Abstract
BACKGROUND
The study aims to determine whether the route of insulin administration influences glycemic variability and inflammatory or neurohormonal markers in patients with type 2 diabetes (T2D) and congestive heart failure (CHF) exacerbation.
METHODS
Patients (N=65) were randomized to intravenous (IV) insulin (duration 48 hours) or subcutaneous (SQ) insulin. Inflammatory cytokines and markers of lipid oxidation; high frequency heart rate variability (HRV, N=27) and cardiac impedance (pre-ejection period, PEP, N=28) were used to estimate parasympathetic and sympathetic tone in patients with valid cardiac data. Glycemic variability was measured using a continuous glucose monitor.
RESULTS
Mean glucose was lower (7.7 +/−1.2 vs. 9.4 +/− 2.7 mmol/L, p=0.004), coefficient of variation (CV) was higher (p=0.03), and glycemic lability index (GLI) was similar on day 1 in the IV group compared to the SQ group, but groups were similar by day 2. The IV group had more confirmed hypoglycemia (p=0.005). There were no differences in hospital readmission or hospital length of stay between groups. There were no differences in CHF biomarkers, HRV or PEP between groups. Increasing log GLI was associated with lower on-treatment PEP (p=0.03) while increasing CV was associated with increasing brain natriuretic peptide (BNP, p=0.004) and paroxonase-1 (p=0.02). Other univariable analyses were not significant.
CONCLUSIONS
There were modest, transient differences in glucose control between IV and SQ insulin in hospitalized CHF patients. However, the analyses do not support a link between insulin route and inflammatory markers or autonomic tone. Further study is needed to assess outcomes in hospitalized CHF patients.
Key Terms: type 2 diabetes, heart failure, insulin
INTRODUCTION
Congestive heart failure (CHF) poses an enormous medical, societal and financial burden in the U.S, affecting 5 million Americans, and leading to $32 billion in costs annually.[1] Over 40% of patients with CHF have diabetes as a discharge diagnosis,[2] and diabetes is an independent predictor of mortality in patients with ischemic CHF.[3] Therefore, patients with CHF exacerbation may be suited for glycemic control measures.
In patients with CHF exacerbation, subcutaneous (SQ) insulin absorption may be significantly impaired.[4] However, there are no studies to demonstrate whether intravenous (IV) insulin results in better glycemic control or other physiologic measures in patients with acute exacerbation. While IV insulin may improve overall mean glucose, this may be at the expense of an increase in relative glucose variability.
Measures of glucose variability are of increasing interest for assessing glucose control, since they may be associated with mortality in in hospitalized patients with CHF [5] or critical illness [6]. However, the mechanism of glycemic variability related harm is unknown, and the effects of glycemic variability must be distinguished from that of hypoglycemia or hyperglycemia. In a euinsulinemic, hyperglycemic clamp study, oscillating glucose levels between 5 and 15 mmol/l resulted in endothelial dysfunction and increased oxidative stress in otherwise healthy patients with type 2 diabetes.[7] These effects exceeded that of sustained hyperglycemia. However, other observations are conflicting with respect to the relationship between glycemic fluctuations and oxidative stress.[8] Moreover, these observations do not necessarily apply to patients with acute illness. In CHF, there are already marked elevations in biomarkers of immune activation, inflammation and oxidative stress.[9,10]
Changes in glucose in controlled settings are also reported to alter autonomic tone.[11,12] Therefore, it is possible that glucose variability may play a role. In the setting of CHF, autonomic perturbations are recognized as contributors to the overall immune activation and inflammatory milieu, [13] and changes predict severity of CHF [14] and mortality.[15] However, it is unknown whether measures of autonomic tone are associated with glycemic control in CHF.
The objectives of this proof-of-concept study are to determine a preliminary effect of IV or SQ insulin on outcomes in hospitalized CHF patients with type 2 diabetes and to determine the role of glycemic variability, systemic inflammation, or autonomic tone.
MATERIALS AND METHODS
Subjects
Patients over age 18 who were admitted at a single academic medical center for CHF exacerbation as the primary diagnosis with significant insulin use (>20 units/day) or hyperglycemia (BG >150 m/dl on at least 2 occasions separated by at least 4 hours apart) were enrolled. Exclusion criteria included type 1 diabetes, hospital stay expected to be less than 48 hr, inability to consent, pregnancy, prisoners, myocardial infarction within the previous 3 months, corticosteroid use, systolic blood pressure <80 mmHg, isolated right heart failure or end stage renal or liver disease. This study was approved by the Ohio State University Institutional Review Board. All patients provided written informed consent prior to any study procedures.
Intervention
Patients were randomized to open label IV insulin (48 hours) or physiologic SQ basal-bolus insulin using a computerized random number generator. All other non-insulin glucose lowering agents were discontinued. In both treatment groups, prandial insulin (lispro or aspart) was delivered according to carbohydrate intake using a carbohydrate to insulin ratio that is based upon the estimated total daily insulin dose in both groups (carbohydrate to insulin ratio=400/total daily dose). In subjects who were insulin naïve, the total dose of insulin was calculated as 0.4 or 0.5 unit/kg if the enrollment glucose was <11.1 mmol/l or >11.1 mmol/l respectively. In subjects who were not insulin naive, the total insulin dose was calculated as 100% or 120% of the total daily insulin dose at admission in subjects with an enrollment glucose of <11.1 mmol/l or >11.1 mmol/l respectively. In the subcutaneous insulin group, all patients received basal insulin glargine, starting at 21:00 the day of enrollment, except one patient who was allowed to remain on pre-existing detemir. Basal and prandial insulin were administered in approximately equal total daily doses with correction dosing and adjustments based upon a published algorithm.[16] The target glucose range was 5.6–8.3 mmol/l.
In patients assigned to IV insulin, SQ basal insulin was discontinued and IV insulin was started at 21:00 on the day of enrollment. Patients continued to receive subcutaneous prandial insulin. All patients receiving IV insulin were managed using our hospital’s universal nursing run guideline, which was adapted from a published protocol [17] and has a target glucose of 6.1–8.3 mmol/l. All floor nurses are trained in its use. Subjects treated with IV insulin also received 5% dextrose in half-normal saline at 10 ml/hour according to hospital guidelines. Patients were transitioned from the infusion beginning 48 hours after initiation using glargine at approximately 70% of the estimated basal insulin infusion requirement with 4 hours of overlap.
Assessments
Continuous subcutaneous glucose monitoring was performed (Medtronic®, Minneapolis, MN) every 5 minutes for up to 72 hours in order to capture changes in mean glucose and glucose variability. The continuous glucose monitor was inserted on the abdomen and downloaded using manufacturer software according to manufacturer instructions. Calibrations were performed using manufacturer’s software, and analyses were performed on the final reported values. Patients with at least 48 hours of data are included in the analyses. Due to variable sensor start times, data were censored on the day of enrollment so that sensor data collection began uniformly on the following calendar day. Any gaps in intervals were handled through time-weighting individual data, using the trapezoidal rule. Glucose values are presented by enrollment day (day zero is defined as the day of enrollment).
Capillary glucoses were analyzed with the Accu-Chek Inform® system (Roche, Indianapolis, IN), and were used to calibrate the sensor according to manufacturer guidelines. Capillary glucoses were collected hourly during insulin infusions and every 4–6 hour otherwise. However, only glucoses at four predetermined time points per day (pre-meal and at bedtime closest to 0700 hr, 1200 hr, 1700 hr, and 2100 hr) within the allowable glucose limits of the software (2.2–22.2 mmol/l) were used to calibrate the continuous glucose monitor device.
Glycemic variability was measured with the coefficient of variation (standard deviation/mean glucose) and glycemic labililty index (which is calculated by first finding the square of the difference between successive glucose measurements, dividing this value by the difference in time between measurements, and then calculating the sum of the quotients).[18] Hypoglycemia was defined as a blood glucose <3.9 mmol/l due to the concern for low accuracy of continuous glucose monitoring in the hypoglycemic range.[19]
Blood draws were performed at baseline and the morning following the transition dose of basal insulin. Plasma samples were collected on ice and immediately processed and frozen. Paraoxonase-1 was measured using a colorimetric assay.[20] Oxidized LDL was measured using an ELISA from Mercodia® (Uppsalla, Sweden). The other laboratory analyses were performed by the OSU Clinical Research Center using standard commercial kits. Interleukin-6 (IL-6, range 0.3–2500 pg/ml), and tumor necrosis factor-α (TNF-α range 0.3–2500 pg/mL) were performed using Meso Scale Discovery kits (Rockville, MD), High sensitivity C-reactive protein (hsCRP, range 0–15 mg/L) was performed using Immunlite 1000 assay (Siemens; Erlangen, Germany), and plasma catecholamines (epinephrine and norepinephrine) were analyzed using high performance liquid chromatography.
Heart rate variability and cardiac impedance were performed at baseline and the morning of days 1–3. Electrocardiographic and impedance measures were obtained using a Bionex system (Mindware, Gahanna, OH). Impedance and HRV were performed with the subject lying supine with the head of the bed raised 45 degrees. The electrocardiogram was performed in the standard lead II configuration and impedance cardiography was performed using a standard tetrapolar arrangement as described previously.[21,22] Measures were performed at baseline and each morning (0800–1000 hour) during and following the intervention for 7 minutes each.
Software (Mindware, Gahanna, OH) was used to derive pre-ejection period, and high frequency HRV. Pre-ejection period is the time between the onset of electrical depolarization of the ventricle and the opening of the aortic valve, which estimates sympathetic tone more exclusively than HRV.[23] The middle five minutes of the recordings were scored minute by minute and the first suitable 1 minute period was used for calculation of high frequency HRV. Five minute epochs were not feasible due to an unexpectedly high frequency of ectopy. One minute intervals allow calculation of high frequency (parasympathetic tone) but not low frequency (combination of sympathetic and parasympathetic tone) HRV.[22, 24] High frequency HRV was calculated using power spectral analysis of the interbeat interval with fast Fourier transformation and integration over the respiratory band (0.12–0.40 Hz) as reported previously.[24] High frequency HRV is reported as the natural log of the heart period variance in the respiratory band (ms2). All patients had respiratory rates within the respiratory band. Pre-ejection period is measured in milliseconds; lower values reflect higher sympathetic tone.
Change in plasma volume was calculated with the hemoglobin and hematocrit from successive days as published previously.[25] Ejection fraction was determined from the clinical record using transthoracic echocardiogram within the previous 3 months, where available. Where a recent echocardiogram was unavailable, a myocardial perfusion scan (N=2) or cardiac magnetic resonance imaging (N=1) result was reported.
Statistical analyses
A sample of 80 patients was targeted for this proof-of-concept study based upon feasible enrollment over 3 years. The primary outcomes of interest were a preliminary assessment of hospital length of stay and hospital readmission; however, the study was not large enough to adequately assess these outcomes. Secondary outcomes were mean glucose, glycemic lability index, coefficient of variation, high frequency heart rate variability, pre-ejection period, high sensitivity C-reactive protein, brain natruiuretic peptide. Other measures were exploratory analyses. Analyses were performed on a per protocol basis. Patients receiving Nesiritide were excluded from analyses involving brain natriuretic peptide (BNP). Continuous variables were reported as mean (standard deviation) for normal distributions or median (interquartile range) for non-normal distributions. Differences between groups were determined with the unpaired student’s t-test or Wilcoxon rank-sum as appropriate. Dichotomous variables were reported as number (percentage) and differences between groups were determined using Fisher’s exact test. P-values less than 0.05 were considered statistically significant. Relationships were evaluated with simple linear regression with log transformation of variables failing to meet the normality assumption. Analyses were performed using JMP 9.0 software.
RESULTS
Baseline Characteristics
Seventy-five patients consented, 65 of whom had available data (3 screen failures (1 prior to randomization, 1 in IV group, 1 in SQ group), 5 withdrawals (3 in IV group, 2 in SQ group), and 2 patients discharged within 24 hours (both in IV group)(Figure 1). Of the remaining patients, 57 subjects had evaluable glucose sensor data. Those without sensor data remained in the study for assessment of other variables. There was a trend for greater mineralocorticoid receptor antagonist use in the SQ group but baseline characteristics were otherwise similar (Table 1).
Figure 1.
Patient flow diagram.
Table 1.
Patient Characteristics
| IV (N=26) | SQ (N=39) | p-value | |
|---|---|---|---|
| Age (years) | 61.3 (9.6) | 63.1 (12) | 0.52 |
| Female | 8 (31%) | 13 (33%) | >0.99 |
| Caucasian | 19 (73%) | 29 (74%) | >0.99 |
| Duration of DM (years) | 14.5 (10–20) | 11 (9–20) | 0.50 |
| Preserved Ejection Fraction | 6 (23%) | 11 (28%) | 0.78 |
| Ejection Fraction* | 0.36 (0.15) | 0.35 (0.18) | 0.82 |
| Hypertension | 22 (85%) | 35 (90%) | 0.70 |
| Coronary Artery Disease | 16 (62%) | 25 (64%) | >0.99 |
| Cebrovascular Accident | 4 (15%) | 5 (13%) | >0.99 |
| Retinopathy | 6 (23%) | 3 (7.7%) | 0.14 |
| Neuropathy | 14 (54%) | 13 (33%) | 0.13 |
| Chronic Kidney Disease | 13 (50%) | 21 (54%) | 0.80 |
| Creatinine (mg/dl) | 1.71 (0.77) | 1.55 (0.51) | 0.37 |
| Atrial Fibrillation | 7 (27%) | 15 (38%) | 0.78 |
| ICD/Pacer | 13 (50%) | 21 (54%) | 0.80 |
| Body Mass Index (kg/m2) | 39.4 (9.99) | 38.8 (8.84 | 0.81 |
| HbA1c (%) | 8.43 (1.97) | 7.70 (1.39) | 0.11 |
| Admission Glucose (mmol/l) | 10.4 (3.0) | 9.7 (3.4) | 0.42 |
| Insulin at admission | 24 (92%) | 33 (85%) | 0.46 |
| ACE Inhibitor/ARB | 13 (50%) | 26 (67%) | 0.21 |
| Aspirin | 21 (81%) | 33 (85%) | 0.74 |
| Beta Blocker | 22 (91.7%) | 34 (89.5%) | >0.99 |
| Mineralocorticoid receptor antagonist | 6 (23%) | 19 (49%) | 0.04 |
| Statin | 23 (88%) | 31 (79.0%) | 0.50 |
| Nesiritide | 5 (19%) | 6 (15%) | 0.74 |
| Milrinone | 1 (3.9%) | 5 (13.8%) | 0.39 |
| Dobutamine | 2 (7.7%) | 6 (15.4%) | 0.46 |
| Total daily insulin (units) | |||
| Day 1 | 24.5 (15–43) | 35 (18–58) | 0.14 |
| Day 2 | 37.5 (16–58) | 38 (24–72) | 0.51 |
| Day 3 | 59 (45–91) | 35 (25–98) | 0.002 |
Dichotomous values are reported as number (%). Continuous variables are reported as mean (standard deviation), except duration of diabetes and insulin dose, which were reported as median (interquartile range). Ejection fraction (EF) was determined by echocardiogram within 3 months where available. In the IV group, all EFs were determined by echocardiogram, whereas in the SQ group, EF was determined by myocardial perfusion scan in 2 subjects and MRI in 1 subject. EF was available within 1 month in 85 and 88% of subjects in the IV and SQ groups respectively. ICD=implantable cardioverter defibrillator, ACE=angiotensin converting enzyme, ARB=angiotensin receptor blocker.
A total of 39 patients received SQ insulin and 26 patients received IV insulin. Continuous glucose monitoring derived mean glucose was lower for the IV group (7.72 +/−1.28 vs. 9.39 +/− 2.72 mmol/l, p=0.004) but differences were negligible after day 1 (Table 2). Continuous glucose monitoring derived coefficient of variation was higher in the IV group on day 1 (p=0.03) compared to SQ, but this was negligible by day 2. Continuous glucose monitoring determined glucose lability index was not significantly different between groups.
Table 2.
Glucose and Laboratory Values
| IV (N=26) | SQ (N=39) | p-value | |
|---|---|---|---|
| Mean Glucose (mmol/L) | |||
| Day 1 | 7.72 (1.28) | 9.39 (2.72) | 0.004 |
| Day 2 | 8.56 (2.39 | 9.11 (2.28) | 0.37 |
| Day 3 | 8.72 (1.56) | 8.72 (2.11) | 0.94 |
| %Coefficient of Variation | |||
| Day 1 | 24.5 (10.2) | 18.6 (8.54) | 0.03 |
| Day 2 | 20.6 (7.94) | 19.4 (7.99) | 0.55 |
| Day 3 | 14.2 (5.9) | 14.8 (7.2) | 0.74 |
| Glycemic lability index#^ | |||
| Day 1 | 0.83 (0.31–1.44) | 0.66 (0.29–2.01) | 0.96 |
| Day 2 | 0.69 (0.29–1.16) | 0.51 (0.30–1.52) | 0.77 |
| Day 3 | 0.57 (0.24–1.16) | 0.24 (0.15–0.67) | 0.21 |
| Blood glucose <3.9 mmol/l | |||
| Day 1 | 7 (27%) | 1 (2.6%) | 0.005 |
| Day 2 | 6 (23%) | 1 (2.6%) | 0.02 |
| Day 3 | 1 (4.2%) | 4 (11%) | 0.65 |
| Sensor Time in Hypoglycemia (hours)* | |||
| Day 1 | 0 (0–3.25) | 0 (0–0.48) | 0.49 |
| Day 2 | 0 (0.0–0.48) | 0 (0.0–0.008) | 0.09 |
| Day 3 | 0 (0.0–0.0) | 0 (0.0–0.21) | >0.99 |
| BNP (pg/ml) Day 1^+ | 794 (168–1090) | 356 (192–910) | 0.35 |
| Change | −267 (628) | −231 (478) | 0.84 |
| IL-6 (pg/ml) Day 1^ | 8.91 (5.27–14.6) | 6.59 (4.02–11.9) | 0.42 |
| Change | 3.42 (10.2) | 7.67 (28.8) | 0.42 |
| TNF-α (pg/ml) Day 1 | 15.6 (6.58) | 14.1 (5.38) | 0.33 |
| Change | 1.07 (4.50) | 0.91 (3.67) | 0.88 |
| hsCRP (mg/dl) Day 1^ | 14.3 (5.51–41.4) | 19.2 (7.45–36.9) | 0.70 |
| Change | −5.69 (34.6) | −1.48 (27.7) | 0.62 |
| Adiponectin Day 1 (ng/ml) | 11.5 (7.2) | 13.7 (7.72) | 0.40 |
| Change | 12.5 (17.5) | 28.7 (30.9) | 0.06 |
| Paraoxonase-1 (U/L) Day 1 | 0.32 (0.19) | 0.41 (0.25) | 0.19 |
| Change | 0.03 (0.15) | −0.015 (0.26) | 0.47 |
| Oxidized LDL (U/L) Day 1 | 42. (19.9) | 43.1 (19.5) | 0.95 |
| Change | −2.63 (6.33) | −0.87 (8.10) | 0.55 |
BNP=brain natriuretic peptide, IL-6=interleukin-6, TNF-α=tumor necrosis factor-α, hsCRP=high sensitivity C-reactive protein, paraoxonase-1, LDL=low density lipoprotein.
Units in (mg/dl)2/hr*day−1,
Data reported as
median (10–90% range),
median (interquartile range, 25–75%); otherwise data reported as mean (SD), or number (%).
Patients receiving Nesiritide were excluded from BNP analysis. Change values were obtained in the morning of day 3 following overnight transition to subcutaneous insulin.
The frequency of hypoglycemia (capillary blood glucose <3.9 mmol/l) was higher on day 1 (27 vs. 2.6%, p=0.005) and day 2 (23 vs. 2.6%, p=0.03) in the IV and SQ groups respectively (Table 2). However, the duration of hypoglycemia on continuous glucose monitoring was no different (p=0.49). No patients had a blood glucose <2.2 mmol/l or severe event. The total daily insulin dose was similar in the IV and SQ groups on day 1 and 2 but was significantly higher in the IV group by day 3 following transition off of the IV infusion (Table 2).
Laboratory Data
Laboratory data are shown in Table 2. Patients had a significant reduction in BNP and increase in total adiponectin (p<0.05 for all) overall but there was no difference between treatment groups. High sensitivity CRP and other cytokines were high at baseline. In the overall sample, IL-6 significantly increased over time and TNF-α showed a trend for increase. Otherwise, there were no differences in the change in biomarkers between study groups. Univariable associations with continuous glucose monitoring measures are shown in Table 3. The estimates did not demonstrate an increasing association between inflammation or oxidative stress and mean glucose or glucose lability index. However, higher BNP, and paradoxically, paraoxonase-1 (reflecting antioxidant activity) were associated with higher coefficient of variation.
Table 3.
Univariable Associations Between CHF Biomarkers and Continuous Glucose Monitoring
| Mean Glucose | Coefficient of Variation | Glycemic Lability Index* | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | p-value | Estimate | SE | p-value | Estimate | SE | p-value | |
| Adiponectin Day 3 | −0.0086* | 0.0054* | 0.13 | 0.025* | 0.022* | 0.26 | 4.50 | 6.02 | 0.46 |
| BNP Day 3^ | −0.0005 | 0.01 | 0.96 | 0.0068 | 0.002 | 0.004 | 6.6×10−5 | 0.0003 | 0.81 |
| IL-6 Day 3 | 0.16 | 0.14 | 0.28 | −0.36 | 0.60 | 0.55 | −7.44 | 5.48 | 0.18 |
| TNF-a Day 3 | 0.043 | 0.025 | 0.10 | −0.11 | 0.11 | 0.32 | −1.41 | 1.00 | 0.16 |
| hsCRP Day 3 | 0.23 | 0.16 | 0.15 | −0.0278* | 0.019* | 0.16 | −0.34* | 0.18* | 0.064 |
| Paraoxonase-1 Day 3 | −0.0001 | 0.001 | 0.94 | 0.0086 | 0.003 | 0.017 | −0.01 | 0.04 | 0.71 |
| Oxidized LDL Day 3 | 0.021 | 0.013 | 0.10 | −0.013* | 0.015* | 0.40 | −0.21* | 0.15* | 0.17 |
| Pre-ejection period 24 hours | −0.10 | 0.10 | 0.32 | −0.51 | 0.48 | 0.29 | −10.4 | 4.33 | 0.02 |
| High frequency HRV 24 hours | 13.2 | 7.33 | 0.09 | −22.7 | 35.6 | 0.53 | 171 | 330 | 0.61 |
Values Log-transformed for better fit.
Patients receiving Nesiritide were excluded from BNP analysis.
BNP=brain natriuretic peptide, IL-6=interleukin-6, TNF-α=tumor necrosis factor-α, hsCRP=high sensitivity C-reactive protein, LDL=low density lipoprotein, HRV= heart rate variability.
Heart Rate Variability (HRV) and Transthoracic Cardiac Impedance
Only 26 subjects had valid HRV and 28 subjects had valid impedance data. Patients were excluded due to medical devices (N=14), conditions that preclude measurement of HRV such as arrhythmias (N=10), a combination of devices and arrhythmia (N=10), or technical problems (N=2). There was no difference in mean glucose, coefficient of variation, or glucose lability index between patients who did or did not have available HRV or impedance data (data not shown). Trends were similar between treatment groups for high frequency HRV and pre-ejection period (Figure 2).
Figure 2.
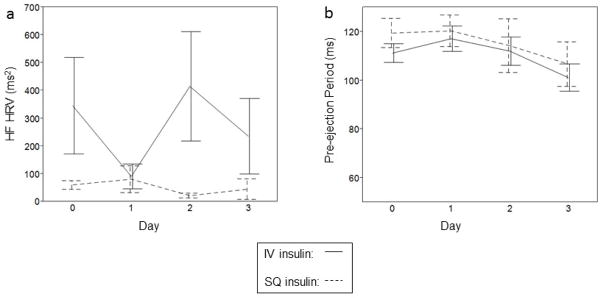
Changes among subjects with CHF exacerbation in (a) high frequency heart rate variability (HF HRV, ms2, P-trend=0.99), and (b) pre-ejection period (PEP, ms, P-trend=0.33), in the intravenous (IV, solid line) and subcutaneous (SQ, dashed line) insulin groups. Each error bar is constructed using 1 standard error of the mean.
Pooled univariable analyses were performed during the period of maximal separation in glucose between groups (in the first 24 hours, Table 3). There was a significant inverse association between pre-ejection period and log glucose lability index at 24 hours (estimate −11.5, SE 4.68, p=0.02). This relationship persisted after adjusting for group assignment and there was no interaction, indicating that the relationship did not differ by group assignment. The relationship persisted after excluding patients (N=7) with preserved ejection fraction (p=0.008). There was no association between pre-ejection period and mean glucose or coefficient of variation and no association between glucose measures and high frequency HRV. Pre-ejection period did not differ among subjects with or without hypoglycemia (defined as a blood glucose <3.9 mmol/l, 126 vs. 117 ms, p=0.54).
In order to further understand predictors of pre-ejection period, additional univariable linear regression analyses were performed. Increasing age, race, duration of diabetes, ejection fraction, and log-transformed epinephrine level, but not other biomarkers, were significantly associated with shorter baseline pre-ejection period (indicating higher cardiac sympathetic tone, Table 4). However, in separate models adjusting for age, only log-transformed epinephrine (p=0.02) and log-transformed glucose lability index (p=0.03) were significant predictors of pre-ejection period.
Table 4.
Univariable Predictors of PEP
| Estimate | SE | p-value | |
|---|---|---|---|
| Age | −0.24 | 0.07 | 0.002 |
| Caucasian | −7.54 | 3.63 | 0.048 |
| Female | −0.270 | 3.77 | 0.94 |
| Duration of diabetes | −0.22 | 0.09 | 0.02 |
| Ejection Fraction* | −0.016 | 0.006 | 0.013 |
| Beta Blocker | −3.24 | 7.04 | 0.65 |
| Total insulin day 1 | −0.103 | 0.241 | 0.73 |
| Creatinine | −0.014 | 0.008 | 0.07 |
| HbA1c | −0.103 | 0.241 | 0.67 |
| Body mass index | −0.058 | 0.093 | 0.54 |
| Brain natriuretic peptide (BNP)^ | 0.007 | 0.005 | 0.19 |
| Adiponectin | 0.17 | 0.12 | 0.18 |
| High sensitivity C-reactive protein* | 0.01 | 0.01 | 0.31 |
| Tumor necrosis factor-α | 0.024 | 0.065 | 0.72 |
| Interleukin-6* | 0.007 | 0.007 | 0.34 |
| Epinephrine* | 0.029 | 0.012 | 0.029 |
| Norepinephrine | 0.0075 | 0.009 | 0.43 |
| Oxidized-LDL* | −0.009 | 0.013 | 0.50 |
| Paraoxonase-1 | −0.001 | 0.002 | 0.56 |
| High frequency heart rate variability | −7.85 | 5.94 | 0.20 |
Variable log-transformed for better fit. Patients on Nesiritide were excluded from BNP analysis.
Hospital Outcomes
There were no differences in hospital length of stay (7 [IQR 5–11] vs. 8 [5–12] in IV vs. SQ group, p=0.76), or all-cause readmission at 30 days (27 vs. 39% in IV vs. SQ group, p=0.42). However, there was only 53% power to detect the observed difference in readmission and 12% power to detect the observed difference in length of stay between groups at a p-value of 0.05. Plasma volume remained stable over time in pooled analysis (p=0.73). There were no differences in change in plasma volume (0.21 [−6.0 to 6.3%] vs 0.59% [−6.1 to 0.59%] in IV vs. SQ group, p=0.87), hospital death (7.7 vs. 5.1% in IV vs. SQ group, p>0.99), or other outcomes (mechanical ventilation, new arrhythmia, acute renal failure, new infection) between treatment groups.
DISCUSSION
There are limited data to support interventions for glycemic control during CHF exacerbation. In this study, IV insulin (48 hour duration) resulted in a more rapid reduction in mean glucose but more hypoglycemia compared to SQ insulin. Intravenous insulin was also associated with greater glucose variability, assessed by coefficient of variation, compared to SQ insulin. Differences between groups generally resolved after the first day. The results suggest that concerns about insulin absorption may not be clinically relevant in non-critically ill patients with CHF exacerbation whose hyperglycemia is managed using a physiologic SQ insulin regimen. Of note, SQ insulin dosing was determined by study investigators, and therefore, the results may not necessarily reflect that achieved with typical inpatient prescribing, particularly with non-physiologic insulin regimens. By comparison, among non-CHF patients undergoing short-term (12 hour) infusion, glucose variability was increased primarily in the transition period following cessation of IV insulin.[26] Altogether, the data suggest that IV insulin is associated with greater glucose variability than SQ insulin, particularly following the initiation or cessation of the infusion. Therefore, it is not clear that a more sophisticated infusion algorithm would adequately address glucose variability. Careful consideration for smooth transitions in therapy for hyperglycemia in general would be of interest for further study.
There were no differences in hospital outcomes between groups. However, both treatment groups were relatively well-controlled, and a larger sample size would be necessary to test these outcomes definitively. Moreover, these patients were very sick, as evidenced by baseline measures and hospital length of stay, and higher frequency of readmission overall than in observational data of all patients with diabetes and CHF exacerbation at this study institution.[8,27] Thus it is not known if the severity of illness in these patients was too advanced to benefit from such an intervention.
The analyses do not support a link between method of insulin administration and inflammatory markers or oxidative stress in hospitalized CHF patients, although the association between BNP and coefficient of variation warrants further study. However, repeat blood draws were not performed during the peak difference in glycemic control. Nevertheless, there was no association between biomarkers and glucose variability in pooled analyses, and if anything, trends were contrary to expectations, either due to chance or due to as yet unexplored mechanisms. Multiple biomarkers are already abnormal in patients with CHF [9,10] and it is not known whether that underlying perturbations in neurohormonal pathways and oxidative stress are amenable to changes in glucose. Furthermore, manifestations of glucose variability in acutely ill patients may not reflect observations in stable outpatients with diabetes. We measured oxidative stress using oxidized LDL (oxLDL) and PON-1 instead of 8-iso-prostaglandin F2α (8-iso-PGF2α), which was more commonly reported in earlier studies, due to the need for tandem mass spectometry for optimal accuracy and other concerns such as the reproducibility and accuracy in the presence of kidney disease.[8]
There was no difference between high frequency HRV or pre-ejection period by glucose lowering strategy. Despite the small number of subjects with adequate HRV data, the observations are consistent with the observations from the immune/inflammatory markers, since parasympathetic tone and immune activation are increasingly recognized to be highly interdependent in CHF.[13] Pre-ejection period is reported to estimate sympathetic tone more exclusively than HRV.[23] While a previous study of patients without heart failure showed that IV insulin was associated with higher glucose lability index and shorter pre-ejection period, [26] it cannot be determined from these studies whether the difference in findings between the CHF and the non-CHF patient is due to differences in study design or due to factors that are intrinsic to the CHF patient. In general, reflex responses are blunted in CHF, particularly in the presence of diabetes [28], raising the possibility that the sympathetic response to glucose variability may be blunted as well. These data are primarily hypothesis-generating; thus further study is needed to determine whether sympathetic tone is a mechanism for harm related to glucose variability in CHF patients.
Our study has several limitations. First, this was a small study using an open label design with per protocol analysis. While limited to patients with congestive heart failure requiring treatment, a variety of concomitant illness and treatment related factors could play a role in the measurement of inflammatory biomarkers and cardiac autonomic activity. In particular, patients with preserved ejection fraction as well as those with systolic dysfunction were included. However, previous observations suggest that symptomatic heart failure is a significant predictor of autonomic changes independent of ejection fraction.[29] Second, both treatment groups were relatively well-controlled, potentially masking important relationships. Third, we were unable to determine the effects of individual CHF therapies on study variables. Fourth, Group sizes were imbalanced, likely due to chance given that drop-out was not significantly different. These issues require additional study in more homogeneous populations. Finally, the noninvasive values for pre-ejection period using bioimpedance methods should be interpreted with caution as it is not used in clinical practice and could be affected by a number of clinical factors [23]. However, this is the first study of its kind to assess responses to IV or SQ insulin in patients with CHF exacerbation.
In conclusion, the study suggests limited benefit in glycemia, and no difference in in markers of inflammation and oxidative stress or autonomic tone with the use of IV insulin compared to a physiologic SQ insulin regimen in hospitalized patients with type 2 diabetes and CHF exacerbation. Further study is warranted to determine the optimal means of lowering glucose while minimizing glucose variability, and whether this impacts outcomes.
Supplementary Material
Acknowledgments
The project described was supported by the OSU Clinical and Translational Research Center, Award Number UL1RR025755 from the National Center for Research Resources and by NIH grant numbers R21DK081877 and K23DK080891. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. The authors wish to thank Colleen Sagrilla, Amanda Lesinski, Rita Burris, Kari Graessle, Tyler Fuller and Kelly Rogers for their assistance with patient enrollment and data collection for this study.
Footnotes
Clinical Trials: NCT00812487
Declaration of interests: KD reports funding from Novo Nordisk for investigator initiated studies and consulting fees from Eli Lilly and Sanofi Aventis. PB, TG, JM and KO have nothing to declare.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.De Groote P, Lamblin N, Mouquet F, Plichon D, McFadden E, Van Belle E, Bauters C. Impact of diabetes mellitus on long-term survival in patients with congestive heart failure. Eur Heart J. 2004;25:656–662. doi: 10.1016/j.ehj.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Ariza-Andraca CR, Altamirano-Bustamante E, Frati-Munari AC, Altamirano-Bustamante P, Graef-Sanchez A. Delayed Insulin absorption due to subcutaneous edema. Arch Invest Med. 1991;22:229–233. [PubMed] [Google Scholar]
- 5.Dungan KM, Binkley P, Nagaraja HN, Schuster DS, Osei K. Assessment of glycemic control and variability in patients hospitalized with congestive heart failure during the implementation of hospital-wide initiatives and effects on mortality. Diabetes Metabolism Research & Reviews. 2011;27:85–93. doi: 10.1002/dmrr.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslami S, Taherzadeh Z, Schultz MJ, Abu-Hanna A. Glucose variability measures and their effect on mortality: a systematic review. Intensive Care Med. 2011;37:583–593. doi: 10.1007/s00134-010-2129-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57:1349–1354. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 8.Siegelaar SE, Holleman F, Hoekstra JB, DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 9.Braunwald E. Biomarkers in Heart Failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 10.Tang WH, Wu Y, Mann S, Pepoy M, Shrestha K, Borowski AG, Hazen SL. Diminished antioxidant activity of high-density lipoprotein-associated proteins in systolic heart failure. Circ Heart Fail. 2011;4:59–64. doi: 10.1161/CIRCHEARTFAILURE.110.958348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santini V, Ciampittiello G, Gigli F, Bracaglia D, Baroni A, Cicconetti E, Verri C, Gambardella S, Frontoni S. QTc and autonomic neuropathy in diabetes: effects of acute hyperglycemia and n-3 PUFA. Nutr Metab Cardiovasc Dis. 2007;17:712–718. doi: 10.1016/j.numecd.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Koivikko ML, Salmela PI, Airaksinen KE, Tapanainen JS, Ruokonen A, Mäkikallio TH, Huikuri HV. Effects of sustained insulin-induced hypoglycemia on cardiovascular autonomic regulation in type 1 diabetes. Diabetes. 2005;54:744–750. doi: 10.2337/diabetes.54.3.744. [DOI] [PubMed] [Google Scholar]
- 13.Jankowska EA, Ponikowski P, Piepoli MF, Banasiak W, Anker SD, Poole-Wilson PA. Autonomic imbalance and immune activation in chronic heart failure - pathophysiological links. Cardiovasc Res. 2006;70:434–445. doi: 10.1016/j.cardiores.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Panina G, Khot UN, Nunziata E, Cody RJ, Binkley PF. Role of spectral measures of heart rate variability as markers of disease progression in patients with chronic congestive heart failure not treated with angiotensin-converting enzyme inhibitors. Am Heart J. 1996;131:153–7. doi: 10.1016/s0002-8703(96)90064-2. [DOI] [PubMed] [Google Scholar]
- 15.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, Baig W, Flapan AD, Cowley A, Prescott RJ, Neilson JM, Fox KA. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 16.Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34:256–61. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg PA. Memoirs of a root canal salesman: the successful implementation of a hospital-wide intravenous insulin infusion protocol. Endocr Pract. 2006;12 (Suppl 3):79–85. doi: 10.4158/EP.12.S3.79. [DOI] [PubMed] [Google Scholar]
- 18.Ryan EA, Shandro T, Green K, Paty BW, Senior PA, Bigam D, Shapiro AM, Vantyghem MC. Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation. Diabetes. 2004;53:955–962. doi: 10.2337/diabetes.53.4.955. [DOI] [PubMed] [Google Scholar]
- 19.Zijlstra E, Heise T, Nosek L, Heinemann L, Heckermann S. Continuous glucose monitoring: quality of hypoglycaemia detection. Diabetes Obes Metab. 2013;15:130–135. doi: 10.1111/dom.12001. [DOI] [PubMed] [Google Scholar]
- 20.Parthasarathy S. In: Modified lipoproteins in the pathogenesis of atherosclerosis. Landers RG, editor. Austin, TX: 1994. pp. 91–119. [Google Scholar]
- 21.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 22.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 23.Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control. II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 24.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–48. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalra PR, Anagnostopoulos C, Bolger AP, Coats AJ, Anker SD. The regulation and measurement of plasma volume in heart failure. J Am Coll Cardiol. 2002;39:1901–8. doi: 10.1016/s0735-1097(02)01903-4. [DOI] [PubMed] [Google Scholar]
- 26.Dungan K, Osei K, Sagrilla C, Binkley P. Effect of the Approach to Insulin Therapy on Glycemic Fluctuations and Autonomic Tone in Hospitalized Patients with Diabetes. Diabetes Obes Metab. 2013;15:558–563. doi: 10.1111/dom.12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dungan KM, Osei K, Nagaraja HN, Schuster DP, Binkley P. Relationship between glycemic control and readmission rates in patients hospitalized with congestive heart failure during implementation of hospital-wide initiatives. Endocr Pract. 2010;16:945–951. doi: 10.4158/EP10093.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger AJ, Aronson D. Blunted sympathetic response in diabetic patients with decompensated congestive heart failure. Int J Cardiol. 2001;81:243–249. doi: 10.1016/s0167-5273(01)00573-3. [DOI] [PubMed] [Google Scholar]
- 29.Patel H, Ozdemir BA, Patel M, Xiao HB, Poole-Wilson PA, Rosen SD. Impairment of autonomic reactivity is a feature of heart failure whether or not the left ventricular ejection fraction is normal. Int J Cardiol. 2011;151:34–39. doi: 10.1016/j.ijcard.2010.04.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.