Abstract
Somatic cells can be reprogrammed to express the features of pluripotent cells, in that they can be differentiated into all three germ layers, and that they have the ability to replicate indefinitely. Recent studies suggest that the efficient induction of pluripotency requires the activation of innate immunity.
The Nobel Prize-winning work of Shinya Yamanaka
Shinya Yamanaka won the Nobel Prize in Physiology or Medicine in November 2012 for his discovery that somatic cells could be induced to form pluripotent stem cells (known as induced pluripotent stem cells, or iPSCs) by forced overexpression of four transcriptional factors (Oct 4, Sox2, KLF4 and cMyc, or OSKM)1. Yamanaka and others subsequently revealed that these transcriptional factors activated a network of genes required for pluripotency.
This discovery has had a transformational effect on the field of regenerative medicine. The IPSCs may be used as a surrogate for the more controversial embryonic stem cells, to study mechanisms of cell self-renewal and pluripotency. The IPSCs may be differentiated into any somatic cell, so as to study pathobiology of disease, to screen drugs for safety or efficacy, or to be used in cell therapy.
In Yamanaka’s original publication, OSKM were overexpressed in somatic cells using retroviral vectors2. Since then, many investigators have improved the technology by using nonintegrating methods to overexpress the Yamanaka factors, and by using small molecules (eg. valproic acid, an inhibitor of histone deacetylase) to accelerate the reprogramming progress and to reduce the number of transcriptional factors3. Most recently, a methodology has been reported that uses only small molecules including epigenetic modifiers.4 These improvements are important for the field, because clinical applications will be more feasible if iPSCs can be generated rapidly and without concerns of integration of foreign DNA into the genome of therapeutic cells.
Nevertheless, the generation of iPSCs remains technically challenging, and requires the activation of an entire network of pluripotency genes, and the parallel suppression of genes enforcing the lineage of the somatic cell that is being reprogrammed. Global changes in the transcriptional profile occur in parallel with genome-wide alterations in histone proteins, noncoding RNAs, and DNA methylation. These genome wide alterations are associated with profound changes in signaling pathways, nuclear structure, metabolism and morphology of the cell that are incompletely characterized5. New insights into the mechanisms of nuclear reprogramming to pluripotency may enhance the efficiency and fidelity of the generation of iPSCs.
Role of innate immune signaling in generation of pluripotent stem cells
In 2012 we reported the surprising observation that the Yamanaka method to generate induced pluripotent stem cells (iPSCs) was dependent on activation of innate immunity6. In a previously published study, we revealed that the retroviral vectors carrying the Yamanaka factors were more than mere vehicles. Indeed, the retroviral vectors, by activating innate immune signaling (in part through TLR3) were absolutely integral to the reprogramming effort. Specifically, when we exposed human fibroblasts to cell permeant peptides of the Yamanaka factors, we were not able to obtain efficient generation of iPSCs. Furthermore, activation of genes in the downstream core pluripotency network was delayed and depressed. Only after activation of innate immunity, using polyinosinic:polycytidylic acid (Poly I:C, a TLR 3 agonist) could we obtain efficient nuclear reprogramming with cell permeant peptides of the Yamanaka factors. Furthermore, when we knocked down expression of the TLR3 receptor or its adaptor TRIF, iPSC colony formation was reduced and delayed when using the retroviral vectors encoding the Yamanaka factors.
We demonstrated that the same mechanisms were operative in other methods of reprogramming somatic cells to pluripotency. Specifically, we documented the importance of innate immune signaling in nuclear reprogramming when we used modified message RNA to express the Yamanaka factors, or when we employed murine embryonic fibroblasts expressing a doxycycline-inducible cassette of the Yamanaka factors. The generation of iPSCs using these methods could also be reduced by TLR3 knockdown, or by interfering with downstream signaling (ie. knocking down IRF3 or using a decoy oligonucleotide against p65).
The effect of innate immune signaling was mediated by global changes in epigenetic modifiers (eg. downregulation of multiple members of the histone deacetylase family, ie. HDAC 1, 5 ,8, 9 and 10; downregulation of Dot1L; upregulation of hastened acetyltransferase 1). These changes in the expression of epigenetic modifiers would be expected to increase the open chromatin state. Indeed, we confirmed an increase in H3K4 trimethylation (an activating histone mark) at the Oct 4 and Sox 2 promoters when cell permeant Sox2 was administered with Poly IC, but not in the absence of Poly IC.
It is very important to note that the contribution of innate immune signaling to nuclear reprogramming is not just a feature of the TLR3 receptor. Recently we have shown that activation of Rig-1 is also involved in the efficient reprogramming induced by retroviral vectors or mmRNA encoding the Yamanaka factors (unpublished data). Furthermore, LPS can also induce similar “epigenetic plasticity” through activation of TLR4.
Beyond Yamanaka
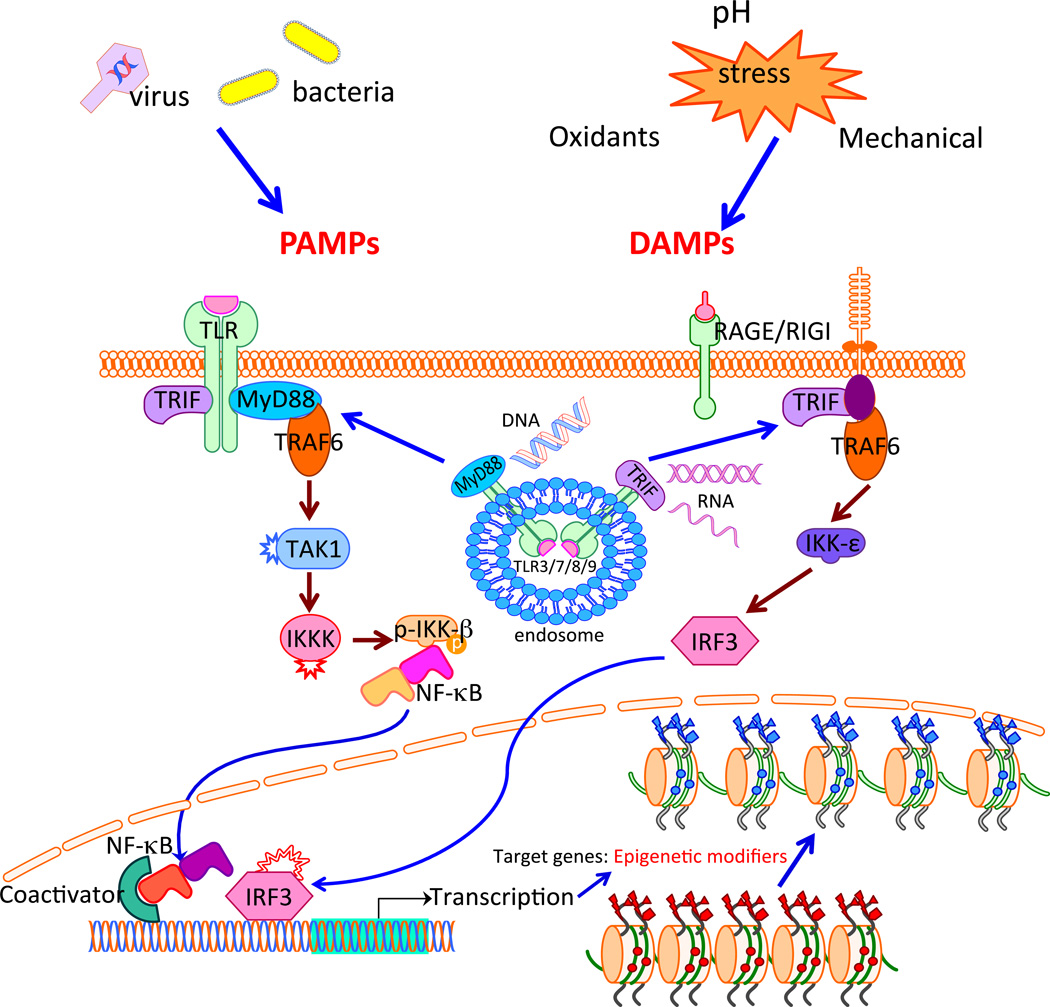
These findings are an important insight into the mechanisms of nuclear reprogramming. But we speculate that these observations have wider implications, with respect to the cellular response to a pathogen or to cellular damage. Damage-associated molecular patterns (DAMPs, also known as alarmins) or pathogen associated molecular patterns (PAMPs) can be sensed by an array of receptors such as toll-like receptors (TLRs), RIG-1, receptor for advanced glycosylation endproducts (RAGE), and others. We have unpublished evidence that activation of a wide range of these pattern recognition receptors (PRRs) causes global changes in epigenetic modifiers that increase the probability for an open chromatin state, in a process we term “transflammation”(Figure)7. In this state of epigenetic plasticity, the cell is poised to respond to microenvironmental cues that may change its phenotype. This is a heretofore unrecognized response to innate immune activation, which results in greater epigenetic plasticity. This response may permit an adaptive form of phenotypic fluidity so as to better respond to environmental stresses. In brief, our data indicates that when a cell is confronted by a pathogen or tissue damage, activation of receptors for PRRs can markedly increase epigenetic plasticity. In this state of epigenetic plasticity, cell phenotype can be transformed by environmental cues (eg. growth factors, small molecule modulators of cell signaling) so that the cell can adjust its phenotype and functions to respond to the challenge.
Figure 1.
Pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) are recognized by PRRs such as Toll-like receptors (TLRs), RIG-1 or receptor for advanced glycosylation endproducts (RAGE) on the cell surface or endosomes11,12. Stimulation of PRRs activates innate immune signaling pathways, leading to mobilization of the transcriptional factors NFkB and IRF-311,12. These transcriptional effects induce changes in epigenetic modifiers (eg. upregulation of the histone acetyltransferases, downregulation of the histone deacetylases), that increases the probability of an open state configuration of the chromatin. The open configuration state increases the epigenetic plasticity, and thereby the phenotypic fluidity of the cell in an adaptive manner to respond to the stress.
The Goldilock’s Zone for Innate Immunity in Nuclear Reprogramming
Because T and B lymphocytes are professional immune cells, they may be poised for epigenetic plasticity. Indeed, the Zambidis group has previously shown that such professional immune cells are easier to reprogram to pluripotency than hematopoietic stem cells, and that this effect is in part dependent upon NFKb expression8. Most recently, Guo et al. demonstrated that nuclear reprogramming is accelerated in CD45+ bone marrow derived progenitors of granulocytes and monocytes9. This ease of reprogramming in immune cells may be due to a baseline level of innate immune activation. In unpublished studies using murine or human fibroblasts, we have learned that there is an optimal zone of innate immune activation for reprogramming (the ‘Goldilock’s zone’). Below or outside of the zone, reprogramming is minimal or absent. We are attempting to elucidate the biological constraints on the Goldilock’s zone. An understanding of these biological constraints could have therapeutic value; small molecule antagonists that remove such constraints would be expected to increase epigenetic plasticity so as to enhance phenotypic fluidity and regeneration.
Plant biologists have long known that, with extreme environmental stress, mature plant cells can be induced to transdifferentiate into new plants in a process known as somatic embryogenesis10. We speculate that this process is at least partially recapitulated in higher organisms, and requires the induction of an epigenetic plasticity that is heavily modulated by innate immune signaling. We suggest that a greater understanding of the mechanisms of transflammation, through careful and rigorous experimentation, will generate scientific insights about regenerative processes that may lead to novel therapeutic avenues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yamanaka S. The winding road to pluripotency (Nobel Lecture) Angew Chem Int Ed Engl. 2013 Dec 23;52(52):13900–13909. doi: 10.1002/anie.201306721. Epub 2013 Nov 19. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. The seminal paper describing the landmark achievement of reprogramming somatic cells to pluripotency, leading to the Nobel prize in Medicine or Physiology just six years later.
- 3.Leeper NJ, Hunter AL, Cooke JP. Stem cell therapy for vascular regeneration: adult, embryonic, and induced pluripotent stem cells. Circulation. 2010 Aug 3;122(5):517–526. doi: 10.1161/CIRCULATIONAHA.109.881441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hou P, Li Y, Zhang X, Liu C, Guan J, Li H, Zhao T, Ye J, Yang W, Liu K, Ge J, Xu J, Zhang Q, Zhao Y, Deng H. Pluripotent stem cells induced from mouse somatic cells by small-molecule compounds. Science. 2013 Aug 9;341(6146):651–654. doi: 10.1126/science.1239278. This was the first paper to induce pluripotency using small molecule compounds alone, in the absence of viral vectors and transcriptional factors. The small molecule cocktail included valproic acid which reduces the activity of histone deacetylase (HDAC) so as to increase epigenetic plasticity.
- 5.Buganim Y, Faddah DA, Jaenisch R. Mechanisms and models of somatic cell reprogramming. Nat Rev Genet. 2013 Jun;14(6):427–439. doi: 10.1038/nrg3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski E, Reijo Pera R, Cooke JP. Activation of innate immunity is required for efficient nuclear reprogramming. Cell. 2012 Oct 26;151(3):547–558. doi: 10.1016/j.cell.2012.09.034. This paper revealed that in Yamanaka’s generation of iPSCs, the retroviral construct itself plays a role in nuclear reprogramming. The retroviral construct activates innate immunity, in part by stimulating toll-like receptor TLR3. Stimulation of TLR 3 activates NFKb and IRF3, which cause global changes in epigenetic modifiers that increases the probability of an open chromatin state. This effect is mediated in part by downregulation of histone deacetylases and upregulation of histone acetyltransferases.
- 7.Cooke JP. Therapeutic transdifferentiation: a novel approach for vascular disease. Circ Res. 2013 Mar 1;112(5):748–750. doi: 10.1161/CIRCRESAHA.113.301053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park TS, Huo JS, Peters A, Talbot CC, Jr, Verma K, Zimmerlin L, Kaplan IM, Zambidis ET. Growth factor-activated stem cell circuits and stromal signals cooperatively accelerate non-integrated iPSC reprogramming of human myeloid progenitors. PLoS One. 2012;7(8):e42838. doi: 10.1371/journal.pone.0042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo S, Zi X, Schulz VP, Cheng J, Zhong M, Koochaki SH, Megyola CM, Pan X, Heydari K, Weissman SM, Gallagher PG, Krause DS, Fan R, Lu J. Nonstochastic reprogramming from a privileged somatic cell state. Cell. 2014 Feb 13;156(4):649–662. doi: 10.1016/j.cell.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steward FC, Mapes MO, Smlth J. Growth and organized development of cultured cells. I. Growth and division of freely suspended cells. Am. J. Bot. 1958;45:693–703. [Google Scholar]
- 11.Newton K, Dixit VM. Signaling in innate immunity and inflammation. Cold Spring Harb Perspect Biol. 2012 Mar 1;4(3) doi: 10.1101/cshperspect.a006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev. 2011 Sep;243(1):174–190. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]