Supplemental Digital Content is Available in the Text.
Key Words: tenofovir, pre-exposure prophylaxis (PrEP), hair, self-report, HIV, medication adherence
Abstract
Objective:
The efficacy of pre-exposure prophylaxis (PrEP) in HIV will diminish with poor adherence; pharmacologic measures of drug exposure have proven critical to PrEP trial interpretation. We assessed drug exposure in hair against other pharmacologic and more routinely used measures to assess pill-taking.
Design:
Participants were randomized to placebo, daily PrEP, or intermittent PrEP to evaluate safety and tolerability of daily versus intermittent tenofovir/emtricitabine (TFV/FTC) in 2 phase II PrEP clinical trials conducted in Africa. Different measures of drug exposure, including self-report, medication event monitoring system (MEMS)-caps openings, and TFV/FTC levels in hair and other biomatrices were compared.
Methods:
At weeks 8 and 16, self-reported pill-taking, MEMS-caps openings, and TFV/FTC levels in hair, plasma, and peripheral blood mononuclear cells (PBMCs) were measured. Regression models evaluated predictors of TFV/FTC concentrations in the 3 biomatrices; correlation coefficients between pharmacologic and nonpharmacologic measures were calculated. Both trials were registered on ClinicalTrials.gov (NCT00931346/NCT00971230).
Results:
Hair collection was highly feasible and acceptable (100% in week 8; 96% in week 16). In multivariate analysis, strong associations were seen between pharmacologic measures and MEMS-caps openings (all P < 0.001); self-report was only weakly associated with pharmacologic measures. TFV/FTC hair concentrations were significantly correlated with levels in plasma and PBMCs (correlation coefficients, 0.41–0.86, all P < 0.001).
Conclusions:
Measuring TFV/FTC exposure in small hair samples in African PrEP trials was feasible and acceptable. Hair levels correlated strongly with PBMC, plasma concentrations, and MEMS-caps openings. As in other PrEP trials, self-report was the weakest measure of exposure. Further study of hair TFV/FTC levels in PrEP trials and demonstration projects to assess adherence/exposure is warranted.
INTRODUCTION
The efficacy of pre-exposure prophylaxis (PrEP) in which at-risk HIV-uninfected individuals take antiretrovirals to prevent HIV acquisition has been demonstrated in several recent trials.1–4,8,9 Based on these results, a combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) taken daily as PrEP was approved by the US Food and Drug Administration for high-risk individuals in 2012 with recently updated guidelines from the Centers of Diseases Control5 and World Health Organization6 citing broad indications. Optimal strategies for PrEP administration are under intense investigation.
Although PrEP was effective in reducing HIV acquisition in some trials,1–4,8,9 2 large trials in sexually active African women (FEM-PrEP7–9 and VOICE10) were unable to demonstrate efficacy of daily TDF/FTC in reducing HIV acquisition. Rates of study product use in major PrEP trials as assessed by self-report or product return counts have been high (88%–95%), but direct measures of drug exposure indicate lower rates of actual use. Of paramount importance to the effective rollout of PrEP in both resource-rich and resource-limited settings is the incorporation of novel, accurate, and feasible ways to estimate adherence.
Although adherence is described as the “behavioral bridge from efficacy to effectiveness”11 in PrEP, each method to quantitate drug-taking has limitations. Self-report can be limited by recall bias, poor recollection, or a desire to please the provider (“social desirability bias”).12,13 Although pill counts and medication event monitoring systems (MEMS) can improve the accuracy of adherence monitoring,14 neither measure can record actual drug consumption12,15–19 nor quantify pharmacokinetic parameters.
Pharmacologic measures of exposure, most often involving the measurement of antiretrovirals in a matrix such as plasma, peripheral blood mononuclear cells (PBMCs), dried blood spots, or hair,20–22 reflect both adherence and pharmacokinetics. Measurement of antiretroviral (ARV) levels in single plasma samples has been frequently used to monitor exposure in PrEP trials, but represent a small window of exposure (2–7 days)23–25 and may be susceptible to “white coat effects,” where adherence improves transiently before visits.26,27 Drug levels in PBMCs have also proven useful in the interpretation of PrEP trial results,1,28 providing information on exposure over longer periods (7–14 days), although procedures to process, isolate, and count PBMCs can be costly and technically challenging. Drug levels in dried blood spots can reflect both recent and cumulative exposure29,30 and were recently used in a large cohort of PrEP users31; although easier to collect and process than PBMCs, dried blood spots require standardization against hemoglobin concentrations and sample volume for interpretation.32 Hair collection is noninvasive and does not require phlebotomy, and hair levels of ARVs reflect uptake from the systemic circulation over weeks to months,33 representing a long-term measure of exposure. Moreover, hair concentrations of tenofovir (TFV) are strongly and linearly related to dose.34
Intermittent PrEP may facilitate adherence, reduce costs, and has demonstrated efficacy in simian models33 and, in a recent trial, in men who have sex with men (MSM)9 although this strategy is not yet clinically approved. Studies investigating intermittent PrEP in humans have demonstrated tolerability, safety, and now efficacy,9,34,35 but adherence to intermittent PrEP, and measuring it accurately, can pose challenges. Comparing pharmacokinetic measures with more “traditional” measures of drug exposure (eg, self-report or MEMS) could provide insight into the utility of these measures across different PrEP dosing patterns and guide the selection of these measures in PrEP studies/settings. Multiple biologic samples for exposure monitoring (plasma, PBMCs, and hair) were collected in 2 phase II trials investigating daily or intermittent PrEP in HIV-negative serodiscordant couples in Uganda34 and MSM in Kenya.35 We report here an analysis comparing hair levels of FTC and TFV with self-report of pill-taking, MEMS-caps openings, and plasma and PBMC drug concentrations in these 2 trials.
METHODS
Description of Trials and Sample Collection
Each trial randomized HIV-negative individuals to oral TDF/FTC or placebo taken daily or intermittently (Mondays, Fridays and within 2 hours after sex other days, not to exceed 1 dose per day) in a 2:1:2:1 ratio (daily active, daily placebo, intermittent active, intermittent placebo); follow-up lasted 16 weeks. These safety trials were completed before the release of any PrEP efficacy results.1–4,8,9 The 2 primary nonbiologic measures of drug exposure collected at weeks 8 and 16 were monthly self-reported pill-taking using a timeline follow-back calendar to prompt recall of recent behavior38 and MEMS-caps openings (Aardex, Switzerland) in which a cap microchip electronically records each bottle opening and closure.
Hair, plasma, and PBMC samples were collected and analyzed for TFV and FTC concentrations at weeks 8 and 16. The timing of blood and hair sampling was random relative to time of dosing. All participants gave written informed consent to participate, and discordant couples were aware of each other's HIV status. The study was approved by the Kenyatta National Hospital Ethics Review Committee, the Kenya Medical Research Institute Ethics Review Committee, the Uganda Virus Research Institute Science and Ethics Committee, and the Uganda National Council for Science and Technology and National Drug Authority. The trials are registered with ClinicalTrials.gov (NCT00931346 and NCT00971230).
Plasma Analysis
Plasma was collected in EDTA tubes for drug concentration measurement and analyzed for TFV/FTC using liquid chromatography/tandem mass spectrometry (LC/MS/MS).39 The low calibration curve standards were linear from 0.31 to 20 ng/mL for both analytes; a second high calibration curve was linear from 1.0 to 1000 ng/mL for TFV and 5.0–5000 ng/mL for FTC. The lower limit of quantification (LLOQ) for both TFV and FTC was 0.31 ng/mL.
PBMC Collection and Analysis
Procedures for processing viable PBMCs have been previously described.28 A validated LC/MS/MS assay analyzed PBMC TFV-diphosphate (TFV-DP) and FTC-triphosphate levels (FTC-TP),40 with a linear range of 2.5–2000 fmol/sample and 0.1–200 pmol/sample, respectively. Approximately 4 million viable cells are typically extracted and results reported as femtomole per million or picomole per million viable cells. The LLOQ values for TFV-DP and FTC-TP are 2.5 fmol per sample and 0.1 pmol per sample, respectively.
Hair Collection and Analysis
Approximately, 50–100 strands of hair were cut as close as possible to the scalp in the occipital region (Fig. 1) and the distal portion labeled.20,34 The proximal section of the hair sample is chopped to ∼1- to 2-mm length segments with scissors and 5 mg weighed, processed, and analyzed using LC/MS/MS. The TFV and FTC in the cut hair sample are extracted with 50% methanol/water containing 1% trifluroacetic acid, 0.5% hydrazine dihydrochloride, and an internal standard in a 37° C shaking water bath overnight (>12 hours) and then analyzed by a modified LC/MS/MS method.39 The relative error (%) and precision (coefficients of variation) for spiked quality control hair samples at low, medium, and high concentrations were all <15%. These assays have been validated from 0.002 to 0.400 ng/mg hair for TFV and 0.02 to 4 ng/mg for FTC, with LLOQ at 0.002 and 0.02 ng/mg, respectively.34,42 The hair, plasma, and PBMC TFV and FTC assays have all been peer-reviewed and approved by the Division of AIDS Clinical Pharmacology and Quality Assurance program.43
FIGURE 1.
Demonstration of hair sampling in African patient (patient consent provided).
Statistical Analysis
Outcomes
Because the target number of pills to be taken varied by treatment arm (intermittent versus daily) and from person-to-person within the intermittent arm, we did not define adherence. Our goal was to measure drug exposure, rather than a particular adherence pattern to a daily or intermittent regimen. Our analytic outcomes were therefore drug levels detected by various measures, including pharmacologic measures as continuous outcomes and traditional measures (eg, MEMS-caps openings and self-reported pill-taking) as count outcomes. Concentrations of TFV and FTC in the various biologic matrices were log transformed for linear regression models. All pharmacological measures below the detection limit were set equal to that limit, and the detection limit was added to all concentrations prior to log transformation to prevent very low levels from becoming large negative values; similarly, 1.0 was added to pill counts before log transformation.
Predictors
The following covariates were assessed as continuous variables given implicated associations with pharmacokinetics: age (in years), height (in centimeters), weight (in kilograms), serum creatinine (in milligrams per deciliter), and creatinine clearance (in milliliter per minute) as calculated by the Cockcroft–Gault equation.44 The following covariates were assessed as categorical variables: biologic sex, dosing frequency (intermittent versus daily), country (Kenya versus Uganda), and self-reported recreational drug use over the past 28 days (yes/no for marijuana or khat, also known as miraa, an herbal stimulant). Of note, only MSM participants at the Kenya center were included in this analysis because very few female sex workers (n = 6) were enrolled. The effect of monthly self-reported number of pills taken as a predictor of hair, plasma, and PBMC levels was assessed per every 10% increase in the number of pills taken, according to the monthly calendar report. Monthly MEMS-caps openings were assessed per every 10% increase in the number of cap openings.
Models
We assessed the linearity assumption for all continuous predictors in all models; although there was evidence of nonlinearity for log-transformed MEMS count as a predictor of hair levels, we preferred it to the nontransformed variable because of the theoretical correspondence (proportionality) with log-transformed outcomes.34 We performed univariate random intercept linear regression models of each outcome by each predictor, accounting for left-censoring as observed in some outcomes, while accounting for repeated measures at weeks 8 and 16.45 Multivariate regression modeling was performed in a forward stepwise manner with predictor variables being added to the model if they demonstrated P values <0.05. Pearson correlation coefficients were calculated between pharmacologic and traditional markers of exposure.
RESULTS
Baseline Characteristics and Feasibility/Acceptability of Hair Collection in Africa
In total, excluding female sex workers, 135 participants underwent randomization in the 2 phase II PrEP trials36,37 from October 2009 to March 2010, with 47 assigned to placebo groups, 42 to daily PrEP groups, and 46 to intermittent PrEP groups. This analysis compares pharmacologic and nonpharmacologic exposure measures for participants in active arms only; Table 1 shows the baseline characteristics of these 88 participants.
TABLE 1.
Baseline Characteristics of Active Arm Participants (n = 88) in the 2 Phase II PrEP Trials
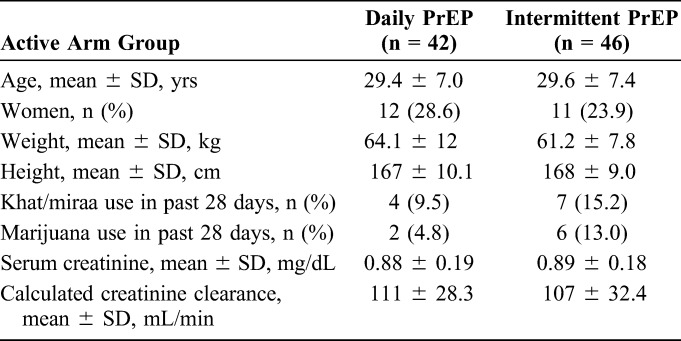
For participants randomized to active arms (176 person-visits at weeks 8 and 16), hair was successfully collected and analyzed for 172 person-visits, with an acceptance rate by participants of 100% at week 8 and 96% at week 16. Hair did not vary in terms of texture and color between participants. Two participants had hair levels below the LLOQ for TFV and 1 below the LLOQ for FTC at week 8, but there were 8 and 4 participants with hair concentrations below the LLOQ of TFV and FTC, respectively, by week 16. Plasma was successfully collected and analyzed for 170 person-visits. PBMC concentrations of TFV-DP/FTC-TP were available for approximately half of person-visits, due to the initial failure to assay the PBMC results accurately. Only 82 of 176 PBMC samples were available for repeat testing after the initial failure using an assay with a different LLOQ.
Univariate Modeling
Table 2 shows univariate associations between hair, plasma, and PBMC concentrations of TFV or FTC and variables of interest through linear regression. Strong associations were seen between each pharmacologic measure and MEMS-caps openings (P < 0.001 for each comparison). There was also an association in univariate analysis between each pharmacologic measure and self-reported pill consumption (P < 0.05 for each comparison). Each pharmacologic measure was also significantly associated with the randomized frequency of dosing: hair levels for those on daily doses averaged more than two-fold higher (×2.6 for FTC; ×2.1 for TFV) than those on intermittent dosing.
TABLE 2.
Estimated Univariate Associations of Various Covariates With Hair, Plasma, and PBMC Concentrations (Fold-effects, 95% Confidence Interval) From 176 Total Person-Visits
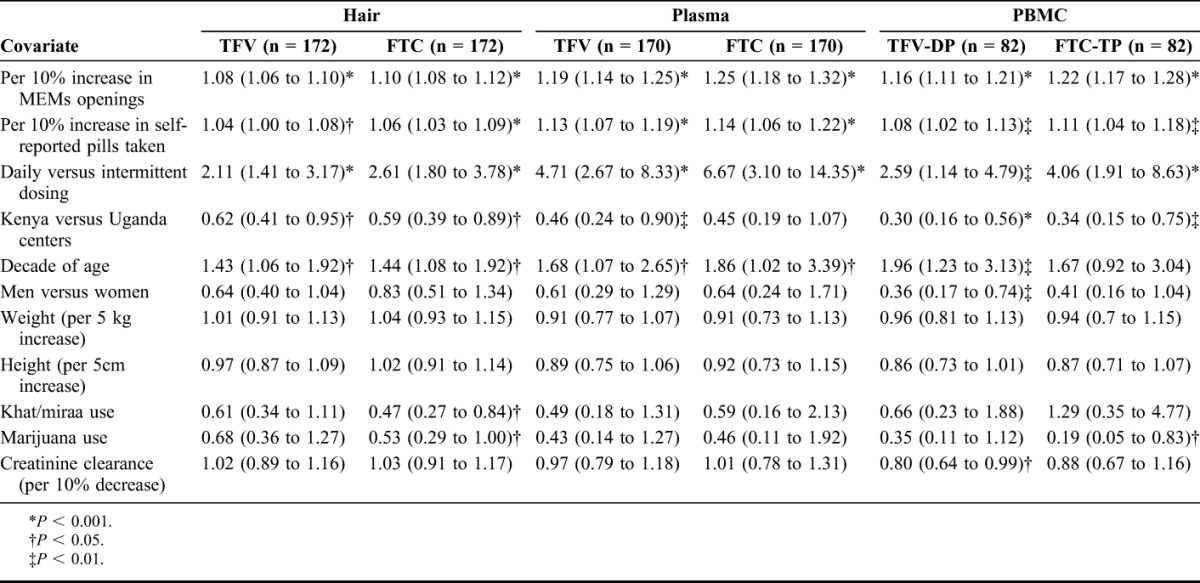
Participants at the Kenya site had statistically significantly lower exposure as assessed by most measures than participants in Uganda. MSM (Kenya) demonstrated ∼40% lower average TFV/FTC hair concentrations than heterosexual participants (Uganda), an effect that was only partially mediated by age and MEMS-caps openings in subsequent models (not shown). Older age was significantly associated with higher exposure as assessed via each pharmacologic measure (P = 0.020 and P = 0.013 per decade of age for hair TFV and FTC concentrations, respectively). There were no consistent gender effects on the pharmacologic measures. Finally, reported consumption of khat or marijuana in the previous 28 days was associated with a reduction in hair FTC concentrations (P = 0.011 and P = 0.048, respectively).
Multivariate Modeling
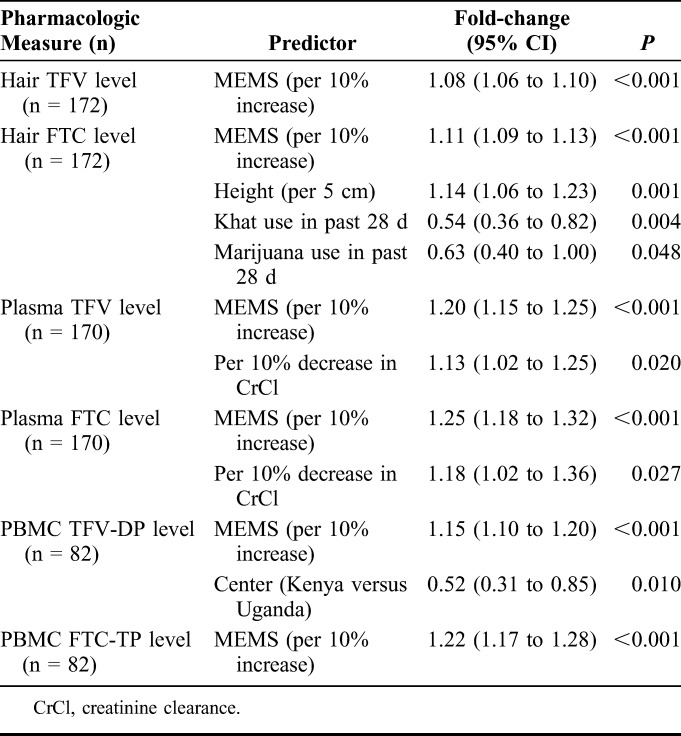
Table 3 demonstrates results of multivariate linear regression models with pharmacologic measures assessed as outcomes using forward stepwise selection. A strong association remained between each pharmacologic measure and MEMS-caps openings (P < 0.001 for each comparison); for every 10% increase in MEMS-caps openings, the concentrations of TFV and FTC in hair increased by 8% and 11%, respectively. The association between self-reported pill-taking and pharmacologic measures was not retained in multivariate models. Increased height was associated with increased hair FTC concentrations (14% increase per 5-cm increase in height); khat or marijuana use in the past 28 days was associated with decreased hair concentrations of FTC (by 46% and 37%, respectively). For every 10% decrease in creatinine clearance, the plasma levels of TFV and FTC increased by 13% and 18%, respectively. Finally, TFV concentrations in PBMCs averaged lower in the Kenyan MSM participants than the Ugandan participants.
TABLE 3.
Estimated Multivariate Associations With Hair, Plasma, and PBMC, TFV, and FTC Concentrations (Fold-Effects, 95% Confidence Interval) Using Forward Stepwise Selection From 176 Total Person-visits
Correlations
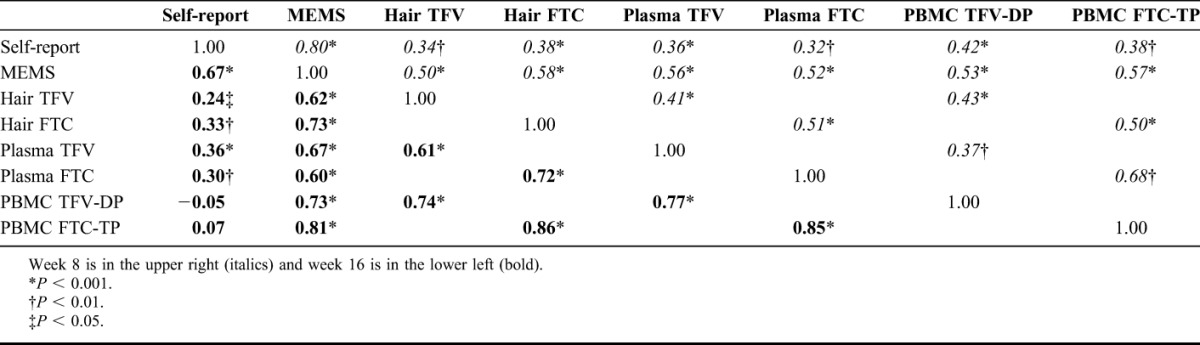
Figure S1 (see Supplemental Digital Content, http://links.lww.com/QAI/A581) depicts correlations between hair concentrations of TFV (top panel) and FTC (bottom panel) with self-reported pill-taking, MEMS-caps openings, PBMC concentrations, and plasma concentrations for the combined intermittent and daily dosing groups at weeks 8 and 16. Table 4 shows correlation coefficients between hair levels of TFV/FTC and the other pharmacologic or traditional exposure measures. In general, hair concentrations of both drugs in both dosing groups were highly correlated with MEMS-caps openings (coefficients ranged from 0.50–0.73, all P values <0.001). Although the correlation between self-reported pill-taking and MEMS-caps openings was high (0.80 at 8 weeks; 0.67 at 16 weeks), the correlations between hair measures and self-reported pill-taking were weaker (range, 0.24–0.38). This same pattern held with the other 2 pharmacologic measures (plasma, PBMCs) in that correlations with MEMS-caps openings (range, 0.52–0.81) were stronger than correlations with self-report (range, −0.05 to 0.42). The correlations between hair measures and the plasma or PBMC measures were high, but generally lower at 8 weeks (range, 0.41–0.51) than 16 weeks (range, 0.61–0.86).
TABLE 4.
Pearson Correlation Coefficients for Log-Transformed Outcomes Arranged by Combined (Daily and Intermittent) PrEP Dosing
DISCUSSION
Because adherence is the “Achilles' heel” to the success of PrEP,44 measuring exposure to the prophylactic agent as objectively as possible is of paramount importance. The main purpose of this report was to assess relationships between hair concentrations of TFV and FTC in 2 intermittent and daily phase II PrEP trials with other measures of drug exposure. We found a strong association between TFV/FTC concentrations in hair, plasma, and PBMCs with MEMS-caps openings in these trials. As has been observed in other HIV prevention and treatment settings, the relationship between pharmacologic measures of exposure and self-reported pill consumption was weak. These trials also showed strong correlations between hair concentrations of TFV and FTC with plasma and PBMC concentrations, with stronger correlations observed after a longer period on drug.
Strong Relationship Between Pharmacologic Measures and MEMS but Not Self-Report
A small study in the HIV treatment setting had similarly shown a significant correlation between MEM-caps openings and plasma drug levels within predefined ranges,47 but no previous study has examined the relationship between MEMS-caps openings and ARV concentrations in PBMCs or hair. Moreover, ours is the first study to comprehensively assess the relationship between pharmacologic measures and MEMS-caps openings in PrEP. In this analysis, the association between pharmacologic measures and self-reported pill-taking did not hold in multivariate analyses, and the correlations were weak or nonexistent.
The finding that self-reported pill-taking did not show strong relationships with biologic measures of exposure is consistent with accumulating data that self-report overestimates pill-taking in HIV prevention trials.7,8 The strong relationship between MEMS-caps openings and pharmacologic measures adds to the literature that MEMS monitoring may be more reflective of actual adherence than self-report.48 The relationship between self-report and MEMS-caps openings (r = 0.80) may reflect similar day-to-day variation in behavior that is not captured by the pharmacologic exposure measures. However, the limitations of electronic monitoring of drug exposure (eg, expense, requirement of database uploading, possibility of tampering, loss or mechanical failure, and requirement for longitudinal monitoring) must be considered in any HIV treatment or prevention setting.
Strong Correlation Between Hair Concentrations and Other Pharmacologic Measures
In HIV treatment, hair concentrations of ARVs are the strongest independent predictor of virologic outcomes.20,21,49 This article reports for the first time a strong correlation between hair concentrations of TFV and FTC and plasma or PBMC drug concentrations in intermittent PrEP. This study and another report42 demonstrated strong correlations between TFV and FTC concentrations in hair, plasma, and PBMCs with daily PrEP. Because plasma and PBMC concentrations of TFV/FTC have both demonstrated associations with PrEP efficacy in clinical trials,1,7,8,28 this analysis suggests that hair concentrations may also serve as a useful pharmacologic monitoring tool in PrEP. Because drug levels in hair reflect exposure over long time periods, hair monitoring may be useful for monitoring long-term PrEP use; the stronger association between hair concentrations and the other pharmacologic measures at 16 weeks (versus 8 weeks) in this trial may represent achievement of steady-state concentrations over time. The higher number of participants with undetectable hair concentrations at 16 versus 8 weeks, however, may represent waning adherence with time.
The use of any exposure monitoring method in PrEP trials or real-world settings will be partially determined by cost, convenience, and acceptability of the method. Hair collection is noninvasive and does not require phlebotomy skills, refrigeration, sterile equipment, or biohazardous precautions,50–52 all features which may enhance the feasibility of hair measures for resource-limited settings. As such, the cost of hair assays may be relatively lower than PBMC or plasma assays, methods that require postcollection processing in the field and training for collection, processing and handling, but such a cost benefit will require validation. Moreover, work is underway on developing low-cost, point-of-care assays to measure ARVs in hair using low-technology methods.53 The feasibility and acceptability of hair collection in these 2 phase II PrEP trials were high, with 100% of participants agreeing to hair collection at week 8 and 96% of participants providing hair samples at week 16. A recent study in a rural Kenyan cohort examining ARV adherence through hair levels demonstrated acceptability rates for hair collection of >95%,52 and a qualitative South African study revealed a general willingness to provide hair samples for HIV research when the purpose behind hair sampling was explained.52 Another trial collecting hair samples from Ugandan HIV-infected mothers and their infants also demonstrated high acceptability rates for collection.51 This report therefore adds to accumulating data that hair collection for antiretroviral monitoring in cohort or trial settings in Africa is feasible, acceptable, and may provide a more “patient-friendly” method for evaluating drug exposure.50
Our study showed a trend between dosing patterns of TDF/FTC and concentrations of drug in hair: hair levels for those assigned daily dosing averaged more than two-fold those assigned to intermittent dosing. These findings correspond to a recent report demonstrating strong linear relationships between TDF dose and hair TFV concentrations in HIV-uninfected volunteers.34 An analysis between hair levels of TFV/FTC and efficacy in the iPrEx Open Label Extension study29 is in progress and should further inform the field.
Pharmacologic Measures Can Assess Pharmacokinetic Information in Addition to Adherence
Pharmacologic measures seem to correlate with behavior, as evidenced by the comparisons with MEMS-caps openings but may also provide information regarding pharmacokinetics. The covariates additionally associated with pharmacologic measures in multivariate analyses (Table 3) are likely to be associated with effects on behavior, pharmacokinetics, or both. For instance, we found that lower rates of creatinine clearance were associated with increases in plasma TFV and FTC concentrations (13% and 18% increase, respectively, for every 10% decrease in clearance), consistent with primary renal clearance of these agents.55 Notably, absolute declines in creatinine clearance in these 2 studies were minimal, were not clinically significant, and were generally transient.
The effects of khat and marijuana use on FTC levels in hair could reflect a detrimental impact on adherence not captured by the MEMS measurement or effects on drug metabolism, although such a relationship was not reflected in the other pharmacologic measures. However, the active intracellular phosphorylated forms of FTC have a shorter half-life than TFV56–58; therefore, lapses in adherence may impact FTC concentrations before TFV concentrations. The participants in serodiscordant heterosexual couples in Uganda had 48% higher TFV concentrations in hair than Kenyan MSM participants. This association was partially mediated by MEMS-caps openings and likely reflects specific challenges that MSM report in taking PrEP59 and higher PrEP adherence rates in HIV-negative partners of individuals with known HIV infection.2,12,46 Finally, the association between increased height and increased FTC concentrations in hair could be secondary to the known association between low body mass index and higher medication levels, although this effect was not seen with other pharmacologic measures, and body mass index did not retain significance in the multivariate model.
Limitations
The biggest limitation of any study evaluating pharmacologic measures of exposure is a lack of a “gold standard” for measuring drug exposure to antiretrovirals. However, this study did provide an opportunity to compare exposure in hair with other biologic measures, self-reported pill-taking, and electronic monitoring. The sample size in each of the active arms was relatively small, although statistically significant associations between key covariates and pharmacologic measures were still detected. Comparison of adherence rates in the active versus placebo arms was not permitted by this analysis because pharmacologic measures cannot assess drug exposure to placebo. Furthermore, we were only able to analyze PBMC concentrations in half of the person-visits, potentially limiting our ability to comprehensively model the impact of various covariates on PBMC measures. Finally, although hair collection was acceptable in this population and hair measures were associated with MEMS measures and other pharmacologic measures, these phase II PrEP trials did not assess the association between drug concentrations and HIV acquisition rates. Moreover, although hair concentrations of drug do not seem to vary significantly by texture or hair color (personal communication, UCSF Drug Studies Unit), further study of the effect of variability in these conditions on hair levels in the field is needed.
CONCLUSIONS
This study demonstrates a number of novel findings, notably that monitoring concentrations of TFV/FTC in hair in the HIV prevention setting was feasible, acceptable, and correlated strongly with MEMS-caps measures and drug concentrations in other biomatrices. The lack of strong correlations between pharmacologic measures of exposure and self-reported pill-taking in this study adds to the growing evidence that self-reported adherence in HIV prevention trials performs poorly. The strong correlation between pharmacologic measures and behavior as assessed by electronic monitoring of bottle openings suggests a role for combining modalities of measuring adherence or exposure to PrEP. Given the urgent need for developing feasible, low-cost, and objective measures of adherence and exposure monitoring in PrEP, these findings pave the way for further exploration of hair concentrations as an exposure monitoring tool in PrEP investigational and implementation projects.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thankfully acknowledge the study volunteers and the clinic staff at the study centers.
Footnotes
Supported and sponsored by the International AIDS Vaccine Initiative (IAVI). IAVI's work is made possible by generous support from many donors including: the Bill & Melinda Gates Foundation; the Ministry of Foreign Affairs of Denmark; Irish Aid; the Ministry of Finance of Japan; the Ministry of Foreign Affairs of the Netherlands; the Norwegian Agency for Development Cooperation (NORAD); the United Kingdom Department for International Development (DFID), and the United States Agency for International Development (USAID). The full list of IAVI donors is available at www.iavi.org. Support in the form of study medication was provided by Gilead Sciences. Funding for the hair assays and statistical analyses was provided by the National Institute of Allergy and Infectious Diseases (NIAID)/National Institutes of Health (NIH) (R01 AI098472 to M.G.). The Women's Interagency HIV Study (NIH U01AI034989) contributed to hair assay development. S.M.B. is supported by the UCSF Traineeship in AIDS Prevention Studies (US National Institutes of Health (NIH) T32 MH-19105). The KWTRP at the Centre for Geographical Medicine Research, Kilifi is supported by core funding from the Wellcome Trust (#077092).
Paper presented in part at the 21st Conference on Retroviruses and Opportunistic Infections, March 3–6, 2014, Boston, MA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
Gilead Sciences provided Truvada (Gilead Sciences, Foster City, CA) and placebo for the study, but did not have access to the data, were not involved in the analysis or interpretation of the results and did not provide input regarding the decision to publish this manuscript. They were granted an opportunity to review the manuscript prior to submission for publication, but no obligation or promise was made to accept any recommended changes.
J.R. is employed by Gilead Sciences. The remaining authors have no funding or conflicts of interest to disclose.
REFERENCES
- 1.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 4.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–2090. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control (CDC). New HIV pre-exposure prophylaxis guidelines. Press Release Issued 2014. Available at: http://www.empr.com/cdc-new-hiv-pre-exposure-prophylaxis-guidelines/article/347053/. Accessed July 15, 2014. [Google Scholar]
- 6.World Health Organization. Consolidated guidelines on HIV prevention, diagnosis, treatment and care for key populations. Available at: http://www.who.int/hiv/pub/guidelines/keypopulations/en/. Accessed July 15, 2014. [PubMed]
- 7.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.PROUD study interim analysis finds pre-exposue prophylaxis (PrEP) is highly protective against HIV for gay men and other men who have sex with men in the UK [press release]. Kingway, London: MRC Press; October 16, 2014. [Google Scholar]
- 9.A Significant Breakthrough in the Fight Against HIV/AIDS: A drug taken at the time of sexual intercourse effectively reduces the risk of infection [IPERGAY press release]. Paris, France: ANRS; October 29, 2014. [Google Scholar]
- 10.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir/emtricitabine, or vaginal tenofovir Gel in the VOICE study (MTN 003). Paper presented at: 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. Paper 26LB.
- 11.Amico KR. Adherence to preexposure chemoprophylaxis: the behavioral bridge from efficacy to effectiveness. Curr Opin HIV AIDS. 2012;7:542–548. [DOI] [PubMed] [Google Scholar]
- 12.Berg KM, Arnsten JH. Practical and conceptual challenges in measuring antiretroviral adherence. J Acquir Immune Defic Syndr. 2006;43(suppl 1):S79–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kagee A, Nel A. Assessing the association between self-report items for HIV pill adherence and biological measures. AIDS Care. 2012;24:1448–1452. [DOI] [PubMed] [Google Scholar]
- 14.Haberer JE, Baeten JM, Campbell J, et al. Adherence to antiretroviral prophylaxis for HIV prevention: a substudy cohort within a clinical trial of serodiscordant couples in East Africa. PLoS Med. 2013;10:e1001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pullar T, Kumar S, Tindall H, et al. Time to stop counting the tablets? Clin Pharmacol Ther. 1989;46:163–168. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JA, Mattson RH, Prevey ML, et al. How often is medication taken as prescribed? A novel assessment technique. JAMA. 1989;261:3273–3277. [PubMed] [Google Scholar]
- 17.Liu H, Golin CE, Miller LG, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134:968–977. [DOI] [PubMed] [Google Scholar]
- 18.Muller AD, Jaspan HB, Myer L, et al. Standard measures are inadequate to monitor pediatric adherence in a resource-limited setting. AIDS Behav. 2011;15:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wendel C, Mohler M, Kroesen K, et al. Barriers to use of electronic adherence monitoring in an HIV clinic. Ann Pharmacother. 2001;35:1010–1015. [DOI] [PubMed] [Google Scholar]
- 20.Gandhi M, Ameli N, Bacchetti P, et al. Atazanavir concentration in hair is the strongest predictor of outcomes on antiretroviral therapy. Clin Infect Dis. 2011;52:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi M, Ameli N, Bacchetti P, et al. Protease inhibitor levels in hair strongly predict virologic response to treatment. AIDS. 2009;23:471–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandhi M, Greenblatt RM. Hair it is: the long and short of monitoring antiretroviral treatment. Ann Intern Med. 2002;137:696–697. [DOI] [PubMed] [Google Scholar]
- 23.Nettles RE, Kieffer TL, Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42:1189–1196. [DOI] [PubMed] [Google Scholar]
- 24.Wertheimer BZ, Freedberg KA, Walensky RP, et al. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006;7:59–69. [DOI] [PubMed] [Google Scholar]
- 25.Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med. 1990;150:1509–1510. [PubMed] [Google Scholar]
- 27.Podsadecki TJ, Vrijens BC, Tousset EP, et al. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9:238–246. [DOI] [PubMed] [Google Scholar]
- 28.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo-Mancilla JR, Zheng JH, Rower JE, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2013;29:384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng JH, Guida LA, Rower C, et al. Quantitation of tenofovir and emtricitabine in dried blood spots (DBS) with LC-MS/MS. J Pharm Biomed Anal. 2014;88:144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14:820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Kesel PM, Sadones N, Capiau S, et al. Hemato-critical issues in quantitative analysis of dried blood spots: challenges and solutions. Bioanalysis. 2013;5:2023–2041. [DOI] [PubMed] [Google Scholar]
- 33.Beumer JH, Bosman IJ, Maes RA. Hair as a biological specimen for therapeutic drug monitoring. Int J Clin Pract. 2001;55:353–357. [PubMed] [Google Scholar]
- 34.Liu AY, Yang Q, Huang Y, et al. Strong relationship between oral dose and tenofovir hair levels in a randomized trial: hair as a Potential adherence measure for pre-exposure prophylaxis (PrEP). PLoS One. 2014;9:e83736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Otten R, Smith D, Adams D, et al. Efficacy of postexposure prophylaxis after intravaginal exposure of pig-tailed macaques to a human-derived retrovirus (human immunodeficiency virus type 2). J Virol. 2000;74:9771–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kibengo FM, Ruzagira E, Katende D, et al. Safety, adherence and acceptability of intermittent tenofovir/emtricitabine as HIV pre-exposure prophylaxis (PrEP) among HIV-uninfected ugandan volunteers living in HIV-serodiscordant relationships: a randomized, clinical trial. PLoS One. 2013;8:e74314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mutua G, Sanders E, Mugo P, et al. Safety and adherence to intermittent pre-exposure prophylaxis (PrEP) for HIV-1 in African men who have sex with men and female sex workers. PLoS One. 2012;7:e33103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weinhardt LS, Carey MP, Maisto SA, et al. Reliability of the timeline follow-back sexual behavior interview. Ann Behav Med. 1998;20:25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell. 2013;155:515–518. [DOI] [PubMed] [Google Scholar]
- 40.Bushman LR, Kiser JJ, Rower JE, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56:390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang Q, Liu A, Gandhi M, et al. LC/LC/MS Assay of Tenofovir in Human Hair for Pre-exposure Prophylaxis. New Orleans, LA: Association of Pharmaceutical Sciences (AAPS) Annual Meeting; 2010. Abstract # T2328. [Google Scholar]
- 42.Liu A, Vittinghoff E, Gandhi M, et al. Validating measures of tenofovir drug exposure in a U.S. Pre-exposure prophylaxis trial. Paper presented at: 17th Conference on Retroviruses and Opportunistic Infections (CROI); 2010; San Francisco, CA. Paper Y-136.
- 43.DiFrancesco R, Tooley K, Rosenkranz SL, et al. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93:479–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. [DOI] [PubMed] [Google Scholar]
- 45.Thiebaut R, Jacqmin-Gadda H. Mixed models for longitudinal left-censored repeated measures. Comput Methods Programs Biomed. 2004;74:255–260. [DOI] [PubMed] [Google Scholar]
- 46.Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? Explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. 2012;59:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hugen PW, Langebeek N, Burger DM, et al. Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. J Acquir Immune Defic Syndr. 2002;30:324–334. [DOI] [PubMed] [Google Scholar]
- 48.Haberer JE, Kiwanuka J, Nansera D, et al. Multiple measures reveal antiretroviral adherence successes and challenges in HIV-infected Ugandan children. PLoS One. 2012;7:e36737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Zyl GU, van Mens TE, McIlleron H, et al. Low lopinavir plasma or hair concentrations explain second line protease inhibitor failures in a resource-limited setting. J Acquir Immune Defic Syndr. 2011;56:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ter Heine R, Beijnen JH, Huitema AD. Bioanalytical issues in patient-friendly sampling methods for therapeutic drug monitoring: focus on antiretroviral drugs. Bioanalysis. 2009;1:1329–1338. [DOI] [PubMed] [Google Scholar]
- 51.Gandhi M, Mwesigwa J, Aweeka F, et al. Hair and plasma data show that lopinavir, ritonavir, and efavirenz all transfer from mother to infant in Utero, but only efavirenz transfers via breastfeeding. J Acquir Immune Defic Syndr. 2013;63:578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hickey MD, Salmen CR, Tessler RA, et al. Antiretroviral concentrations in small hair samples as a feasible marker of adherence in rural Kenya. J Acquir Immune Defic Syndr. 2014;66:311–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi M, Yang Q, Bacchetti P, et al. Short communication: a low-cost method for analyzing nevirapine levels in hair as a marker of adherence in resource-limited settings. AIDS Res Hum Retroviruses. 2014;30:25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coetzee B, Kagee A, Tomlinson M, et al. Reactions, beliefs and concerns associated with providing hair specimens for medical research among a South African sample: a qualitative approach. Future Virol. 2012;7:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baxi SM, Greenblatt RM, Bacchetti P, et al. Common clinical conditions—age, low BMI, ritonavir use, mild renal impairment—affect tenofovir pharmacokinetics in a large cohort of HIV-infected women. AIDS. 2014;28:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anderson PL, Kakuda TN, Lichtenstein KA. The cellular pharmacology of nucleoside- and nucleotide-analogue reverse-transcriptase inhibitors and its relationship to clinical toxicities. Clin Infect Dis. 2004;38:743–753. [DOI] [PubMed] [Google Scholar]
- 57.Stevens RC, Blum MR, Rousseau FS, et al. Intracellular pharmacology of emtricitabine and tenofovir. Clin Infect Dis. 2004;39:877–878; author reply 878–879. [DOI] [PubMed] [Google Scholar]
- 58.Jackson A, Moyle G, Watson V, et al. Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: implications for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2013;62:275–281. [DOI] [PubMed] [Google Scholar]
- 59.Van der Elst EM, Mbogua J, Operario D, et al. High acceptability of HIV pre-exposure prophylaxis but challenges in adherence and use: qualitative insights from a phase I trial of intermittent and daily PrEP in at-risk populations in Kenya. AIDS Behav. 2013;17:2162–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]


