Abstract
Objectives
We investigated whether annexin-A8 (A-A8), a Ca2+-binding protein overexpressed in pancreatic cancer, plays a role in cell growth and migration, and its association with pancreatic cancer prognosis.
Methods
Clinicopathological features and associations between increased A-A8 expression (determined by immunohistochemistry) and histologic grade were studied in a tissue microarray of 90 resected stage I/II pancreatic cancer patients. We investigated A-A8’s effect on cell migration, proliferation, and colony formation in 2 pancreatic cancer cells (BXPC-3, Panc-1). Statistical analyses included Fisher’s exact test, t-test, ANOVA, and survival analysis.
Results
Western blot showed increased A-A8 expression in human pancreatic cancer cells, with A-A8 knockdown in BXPC-3 and Panc-1 cells demonstrating decreased cell viability (P=0.017, P=0.001), migration (2.5 vs. 0.9 and 1.6 vs. 1 mm at 96 h; P=0.048, P=0.004), and colony formation (~75% and 40% from scramble; P≤0.01), respectively. In our tissue microarray, A-A8 expression increased 5.9-fold (r=0.31; P=0.019) from low- to high-grade tumors, correlating with tumor grade (r=0.23; P=0.027). Additionally, high A-A8 expression was associated with decreased 5-year survival (P=0.042).
Conclusion
Our study is the first showing increased A-A8 expression is associated with poor prognosis in early stage pancreatic cancer; thus supporting further investigation as a future therapeutic target and prognostic marker.
Keywords: Pancreas, pancreatic cancer, annexin, markers, therapeutic target
INTRODUCTION
Pancreatic cancer is a formidable health care problem, corresponding to the 4th leading cause of cancer death1 in the United States in 2013. Although the only curative treatment is surgical resection, the 5-year survival rate after pancreatectomy remains ~20%.2 Moreover, AJCC staging for early (stages I and II) disease is not informative of the long-term outcomes for the majority of patients outside stage Ia pancreatic cancer.3 Therefore, better markers of biological behavior are urgently needed.4–6
Annexins are a type of Ca2+-binding protein involved in the organization and structure of the phospholipid membrane and cytoskeletal proteins. Their molecular structure is composed of two principal domains: the COOH-terminal annexin core and NH2-terminal domain. The annexin core is conserved throughout the annexin family and consists of four annexin repeats (except for annexin-6, which has an 8-protein core), with each being 70–80 amino acids long and having a highly alpha-helical shape.7 The annexin core serves as a membrane-docking site that mediates binding to phospholipids and harbors the Ca2+-binding site. The NH2-terminal domain, known as the head region, is unique for each annexin type and has a role in interacting with protein ligands.8 It is thought to confer specific properties to each annexin type by specifying both the cellular target and the type of membrane interaction.9
Annexin-A8 (A-A8) is the most poorly studied annexin within the family but appears to demonstrate similar roles in membrane-cytoskeleton dynamics. Dysregulations in annexin expression are associated with human disease, specifically cancer. Previous studies have reported that there is high expression of A-A8 in acute promyelocytic leukemia, with similar findings in mammary tissue in which high-grade tumors have higher protein expression, correlating with worse overall survival.10 An association with high expression of A-A8 has also been shown in pancreatic cancer. In a study by Karanjawala et al., Affymetrix analysis was performed on a series of pancreatic cancers, revealing A-A8 as one of four gene fragments overexpressed. A-A8 mRNA was expressed in 92% of pancreatic cancers and in only 11% of normal tissue.11 A-A8 protein was also focally expressed in 149/154 (97%) carcinomas11; of these, 67% had strong and diffuse A-A8 staining in neoplastic glands, with nuclear and cytoplasmic labeling.11 However, A-A8’s role as a potential prognostic marker and therapeutic target for pancreatic cancer has not yet been thoroughly elucidated.
Previously, our institution and others have identified histologic grade as an independent prognostic factor in resected pancreatic cancer, with patients with poorly differentiated tumors having worse survival than patients with well-differentiated tumors,3–5 indicating that high-histologic grade is associated with poor biologic behavior. In this study, our aim was to examine the role of A-A8 expression in pancreatic cancer cell growth and migration. We also wanted to determine the relationship between A-A8 and histological grade in pancreatic cancer. Finally, we investigated the role of increased A-A8 expression in pancreatic cancer prognosis.
MATERIALS AND METHODS
Tissues
Samples for mRNA analyses using Affymetrix chips were obtained from primary pancreatic cancer samples from 58 patients who had surgical resection for histologically verified pancreatic ductal adenocarcinoma. These samples were obtained from Moffitt’s Total Cancer Care (TCC™) biorepository, a comprehensive clinical database with a large tumor bio-bank of >20,000 samples, as well as patient clinical data, where custom Affymetrix global GeneChip gene expression is analyzed. Patients prospectively provided written informed consent to participate in the IRB-approved TCC™ protocol. Clinicopathological findings were obtained from surgical records and included age, gender, tumor type, histological classification, level of lymph node metastasis, presence or absence of lymphatic and vascular invasion, and disease stage. Patients with other pancreatic malignancies such as intraductal papillary mucinous adenocarcinoma, acinar cell carcinoma, and malignant endocrine tumors were excluded. All tumor specimens were reclassified on hematoxylin- and eosin-stained slides, and histologic type and tumor grade were reassessed by a pathologist. Histologic grade was defined by the American College of Pathology as follows12: grade 1 = well differentiated (>95% of tumor composed of glands); grade 2 = moderately differentiated (50% to 95% of tumor composed of glands); and grade 3 = poorly differentiated (49% or less of tumor composed of glands). Overall survival was defined as time elapsed from surgery to death of patients with pancreatic cancer or last contact, which would be date of last follow-up visit. Disease-free survival was defined as time elapsed from surgery with curative intent to appearance of local disease recurrence, evidence of metastatic lesions detected by computed tomography, or death from any cause without documentation of a cancer-related event.
Immunohistochemical Labeling
A-A8 protein expression was determined by immunohistochemistry using an A-A8 Human Antibody (ABCAM, Cambridge, MA) in a tissue microarray of 90 resected pancreatic cancer patients. Antigen expression was defined as the presence of specific staining on surface membranes of tumor cells. Histoscore was defined as strength of staining as follows: 0 = no staining, 1 = mild, 2 = moderate, and 3 = strong, multiplied by the percentage of cells stained.
Cell Culture
Human pancreatic cancer cells BXPC-3, Panc-1, and MIAPaCa-2 (ATCC, Manassas, VA) were cultured in 10% serum media per ATCC guidelines. RNA inhibition was accomplished using A-A8 siRNA (SASI_Hs01_00091429; Sigma-Aldrich, St. Louis, MO) and Lipofectamine 2000 (Invitrogen, Grand Island, NY) and by following the manufacturer’s protocol for transfection of mammalian cell lines.
Western Blot Analysis
Western blot analysis was performed to confirm the expression of A-A8 in the BXPC-3, PANC-1, and MIAPaCa-2 cell lines. Thirty micrograms of protein were loaded in each well, and A-A8 antibody (R-18; Santa Cruz, Santa Cruz, CA) and vinculin antibody (Cell Signaling, Danvers, MA) for protein load control were used. Proliferation was determined using the MTT [3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] colorimetric assay for cytotoxicity according to manufacturer’s instructions. Optical densities were read using a microtiter plate reader at 562 nm. The scratch test was measured in 6-well plates, 10×103 cells were plated after pretreatment with regular media, scrambled siRNA, or A-A8 siRNA, and cells were allowed to grow to confluence, after which plates were subjected to a 1-cm scratch with a sterile instrument. Plates were examined every 24 hours, with growth measured under microscopic examination.
Cell Migration Assay
In each migration chamber (8 μm; BD Falcon, Sparks, MD), 2 × 104 cells were plated along with serum-free media, with 10% FBS used in the lower plate. After 12 hours, membranes were washed and cells were fixed with 4% paraformaldehyde and stained with 0.05% crystal violet. The filter was washed again, and cells were counted.
Colony Formation Assay
Standard soft agar colony formation assays were performed in BXPC-3, MIAPaCa-2, and Panc-1 cells. Cells were treated with serum-free media, scrambled siRNA, or A-A8 siRNA and subsequently seeded at a density of 5 × 103 per well in 12-well plates with 0.3% agar over a 0.6% bottom agar layer. Colonies were fed with 10% enriched media and then observed for 14 days. Colonies were also photographed after overnight incubation with 1 mg/mL MTT in the wells. Colonies were then counted under stereomicroscope and compared with control. The experiments were done at least twice, each in triplicate.
Statistical Analysis
Associations between A-A8 protein expression and cellular effects such as cell viability, migration, and colony formation were analyzed by Fisher’s exact test, t-test, and ANOVA. Survival curves were estimated by the Kaplan-Meier method; to this end, R and SPSS 20® (IBM Corporation, Somers, NY) statistical softwares were used. For all analyses, a P value of 0.05 or less was considered to be statistically significant.
RESULTS
Expression of Annexin-A8
Gene expression analysis revealed up-regulation of A-A8 expression in pancreatic cancer cells, with a significant 5.9-fold increase in expression of A-A8 from low- to high-grade tumors (r=0.31; P=0.019) (Figure 1). Immunohistochemical staining of A-A8 predominantly presented in the cytoplasm. The stroma surrounding the low grade and high grade adenocarcinoma demonstrated expression for A-A8 in fibroblasts or stellate cells and also some lymphocytes (Figure 2A). The A-A8 was constitutively expressed in these cells. As shown in Figure 2B, differences in A-A8 protein staining between low-grade and high-grade pancreatic cancer were statistically significant, with a 45% increase from well-differentiated and moderately differentiated to high-grade tumor (r=0.23; P=0.027; Figure 2B). The largest change in A-A8 protein expression was seen when comparing moderate to poor grade, whereas mRNA expression was similar between these two groups. In contrast, the mRNA expression data displayed the largest difference between moderate to poor grade, but immunohistochemical data had similar ranges and means.
Figure 1.
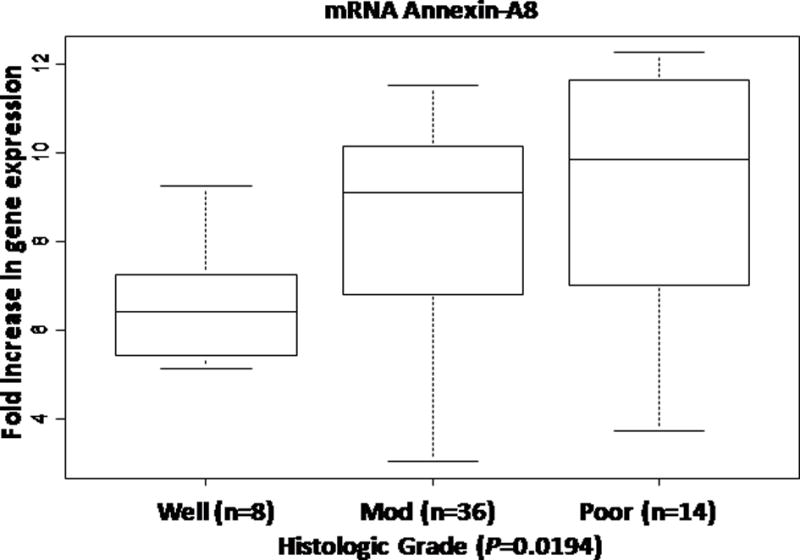
Annexin-A8 gene expression increases as tumor grade increases in pancreatic cancer.
Figure 2.
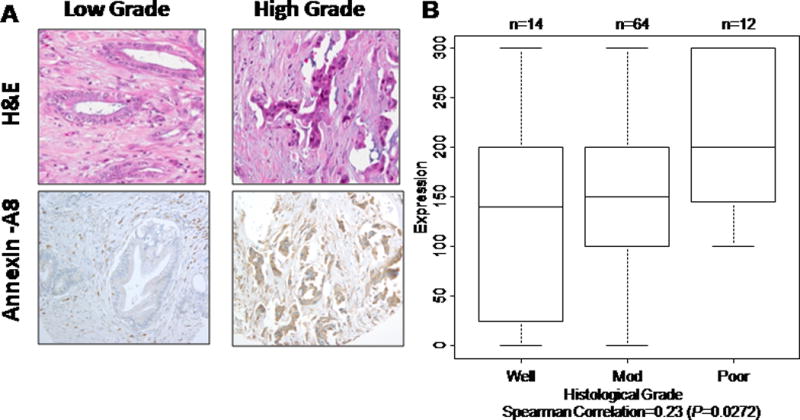
Annexin-A8 protein expression increases as tumor grade increases in pancreatic cancer. A, Immunohistochemical staining of A-A8 predominantly presented in the cytoplasm. The stroma surrounding the low-grade and high-grade adenocarcinoma demonstrate expression for A-A8 in fibroblasts or stellate cells and also some lymphocytes. B, Differences in annexin-A8 protein staining by immunohistochemical score between low-grade and high-grade pancreatic cancer.
Western blot analyses of A-A8 expression in 3 human pancreatic cancer cell lines revealed the presence of an immunopositive protein band in BXPC-3 and Panc-1 cells, but not in MIAPaCa-2 cells (Figure 3). When A-A8 siRNA was added to decrease the expression of A-A8, the expression of A-A8 was decreased in BXPC-3 and Panc-1 cell lines as expected (Figure 3).
Figure 3.
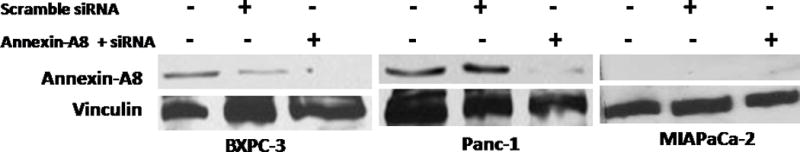
Annexin-A8 knockdown in 3 pancreatic cell lines (BXPC-3, Panc-1, and MIAPaCa-2).
Correlation Between A-A8 Expression and Cellular Characteristics
We examined the effect of A-A8 knockdown on the cellular characteristics of pancreatic cancer cells. With the use of Fisher’s exact test, t-test, and ANOVA, A-A8 expression was shown to be correlated with cell viability, migration, and colony formation. As measured by MTT test, cell viability was significantly decreased in BXPC-3 cells (P=0.017) and Panc-1 cells (P=0.001). In contrast, as expected, A-A8 siRNA had no effect on MIAPaCa-2 cells (Figure 4A). Cell migration was also decreased as measured by a scratch test at 96 hours in both BXPC-3 (2.5 vs. 0.9 mm; P=0.048) and Panc-1 (1.6 vs. 1 mm; P=0.004) (Figure 4B) cells. Colony formation was decreased after A-A8 siRNA treatment in both BXPC-3 (~75% from scramble; P≤0.01) and Panc-1 cells (~40% from scramble; P≤0.01) (Figure 4C). A-A8 knockdown resulted in a 50% decrease in BXPC-3 (P=0.03) and 40% decrease in Panc-1 (P=0.01) cell migration as measured using the migration chamber (Figure 4D).
Figure 4.
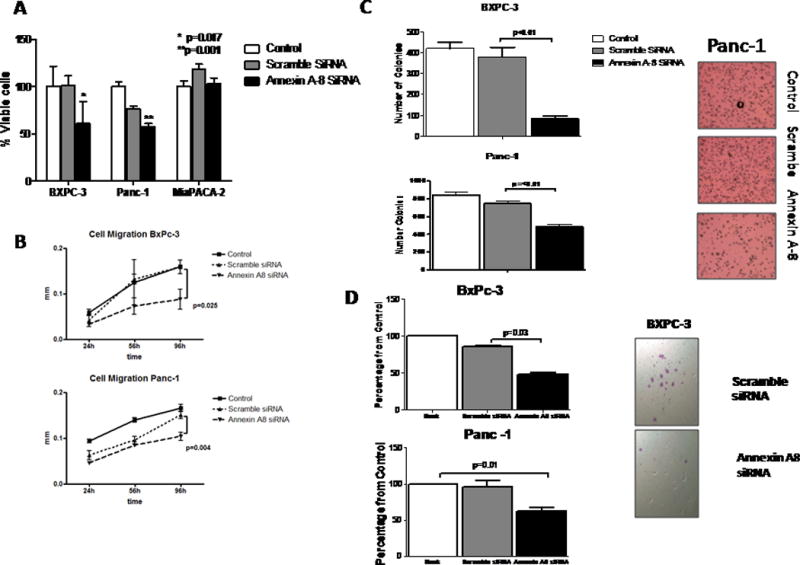
Annexin-A8 knockdown suppresses oncogenesis in pancreatic cancer cell lines. A, percent viable cells. B, cell migration results. C, number of colonies at A-A8 knockdown. D, colony formation after A-A8 siRNA treatment.
A-A8 Expression Is Associated with Overall Survival and Disease-free Survival in Patients with Pancreatic Cancer
Because of the significant correlation between high expression of A-A8 and histologic grade and knowing that histologic grade is commonly associated with poorer prognosis, we examined the effect of A-A8 expression on the survival of patients following curative resection of pancreatic cancer. We found that the median overall survival for patients with high expression of A-A8 was significantly shorter at 1.52 years compared to 5.23 years in patients with low expression of A-A8 (P=0.042) (Figure 5, left). In addition, the median disease-free survival for patients with low expression of A-A8 was significantly longer at 5.23 years than median disease-free survival of patients with high expression of A-A8 at 1.52 years (P=0.0098) (Figure 5, right).
Figure 5.
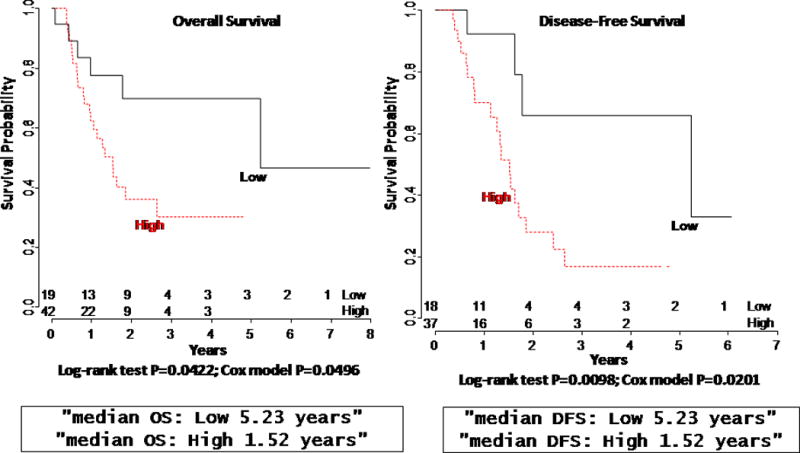
Decreased expression of annexin A8 correlates with improved disease free (right) and overall survival (left) in stage I–II pancreatic cancer.
DISCUSSION
With a 5-year survival of 5.8%,2 pancreatic cancer has the lowest survival rate among all types of cancers. Its presentation at an often late and incurable stage results in poor outcome for most patients. Therefore, dependable tumor molecular biomarkers are needed for pancreatic cancer to help design personalized treatments and for predicting prognosis. Increased expression of A-A8, a type of Ca2+-binding protein, has been shown in different types of cancers, including pancreatic cancer.
Although first described as an anticoagulation factor and shown to have many of the same roles in membrane-cytoskeleton dynamics as other members of its family, the biological function of A-A8 has not yet been completely elucidated. With the use of gene silencing of A-A8 in pancreatic cancer cells using siRNA, we found that cell viability, proliferation, migration, and colony formation were decreased. There is much evidence available to support the notion that A-A8 plays roles in these cellular activities. It has been shown that A-A8 binds F-actin and plasma membrane phospholipids with regulation from Ca2+ fluctuations occurring within the cell.13 A-A8 was demonstrated to localize to phosphatidylinositol 4,5-bisphosphate-rich membrane sites of F-actin accumulation in HeLa cells infected with enteropathogenic Escherichia coli, thus further supporting its potential role in the organization of specific membrane and cytoskeleton contacts.13 Moreover, there seems to be a specific role of A-A8 in the intracellular endocytic pathways, although the specific biologic consequences of overabundance of this Ca2+-regulated protein are unknown.14 A-A8 has been shown to be associated with late endosomes, which require associated actin filaments for localization and function. Depletion of A-A8 resulted in reduced association of late endosomes with actin filaments, leading to impaired actin-based late endosome motility.15 These biologic studies support A-A8’s role in cytoskeleton function, which might explain its impact on cell proliferation and migration.
Embryologic studies in mice have suggested that A-A8 may have a specific role in the terminal differentiation of epithelial cells due to its strong expression in the suprabasal layers of the epidermis of the skin, eye, and tongue.16 Our study displayed that A-A8 may have a role in cellular differentiation, and we found that A-A8 was remarkably upregulated in pancreatic cancer cells, with a 5.9-fold increased expression of A-A8 from low- to high-grade tumors. Additionally, our study showed that immunohistochemical expression of A-A8 was correlated with tumor grade in a human pancreatic tumor tissue microarray and that increased levels of A-A8 protein expression were significantly associated with high-grade tumors. A-A8 also displays exceptionally high levels in acute promyelocytic leukemia (APL). When APL is treated with differentiating agents such as vitamin A, it inhibits A-A8 expression, suggesting a role in cellular differentiation in the hematopoietic system.17 Similar findings were reported with A-A8 expression in mammary tissue, with high-grade tumors having higher protein expression and correlating with worse survival. Analysis of expression of this protein may also provide one component of a panel of markers to define a poor-acting breast cancer subgroup.10 A-A8 appears to play a role in cellular differentiation, which is further supported by our study.
Pancreatic cancer genetic expression analysis results have suggested an up-regulation of A-A8 in this lethal disease.18 Evidence that A-A8 may also play a role in tumor development, progression, and resistance to chemotherapeutic agents is increasing. Our study showed that knockdown of A-A8 resulted in decreased cellular migration, proliferation, and colony formation. However, similar to our study, a 2012 study that examined the relationship between A-A8 and pancreatic cancer also demonstrated that A-A8 is associated with cell viability and migration. BXPC-3 pancreatic cells were also used, and results corresponded to our study by displaying that knockdown of A-A8 is associated with decreased cell migration.19 These investigators also increased A-A8 expression in Panc-1 cells using cell transfection, resulting in increased cell viability.19 Additionally, their study demonstrated that A-A8 is associated with resistance against nutrient deprivation in pancreatic cancer cells and discovered that expression of A-A8 involves an epigenetic mechanism.19 In normal pancreatic cells, A-A8 expression is suppressed by hypermethylation of CpG in promoter exon-1 region.19 However, in pancreatic cancer cells, selective demethylation occurs in that region, resulting in up-regulation of A-A8 instead.19 Pancreatic cancers are unique in that they are a hypovascular tumor type and can survive in conditions of hypoxia and low-nutrient supply. A-A8 is induced by nutrient starvation, suggesting that the unique characteristics may be partly due to its overexpression of A-A8.19 These data support our hypothesis that A-A8 might play a pivotal role in pancreatic cancer processes and could be a potential therapeutic target and prognostic biomarker of pancreatic cancer.
In summary, A-A8 expression is important in pancreatic cancer cell growth and migration. To our knowledge, this is the first study displaying A-A8 expression is associated with increased histologic grade and decreased 5-year survival following resection of stage I and II pancreatic cancer in human patients. We determined that A-A8 may not only be a potential prognostic biomarker for pancreatic cancer but also an effective tool for identifying patients with worse or better prognosis and for providing personalized adjuvant therapy. Overall, we have demonstrated that high A-A8 expression is associated with poor disease outcome in pancreatic cancer patients. Our results support further investigation of A-A8 as a potential therapeutic target and prognostic marker for human pancreatic cancer.
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Source of Funding: The study was supported in part by National Cancer Institute/USPHS Grant 1RO1 CA-129227-01A1.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2009. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 3.Helm J, Centeno BA, Coppola D, et al. Histologic characteristics enhance predictive value of American Joint Committee on Cancer staging in resectable pancreas cancer. Cancer. 2009;115:4080–4089. doi: 10.1002/cncr.24503. [DOI] [PubMed] [Google Scholar]
- 4.Lenz J, Karasek P, Jarkovsky J, et al. Clinicopathological correlations of nestin expression in surgically resectable pancreatic cancer including an analysis of perineural invasion. J Gastrointestin Liver Dis. 2011;20:389–396. [PubMed] [Google Scholar]
- 5.Cleary SP, Gryfe R, Guindi M, et al. Prognostic factors in resected pancreatic adenocarcinoma: analysis of actual 5-year survivors. J Am Coll Surg. 2004;198:722–731. doi: 10.1016/j.jamcollsurg.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Eschrich S, Pramana J, Zhang H, et al. A robust multigene expression assay to predict clinical response to chemoradiotherapy. AACR Meeting Abstracts. 2008;2008:B38-. [Google Scholar]
- 7.Gerke V, Creutz CE, Moss SE. Annexins: linking Ca2+ signalling to membrane dynamics. Nat Rev Mol Cell Biol. 2005;6:449–461. doi: 10.1038/nrm1661. [DOI] [PubMed] [Google Scholar]
- 8.Monastyrskaya K, Babiychuk EB, Draeger A. The annexins: spatial and temporal coordination of signaling events during cellular stress. Cell Mol Life Sci. 2009;66:2623–2642. doi: 10.1007/s00018-009-0027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes MJ, Rescher U, Gerke V, et al. Annexin-actin interactions. Traffic. 2004;5:571–576. doi: 10.1111/j.1600-0854.2004.00210.x. [DOI] [PubMed] [Google Scholar]
- 10.Stein T, Price KN, Morris JS, et al. Annexin A8 is up-regulated during mouse mammary gland involution and predicts poor survival in breast cancer. Clin Cancer Res. 2005;11:6872–6879. doi: 10.1158/1078-0432.CCR-05-0547. [DOI] [PubMed] [Google Scholar]
- 11.Karanjawala ZE, Illei PB, Ashfaq R, et al. New markers of pancreatic cancer identified through differential gene expression analyses: claudin 18 and annexin A8. Am J Surg Pathol. 2008;32:188–196. doi: 10.1097/PAS.0b013e31815701f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Washington MK, Berlin J, Branton PA, et al. Protocol for the Examination of Specimens From Patients With Carcinoma of the Exocrine Pancreas. College of American Pathologists; 2009. [Google Scholar]
- 13.Goebeler V, Ruhe D, Gerke V, et al. Annexin A8 displays unique phospholipid and F-actin binding properties. FEBS Lett. 2006;580:2430–2434. doi: 10.1016/j.febslet.2006.03.076. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Martinez MT, Porte F, Liautard JP, et al. Effects of profilin-annexin I association on some properties of both profilin and annexin I: modification of the inhibitory activity of profilin on actin polymerization and inhibition of the self-association of annexin I and its interactions with liposomes. Biochim Biophys Acta. 1997;1339:331–340. doi: 10.1016/s0167-4838(97)00018-6. [DOI] [PubMed] [Google Scholar]
- 15.Goebeler V, Poeter M, Zeuschner D, et al. Annexin A8 regulates late endosome organization and function. Mol Biol Cell. 2008;19:5267–5278. doi: 10.1091/mbc.E08-04-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Runkel F, Michels M, Franken S, et al. Specific expression of annexin A8 in adult murine stratified epithelia. J Mol Histol. 2006;37:353–359. doi: 10.1007/s10735-006-9063-4. [DOI] [PubMed] [Google Scholar]
- 17.Liu JH, Stass SA, Chang KS. Expression of the annexin VIII gene in acute promyelocytic leukemia. Leuk Lymphoma. 1994;13:381–386. doi: 10.3109/10428199409049626. [DOI] [PubMed] [Google Scholar]
- 18.Logsdon CD, Simeone DM, Binkley C, et al. Molecular profiling of pancreatic adenocarcinoma and chronic pancreatitis identifies multiple genes differentially regulated in pancreatic cancer. Cancer Res. 2003;63:2649–2657. [PubMed] [Google Scholar]
- 19.Hata H, Tatemichi M, Nakadate T. Involvement of Annexin A8 in the properties of pancreatic cancer. Mol Carcinog. 2012;21:21961. doi: 10.1002/mc.21961. [DOI] [PubMed] [Google Scholar]


