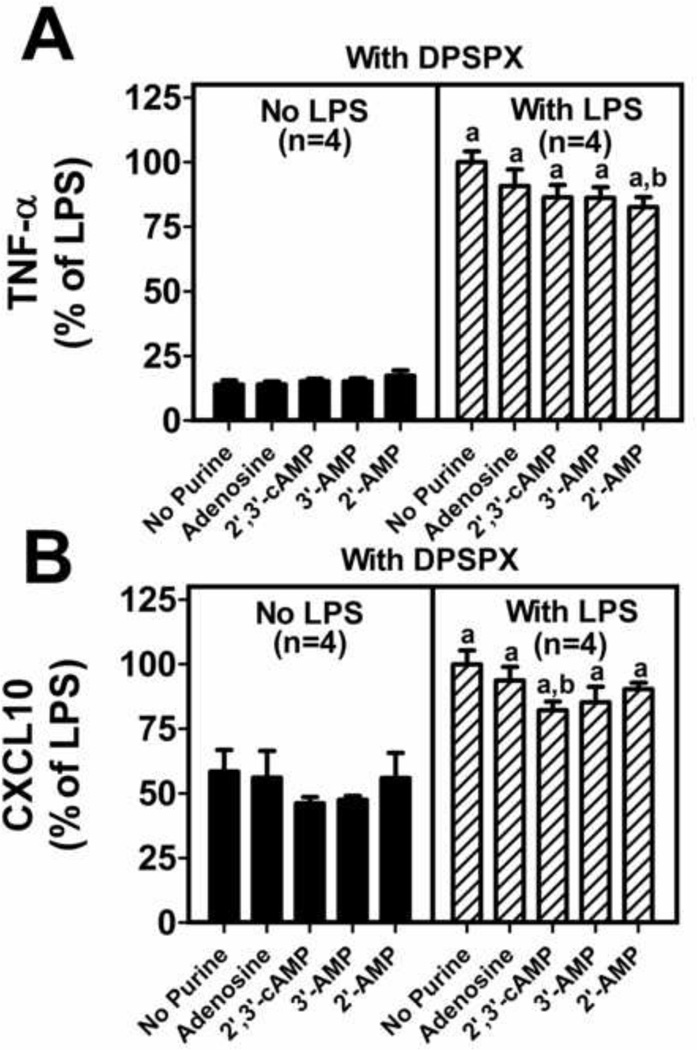
To determine whether the inhibitory effects of adenosine, 2’,3’-cAMP, 3’-AMP or 2’-AMP on LPS-induced TNF-α and CXCL10 production is adenosine-receptor-mediated, the experiment described above was repeated in the presence of 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX) (0.1 µM; a receptor antagonist that blocks all four adenosine receptor subtypes). In the absence of LPS but in the presense of DPSPX, adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP did not affect TNF-α or CXCL10 production by microglia (Figure 3). LPS per se significantly stimulated TNF-α and CXCL10 production in the absence or presence of purines; however, the stimulation of the production of TNF-α and CXCL10 by LPS was not attenuated by adenosine, 2’,3’-cAMP, 3’-AMP or 2’-AMP in DPSPX-treated microglia to the degree seen in the absence of adenosine receptor blockade (Figure 3). While there was a statistically significant decrease in TNF-α by 2’-AMP and a statistically significant decrease in CXCL10 by 2’,3’-cAMP despite blockade of adenosine receptors with DPSPX, these effects were stastitically (p<0.0001) smaller compared to the magnitude of suppression seen in the absence of adenosine receptor blockade (compare Figures 2 and 3). These results suggest that adenosine blocks LPS-induced activation via an adenosine-receptor-mediated mechanism and that likely 2’,3’-cAMP, 3’-AMP or 2’-AMP also inhibit LPS-induced activation via conversion to adenosine (as previously shown [23]) and activation of adenosine receptors.
Figure 3. 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX) blocks the effects of adenosine, 2’,3’-cAMP, 3’-AMP and 2’-AMP on lipopolysaccharide (LPS) induced TNF-α and CXCL10 production in primary murine microglia.
Primary microglia were treated with DPSPX (0.1µM) and without and with LPS (100 ng/mL) and without and with adenosine (30 µM), 2’,3’-cAMP (30 µM), 3’-AMP (30 µM), or 2’-AMP (30 µM) for 24 hours. TNF-α and CXCL10 production were measured by ELISA. Data represent means and SEMs and are expressed as % of LPS treated control. LPS treatment in the presence of DPSPX resulted in TNF-α levels of 512±22 pg/ml and CXCL10 levels of 11,246±533 pg/ml. “a” indicates significantly different from corresponding group without LPS and “b” indicates significantly different from “No Purine” LPS-treated group.
2.3 A2A Receptors Mediate Inhibition of LPS-Induced TNF-α and CXCL10 Production
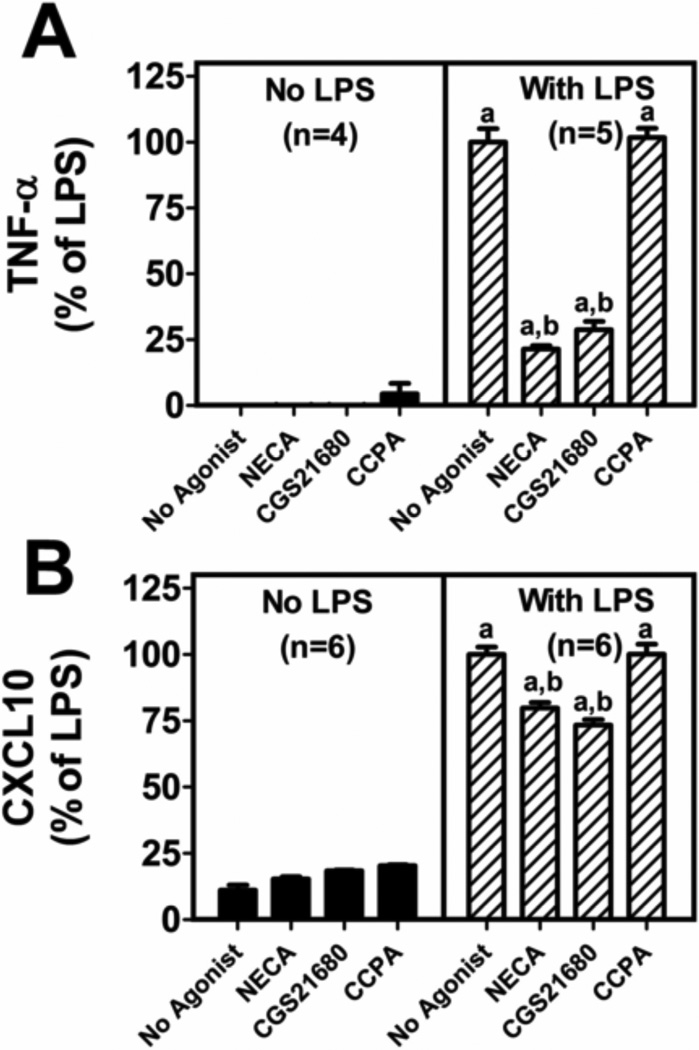
Next, we sought to explore the adenosine receptor subtype involved in inhibition of microglia TNF-α and CXCL10 production. To do this, we treated LPS activated microglia with the nonselective adenosine receptor agonist 5’-N-ethylcarboxamide adenosine (NECA), the highly selective A1 receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA), or the highly selective A2A receptor agonist CGS21680. For each agonist we employed a high concentration (10 µM) to assure that receptors were maximally activated. CCPA had no effect on LPS-induced TNF-α or CXCL10 production (Figure 4) suggesting no role for A1 receptors in attenuating LPS-induced TNF-α or CXCL10 production in primary microglia. In contrast, CGS21680 significantly reduced LPS-induced TNF-α and CXCL10 synthesis by primary microglia (Figure 4) suggesting that activation of A2A receptors does indeed attenuate LPS-induced TNF-α and CXCL10 production. NECA activates not only A2A receptors, but also A2B and A3 receptors. Therefore, if A2B or A3 receptors affect TNF-α or CXCL10 production, then the inhibitory effects of NECA should substantially exceed the inhibitory effects of CGS21680. However, this was not the case, i.e., NECA and CGS21680 similarly inhibited LPS-induced TNF-α and CXCL10 production (Figure 4).
Figure 4. Activation of A2A, but not A1, adenosine receptors results in inhibition of lipopolysaccharide (LPS) induced TNF-α and CXCL10 production.
Primary microglia were co-treated with LPS (100 ng/mL) and NECA (10 µM), CGS21680 (10 µM), or CCPA (10 µM). TNF-α and CXCL10 production were measured by ELISA. Data represent means and SEMs and are expressed as % of LPS treated control. LPS treatment in the presence of ADA resulted in TNF-α levels of 1291±67 pg/ml and CXCL10 levels of 2238±158 pg/ml. “a” indicates significantly different from corresponding group without LPS and “b” indicates significantly different from “No Purine” LPS-treated group.
3. DISCUSSION
A novel and important finding of the present study is the ability of the adenosine precursor, 2’,3’-cAMP and its metabolites, to inhibit LPS-induced microglial activation. 2’,3’-cAMP has been increasingly recognized as an important source of adenosine at times of cellular injury. In this regard, recent studies demonstrate that the 2’,3’-cAMP-adenosine pathway exists in the brain in vivo, that it is more efficient than the 3’,5’-cAMP-adenosine pathway at generating extracellular adenosine and that following experimental TBI the brain generates endogenous 2’,3’-cAMP [25]. Also, findings from our lab show that primary microglia and astrocytes metabolize 2’,3’-cAMP to adenosine [23]. An important finding of the present study is that the 2’,3’-cAMP-adenosine pathway effectively inhibits a key component of microglial activation, namely inflammatory cytokine and chemokine production. In this regard, 2’,3’-cAMP inhibits the production of TNF-α and CXCL10 as efficiently as adenosine. Furthermore, because the inhibitory effects of 2’,3’-cAMP, 3’-AMP and 2’-AMP are diminished in the presence of DPSPX, an adenosine receptor antagonist, our results suggest that these adenosine precursors likely work following their conversion to adenosine. Nonetheless, 2’-AMP has a slightly greater suppressive effect on TNF-α production compared with adenosine, and not all of the suppressive effects of 2’-AMP on TNF-α production are blocked by DPSPX. Therefore, it is possible that 2’-AMP possess additional pharmacological effects independent of adenosine receptors. Also the suppressive effects of 2’,3’-cAMP on CXCL10 production are not entirely blocked by DPSPX, suggesting that 2’,3’-cAMP, perhaps via conversion to its downstream AMPs, has non-adenosine receptor mediated actions.
While it has been shown that adenosine decreases TNF-α secretion from LPS-stimulated non-human primate microglia [26] and from LPS-activated murine BV-2 cells [27], this is the first study to document the ability of adenosine to inhibit CXCL10 production in primary microglia. CXCL10 is a chemokine produced in large amounts by activated microglia and of growing interest in both infectious and non-infectious neurologic insults due to increased recognition of its role in neuronal injury. As the resident innate immune effectors of the CNS, microglia are important in mounting an immediate response to CNS insults as well as in directing the subsequent cellular immune response. Production of CXCL10 by activated microglia is likely critical in both of these roles because CXCL10/CXCR3 signalling is needed for microglial migration to the site of injury and CXCL10 is a recognized chemo-attractant for a variety of peripheral immune cells [28–30]. The importance of our finding regarding the reduction in CXCL10 production by LPS activated microglia is underscored by the observations that manipulation of CXCL10/CXCR3 signaling alters neuronal survival after injury [31]. Additional support for a role of CXCL10 is that levels of CXCL10 in the CNS are elevated in patients with a number of neurologic diseases. Of particular interest is the elevation of CXCL10 in the cerebrospinal fluid of patients with amnestic mild cognitive impairment, many of whom progress to Alzheimer disease, suggesting a role for CXCL10 in early neurodegeneration [32]. Interestingly, tissue levels of CXCL10 are increased in the hippocampus of transgenic mice over-expressing a mutated form of human amyloid precursor protein [33].
Taken together, our data and previous publications suggest that drugs modulating CXCL10 production by activated microglia may benefit patients with various CNS insults and neurologic diseases [34, 35]. Interestingly, atorvastatin, an FDA approved HMG-CoA reductase inhibitor, has been shown to decrease plasma levels of CXCL10 in patients with inflammatory disease [36]. As elevated plasma and CSF levels of CXCL10 were found to be associated with the development of fatal cerebral malaria in children and adults, atorvastatin was studied as an adjunct to antimalarial therapy in a mouse model of cerebral malaria [37]. In this model, atorvastatin led to significant reduction in mortality and in neuroinflammation [38]. Atorvastatin has also been used in experimental TBI and has been shown to improve outcome, although CXCL10 was not evaluated in these studies [39, 40]. Ongoing evaluation of the role of CXCL10 in various neurologic diseases is warranted as well as the identification of potential drugs that modulate the CXCL10 response.
Like CXCL10, elevated TNF-α has also been implicated in the pathophysiology of various neurologic diseases, including TBI. TNF-α is known to be a classic proinflammatory cytokine, and within the CNS is produced primarily by microglia and astrocytes. Both experimental and human data have demonstrated an increase in TNF following TBI [41–43]. Determining the effect of TNF on outcome following neurologic injury, however, has proven to be complex. In a number of experimental TBI studies in which anti-TNFα therapies were utilized, improvement in outcome was demonstrated [44, 45]. However, in a study utilizing TNF-α knockout mice exposed to experimental brain injury, TNF-α deficiency resulted in improved functional outcome in the short term, but worsened motor and histologic outcome when followed for up to four weeks following injury [46]. Additionally, various in vitro studies have shown a neuroprotective role of TNF-α [47]. To date, there have been no clinical studies that demonstrate a correlation between serum or CSF TNF-α elevation and overall outcome. However, a TNF-α gene polymorphism that has been associated with increased TNF-α expression was found to be associated with worse overall outcome at six months following TBI [48]. Due to the growing evidence demonstrating a role of TNF-α in the pathophysiology of neurologic injury, treatment strategies that modulate TNF-α activity have been explored, including the use of Etanercept, a FDA approved TNF-α antagonist [45, 49]. It is clear that ongoing study of the role of TNF-α in TBI is indicated including investigation of endogenous factors that modulate TNF-α production.
Besides showing inhibition of microglial TNF-α and CXCL10 production by adenosine, the present study also demonstrates that activation of A2A receptors (with CGS21680), but not A1 receptors (with CCPA), inhibits microglial TNF-α and CXCL10 production. These receptor subtypes were of interest as they are the most highly expressed adenosine receptor subtypes in the brain and both are implicated in inhibiting inflammation. Moreover, the fact that CGS21680 inhibits TNF-α and CXCL10 production equivalently to NECA strongly suggests that A2A receptors, rather than A2B or A3 receptors, mediate suppression of LPS-induced TNF-α and CXCL10. Although additional experiments with receptor subtype selective antagonists would be required to confirm this inference, the facts that A2A receptor-activation causes maximum suppression and A2A receptors have a much higher affinity for adenosine compared with A2B or A3 receptors suggests that even if A2B or A3 receptors suppress cytokine/chemokine production by activated microglia these effects would be redundant with those of the A2A receptor.
Deciphering the role of A1 receptors in neurologic injury is challenging. Although the present study suggests that A1 receptors do not mediate suppression of LPS-induced TNF-α and CXCL10, other studies do suggest a role for A1 receptors in protecting against brain insults. For example, a study of experimental autoimmune encephalitis shows that A1 receptor knock-out mice have more severe impairment, increased spinal cord microglia/macrophage reactivity and increased expression of proinflammatory molecules in the spinal cord compared to wild-type controls [50]. Also, in an experimental TBI model, the microglial response is enhanced in A1 receptor knockout compared to wild type mice [51]. Thus although A1 receptors do not mediate suppression of LPS-induced TNF-α and CXCL10 production by microglia, A1 receptors most likely are neuroprotective. However, in TBI, we cannot rule out the possibility that exacerbation of damage by enhanced excitotoxicity in A1 receptor knock-out mice is not indirectly mediating the enhanced microglial response.
Deciphering the role of A2A receptors in neurologic injury is even more difficult. Unlike in peripheral tissue injury models and in peripheral immune cells where A2A-receptor activation consistently results in protective, anti-inflammatory effects, conflicting results are obtained in neurologic injury models [52, 53]. Following a wide range of injury types, neuroprotection occurs with both activation and blockade of A2A receptors [54].
Although the issue remains unresolved, a number of explanations regarding the conflicting results with A2A receptor manipulation are possible. Maturational stage is one factor that may play an important role. For example, A2A receptor knock-out results in neuroprotection in adult mice following ischemic brain injury whereas A2A receptor knock-out worsens outcome in neonatal mice following hypoxic ischemic brain injury [55, 56]. The timing of A2A receptor manipulation following injury is also likely critical. A recent study of experimental TBI demonstrates that an A2A receptor stimulation and antagonism are either protective or harmful depending on the time of administration following injury [57].
The cell type targeted is an additional consideration. In the CNS, A2A receptors are found on neurons and glia with highest expression in striatal neurons and lower levels of expression in other neurons and glial cells. Additionally, infiltrating peripheral leukocytes highly express A2A receptors. Achieving neuroprotection may thus require differential targeting of the A2A-receptor-expressing cell populations; and to better assess the contribution of peripheral versus CNS-derived cells, mouse chimera models can be deployed. Work by Yu et al. strongly suggests that A2A receptor knock-out is neuroprotective in stroke via effects on bone marrow rather than CNS-derived cells, as selective reconstitution of bone-marrow-derived cells in A2A receptor knock-out mice largely reinstates ischemic brain injury [58]. However, studies in experimental TBI suggest a detrimental role for A2A receptors on both bone marrow and CNS-derived cells [59]. While chimera studies are informative, to fully elucidate the role of A2A-receptor-manipulation, targeting of individual cell types is likely needed as activation or blockade may have opposing effects at the cellular level.
Finally, recent work points to the importance of the cellular activation state and the local environment in determining the effect of A2A-receptor-activation on neuroinflammation and outcome following acute neurologic injury. Studies by Van der Puten et al. demonstrate in non-human primate microglia that A2A-receptor-activation leads to more significant inhibition of TNF-α and IL-12p40/p70 in activated microglia compared to resting microglia [26]. Moreover, studies by Dai et al. show that microglial A2A-receptor-activation in the presence of low glutamate results in decreased inflammation as measured by NOS activity. However, when primary microglia are exposed to high glutamate concentrations, A2A-receptor-activation results in increased NOS activity. An in vivo TBI model confirms this conclusion. Administration of an A2A-receptor-agonist results in decreased neuroinflammation and improved outcomes if given at times of low local glutamate concentrations or if given following pretreatment with a glutamate release inhibitor. When administered at times of high CNS glutamate, however, A2A-receptor-activation worsens outcomes [57]. This may be important in explaining the variable results following A2A-receptor-manipulation in the context of acute neurologic injury.
In the present study, we derived microglia from neonatal mice and maintained cells in culture medium containing an extremely low glutamate concentration (0.05 mM), less than the lower concentration used by Dai and coworkers. Using this method, we felt confident that the results reflected only single subtype receptor activation and that activation of A2A, but not A1, receptors, inhibited TNF-α and CXCL10 production equivalently to the non-selective adenosine receptor agonist, NECA. Therefore, activation of microglial A2A receptors clearly has potential for anti-inflammatory effects, but the conditions under which it behaves in this manner are complex and must be better understood.
4. CONCLUSIONS
In summary, the 2’,3’-cAMP-adenosine pathway inhibited production of TNF-α and CXCL10 at least as effectively as adenosine alone. This occurred mostly via an adenosine receptor dependent mechanism. Inhibition of TNF-α and CXCL10 production occurs upon activation of A2A, but not A1 receptors. Further study of the impact of the 2’,3’-cAMP pathway on microglia activation and outcome following experimental TBI is ongoing.
5. EXPERIMENTAL PROCEDURE
5.1. Drugs
Lipopolysaccharide (LPS) from E. coli 026:B6, glutamate, adenosine, 2’,3’-cAMP, 3’-AMP, 2’-AMP, 1,3-dipropyl-8-p-sulfophenylxanthine (DPSPX), 5’-N-ethylcarboxamide adenosine (NECA), CGS21680, and 2-chloro-N6-cyclopentyladenosine (CCPA) were obtained from Sigma-Aldrich (St. Louis, MO).
5.2. Isolation of Primary Murine Microglia
Primary murine microglia were isolated from mixed glial cultures as previously described [23]. Briefly, brains of postnatal day 1–3 C57BL/6 mice were removed. Cerebral cortices were isolated and meninges were carefully removed. Cerebral cortices were dissociated by trituration in 0.25% trypsin. Typsin was inactivated by addition of growth media (DMEM/F12 and 10% FCS) and the mixture was passed through a 40 µm filter followed by centrifugation at 1200 RPM for 10 minutes. The cell pellet was resuspended in growth media (DMEM/F12 and 10% FCS) and cells were plated on poly-L-lysine coated T-75 flasks. Media was changed every 4–5 days. Mixed glial cultures were used between 18–21 days when cultures were confluent. Microglia were isolated by the mild trypsinization method using 0.25% trypsin diluted 1:4 in DMEM/F12 [60]. This resulted in detachment of an intact layer of astrocytes and an adherent population of microglia. Microglia were then removed from the flask using 0.25% trypsin and seeded into plates. After 24 hours of recovery, microglia were used in various experiments. We have previously demonstrated purity of microglial cultures obtained by this method [23].
5.3. Global Analysis of Cytokine and Chemokine Production
Microglia were treated with phosphate-buffered saline (PBS) or LPS (100 ng/mL) for 24 hours. Supernatants were collected to measure the concentrations of 20 cytokines/chemokines using multianalyte profiling (LabMAP™ system; Luminex Corporation, Austin, TX) with a Milliplex® kit (EMD Millipore, Billerica, MA).
5.4. Targeted Analysis of TNF-α and CXCL10 Production
Supernatant was collected following various experimental conditions described below and TNF-α and CXCL10 production were measured using murine TNF-α and CXCL10 ELISA kits from R&D Systems (Minneapolis, MN).
To determine the effect of adenosine and its precursors on TNF-α and CXCL10 production from activated microglia, microglia were co-treated with LPS (100 ng/mL) and adenosine (30 µM), 2,’3’-cAMP (30 µM), 3’-AMP (30 µM) or 2’-AMP (30 µM) for 24 hours. To determine if adenosine and its precursors were acting via adenosine receptors, the above described experiment was repeated, but also in the presence of DPSPX (100 nM), a non-selective adenosine receptor antagonist.
To evaluate the role of specific adenosine receptor subtypes, microglia were co-treated with LPS and various adenosine receptor agonists. Agonists included NECA (10 µM; a non-selective adenosine receptor agonist), CCPA (10 µM; a selective A1 adenosine receptor agonist), and CGS21680 (10 µM: a selective A2A adenosine receptor agonist).
5.5. Statistical Analysis
Specific comparisons were conducted with 2-tailed Student’s t-tests with a criterion of significance of p<0.05. Data are shown as mean and standard errors of the mean (SEM).
Highlights.
The 2’,3’-cAMP-adenosine pathway is: 2’,3’-cAMP →2’-AMP + 3’-AMP →adenosine
Microglia express a 2’,3’-cAMP-adenosine pathway
The 2’,3’-cAMP-adenosine pathway inhibits LPS-induced cytokine release
A2A receptors mediate inhibition of cytokines by the 2’,3’-cAMP-adenosine pathway
The 2’,3’-cAMP-adenosine- A2A receptor axis may regulate neuroinflammation
ACKNOWLEDGEMENTS
Supported by grants from the National Institutes of Health [NS087978 (PMK/EKJ); HD040686 (JLE); HL109002, DK091190, HL069846, DK068575 and DK079307 (EKJ)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
KJ-F bred and provided the postnatal day 1–3 mice. JLE, EAN, DGG, JDV, and TCJ developed and refined the primary murine microglia culture model. JLE performed the multianalyte profiling experiments. EAN and DGG performed the CXCL10 release experiments. EKJ provided the mass spectrometry measurements. PMK and EKJ provided funding. All authors participated in the design, analysis and interpretation of the experiments as well as the preparation of the manuscript.
Contributor Information
Elizabeth A. Newell, Email: elizabeth-newell@uiowa.edu.
Jennifer L. Exo, Email: jnnfrx0@gmail.com.
Jonathan D. Verrier, Email: jdv17@pitt.edu.
Travis C. Jackson, Email: tcj10@pitt.edu.
Delbert G. Gillespie, Email: dgg3@pitt.edu.
Keri Janesko-Feldman, Email: JaneskoKL@anes.upmc.edu.
Patrick M. Kochanek, Email: kochanekpm@ccm.upmc.edu.
Edwin K. Jackson, Email: edj@pitt.edu.
REFERENCES
- 1.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 2.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 3.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 4.Butovsky O, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31(1):149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Helmy A, et al. The cytokine response to human traumatic brain injury: temporal profiles and evidence for cerebral parenchymal production. J Cereb Blood Flow Metab. 2011;31(2):658–670. doi: 10.1038/jcbfm.2010.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochanek PM, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26(4):565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- 7.Dare E, et al. Modulation of glial cell functions by adenosine receptors. Physiol Behav. 2007;92(1–2):15–20. doi: 10.1016/j.physbeh.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 8.Kochanek PM, et al. Characterization of the effects of adenosine receptor agonists on cerebral blood flow in uninjured and traumatically injured rat brain using continuous arterial spin-labeled magnetic resonance imaging. J Cereb Blood Flow Metab. 2005;25(12):1596–1612. doi: 10.1038/sj.jcbfm.9600154. [DOI] [PubMed] [Google Scholar]
- 9.Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79(3):463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111(4):904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. New England Journal of Medicine. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mi Z, et al. 3-isobutyl-1-methylxanthine decreases renal cortical interstitial levels of adenosine and inosine. Life Sciences. 1994;54(16):277–282. doi: 10.1016/0024-3205(94)00846-9. [DOI] [PubMed] [Google Scholar]
- 13.Mi Z, Jackson EK. Metabolism of exogenous cyclic AMP to adenosine in the rat kidney. Journal of Pharmacology & Experimental Therapeutics. 1995;273(2):728–733. [PubMed] [Google Scholar]
- 14.Mi Z, Jackson EK. Evidence for an endogenous cAMP-adenosine pathway in the rat kidney. Journal of Pharmacology & Experimental Therapeutics. 1998;287(3):926–930. [PubMed] [Google Scholar]
- 15.Dubey RK, et al. Cyclic AMP-adenosine pathway inhibits vascular smooth muscle cell growth. Hypertension. 1996;28(5):765–771. doi: 10.1161/01.hyp.28.5.765. [DOI] [PubMed] [Google Scholar]
- 16.Dubey RK, Gillespie DG, Jackson EK. Cyclic AMP-adenosine pathway induces nitric oxide synthesis in aortic smooth muscle cells. Hypertension. 1998;31(1 Pt 2):296–302. doi: 10.1161/01.hyp.31.1.296. [DOI] [PubMed] [Google Scholar]
- 17.Dubey RK, et al. Cardiac fibroblasts express the cAMP-adenosine pathway. Hypertension. 2000;36(3):337–342. doi: 10.1161/01.hyp.36.3.337. [DOI] [PubMed] [Google Scholar]
- 18.Dubey RK, et al. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension. 2001;37(4):1095–1100. doi: 10.1161/01.hyp.37.4.1095. [DOI] [PubMed] [Google Scholar]
- 19.Jackson EK, Ren J, Mi Z. Extracellular 2’,3’-cAMP is a source of adenosine. Journal of Biological Chemistry. 2009;284(48):33097–33106. doi: 10.1074/jbc.M109.053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jackson EK, et al. Extracellular 2’,3’-cyclic adenosine 5’-monophosphate is a potent inhibitor of preglomerular vascular smooth muscle cell and mesangial cell growth. Hypertension. 2010;56(1):151–158. doi: 10.1161/HYPERTENSIONAHA.110.152454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson EK, Gillespie DG, Dubey RK. 2’-AMP and 3’-AMP inhibit proliferation of preglomerular vascular smooth muscle cells and glomerular mesangial cells via A2B receptors. Journal of Pharmacology and Experimental Therapeutics. 2011;337(2):444–450. doi: 10.1124/jpet.110.178137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson EK, et al. Extracellular cAMP-adenosine pathways in the mouse kidney. Am. J. Physiol. Renal Physiol. 2011;301:F565–F573. doi: 10.1152/ajprenal.00094.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verrier JD, et al. Expression of the 2’,3’-cAMP-adenosine pathway in astrocytes and microglia. J Neurochem. 2011;118(6):979–987. doi: 10.1111/j.1471-4159.2011.07392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson EK, Gillespie DG. Extracellular 2’,3’-cAMP and 3’,5’-cAMP stimulate proliferation of preglomerular vascular endothelial cells and renal epithelial cells. Am. J. Physiol. Renal Physiol. 2012;303:F954–F962. doi: 10.1152/ajprenal.00335.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verrier JD, et al. The brain in vivo expresses the 2’,3’-cAMP-adenosine pathway. J Neurochem. 2012;122(1):115–125. doi: 10.1111/j.1471-4159.2012.07705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Putten C, et al. Differential expression of adenosine A3 receptors controls adenosine A2A receptor-mediated inhibition of TLR responses in microglia. J Immunol. 2009;182(12):7603–7612. doi: 10.4049/jimmunol.0803383. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, et al. Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-α production through inhibition of PI 3-kinase/Akt and NF-kB activation in murine BV2 microglial cells. Neurosci Lett. 2006;396(1):1–6. doi: 10.1016/j.neulet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 28.Biber K, et al. Functional expression of CXCR3 in cultured mouse and human astrocytes and microglia. Neuroscience. 2002;112(3):487–497. doi: 10.1016/s0306-4522(02)00114-8. [DOI] [PubMed] [Google Scholar]
- 29.Rappert A, et al. CXCR3-dependent microglial recruitment is essential for dendrite loss after brain lesion. J Neurosci. 2004;24(39):8500–8509. doi: 10.1523/JNEUROSCI.2451-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller M, et al. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity--a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36(5):368–387. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 31.van Weering HR, et al. CXCL10/CXCR3 signaling in glia cells differentially affects NMDA-induced cell death in CA and DG neurons of the mouse hippocampus. Hippocampus. 2011;21(2):220–232. doi: 10.1002/hipo.20742. [DOI] [PubMed] [Google Scholar]
- 32.Galimberti D, et al. Intrathecal chemokine synthesis in mild cognitive impairment and Alzheimer disease. Archives of Neurology. 2006;63(4):538–543. doi: 10.1001/archneur.63.4.538. [DOI] [PubMed] [Google Scholar]
- 33.Duan R-S, et al. Decreased fractalkine and increased IP-10 expression in aged brain of APP(swe) transgenic mice. Neurochemical Research. 2008;33(6):1085–1089. doi: 10.1007/s11064-007-9554-z. [DOI] [PubMed] [Google Scholar]
- 34.Giunti D, et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol. 2003;73(5):584–590. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- 35.Kolb SA, et al. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-y inducible protein 10. J Neuroimmunol. 1999;93(1–2):172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- 36.Grip O, Janciauskiene S. Atorvastatin reduces plasma levels of chemokine (CXCL10) in patients with Crohn’s disease. PLoS One. 2009;4(5):e5263. doi: 10.1371/journal.pone.0005263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson NO, et al. CXCL4 and CXCL10 predict risk of fatal cerebral malaria. Dis Markers. 2011;30(1):39–49. doi: 10.3233/DMA-2011-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson NO, et al. Pharmacologic inhibition of CXCL10 in combination with antimalarial therapy eliminates mortality associated with murine model of cerebral malaria. PLoS One. 2013;8(4):e60898. doi: 10.1371/journal.pone.0060898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, et al. Simvastatin and atorvastatin improve behavioral outcome, reduce hippocampal degeneration, and improve cerebral blood flow after experimental traumatic brain injury. Exp Neurol. 2007;206(1):59–69. doi: 10.1016/j.expneurol.2007.03.031. [DOI] [PubMed] [Google Scholar]
- 40.Lu D, et al. Statins increase neurogenesis in the dentate gyrus, reduce delayed neuronal death in the hippocampal CA3 region, and improve spatial learning in rat after traumatic brain injury. J Neurotrauma. 2007;24(7):1132–1146. doi: 10.1089/neu.2007.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross SA, et al. The presence of tumour necrosis factor in CSF and plasma after severe head injury. Br J Neurosurg. 1994;8(4):419–425. doi: 10.3109/02688699408995109. [DOI] [PubMed] [Google Scholar]
- 42.Frugier T, et al. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27(3):497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- 43.Fan L, et al. Experimental brain injury induces differential expression of tumor necrosis factor-alpha mRNA in the CNS. Brain Res Mol Brain Res. 1996;36(2):287–291. doi: 10.1016/0169-328x(95)00274-v. [DOI] [PubMed] [Google Scholar]
- 44.Bermpohl D, et al. TNF alpha and Fas mediate tissue damage and functional outcome after traumatic brain injury in mice. J Cereb Blood Flow Metab. 2007;27(11):1806–1818. doi: 10.1038/sj.jcbfm.9600487. [DOI] [PubMed] [Google Scholar]
- 45.Cheong CU, et al. Etanercept attenuates traumatic brain injury in rats by reducing brain TNF- alpha contents and by stimulating newly formed neurogenesis. Mediators Inflamm. 2013;2013:620837. doi: 10.1155/2013/620837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherbel U, et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proc Natl Acad Sci U S A. 1999;96(15):8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shohami E, Ginis I, Hallenbeck JM. Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 1999;10(2):119–130. doi: 10.1016/s1359-6101(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 48.Waters RJ, et al. Cytokine gene polymorphisms and outcome after traumatic brain injury. J Neurotrauma. 2013;30(20):1710–1716. doi: 10.1089/neu.2012.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chio CC, et al. Etanercept attenuates traumatic brain injury in rats by reducing early microglial expression of tumor necrosis factor-alpha. BMC Neurosci. 2013;14:33. doi: 10.1186/1471-2202-14-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsutsui S, et al. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J Neurosci. 2004;24(6):1521–1529. doi: 10.1523/JNEUROSCI.4271-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haselkorn ML, et al. Adenosine A1 receptor activation as a brake on the microglial response after experimental traumatic brain injury in mice. J Neurotrauma. 2010;27(5):901–910. doi: 10.1089/neu.2009.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasko G, et al. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14(13):2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 53.Link AA, et al. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J Immunol. 2000;164(1):436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- 54.Dai SS, Zhou YG. Adenosine 2A receptor: a crucial neuromodulator with bidirectional effect in neuroinflammation and brain injury. Rev Neurosci. 2011;22(2):231–239. doi: 10.1515/RNS.2011.020. [DOI] [PubMed] [Google Scholar]
- 55.Aden U, et al. Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke. 2003;34(3):739–744. doi: 10.1161/01.STR.0000060204.67672.8B. [DOI] [PubMed] [Google Scholar]
- 56.Chen JF, et al. A2A adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci. 1999;19(21):9192–9200. doi: 10.1523/JNEUROSCI.19-21-09192.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai SS, et al. Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci. 2010;30(16):5802–5810. doi: 10.1523/JNEUROSCI.0268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yu L, et al. Selective inactivation or reconstitution of adenosine A2A receptors in bone marrow cells reveals their significant contribution to the development of ischemic brain injury. Nat Med. 2004;10(10):1081–1087. doi: 10.1038/nm1103. [DOI] [PubMed] [Google Scholar]
- 59.Dai SS, et al. Adenosine A2A receptors in both bone marrow cells and non-bone marrow cells contribute to traumatic brain injury. J Neurochem. 2010;113(6):1536–1544. doi: 10.1111/j.1471-4159.2010.06716.x. [DOI] [PubMed] [Google Scholar]
- 60.Saura J, Tusell JM, Serratosa J. High-yield isolation of murine microglia by mild trypsinization. Glia. 2003;44(3):183–189. doi: 10.1002/glia.10274. [DOI] [PubMed] [Google Scholar]