Abstract
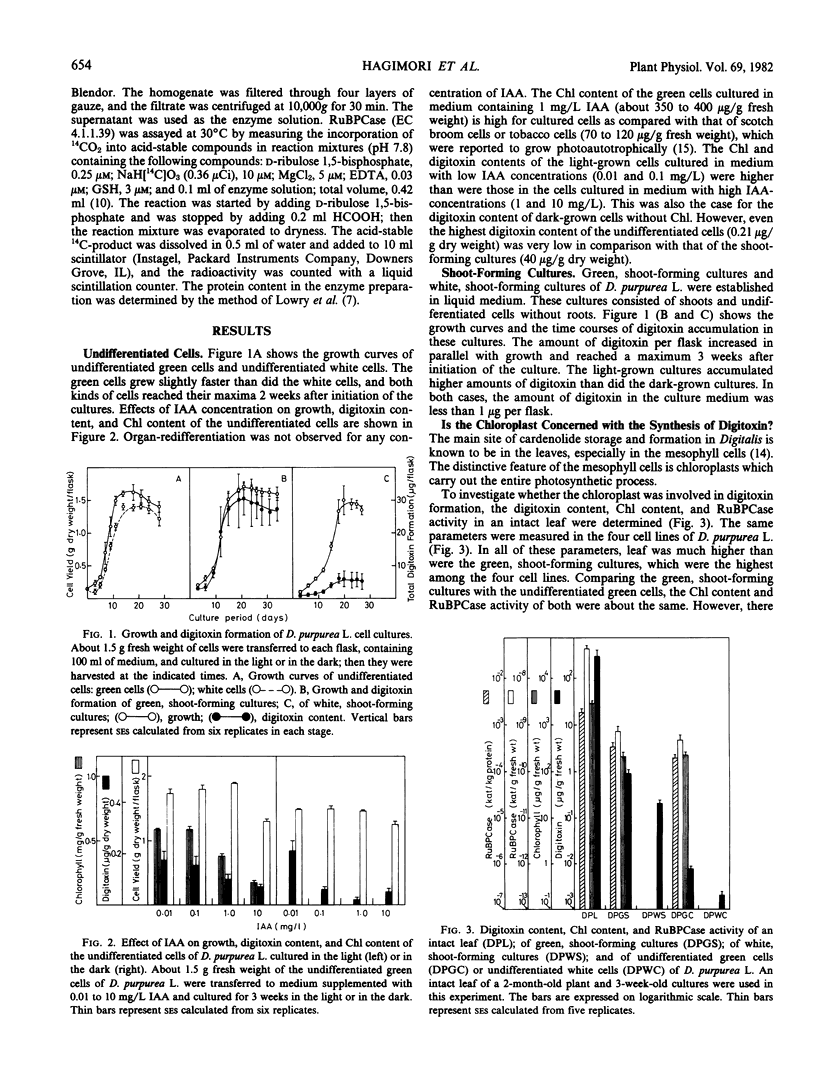
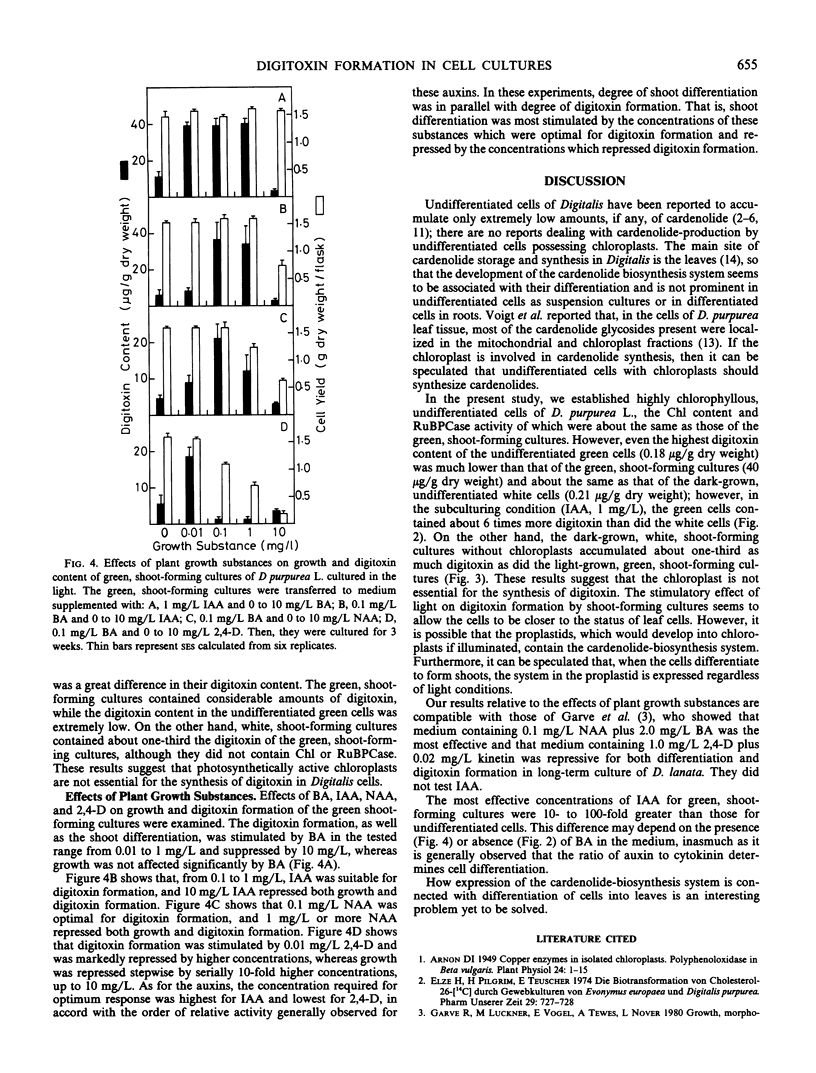
Undifferentiated, highly chlorophyllous cell cultures; undifferentiated white cell cultures; green, shoot-forming cultures; and white, shoot-forming cultures of Digitalis purpurea L. were established and subcultured every 3 weeks in liquid media in the light or in the dark. The digitoxin content, the chlorophyll content, and the ribulose bisphosphate carboxylase activity of these cultures were assayed. The light-grown, green, shoot-forming cultures accumulated considerable amounts of digitoxin (about 20 to 40 micrograms per gram dry weight), and the white, shoot-forming cultures without chloroplasts accumulated about one-third that amount of digitoxin. The chlorophyll content and the ribulose bisphosphate carboxylase activity of the undifferentiated green cells were about the same as they were in the green, shoot-forming cultures, but the digitoxin content of the former was extremely low (about 0.05 to 0.2 microgram per gram dry weight), which is about the same as that in undifferentiated white cells without chloroplasts. Thus, it was concluded that the chloroplasts are not essential for the synthesis of digitoxin in Digitalis cells. The optimum concentrations of the tested compounds for accumulation of digitoxin were: benzyladenine, 0.01 to 1 milligram per liter; indoleacetic acid, 0.1 to 1 milligram per liter; α-naphthaleneacetic acid; 0.1 milligram per liter; and 2,4-dichlorophenoxyacetic acid, 0.01 milligram per liter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elze H., Pilgrim H., Teuscher E. Die Biotransformation von Cholesterol-26-14C durch Gewebekulturen von Evonymus europaea und Digitalis purpurea. Pharmazie. 1974 Oct-Nov;29(10-11):727–728. [PubMed] [Google Scholar]
- Graves J. M., Smith W. K. Transformation of pregnenolone and progesterone by cultured plant cells. Nature. 1967 Jun 17;214(5094):1248–1249. doi: 10.1038/2141248a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Paulsen J. M., Lane M. D. Spinach ribulose diphosphate carboxylase. I. Purification and properties of the enzyme. Biochemistry. 1966 Jul;5(7):2350–2357. doi: 10.1021/bi00871a025. [DOI] [PubMed] [Google Scholar]
- Voigt W., Reissbrodt R., Baumgarten G. Zur Frage der Bindung von Cardenoiden an pfanzliche Zellpartikel. Pharmazie. 1969 Jul;24(7):422–422. [PubMed] [Google Scholar]



