Abstract
Objective
To develop and validate rheumatoid arthritis (RA) risk models based on family history, epidemiologic factors, and known genetic risk factors.
Methods
We developed and validated models for RA based on known RA risk factors, among women in two cohorts: the Nurses’ Health Study (NHS, 381 RA cases and 410 controls) and the Epidemiological Investigation of RA (EIRA, 1244 RA cases and 971 controls). Model discrimination was evaluated using the area under the receiver operating characteristic curve (AUC) in logistic regression models for the study population and for those with positive family history. The joint effect of family history with genetics, smoking, and body mass index (BMI) was evaluated using logistic regression models to estimate odds ratios (OR) for RA.
Results
The complete model including family history, epidemiologic risk factors, and genetics demonstrated AUCs of 0.74 for seropositive RA in NHS and 0.77 for anti-citrullinated protein antibody (ACPA)-positive RA in EIRA. Among women with positive family history, discrimination was excellent for complete models for seropositive RA in NHS (AUC 0.82) and ACPA-positive RA in EIRA (AUC 0.83). Positive family history, high genetic susceptibility, smoking, and increased BMI had an OR of 21.73 for ACPA-positive RA.
Conclusions
We developed models for seropositive and seronegative RA phenotypes based on family history, epidemiologic and genetic factors. Among those with positive family history, models utilizing epidemiologic and genetic factors were highly discriminatory for seropositive and seronegative RA. Assessing epidemiological and genetic factors among those with positive family history may identify individuals suitable for RA prevention strategies.
Keywords: rheumatoid arthritis, family history, epidemiology, genetics
INTRODUCTION
Rheumatoid arthritis (RA) develops in individuals at increased genetic risk after certain environmental exposures.1,2 Epidemiologic factors associated with RA include cigarette smoking, alcohol intake, excess body weight, low socioeconomic status, and female reproductive factors.3–17 Genome-wide association studies (GWAS) and meta-analyses have identified RA-associated alleles, and an interaction between HLA-DRB1 and smoking.18–30 Individuals with family history (FH) of autoimmunity are at especially elevated RA risk, likely due to shared environment and genetics.31–33
RA prevention remains an elusive goal given its relatively low prevalence and unclear transitions between pre-clinical phases and clinical disease.34,35 Pre-clinical RA prevention efforts targeted for individuals at increased risk may overcome these challenges. The identification of high risk individuals using RA risk models is therefore an important goal.36 RA models incorporating genetic and epidemiologic factors have been developed.37 However, these models did not incorporate FH, a potent RA risk factor.31,32,34 Previous risk models have evaluated only autoantibody-positive RA or have utilized a limited set of epidemiologic factors.37–40 Studies of RA clinical prediction rules limited to patients with symptomatic, undifferentiated arthritis have utilized clinical, epidemiologic, genetic and autoantibody factors, but this population is further towards RA development than are pre-clinical, asymptomatic cohorts.35,41–44
Our goal was to develop and validate risk models incorporating FH, genetic and epidemiologic factors, for RA and its serologic subtypes among asymptomatic individuals. We aimed to evaluate model performance among those with and without FH. We quantified the joint effects of FH with high-risk genetics and epidemiologic factors and hypothesized that, among those with positive FH, models would be highly discriminatory for RA.
MATERIALS AND METHODS
Study design and populations
We developed models in a nested case-control study in the Nurses’ Health Study (NHS). NHS is a prospective cohort of 121,700 female nurses in the United States aged 30 to 55 years at baseline in 1976. Of these, 32,826 (27%) provided blood and another 33,040 (27%) provided buccal samples. Women who self-reported RA were screened for RA symptoms; chart review confirmed RA according to the 1987 American College of Rheumatology (ACR) classification criteria.45,46 Seropositive was defined as positive rheumatoid factor or anti-citrullinated peptide antibody (ACPA) by chart review after RA diagnosis, or by assay among a subset of cases with plasma collected prior to onset.47 Genotyped cases and healthy controls were matched 1:1 at index date of diagnosis by age, menopausal status, and post-menopausal hormone use. Women with non-white race or missing FH were excluded.
We validated our models in Epidemiological Investigation of RA (EIRA), a Swedish population-based case-control study that enrolls RA cases at diagnosis aged 18–70 years, enrolled between May 1996 and December 2009. RA was diagnosed by a rheumatologist and met the 1987 ACR classification criteria.46 ACPA assays were performed on all cases at enrollment. Cases were matched to controls on age, sex, and region at index date of diagnosis.26 A subset was randomly selected for genotyping. Participants with kinship, non-white race, or missing FH were excluded.
FH assessment
In NHS, women completed a single question on FH of RA or systemic lupus erythematosus (SLE) in first-degree relatives in 2008. We dichotomized responses as any or no FH of RA or SLE in first-degree relatives.
In EIRA, FH of RA in first-degree relatives among RA cases and controls was determined through the Swedish Patient and Multi-Generation registers, described in detail elsewhere.1 We dichotomized data as any or no FH of RA in first-degree relatives.
Epidemiological factors
Selection of epidemiologic factors
We included epidemiologic factors in our RA risk models that were significantly associated with RA in previous studies and our datasets.3–5,7,9–13,48 Our group previously developed RA models utilizing epidemiologic factors, genetics, and gene-environment interactions.37 The primary model in those analyses consisted of risk factors (cigarette smoking, alcohol, education, and parity) easily attained and significantly contributed to the overall model. Based on recent literature, we included body mass index (BMI) for these analyses.6,9,48,49
Covariates
Age was included as a continuous variable. Categorical variables were defined as follows: smoking as never, <10 pack-years, 10 to <20 pack-years, or ≥20 pack-years; cumulative average alcohol intake as none, 1 to <5 grams per day, 5 to <10 grams per day, 10 to <20 grams per day, or ≥20 grams per day; education as high school or some college / college graduate or more education (husband’s education in NHS); parity as nulliparous or parous. BMI was dichotomized at 25 kg/m2 (underweight or normal / overweight or obese, according to the World Health Organization).50 For NHS, data were updated through biennial questionnaires until the index date. For EIRA, data were collected at index date and pertained to exposures prior to RA onset.
Genetic risk scores and gene-environment interaction
RA risk alleles were combined to form genetic risk scores (GRS), weighted by the natural logarithm of published odds ratios for RA in GWAS or meta-analyses (Supplementary Table 1).40 We included 39 independent RA risk alleles (8 HLA-DRB1 and 31 non-HLA alleles) validated at the time of genotyping that were available in both datasets for our complete GRS.
Since HLA-DRB1 and smoking interact in prior studies, we utilized two GRS: one for 8 HLA-DRB1 alleles (GRS-HLA) and another for 31 non-HLA alleles (GRS-non-HLA), when HLA × smoking was considered.23,26 Genotyping and quality control procedures for NHS and EIRA have been previously described in detail.20,23,27,28
Statistical analysis
RA risk models for women
Risk models for women were developed in NHS and validated in EIRA. We estimated the area under the receiver operating characteristic curve (AUC) and 95% confidence intervals (CI) based on model components using logistic regression and discrimination interpreted by Hosmer and Lemeshow’s rules (AUC ≥0.7 acceptable; ≥0.8 excellent).51 We performed separate analyses for seropositive and seronegative RA in NHS, and for ACPA-positive and ACPA-negative RA in EIRA. We used the following model components: family history (FH), epidemiologic factors (E), genetics (G), FH+E, FH+E+G, and FH+E+G+GEI (the complete model). Models were compared using the integrated discrimination index (IDI), a measure of overall improvement in sensitivity and ‘1 – specificity’ between models in the same case-control dataset.49 IDI is not comparable across populations due to different event rates.52 IDI can be more sensitive to addition of new variables than AUC, and is more stable as a function of the baseline model than AUC.52 Reclassification of cases between models within datasets was assessed using the continuous net reclassification improvement (cNRI), a positive value indicating correct reclassification of cases as higher risk and controls as lower risk by the new model compared to the original model.53
RA risk models for women stratified by FH
We stratified analyses based on any or no FH and used logistic regression models to estimate AUC and 95% CI. After stratification by FH, we used the following model components: E, G, E+G, and E+G+GEI (the complete model).
RA risk models for men
We performed analyses for men in EIRA using the same methods. For men in EIRA, E models did not include parity, but were otherwise identical to models for women.
Joint effect of FH with genetics, smoking, and BMI
We focused on two modifiable risk factors for RA, smoking and BMI, to assess risk of RA among subgroups stratified by FH and GRS. We dichotomized GRS based on the 75th percentile of the GRS distribution of controls in each study. Smoking was dichotomized as never/≤10 pack-years or >10 pack-years and BMI as <25 kg/m2 or ≥25 kg/m2. The joint effect of FH with genetics, smoking, and BMI was examined using logistic regression models to estimate odds ratios (OR) and 95% CI for each RA phenotype, with the reference of no FH and low genetic risk, never/low smoking, or normal/underweight BMI. Logistic regression models estimated OR and 95% CI for RA phenotypes from multiple risk factors (positive FH, smoking >10 pack-years, BMI ≥25 kg/m2, and high GRS). All models were adjusted for alcohol intake, education, parity, and matching factors (age, menopausal status, and post-menopausal hormone usage for NHS; age and region for EIRA).
RESULTS
Population characteristics
Among women in NHS, there were 221 seropositive RA cases, 160 seronegative RA cases, and 517 controls. Among women in EIRA, there were 733 ACPA-positive RA cases, 511 ACPA-negative RA cases, and 971 controls. Among men in EIRA, there were 295 ACPA-positive RA cases, 213 ACPA-negative RA cases, and 390 controls. Characteristics for women in NHS and EIRA at index date are shown in Table 1. Positive FH was more common in NHS (34% of cases; 8% of controls) than EIRA (10% of cases; 4% of controls) likely due to study differences in FH ascertainment.
Table 1.
Characteristics of women at index date in NHS and EIRA according to case or control status.
| NHS | EIRA women | |||||
|---|---|---|---|---|---|---|
| RA cases | RA cases | |||||
| Seropositive RA | Seronegative RA | Controls | ACPA-positive RA | ACPA-negative RA | Controls | |
| (n = 221) | (n = 160) | (n = 410) | (n = 733) | (n = 511) | (n = 971) | |
| Age at blood draw, mean (years) (SD) | 55.1 (6.5) | 56.4 (6.5) | 56.4 (6.5) | 50.8 (12.4) | 51.4 (13) | 53.1 (11.4) |
| Positive family history1, no. (%) | 74 (33) | 54 (34) | 39 (10) | 78 (11) | 27 (5) | 36 (4) |
| Cigarette smoking, no. (%) | ||||||
| Never smoker | 85 (38) | 74 (46) | 172 (42) | 212 (29) | 205 (40) | 402 (41) |
| ≤10 pack-years | 31 (14) | 27 (17) | 97 (24) | 164 (22) | 100 (20) | 236 (24) |
| 10 to <20 pack-years | 34 (15) | 16 (10) | 38 (9) | 130 (18) | 86 (17) | 129 (13) |
| ≥20 pack-years | 66 (30) | 40 (25) | 98 (24) | 190 (26) | 79 (15) | 156 (16) |
| Alcohol intake, no. (%) | ||||||
| None | 51 (23) | 42 (26) | 102 (25) | 285 (39) | 184 (36) | 194 (20) |
| 1 to <5 g/day | 103 (47) | 65 (41) | 145 (35) | 381 (52) | 261 (51) | 638 (66) |
| 5 to <10 g/day | 18 (8) | 22 (14) | 62 (15) | 48 (7) | 44 (9) | 99 (10) |
| 10 to <20 g/day | 27 (12) | 18 (11) | 51 (12) | 10 (1) | 12 (2) | 26 (3) |
| ≥20 g/day | 14 (6) | 9 (6) | 39 (10) | 2 (0) | 2 (0) | 2 (0) |
| Overweight or obese2, no. (%) | 111 (49) | 81 (51) | 187 (46) | 246 (34) | 192 (38) | 263 (27) |
| College educated or greater3, no. (%) | 98 (44) | 69 (43) | 192 (47) | 203 (28) | 149 (29) | 343 (35) |
| Parous, no. (%) | 201 (91) | 152 (95) | 391 (95) | 594 (81) | 435 (85) | 815 (84) |
| GRS4, mean (SD) | 5.07 (0.89) | 4.45 (0.77) | 4.32 (0.74) | |||
| RA features | ||||||
| Age at symptom onset, mean (years) (SD) | 4.86 (0.89) | 4.58 (0.81) | 4.44 (0.80) | 50.0 (12.5) | 50.5 (13.1) | - |
| Age at diagnosis, mean (years) (SD) | 50.8 (12.4) | 51.4 (13.0) | - | |||
Family history was defined as self-reported RA or lupus in first-degree relatives in NHS and register data indicating first-degree relative with RA in EIRA.
Defined as body mass index ≥25 mg/m2.
Husband’s education considered for NHS.
GRS consisted of 39 alleles (8 HLA-DRB1 and 31 non-HLA alleles).
ACPA, anti-citrullinated peptide antibody; EIRA, Epidemiological Investigation of Rheumatoid Arthritis; GRS, genetic risk score; NHS, Nurses’ Health Study; RA, rheumatoid arthritis; SD, standard deviation.
Model validation and performance among women
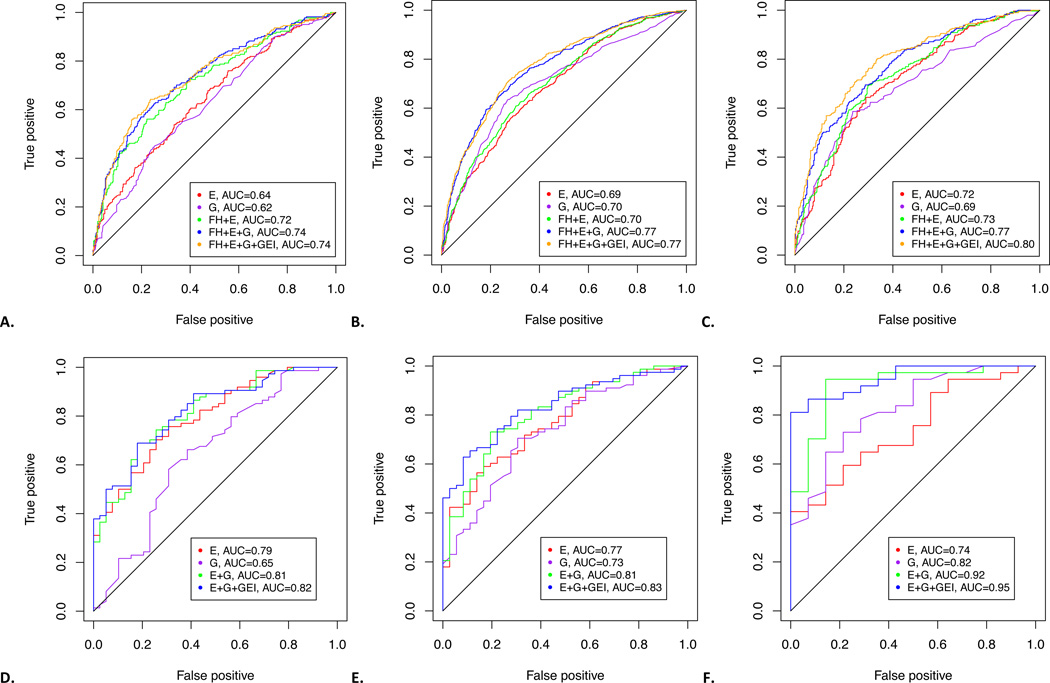
AUCs for RA risk models for women in NHS and EIRA are shown in Table 2. FH models had AUCs of 0.64 (95% CI 0.60–0.69)/0.66 (95% CI 0.61–0.71) in NHS for seropositive/seronegative RA and 0.58 (95% CI 0.55–0.60)/0.53 (95% CI 0.50–0.57) for ACPA-positive/ACPA-negative RA in EIRA. E models had higher AUCs for autoantibody-positive RA: 0.64 (95% CI 0.60–0.69) in NHS and 0.69 (95% CI 0.67–0.72) in EIRA, than for autoantibody-negative RA. G models had modest discrimination for RA serotypes with AUCs of 0.62 (95% CI 0.58–0.67) in NHS for seropositive RA and 0.70 (95% CI 0.68–0.73) in EIRA for ACPA-positive RA. AUCs for complete models (FH+E+G+GEI) were 0.74 (95% CI 0.70–0.78) for seropositive RA in NHS and 0.77 (95% CI 0.75–0.80) for ACPA-positive RA in EIRA, and lower in autoantibody-negative RA.
Table 2.
Areas under the receiver operating characteristic curves (AUC) for rheumatoid arthritis (RA) models among women using family history (FH), epidemiologic factors (E), genetics (G), and gene-environment interaction (GEI) in NHS and EIRA.
| AUC (95% CI) NHS |
AUC (95% CI) EIRA women |
|||
|---|---|---|---|---|
| Models | Seropositive RA | Seronegative RA | ACPA-positive RA | ACPA-negative RA |
| FH | 0.64 (0.60–0.69) | 0.66 (0.61–0.71) | 0.58 (0.55–0.60) | 0.53 (0.50–0.57) |
| E | 0.64 (0.60–0.69) | 0.60 (0.55–0.65) | 0.69 (0.67–0.72) | 0.65 (0.62–0.68) |
| G | 0.62 (0.58–0.67) | 0.54 (0.49–0.60) | 0.70 (0.68–0.73) | 0.56 (0.53–0.59) |
| FH+E+G+GEI | 0.74 (0.70–0.78) | 0.70 (0.65–0.75) | 0.77 (0.75–0.80) | 0.66 (0.63–0.69) |
| NHS, FH Positive | EIRA women, FH Positive | |||
| Seropositive RA | Seronegative RA | ACPA-positive RA | ACPA-negative RA | |
| E | 0.79 (0.71–0.88) | 0.79 (0.70–0.89) | 0.77 (0.68–0.86) | 0.79 (0.67–0.90) |
| G | 0.65 (0.53–0.76) | 0.62 (0.51–0.74) | 0.73 (0.64–0.83) | 0.62 (0.48–0.76) |
| E+G+GEI | 0.82 (0.74–0.90) | 0.83 (0.74–0.91) | 0.83 (0.76–0.91) | 0.78 (0.67–0.90) |
| NHS, FH Negative | EIRA women, FH Negative | |||
| Seropositive RA | Seronegative RA | ACPA-positive RA | ACPA-negative RA | |
| E | 0.67 (0.61–0.72) | 0.64 (0.57–0.70) | 0.69 (0.67–0.72) | 0.65 (0.62–0.68) |
| G | 0.62 (0.57–0.68) | 0.58 (0.52–0.64) | 0.70 (0.67–0.72) | 0.56 (0.53–0.59) |
| E+G+GEI | 0.69 (0.64–0.75) | 0.64 (0.58–0.70) | 0.77 (0.74–0.79) | 0.66 (0.63–0.69) |
FH models: family history
E models: cigarette smoking pack-years, alcohol intake, education, parity, and body mass index
G models: weighted genetic risk score based on 39 RA associated alleles
GEI models: HLA shared epitope × cigarette smoking interaction
All models also included matching factors (age, menopausal status and post-menopausal hormone use in NHS and age and Swedish region in EIRA).
ACPA, anti-citrullinated peptide antibody; AUC, area under the receiver operating characteristic curve; CI, confidence interval; E, epidemiologic; EIRA, Epidemiological Investigation of Rheumatoid Arthritis; FH, family history; G, genetics; GEI, gene-environment interaction; NHS, Nurses’ Health Study; RA, rheumatoid arthritis.
Among women with positive FH in NHS, E models had AUCs of 0.79 for seropositive and seronegative RA (Table 2). E models for women in EIRA with positive FH had AUCs of 0.77 (95% CI 0.68–0.86) and 0.79 (95% CI 0.67–0.90) for ACPA-positive and ACPA-negative RA, respectively. Among women in NHS with positive FH, G models had modest AUCs: 0.65 (95% CI 0.53–0.76) and 0.62 (95% CI 0.51–0.74) for seropositive/seronegative RA. For women with positive FH in EIRA, the G model for ACPA-positive RA had a higher AUC than NHS (0.73, 95% CI 0.64–0.83). The complete models (E+G+GEI) were highly discriminatory in both studies. AUCs were 0.82 (95% CI 0.74–0.90) and 0.83 (95% CI 0.74–0.91) for seropositive and seronegative RA in NHS. For women in EIRA with positive FH, complete models had excellent discrimination, with AUCs of 0.83 (95% CI 0.76–0.91) for ACPA-positive RA and 0.78 (95% 0.67–0.90) for ACPA-negative RA.
Receiver operating characteristic (ROC) curves are shown in Figure 1 for seropositive/ACPA-positive RA. Supplemental Figure 1 shows ROCs for seronegative/ACPA-negative RA.
Figure 1.
Receiver operating characteristic curves for (A) seropositive rheumatoid arthritis (RA) for women in Nurses’ Health Study (NHS), (B) ACPA-positive RA for women in the Epidemiologic Investigation of RA (EIRA), (C) ACPA-positive RA for men in EIRA, (D) seropositive RA among women with positive family history in NHS (E) ACPA-positive RA among women with positive family history in EIRA, and (F) ACPA-positive RA among men with positive family history in EIRA.
Performance of complete models
Comparisons of complete (FH+G+E+GEI) to each model with single factors (FH, E, or G) for autoantibody-positive RA showed improved discrimination by IDI (0.08–0.20) that was highly significant (Table 3) , suggesting marked model improvement. IDI improvements of 0.03–0.10 between complete and FH+E models suggest that genetics significantly improved discrimination. A positive cNRI that was highly statistically significant suggest improved reclassification to all other models except the FH+E+G. The lack of improvement in IDI or cNRI when adding GEI to the FH+E+G model shows little benefit to including GEI.
Table 3.
Comparisons of partial models to complete models for ACPA-positive/seropositive RA including family history (FH), epidemiologic factors (E), genetics (G), and gene-environment interaction (GEI) for women in NHS and EIRA.
| Population Outcome |
Complete model |
Model comparison |
cNRI | P value | IDI | P value |
|---|---|---|---|---|---|---|
| NHS | FH | 0.52 | 1.11×10−10 | 0.08 | 4.23×10−13 | |
| Seropositive RA | E | 0.60 | 3.18×10−14 | 0.11 | 4.44×10−16 | |
| FH+E+G+GEI | G | 0.72 | <10−16 | 0.13 | 2.22×10−16 | |
| FH+E | 0.35 | 1.63×10−5 | 0.03 | 5.11×10−6 | ||
| FH+E+G | 0.08 | 0.347 | 0.01 | 0.294 | ||
| EIRA women | FH | 0.85 | <10−16 | 0.20 | <10−16 | |
| ACPA-positive RA | E | 0.65 | <10−16 | 0.12 | <10−16 | |
| FH+E+G+GEI | G | 0.43 | <10−16 | 0.09 | <10−16 | |
| FH+E | 0.63 | <10−16 | 0.10 | <10−16 | ||
| FH+E+G | 0.22 | 6.76×10−6 | 0.01 | 0.0552 | ||
| NHS with +FH | E | 0.29 | 0.123 | 0.04 | 0.054 | |
| Seropositive RA | E+G+GEI | G | 0.84 | 2.85×10−6 | 0.22 | 4.08×10−7 |
| E+G | 0.35 | 0.068 | 0.01 | 0.536 | ||
| EIRA women with +FH | E | 0.62 | 1.92 ×10−3 | 0.09 | 2.79×10−3 | |
| ACPA-positive RA | E+G+GEI | G | 0.73 | 2.87 ×10−4 | 0.15 | 2.14×10−5 |
| E+G | 0.42 | 0.0354 | 0.03 | 0.108 | ||
ACPA, anti-citrullinated peptide antibody; cNRI, continuous net reclassification improvement; EIRA, Epidemiological Investigation of Rheumatoid Arthritis; CI, confidence interval; E, epidemiologic; EIRA, Epidemiological Investigation of Rheumatoid Arthritis; FH, family history; G, genetics; GEI, gene-environment interaction; IDI, integrated discrimination index; NHS, Nurses’ Health Study; RA, rheumatoid arthritis.
Among those with positive FH, IDI showed significantly improved discrimination for complete (E+G+GEI) compared to G models in NHS (0.22) and EIRA (0.15) and all E models. Among women with positive FH, significantly positive cNRI values (0.73–0.84) suggests that complete models improved case reclassification compared to other models.
The addition of GEI to complete models improved case reclassification by cNRI (0.35–0.42) but only slightly improved discrimination by IDI (0.01–0.03) that was not statistically significant, suggesting only marginal improvement with GEI.
Complete comparisons for RA risk models are in Supplemental Tables 4–6.
Model performance among men
AUCs for models among men in EIRA are shown in Supplemental Tables 2 and 3. The complete model (FH+E+G+GEI) among men in EIRA had excellent discrimination for ACPA-positive RA (AUC 0.80, 95% CI 0.76–0.83). ROC curves are shown in Figure 1 for ACPA-positive RA and Supplemental Figure 1 for ACPA-negative RA.
Joint effect of FH with genetics, smoking, and BMI
The joint effects of FH and GRS are shown in Table 4. In NHS, positive FH and high-risk genetics had an OR of 10.30 (95% CI 4.98–21.67) for seropositive RA. In EIRA, positive FH/high GRS had an OR of 13.04, 95% CI 6.56–25.91).
Table 4.
Joint effect of family history (FH) with genetics, smoking, and BMI for RA phenotypes in NHS and EIRA.
| Low GRS* | High GRS* | ||||
|---|---|---|---|---|---|
| Population Outcome |
FH | Cases / controls |
OR (95% CI) | Cases / controls |
OR (95% CI) |
| NHS Seropositive RA |
None | 83 / 280 | 1.0 (Ref) | 64 / 91 | 2.40 (1.56–3.70) |
| Any | 39 / 27 | 4.98 (2.81–8.98) | 35 / 12 | 10.30 (4.98–21.67) | |
| EIRA women ACPA-positive RA |
None | 257 / 698 | 1.0 (Ref) | 398 / 237 | 4.12 (3.28–5.18) |
| Any | 24 / 25 | 2.72 (1.47–5.01) | 54 / 11 | 13.04 (6.56–25.91) | |
| NHS Seronegative RA |
None | 80 / 280 | 1.0 (Ref) | 26 / 91 | 0.96 (0.56–1.59) |
| Any | 36 / 27 | 5.42 (3.01–9.90) | 18 / 12 | 5.97 (2.68–13.30) | |
| EIRA women ACPA-negative RA |
None | 325 / 698 | 1.0 (Ref) | 159 / 237 | 1.28 (0.99–1.64) |
| Any | 17 / 25 | 1.46 (0.75–2.79) | 10 / 11 | 2.12 (0.88–5.14) | |
| Low smoking (never or ≤10 pack-years) | High smoking (>10 pack-years) | ||||
|
Population Outcome |
FH |
Cases / controls |
OR (95% CI) |
Cases / controls |
OR (95% CI) |
| NHS Seropositive RA |
None | 79 / 243 | 1.0 (Ref) | 67 / 123 | 1.81 (1.20–2.73) |
| Any | 37 / 26 | 4.59 (2.58–8.29) | 33 / 13 | 8.42 (4.06–17.46) | |
| EIRA women ACPA-positive RA |
None | 336 / 616 | 1.0 (Ref) | 285 / 271 | 2.08 (1.65–2.63) |
| Any | 40 / 22 | 3.40 (1.95–6.06) | 34 / 14 | 5.43 (2.79–10.59) | |
| NHS Seronegative RA |
None | 66 / 243 | 1.0 (Ref) | 38 / 123 | 1.22 (0.76–1.95) |
| Any | 35 / 26 | 5.74 (3.16–10.62) | 18 / 13 | 6.27 (2.81–13.98) | |
| EIRA women ACPA-negative RA |
None | 291 / 616 | 1.0 (Ref) | 154 / 271 | 1.26 (0.97–1.63) |
| Any | 14 / 22 | 1.46 (0.71–2.93) | 11 / 14 | 1.80 (0.79–4.13) | |
|
Normal or underweight BMI (<25 kg/m2) |
Overweight or obese BMI (≥25 kg/m2) | ||||
|
Population Outcome |
FH |
Cases / controls |
OR (95% CI) |
Cases / controls |
OR (95% CI) |
| NHS Seropositive RA |
None | 74 / 196 | 1.0 (Ref) | 72 / 174 | 1.05 (0.71–1.56) |
| Any | 37 / 26 | 3.65 (2.03–6.63) | 37 / 13 | 7.44 (3.66–15.13) | |
| EIRA women ACPA-positive RA |
None | 323 / 359 | 1.0 (Ref) | 221 / 252 | 0.92 (0.71–1.18) |
| Any | 42 / 10 | 5.09 (2.55–11.11) | 25 / 11 | 2.43 (1.15–5.14) | |
| NHS Seronegative RA |
None | 55 / 196 | 1.0 (Ref) | 51 / 174 | 1.02 (0.65–1.58) |
| Any | 24 / 26 | 3.67 (1.89–7.15) | 30 / 13 | 9.71 (4.58–20.60) | |
| EIRA women ACPA-negative RA |
None | 206 / 359 | 1.0 (Ref) | 181 / 252 | 1.15 (0.87–1.51) |
| Any | 11 / 10 | 2.39 (0.98–5.91) | 11 / 11 | 1.53 (0.64–3.65) | |
GRS was dichotomized as high or low based on 75th percentile in the GRS distribution of the controls.
All models were adjusted for alcohol intake, education, parity, and matching factors (age, menopausal status and post-menopausal hormone usage in NHS; age and region for EIRA).
ACPA, anti-citrullinated peptide antibody; CI, confidence interval; EIRA, Epidemiological Investigation of Rheumatoid Arthritis; FH, family history; GRS, genetic risk score; NHS, Nurses’ Health Study; OR, odds ratio; RA, rheumatoid arthritis.
Positive FH and high smoking had ORs of 8.42 (95% CI 4.06–17.46) for seropositive RA in NHS and 5.43 (95% CI 2.79–10.59) in EIRA for ACPA-positive RA compared to no FH and low smoking. Positive FH and high BMI had ORs for seropositive/ACPA-positive RA of 7.44 (95% CI 3.66–15.13) and 2.43 (95% CI 1.15–5.14), respectively.
The ORs for RA from multiple risk factors are shown in Table 5. In NHS, women with positive FH, high smoking, and high BMI had an OR of 9.42 (95% CI 4.59–19.35) for seropositive RA, which increased to 20.89 (95% CI 9.04–48.29) with the addition of high GRS. Women in EIRA had similarly elevated ORs for ACPA-positive RA with positive FH, high smoking, high BMI, and high GRS (OR 21.73, 95% CI 10.69–44.19). Compared with autoantibody-positive RA, multiple positive risk factors conferred relatively less risk for seronegative (OR 8.03, 95% CI 3.29–19.63) and ACPA-negative RA (OR 3.23, 95% CI 1.48–7.06).
Table 5.
Odds ratios for RA serologic phenotypes with positive family history (FH), high genetic risk scores (GRS), elevated BMI, and smoking >10 pack-years in NHS and EIRA.
| OR (95% CI)1 | |
|---|---|
| Seropositive RA (NHS) | |
| Positive FH and high GRS2 | 10.30 (4.98–21.67) |
| Positive FH and high smoking | 8.42 (4.06–17.46) |
| Positive FH and high BMI | 7.44 (3.66–15.13) |
| Positive FH, high smoking, and high BMI | 9.42 (4.59–19.35) |
| Positive FH, high smoking, high BMI, and high GRS | 20.89 (9.04–48.29) |
| ACPA-positive RA (EIRA women) | |
| Positive FH and high GRS | 13.04 (6.56–25.91) |
| Positive FH and high smoking | 5.43 (2.79–10.59) |
| Positive FH and high BMI | 2.43 (1.15–5.14) |
| Positive FH, high smoking, and high BMI | 6.06 (3.20–11.46) |
| Positive FH, high smoking, high BMI, and high GRS | 21.73 (10.69–44.19) |
| Seronegative RA (NHS) | |
| Positive FH and high GRS | 5.97 (2.68–13.30) |
| Positive FH and high smoking | 6.27 (2.81–13.98) |
| Positive FH and high BMI | 9.71 (4.58–20.60) |
| Positive FH, high smoking, and high BMI | 8.29 (3.78–18.20) |
| Positive FH, high smoking, high BMI, and high GRS | 8.03 (3.29–19.63) |
| ACPA-negative RA (EIRA women) | |
| Positive FH and high GRS | 2.12 (0.88–5.14) |
| Positive FH and high smoking | 1.80 (0.79–4.13) |
| Positive FH and high BMI | 1.53 (0.64–3.65) |
| Positive FH, high smoking, and high BMI | 2.31 (1.17–4.83) |
| Positive FH, high smoking, high BMI, and high GRS | 3.23 (1.48–7.06) |
Adjusted for alcohol intake, education, parity, and matching factors (age, menopausal status and post-menopausal hormone usage for NHS; age and region for EIRA).
Reference was no family history, low smoking (never or ≤10 pack-years), normal/underweight BMI (≤25 kg/m2), and low GRS-HLA or GRS-all, as appropriate for each model.
Smoking was dichotomized as never/≤10 pack-years (low) or >10 pack-years (high). BMI was dichotomized as normal/underweight BMI (≤25 kg/m2) or overweight/obese (>25 kg/m2). GRS was dichotomized as high or low based on the 75th percentile in the GRS distribution of the controls.
ACPA, anti-citrullinated peptide antibody; CI, confidence interval; EIRA, Epidemiological Investigation of Rheumatoid Arthritis; FH, family history; GRS, genetic risk score; NHS, Nurses’ Health Study; OR, odds ratio; RA, rheumatoid arthritis.
DISCUSSION
We developed and validated models for RA serotypes among women enrolled in studies where epidemiologic factors were assessed in the asymptomatic, pre-clinical period prior to RA onset. RA risk models were highly discriminatory among those with positive FH, with AUCs of 0.82 in NHS and 0.83 in EIRA. We found that complete models incorporating FH, epidemiologic factors, and genetics improved discrimination of RA cases from controls, especially for autoantibody-positive RA. We found that women with positive FH, high-risk genetics, high smoking, and high BMI had up to 22-fold increased odds for ACPA-positive RA. Our models utilized easily obtained clinical information (FH, smoking, BMI, alcohol, parity, and education) and validated RA genetic markers.
Models utilizing combinations of FH, epidemiologic factors, and genetics improved discrimination by IDI compared to models using components alone. Reclassification, measured by cNRI, was improved in complete models. Models using only FH did not discriminate well, despite the potent association of FH with RA, likely due to the low prevalence of FH, especially in EIRA, where IDI was highest for the complete model compared to the FH model.31 E models generally had better discrimination for RA compared to G and FH models. This highlights both the importance of epidemiologic factors in RA pathogenesis and that known genetic factors currently offer modest discrimination. Several studies have recently evaluated the performance of RA models with genetics, but have used a limited number of environmental factors, typically smoking, for ACPA-positive RA. Genetic models in these studies provided less discrimination than models that also used smoking.38,39 EIRA complete models for ACPA-positive RA (AUC 0.77) performed better than NHS models for seropositive RA (AUC 0.74), perhaps due to more homogenous classification by ACPA in EIRA.
Among those with positive FH, RA models had excellent discrimination for autoantibody-positive RA (AUCs 0.82–0.83). This suggests that evaluating epidemiologic and genetic factors among those with positive FH may be able to identify asymptomatic individuals at increased RA risk. Among women with positive FH, the complete model showed significant improvement in discrimination compared to G models. Environmental factors may be especially important in the etiology of RA among those at high risk. In a recent study, among a population with arthralgias and RA-related autoantibodies, those that smoked and were overweight were seven-fold more likely to develop RA.36 In our study, those that smoked, had high BMI, positive FH, and high-risk genetics had 22-fold higher odds for RA. Furthermore, these findings suggest that utilizing these risk models among an asymptomatic population may be useful in screening for high risk subjects to enroll in RA prevention trials. Since many of the epidemiologic factors in our RA risk models are modifiable and E models had higher AUCs in FH-positive models than G models, this also suggests that a proportion of RA may be preventable. Prior reports suggest that cigarette smoking may account for 25–35% of population attributable RA risk perhaps due to interaction with HLA-DRB1.14,16 Our findings offer more evidence that environmental factors are important to the etiology of RA, even among those with positive FH.
We acknowledge limitations in our study. Our models were developed and validated among women without RA symptoms and do not address the progression of symptomatic, undifferentiated arthritis to RA. Exploratory analyses using males with similar models had excellent discrimination for ACPA-positive RA (AUC of 0.80), but this needs replication. ACPA testing was not performed on all NHS samples due to lack of plasma for those with buccal samples. ACPA testing prior to RA onset was unavailable in EIRA. Thus, we were unable to include pre-clinical ACPA in our models.35
Autoantibody-negative RA models generally performed worse than autoantibody-positive RA models. AUCs for complete models were modest for seronegative RA in NHS (0.70) and ACPA-negative RA in EIRA (0.66). Epidemiologic factors have different associations for seropositive and seronegative RA, often with weakened or null associations in seronegative RA compared to seropositive RA.6,8,54 In our study, the odds for autoantibody-negative RA with multiple risk factors were only 3-to-9-fold increased compared to 21-to-22-fold increased odds for autoantibody-positive RA (Table 5). There may be less heritability in ACPA-negative RA, which might also explain the underperformance of risk models for autoantibody-negative RA.1 Our study used 39 genetic RA risk alleles validated at the time of our genotyping, less than the currently reported 101 loci.55 These newly discovered SNPs have modest ORs, so it is unlikely that these SNPs would change discrimination appreciably.18,55 Autoantibody-negative RA risk models performed better among FH-positive women (AUCs 0.83 in NHS and 0.77 in EIRA). The higher AUC in NHS might reflect some misclassification by serostatus for RA cases diagnosed prior to the development of ACPA testing.
The NHS and EIRA study designs were also different. In NHS, we performed a nested case-control study within a prospective cohort of US women. Many RA cases in NHS were diagnosed prior to routine clinical ACPA testing, so there is potential for misclassification of serologic status. Women in NHS were followed prospectively prior to RA diagnosis, so epidemiologic data were collected without differential bias between cases and controls. EIRA is a Swedish case-control study, and all cases were classified by ACPA at diagnosis. Epidemiologic data before RA diagnosis were assessed retrospectively, introducing the potential for recall bias.
Finally, FH ascertainment was different in the studies. In NHS, FH was collected by self-report, usually after RA diagnosis, and included SLE. In EIRA, FH was validated using register linkages that provided close to complete coverage of FH irrespective of its temporal association to the index case/control, but only for birth cohorts covered by the Multi-Generation registers. The prevalence of FH among RA patients in prior studies ranged from 7–22%, with higher FH prevalence in studies utilizing self-report (18–22%).30 Since NHS data on FH were collected by self-report and included SLE, and was usually collected after RA diagnosis, the high prevalence of FH in this study (34%) is likely overestimated. However, controls also had a high prevalence of FH (10%), suggesting that overestimation of FH occurred in both cases and controls. Since women in NHS were advanced in age when FH was collected, family members might have been more likely to develop RA or SLE compared to other studies. The prevalence of FH in EIRA (10% in cases, 4% in controls), may have underestimated the true FH prevalence, though not the relative prevalence of FH between cases and controls for reasons mentioned above. Despite these differences, our models performed similarly in both studies enhancing the generalizability of our models.
In conclusion, models based on FH, RA risk factors (smoking, BMI, alcohol, education, and parity), and validated RA genetic markers, classified RA risk well among women. Among those with positive FH, RA risk models utilizing known risk factors and genetics provided excellent discrimination between RA cases and controls. Our results suggest that using risk models with epidemiologic and genetic factors among those with FH may enable identification of individuals suitable for RA prevention strategies.
Supplementary Material
ACKNOWLEDGMENTS
We thank May Al-Daabil, MD for her assistance in reviewing medical records in NHS. We thank Lori Chibnik, PhD for critical manuscript review. Finally, we thank all the participants and staff of NHS in the US and EIRA in Sweden for their contributions.
Funding: This work was supported by grants from the National Institutes of Health (grants CA087969, CA049449, CA050385, and CA067262) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grants AR049880, AR052403, and AR047782). The EIRA study was supported by grants from the Swedish Medical Research Council, from the Swedish Research Council for Health, Working Life and Welfare (FORTE), from King Gustaf V’s 80-year foundation, from the Swedish Rheumatism Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Competing interests: None.
Ethics approval: All aspects of this study were approved by the Partners Healthcare and Karolinska Institutet Institutional Review Boards.
REFERENCES
- 1.Frisell T, Holmqvist M, Kallberg H, Klareskog L, Alfredsson L, Askling J. Familial risks and heritability of rheumatoid arthritis: role of rheumatoid factor/anti-citrullinated protein antibody status, number and type of affected relatives, sex, and age. Arthritis Rheum. 2013;65(11):2773–2782. doi: 10.1002/art.38097. [DOI] [PubMed] [Google Scholar]
- 2.Majka DS, Holers VM. Can we accurately predict the development of rheumatoid arthritis in the preclinical phase? Arthritis Rheum. 2003;48(10):2701–2705. doi: 10.1002/art.11224. [DOI] [PubMed] [Google Scholar]
- 3.Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- 4.Costenbader KH, Feskanich D, Mandl LA, Karlson EW. Smoking intensity, duration, and cessation, and the risk of rheumatoid arthritis in women. Am J Med. 2006;119(6):503 e1–503 e9. doi: 10.1016/j.amjmed.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Kallberg H, Jacobsen S, Bengtsson C, Pedersen M, Padyukov L, Garred P, et al. Alcohol consumption is associated with decreased risk of rheumatoid arthritis: results from two Scandinavian case-control studies. Ann Rheum Dis. 2009;68(2):222–227. doi: 10.1136/ard.2007.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wesley A, Bengtsson C, Elkan AC, Klareskog L, Alfredsson L, Wedren S. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: results from a population-based case-control study. Arthritis Care Res (Hoboken) 2013;65(1):107–112. doi: 10.1002/acr.21749. [DOI] [PubMed] [Google Scholar]
- 7.Lahiri M, Morgan C, Symmons DP, Bruce IN. Modifiable risk factors for RA: prevention, better than cure? Rheumatology (Oxford) 2012;51(3):499–512. doi: 10.1093/rheumatology/ker299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen M, Jacobsen S, Klarlund M, Pedersen BV, Wiik A, Wohlfahrt J, et al. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res Ther. 2006;8(4):R133. doi: 10.1186/ar2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hazes JM, Dijkmans BA, Vandenbroucke JP, de Vries RR, Cats A. Lifestyle and the risk of rheumatoid arthritis: cigarette smoking and alcohol consumption. Ann Rheum Dis. 1990;49(12):980–982. doi: 10.1136/ard.49.12.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerhan JR, Saag KG, Criswell LA, Merlino LA, Mikuls TR. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J Rheumatol. 2002;29(2):246–254. [PubMed] [Google Scholar]
- 11.Spector TD, Roman E, Silman AJ. The pill, parity, and rheumatoid arthritis. Arthritis Rheum. 1990;33(6):782–789. doi: 10.1002/art.1780330604. [DOI] [PubMed] [Google Scholar]
- 12.Jorgensen C, Picot MC, Bologna C, Sany J. Oral contraception, parity, breast feeding, and severity of rheumatoid arthritis. Ann Rheum Dis. 1996;55(2):94–98. doi: 10.1136/ard.55.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breast-feeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurses' Health Study. Arthritis Rheum. 2004;50(11):3458–3467. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Deane K. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum Dis Clin North Am. 2012;38(2):405–426. doi: 10.1016/j.rdc.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bengtsson C, Nordmark B, Klareskog L, Lundberg I, Alfredsson L. Socioeconomic status and the risk of developing rheumatoid arthritis: results from the Swedish EIRA study. Ann Rheum Dis. 2005;64(11):1588–1594. doi: 10.1136/ard.2004.031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kallberg H, Ding B, Padyukov L, Bengtsson C, Ronnelid J, Klareskog L, et al. Smoking is a major preventable risk factor for rheumatoid arthritis: estimations of risks after various exposures to cigarette smoke. Ann Rheum Dis. 2011;70(3):508–511. doi: 10.1136/ard.2009.120899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orellana C, Wedren S, Kallberg H, Holmqvist M, Karlson EW, Alfredsson L, et al. Parity and the risk of developing rheumatoid arthritis: results from the Swedish Epidemiological Investigation of Rheumatoid Arthritis study. Ann Rheum Dis. 2014;73:752–755. doi: 10.1136/annrheumdis-2013-203567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44(12):1336–1340. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, et al. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42(6):508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raychaudhuri S, Remmers EF, Lee AT, Hackett R, Guiducci C, Burtt NP, et al. Common variants at CD40 and other loci confer risk of rheumatoid arthritis. Nat Genet. 2008;40(10):1216–1223. doi: 10.1038/ng.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernando MM, Stevens CR, Walsh EC, De Jager PL, Goyette P, Plenge RM, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4(4):e1000024. doi: 10.1371/journal.pgen.1000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75(2):330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karlson EW, Chang SC, Cui J, Chibnik LB, Fraser PA, De Vivo I, et al. Gene-environment interaction between HLA-DRB1 shared epitope and heavy cigarette smoking in predicting incident rheumatoid arthritis. Ann Rheum Dis. 2010;69(1):54–60. doi: 10.1136/ard.2008.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remmers EF, Plenge RM, Lee AT, Graham RR, Hom G, Behrens TW, et al. STAT4 and the risk of rheumatoid arthritis and systemic lupus erythematosus. N Engl J Med. 2007;357(10):977–986. doi: 10.1056/NEJMoa073003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raychaudhuri S, Thomson BP, Remmers EF, Eyre S, Hinks A, Guiducci C, et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41(12):1313–1318. doi: 10.1038/ng.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50(10):3085–3092. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 27.Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006;54(1):38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 28.Keenan BT, Chibnik LB, Cui J, Ding B, Padyukov L, Kallberg H, et al. Effect of interactions of glutathione S-transferase T1, M1, and P1 and HMOX1 gene promoter polymorphisms with heavy smoking on the risk of rheumatoid arthritis. Arthritis Rheum. 2010;62(11):3196–3210. doi: 10.1002/art.27639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plenge RM, Cotsapas C, Davies L, Price AL, de Bakker PI, Maller J, et al. Two independent alleles at 6q23 associated with risk of rheumatoid arthritis. Nat Genet. 2007;39(12):1477–1482. doi: 10.1038/ng.2007.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plenge RM, Seielstad M, Padyukov L, Lee AT, Remmers EF, Ding B, et al. TRAF1-C5 as a risk locus for rheumatoid arthritis--a genomewide study. N Engl J Med. 2007;357(12):1199–1209. doi: 10.1056/NEJMoa073491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frisell T, Holmqvist M, Kallberg H, Klareskog L, Alfredsson L, Askling J. Familial risks and heritability of RA in relation to RF/ACPA status, number and type of affected relatives, sex, and age. Arthritis Rheum. 2013;65:2773–2782. doi: 10.1002/art.38097. [DOI] [PubMed] [Google Scholar]
- 32.Hemminki K, Li X, Sundquist J, Sundquist K. Familial associations of rheumatoid arthritis with autoimmune diseases and related conditions. Arthritis Rheum. 2009;60(3):661–668. doi: 10.1002/art.24328. [DOI] [PubMed] [Google Scholar]
- 33.Somers EC, Antonsen S, Pedersen L, Sorensen HT. Parental history of lupus and rheumatoid arthritis and risk in offspring in a nationwide cohort study: does sex matter? Ann Rheum Dis. 2013;72(4):525–529. doi: 10.1136/annrheumdis-2011-201165. [DOI] [PubMed] [Google Scholar]
- 34.Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am. 2010;36(2):213–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerlag DM, Raza K, van Baarsen LG, Brouwer E, Buckley CD, Burmester GR, et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann Rheum Dis. 2012;71(5):638–641. doi: 10.1136/annrheumdis-2011-200990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Hair MJ, Landewe RB, van de Sande MG, van Schaardenburg D, van Baarsen LG, Gerlag DM, et al. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann Rheum Dis. 2013;72:1654–1658. doi: 10.1136/annrheumdis-2012-202254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlson EW, Ding B, Keenan BT, Liao K, Costenbader KH, Klareskog L, et al. Association of environmental and genetic factors and gene-environment interactions with risk of developing rheumatoid arthritis. Arthritis Care Res (Hoboken) 2013;65(7):1147–1156. doi: 10.1002/acr.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarwood A, Han B, Raychaudhuri S, Bowes J, Lunt M, Pappas DA, et al. A weighted genetic risk score using all known susceptibility variants to estimate rheumatoid arthritis risk. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2013-204133. Published Online First: 3 Oct 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott IC, Seegobin SD, Steer S, Tan R, Forabosco P, Hinks A, et al. Predicting the Risk of Rheumatoid Arthritis and Its Age of Onset through Modelling Genetic Risk Variants with Smoking. PLoS Genet. 2013;9(9):e1003808. doi: 10.1371/journal.pgen.1003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karlson EW, Chibnik LB, Kraft P, Cui J, Keenan BT, Ding B, et al. Cumulative association of 22 genetic variants with seropositive rheumatoid arthritis risk. Ann Rheum Dis. 2010;69(6):1077–1085. doi: 10.1136/ard.2009.120170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Helm-van Mil AH, Toes RE, Huizinga TW. Genetic variants in the prediction of rheumatoid arthritis. Ann Rheum Dis. 2010;69(9):1694–1696. doi: 10.1136/ard.2009.123828. [DOI] [PubMed] [Google Scholar]
- 42.van der Helm-van Mil AH, Detert J, le Cessie S, Filer A, Bastian H, Burmester GR, et al. Validation of a prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: moving toward individualized treatment decision-making. Arthritis Rheum. 2008;58(8):2241–2247. doi: 10.1002/art.23681. [DOI] [PubMed] [Google Scholar]
- 43.van der Helm-van Mil AH, le Cessie S, van Dongen H, Breedveld FC, Toes RE, Huizinga TW. A prediction rule for disease outcome in patients with recent-onset undifferentiated arthritis: how to guide individual treatment decisions. Arthritis Rheum. 2007;56(2):433–440. doi: 10.1002/art.22380. [DOI] [PubMed] [Google Scholar]
- 44.Lahiri M, Luben RN, Morgan C, Bunn DK, Marshall T, Lunt M, et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register--the EPIC-2-NOAR Study) Ann Rheum Dis. 2014;73:219–226. doi: 10.1136/annrheumdis-2012-202481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 46.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 47.Chibnik LB, Mandl LA, Costenbader KH, Schur PH, Karlson EW. Comparison of threshold cutpoints and continuous measures of anti-cyclic citrullinated peptide antibodies in predicting future rheumatoid arthritis. J Rheumatol. 2009;36(4):706–711. doi: 10.3899/jrheum.080895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voigt LF, Koepsell TD, Nelson JL, Dugowson CE, Daling JR. Smoking, obesity, alcohol consumption, and the risk of rheumatoid arthritis. Epidemiology. 1994;5(5):525–532. [PubMed] [Google Scholar]
- 49.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 50.de Onis M, Habicht JP. Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr. 1996;64(4):650–658. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 51.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed. John Wiley & Sons, Inc.; 2002. pp. 156–164. [Google Scholar]
- 52.Pencina MJ, D'Agostino RB, Sr, Demler OV. Novel metrics for evaluating improvement in discrimination: net reclassification and integrated discrimination improvement for normal variables and nested models. Stat Med. 2012;31(2):101–113. doi: 10.1002/sim.4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pencina MJ, D'Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30(1):11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klareskog L, Malmstrom V, Lundberg K, Padyukov L, Alfredsson L. Smoking, citrullination and genetic variability in the immunopathogenesis of rheumatoid arthritis. Semin Immunol. 2011;23(2):92–98. doi: 10.1016/j.smim.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 55.Okada Y, Wu D, Trynka G, Raj T, Terao C, Ikari K, et al. Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature. 2013;506:376–381. doi: 10.1038/nature12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.