Abstract
Hospitalization for heart failure (HF) is frequently related to dyspnea, yet associations between dyspnea severity, outcomes, and health care costs are unknown. We aimed to describe characteristics of patients hospitalized for acute HF by dyspnea severity and to examine associations between dyspnea severity, outcomes and costs. We linked registry data for patients hospitalized for HF with Medicare claims to evaluate dyspnea and outcomes among patients 65 years and older. We classified patients by patient-reported dyspnea severity at admission. Outcomes included length of stay, mortality 30 days after admission, and days alive and out of the hospital, readmission, and Medicare payments 30 days after discharge. Of 48,616 patients with acute HF and dyspnea, 4022 (8.3%) had dyspnea with moderate activity, 19,619 (40.3%) with minimal activity, and 24,975 (51.4%) at rest. Patients with dyspnea with minimal activity or at rest had greater comorbidity, including renal insufficiency. Greater severity of baseline dyspnea was associated with mortality (moderate activity, 6.3%; minimal activity, 7.6%; at rest, 11.6%) and heart failure readmission (7.2%, 9.0%, and 9.4%). After multivariable adjustment, dyspnea at rest was associated with greater 30-day mortality and heart failure readmission, fewer days alive and out of the hospital, longer length of stay, and higher Medicare payments, compared to dyspnea with moderate activity. In conclusion, dyspnea at rest on presentation was associated with greater mortality, readmission, length of stay, and health care costs among patients hospitalized with acute HF.
Keywords: Dyspnea, Heart Failure, Mortality, Patient Outcome Assessment, Patient Readmission
Heart failure (HF) is a common and costly condition and a leading cause of hospitalization worldwide.1–3 Many patients hospitalized with acute HF have dyspnea either at rest or with minimal exertion.4 Despite dyspnea being a common presenting symptom, little is known about how it relates to patient outcomes. Investigations related to dyspnea in hospitalized patients with HF have focused primarily on symptom relief. Dyspnea relief has been used as an end point for establishing regulatory approval for therapies, and in some analyses has been associated with improved outcomes.5–7 Notably, dyspnea relief has been linked with improved clinical outcomes in clinical trials of therapeutic agents,8 but in other trials there has been a disconnect between dyspnea relief and clinical outcomes.9 Characteristics, outcomes, and associated costs among patients with acute HF in clinical practice have not been well characterized by baseline dyspnea severity.
Methods
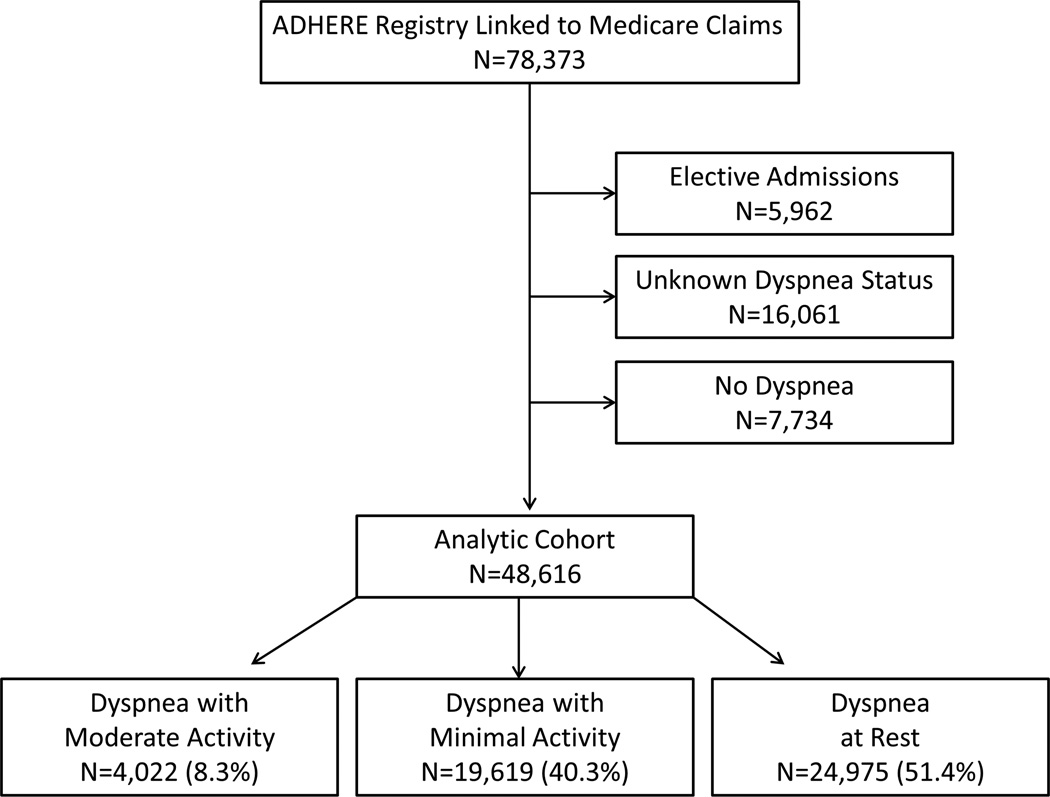
We obtained hospitalization data from the Acute Decompensated Heart Failure National Registry (ADHERE) of patients hospitalized with acute HF.10 We linked the ADHERE data to Medicare inpatient and denominator files11 using methods described previously.12,13 We included patients 65 years or older who had a registry hospitalization for acute HF between January 1, 2001, and March 31, 2006, that was linked to fee-for-service Medicare claims data. Eligible patients lived in the United States at the time of the index admission and were enrolled in fee-for-service Medicare for at least 6 months before the index admission (n=78,373). We excluded patients who were admitted on an elective basis (n=5962). Patients for whom dyspnea severity at admission was not documented in the registry (n=16,061) and those who did not have dyspnea at admission (n=7734) were excluded from the analysis (Figure 1). These excluded patients were generally similar to the study population (Supplemental Table 1). Patients who died in the hospital, left against medical advice, or were discharged or transferred to another short-term hospital or hospice were excluded from the measurement of 30-day readmission and clinical status at discharge. Patients in the postdischarge cohort who enrolled in a Medicare managed care plan during the 30 days after discharge from the index hospitalization were excluded from the measurement of days alive and out of the hospital and Medicare payments.
Figure 1.
Consort Diagram of the Study Population
The study variable of interest was dyspnea severity on admission (at time of initial presentation). The dyspnea severity characterization in ADHERE was based on patient self-reported symptom severity (dyspnea with moderate activity, dyspnea with minimal activity, or dyspnea at rest) as obtained by the clinicians directly involved in their routine clinical care and as documented in the medical record. A specific research instrument or standardized questionnaire was not utilized. The outcomes of interest were mortality during the index hospitalization and at 30 days after admission; length of stay and clinical status at discharge; and 30-day postdischarge days alive and out of the hospital, readmission (HF and all-cause), and Medicare payments. We determined all-cause mortality based on death dates in the Medicare denominator files. Length of stay and in-hospital mortality were based on Medicare claims for the index hospitalization. Clinical status at discharge was categorized as asymptomatic, improved but still symptomatic, or other/unknown, as recorded in the registry based on patient-report. Total days alive and out of the hospital in the 30 days after discharge was determined based on the date of death in the Medicare denominator files and hospitalization dates in Medicare inpatient files. We identified readmission based on subsequent inpatient Medicare claims. Readmission for HF was based on subsequent inpatient claims with a primary diagnosis of HF (ICD-9 diagnosis code 428.x, 402.x1, 404.x1, or 404.x3). We calculated time to readmission as the number of days from the index discharge date to the subsequent admission date. Medicare payments in the 30 days after discharge were determined based on payments for inpatient, outpatient, and carrier claims. Payments were reported in 2010 US dollars with inflation adjustment using the Consumer Price Index for medical care.
We described baseline characteristics of the study population by dyspnea severity at admission using frequencies with percentages for categorical variables and medians with interquartile ranges (IQRs) for continuous variables. We tested for differences between the groups using chi-square tests for categorical variables and Kruskal-Wallis tests for continuous variables. For variables that had low rates of missingness (ie, less than 5% of records), we imputed continuous variables to the overall median value, dichotomous variables to “no,” and multichotomous variables to the most frequent categorical value. For variables with greater than 5% missingness (ie, smoking status, BNP level, race, and ejection fraction), we treated the missing values as a separate category.
We present the observed outcomes by dyspnea severity at admission. For in-hospital mortality and clinical status at discharge, we tested for differences between groups using chi-square tests. For length of stay, days alive out of the hospital at 30 days, and Medicare payments at 30 days, we tested for differences between groups using Kruskal-Wallis tests. For 30-day mortality, we calculated cumulative incidence based on Kaplan-Meier estimates and tested for differences between groups using log-rank tests. For readmission, we calculated cumulative incidence at 30 days based on estimates from the cumulative incidence function, which accounts for the competing risk of mortality, and we tested for differences between groups using Gray tests.
We estimated the unadjusted and adjusted associations between dyspnea severity at admission and the outcomes of interest. In the unadjusted model, dyspnea severity was the only predictor. In the adjusted model, we controlled for baseline covariates, medications at discharge, and the year of the index admission (see Table 3 footnote for variable list). With the large number of events in each analysis, there was no over-fitting problem with the adjustment variables. We used a linear mixed model for days alive out of the hospital at 30 days, Cox proportional hazard models for 30-day mortality and readmission, generalized linear mixed models with a Poisson distribution and log link for Medicare payments, and a logistic regression model for in-hospital mortality. Finally, we assessed associations between other baseline covariates and dyspnea severity using a generalized logistic regression model. We report the estimated odds ratios (ORs) associated with each characteristic for comparisons of the dyspnea categories. For all models, significance tests and confidence intervals (CIs) were based on robust standard errors to account for the clustering of patients by hospital. Because of the large number of comparisons in the analysis, we used a 2-tailed α = 0.01 to establish statistical significance and we report 99% CIs. We used SAS version 9.3 (SAS Institute Inc, Cary, NC) for all analyses. The institutional review board of the Duke University Health System approved the study.
Table 3.
Unadjusted and Adjusted Associations Between Dyspnea Severity at Admission and Outcomes
| Outcome | Unadjusted Association | Adjusted Association§ | ||
|---|---|---|---|---|
| HR (99% CI) | p Value | HR (99% CI) | p Value | |
| Mortality within 30 days after admission* | ||||
| Dyspnea with minimal activity vs moderate activity | 1.21 (1.02 to 1.44) | 0.004 | 1.11 (0.93 to 1.32) | 0.14 |
| Dyspnea at rest vs with moderate activity | 1.89 (1.60 to 2.24) | < 0.001 | 1.72 (1.45 to 2.03) | < 0.001 |
| Dyspnea at rest vs with minimal activity | 1.56 (1.42 to 1.72) | < 0.001 | 1.55 (1.41 to 1.71) | < 0.001 |
| All-cause readmission within 30 days after discharge† | ||||
| Dyspnea with minimal activity vs moderate activity | 1.10 (1.00 to 1.23) | 0.01 | 1.06 (0.95 to 1.18) | 0.18 |
| Dyspnea at rest vs with moderate activity | 1.17 (1.04 to 1.30) | < 0.001 | 1.12 (1.00 to 1.25) | 0.01 |
| Dyspnea at rest vs with minimal activity | 1.05 (0.99 to 1.12) | 0.02 | 1.06 (1.00 to 1.12) | 0.01 |
| Heart failure readmission within 30 days after discharge† | ||||
| Dyspnea with minimal activity vs moderate activity | 1.26 (1.05 to 1.51) | < 0.001 | 1.19 (0.99 to 1.42) | 0.01 |
| Dyspnea at rest vs with moderate activity | 1.35 (1.14 to 1.59) | < 0.001 | 1.26 (1.07 to 1.49) | < 0.001 |
| Dyspnea at rest vs with minimal activity | 1.07 (0.98 to 1.16) | 0.05 | 1.06 (0.98 to 1.16) | 0.07 |
| Days alive and out of hospital at 30 days after discharge‡ | ||||
| Dyspnea with minimal activity vs moderate activity | −0.281 (−0.553 to −0.009) | 0.008 | −0.129 (−0.396 to 0.139) | 0.21 |
| Dyspnea at rest vs with moderate activity | −0.656 (−0.924 to −0.388) | < 0.001 | −0.492 (−0.757 to −0.227) | < 0.001 |
| Dyspnea at rest vs with minimal activity | −0.375 (−0.528 to −0.222) | < 0.001 | −0.363 (−0.515 to −0.211) | < 0.001 |
| Length of stay for the index hospitalization* | ||||
| Dyspnea with minimal activity vs moderate activity | 0.415 (0.189 to 0.642) | < 0.001 | 0.251 (0.029 to 0.474) | 0.004 |
| Dyspnea at rest vs with moderate activity | 0.677 (0.454 to 0.900) | < 0.001 | 0.515 (0.295 to 0.736) | < 0.001 |
| Dyspnea at rest vs with minimal activity | 0.262 (0.135 to 0.389) | < 0.001 | 0.264 (0.138 to 0.390) | < 0.001 |
| Medicare payments at 30 days after discharge‡ | ||||
| Dyspnea with minimal activity vs moderate activity | 1.105 (0.981 to 1.246) | 0.03 | 1.072 (0.955 to 1.203) | 0.12 |
| Dyspnea at rest vs with moderate activity | 1.176 (1.046 to 1.323) | < 0.001 | 1.149 (1.025 to 1.288) | 0.002 |
| Dyspnea at rest vs with minimal activity | 1.064 (0.997 to 1.134) | 0.01 | 1.071 (1.006 to 1.141) | 0.005 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
Among all patients in the study population.
Among patients discharged alive.
Among patients discharged alive, not censored, at 30 days after discharge.
Adjusted for demographics (age, gender, race), medical history (anemia, atrial fibrillation, coronary artery disease, chronic renal insufficiency, COPD, diabetes mellitus, HF, hyperlipidemia, hypertension, prior myocardial infarction, peripheral vascular disease, prior stroke/TIA, smoking status, pacemaker type, implantable cardioverter defibrillator), initial evaluation/vital signs/lab results (fatigue, rales, pulmonary edema, ejection fraction, pulse, systolic blood pressure, serum sodium, hemoglobin, serum creatinine), medications (ACE inhibitor or ARB, aspirin, beta blocker, diuretic, clopidogrel, lipid-lowering, warfarin), and year of index hospitalization. Post-discharge models include medication information from discharge.
Results
Of 48,616 patients with acute HF and dyspnea, 4022 (8.3%) had dyspnea with moderate activity, 19,619 (40.3%) had dyspnea with minimal activity, and 24,975 (51.4%) had dyspnea at rest. Thus, the group with dyspnea with moderate activity represented a comparatively lower percentage of the study population. Table 1 shows the baseline characteristics of the 3 groups. Patients with dyspnea at rest had more advanced chronic kidney disease (ie, stages 3–5) than patients with dyspnea with moderate activity. The dyspnea at rest group also had the highest percentage of patients with a baseline systolic blood pressure of 140 mm Hg or greater (Supplemental Table 2).
Table 1.
Baseline Characteristics of the Study Population by Dyspnea Severity at Admission
| Characteristic | Dyspnea Severity | p Value | ||
|---|---|---|---|---|
| Moderate Activity (n = 4022) |
Minimal Activity (n = 19,619) |
At Rest (n = 24,975) |
||
| Age (years) | 79.6 (73.9–85.0) | 79.7 (73.9–85.1) | 80.1 (74.2–85.8) | < 0.001 |
| Men | 1864 (46.3%) | 8840 (45.1%) | 10,636 (42.6%) | < 0.001 |
| Race | < 0.001 | |||
| Black | 517 (12.9%) | 2,055 (10.5%) | 2745 (11.0%) | |
| White | 3304 (82.1%) | 16,181 (82.5%) | 20,430 (81.8%) | |
| Other/unknown | 201 (5.0%) | 1383 (7.0%) | 1800 (7.2%) | |
| Medical history | ||||
| Anemia | 2082 (51.8%) | 10,826 (55.2%) | 13,315 (53.3%) | < 0.001 |
| Atrial fibrillation | 1367 (34.0%) | 7264 (37.0%) | 8650 (34.6%) | < 0.001 |
| Coronary artery disease | 2375 (59.1%) | 12,059 (61.5%) | 15,217 (60.9%) | 0.02 |
| Chronic renal insufficiency | 949 (23.6%) | 5623 (28.7%) | 7095 (28.4%) | < 0.001 |
| Chronic obstructive pulmonary disease | 1128 (28.0%) | 6095 (31.1%) | 7965 (31.9%) | < 0.001 |
| Diabetes mellitus | 1522 (37.8%) | 8057 (41.1%) | 10,326 (41.3%) | 0.001 |
| Heart failure admission in prior 6 months | 487 (12.1%) | 2760 (14.1%) | 3706 (14.8%) | < 0.001 |
| Hyperlipidemia | 1511 (37.6%) | 7770 (39.6%) | 9216 (36.9%) | < 0.001 |
| Hypertension | 2982 (74.1%) | 14,777 (75.3%) | 18,786 (75.2%) | 0.28 |
| Myocardial infarction | 1168 (29.0%) | 6220 (31.7%) | 7977 (31.9%) | 0.001 |
| Peripheral vascular disease | 743 (18.5%) | 3804 (19.4%) | 4960 (19.9%) | 0.09 |
| Stroke or transient ischemic attack | 680 (16.9%) | 3582 (18.3%) | 4923 (19.7%) | < 0.001 |
| Smoker | < 0.001 | |||
| Never | 1832 (45.5%) | 8251 (42.1%) | 10,950 (43.8%) | |
| Former | 1551 (38.6%) | 7627 (38.9%) | 9286 (37.2%) | |
| Current | 307 (7.6%) | 1448 (7.4%) | 1909 (7.6%) | |
| Missing | 332 (8.3%) | 2293 (11.7%) | 2830 (11.3%) | |
| Devices | ||||
| Cardiac resynchronization therapy | 72 (1.8%) | 365 (1.9%) | 377 (1.5%) | 0.01 |
| Implantable cardioverter-defibrillator | 208 (5.2%) | 1143 (5.8%) | 1326 (5.3%) | 0.04 |
| Pacemaker | 738 (18.3%) | 3899 (19.9%) | 4613 (18.5%) | < 0.001 |
| Initial evaluation | ||||
| Ejection fraction | < 0.001 | |||
| ≥ 40% | 2007 (49.9%) | 9438 (48.1%) | 11,320 (45.3%) | |
| < 40% | 1417 (35.2%) | 7298 (37.2%) | 9318 (37.3%) | |
| Missing | 598 (14.9%) | 2883 (14.7%) | 4337 (17.4%) | |
| Fatigue | 1240 (30.8%) | 7475 (38.1%) | 8758 (35.1%) | < 0.001 |
| Pulmonary edema | 3385 (84.2%) | 17,224 (87.8%) | 22,931 (91.8%) | < 0.001 |
| Rales | 2632 (65.4%) | 13,805 (70.4%) | 19,266 (77.1%) | < 0.001 |
| Initial vital signs | ||||
| BNP level (pg/mL) | 739 (397–1307) | 839 (437–1440) | 924 (470–1540) | < 0.001 |
| Missing | 1705 (42.4%) | 8406 (42.8%) | 10,485 (42.0%) | |
| Pulse (bpm) | 82.0 (70.0–96.0) | 82.0 (70.0–98.0) | 86.0 (73.0–103) | < 0.001 |
| Systolic blood pressure (mm Hg) | 143 (124–165) | 142 (122–163) | 145 (124–168) | < 0.001 |
| Initial laboratory test results | ||||
| eGFR (mL/min/1.73 m2) | 51.2 (36.0–67.3) | 48.2 (33.7–63.7) | 47.7 (33.3–63.6) | < 0.001 |
| Hemoglobin (g/dL) | 12.2 (10.9–13.5) | 12.1 (10.7–13.4) | 12.1 (10.8–13.5) | < 0.001 |
| Serum creatinine (mg/dL) | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) | 1.3 (1.0–1.8) | < 0.001 |
| Serum sodium (mEq/L) | 139 (136–141) | 139 (136–141) | 139 (136–141) | 0.04 |
| Medication at discharge† | ||||
| ACE inhibitor or ARB | 2511 (63.8%) | 12,080 (63.6%) | 14,430 (61.3%) | < 0.001 |
| Aspirin | 1972 (50.1%) | 9222 (48.5%) | 11,297 (48.0%) | 0.05 |
| β-Blocker | 2329 (59.2%) | 11,476 (60.4%) | 14,057 (59.8%) | 0.26 |
| Clopidogrel | 510 (13.0%) | 2660 (14.0%) | 3366 (14.3%) | 0.08 |
| Diuretic | 2959 (75.2%) | 14,887 (78.3%) | 17,404 (74.0%) | < 0.001 |
| Lipid-lowering agent | 1482 (37.7%) | 6979 (36.7%) | 8233 (35.0%) | < 0.001 |
| Warfarin | 1126 (28.6%) | 5671 (29.8%) | 6286 (26.7%) | < 0.001 |
| Index hospitalization year | < 0.001 | |||
| 2001 | 86 (2.1%) | 549 (2.8%) | 743 (3.0%) | |
| 2002 | 1141 (28.4%) | 5605 (28.6%) | 7561 (30.3%) | |
| 2003 | 1353 (33.6%) | 6607 (33.7%) | 7914 (31.7%) | |
| 2004 | 980 (24.4%) | 5055 (25.8%) | 5917 (23.7%) | |
| 2005 | 420 (10.4%) | 1646 (8.4%) | 2597 (10.4%) | |
| 2006 | 42 (1.0%) | 157 (0.8%) | 243 (1.0%) | |
Definitions: Anemia, hypertension, and hyperlipidemia were based on documentation of these clinical diagnoses in the medical history.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; eGFR, estimated glomerular filtration rate.
Within the previous 6 months.
Data are presented only for patients discharged alive, including 3933 patients with dyspnea with moderate activity, 19,006 patients with dyspnea with minimal activity, and 23,524 patients with dyspnea at rest.
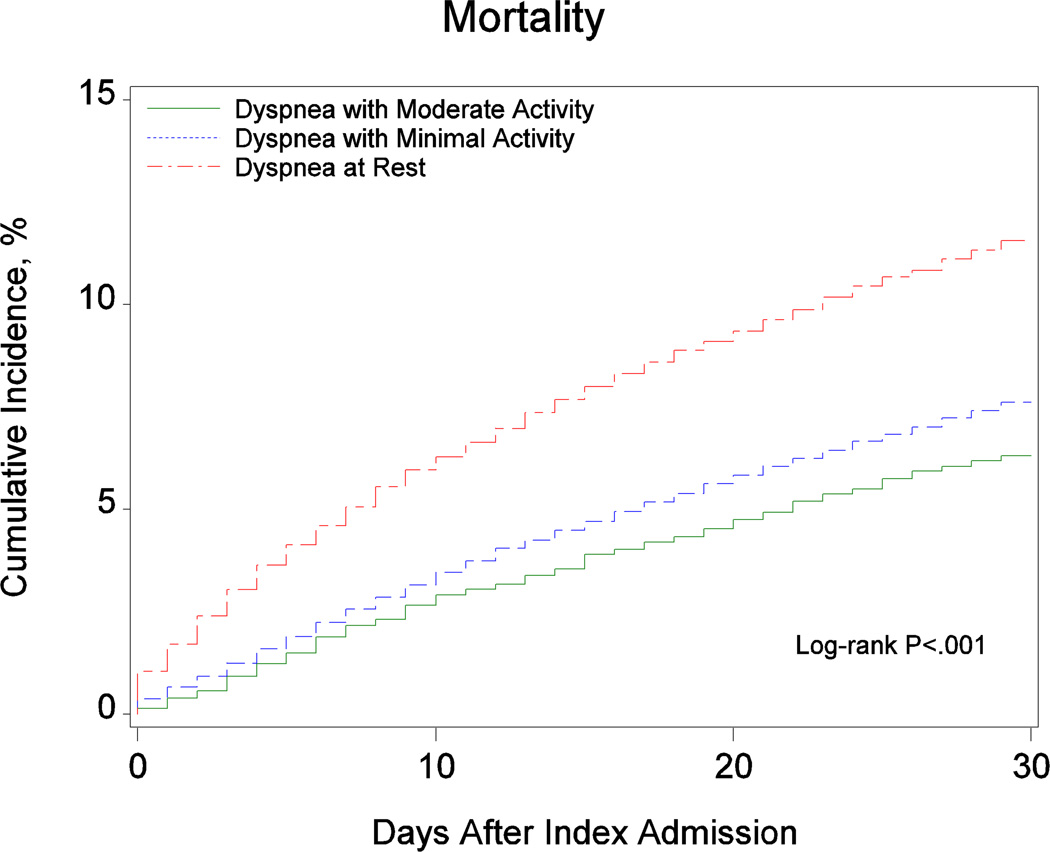
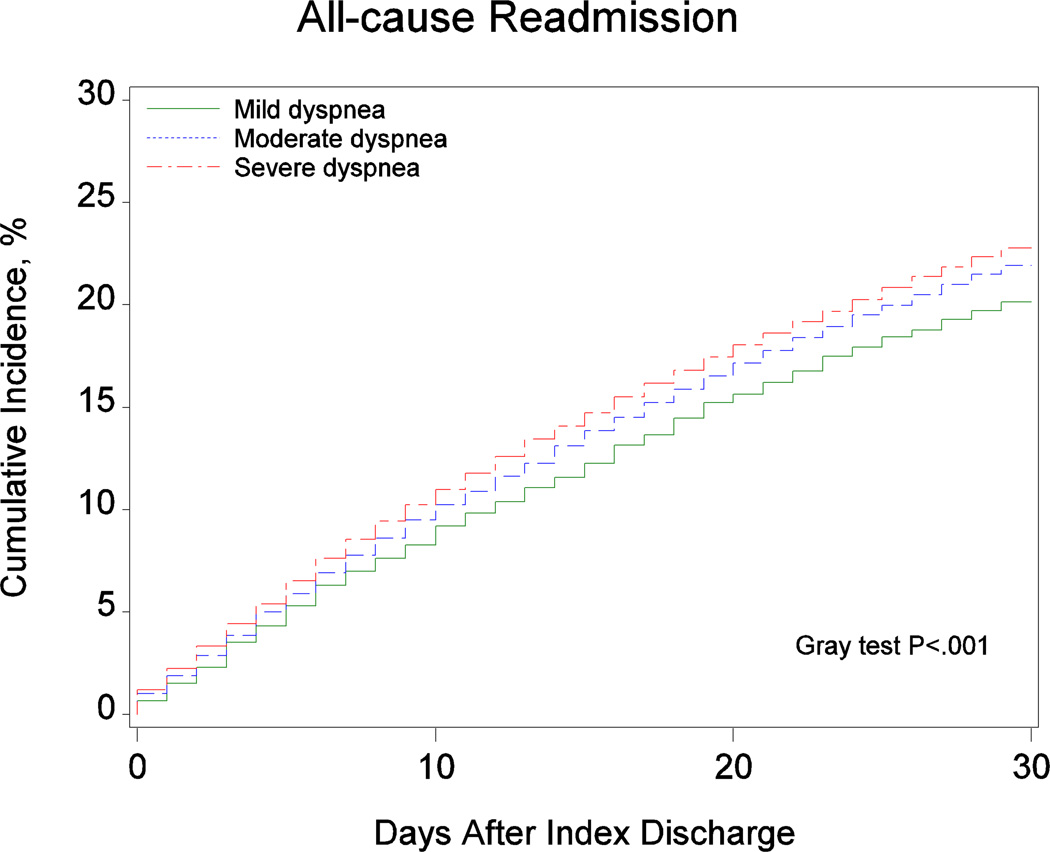
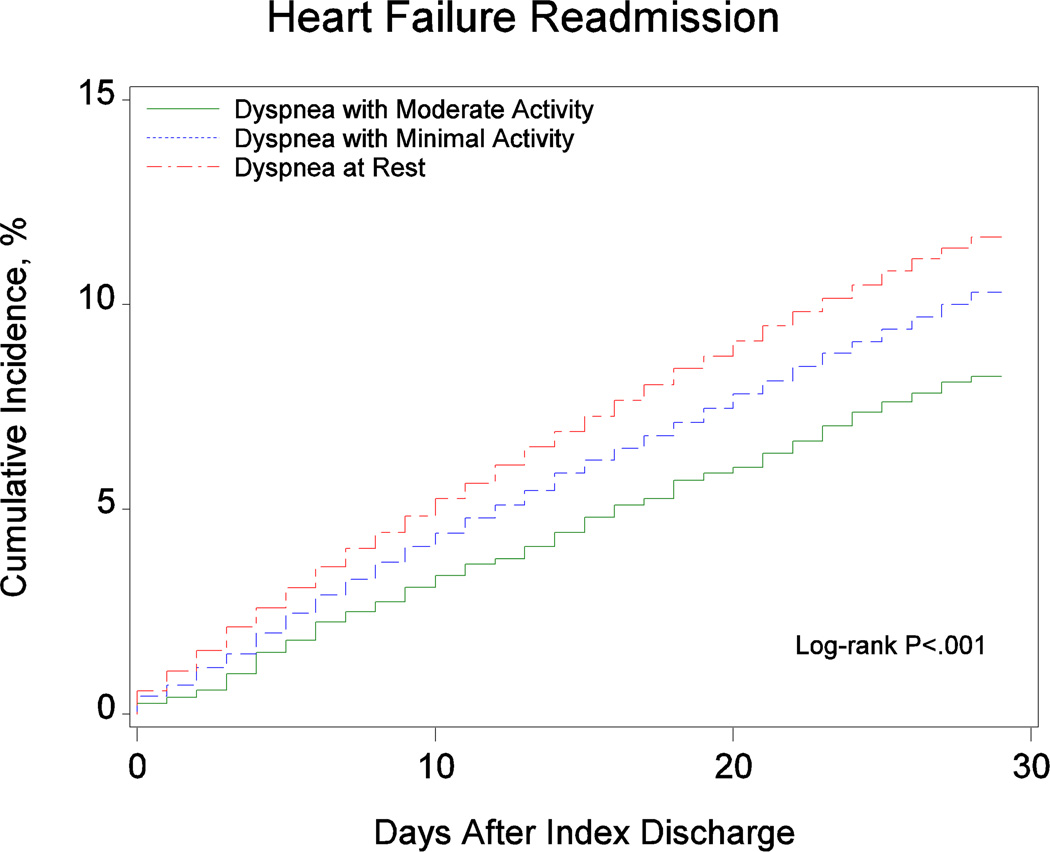
Table 2 presents the observed outcomes based on dyspnea severity at admission. Figure 2 shows the cumulative incidence of 30-day mortality and readmission based on dyspnea severity at admission. There was a graded increase in the cumulative incidence of mortality from patients with dyspnea with moderate activity to patients with dyspnea with minimal activity to patients with dyspnea at rest. We observed similar trends for the readmission outcomes.
Table 2.
Observed Outcomes by Dyspnea Severity at Admission
| Outcomes | Dyspnea With Moderate Activity (n = 4022) |
Dyspnea With Minimal Activity (n = 19,619) |
Dyspnea at Rest (n = 24,975) |
p Value |
|---|---|---|---|---|
| Length of stay, median (IQR) (days) | 4.0 (3.0–6.0) | 4.0 (3.0–7.0) | 4.0 (3.0–7.0) | < 0.001 |
| In-hospital mortality | 89 (2.2%) | 613 (3.1%) | 1451 (5.8%) | < 0.001 |
| Discharged alive | 3731 (92.8%) | 18,149 (92.5) | 22,143 (88.7%) | |
| Clinical status at discharge | < 0.001 | |||
| Asymptomatic | 2097 (56.2%) | 9074 (50.0%) | 10,856 (49.1%) | |
| Still symptomatic | 1390 (37.3%) | 7579 (41.8%) | 9371 (42.4%) | |
| Other/unknown | 242 (6.5%) | 1479 (8.2%) | 1895 (8.6%) | |
| Discharged alive, not censored at 30 days | 3729 (92.7%) | 18,132 (92.4%) | 22,122 (88.6%) | |
| Days alive and out of hospital at 30 days after discharge (days) | 30.0 (30.0–30.0) | 30.0 (29.0–30.0) | 30.0 (28.0–30.0) | < 0.001 |
| Mortality at 30 days | 254 (6.3%) | 1493 (7.6%) | 2888 (11.6%) | < 0.001 |
| All-cause readmission at 30 days after discharge† | 751 (20.1%) | 3974 (21.9%) | 5037 (22.8%) | < 0.001 |
| Heart failure readmission at 30 days† | 252 (7.2%) | 1527 (9.0%) | 1964 (9.4%) | < 0.001 |
| Medicare payments at 30 days after discharge ($)‡ | 520 (253–2421) | 569 (264–3693) | 572 (268–4621) | < 0.001 |
Abbreviations: IQR, interquartile range.
Presented as the number of patients (cumulative incidence per 100 patients at risk) who died within 30 days after admission to the index hospitalization.
Presented as the number of patients (cumulative incidence per 100 patients at risk) who were readmitted within 30 days after discharge from the index hospitalization.
Presented in 2010 US dollars.
Figure 2.
Cumulative Incidence of (A) Mortality, (B) All-Cause Readmission, and (C) Heart Failure Readmission by Dyspnea Severity at Admission
In the unadjusted analysis, dyspnea at rest was associated with greater length of stay, 30-day mortality, all-cause and heart failure readmission, and Medicare payments and fewer days alive and out of the hospital, compared to dyspnea with moderate activity (Table 3). After multivariable adjustment, dyspnea at rest was associated with greater 30-day mortality and heart failure readmission, fewer days alive and out of the hospital at 30 days, longer length of stay, and higher Medicare payments, compared to dyspnea with moderate activity. Other independent predictors of the outcomes are presented in Supplemental Table 3.
The odds of having dyspnea at rest or with minimal activity compared with dyspnea with moderate activity were higher for patients who currently smoked and or had chronic obstructive pulmonary disease or diabetes (p < 0.01 for both) (Supplemental Table 4).
Discussion
In a large US HF registry, approximately 50% of patients with acute HF and dyspnea on hospital admission reported dyspnea at rest. These patients had a distinct, severe clinical profile with higher blood pressure, heart rate, and BNP level and worse renal function compared with patients with less severe dyspnea on presentation. Dyspnea at rest was independently associated with a 72% greater risk of 30-day mortality and a 26% greater risk of 30-day HF readmission, compared with dyspnea with moderate activity. Overall, dyspnea at rest was associated with fewer days alive and out of the hospital than less severe dyspnea. In addition, dyspnea at rest was associated with 14.9% higher costs than dyspnea with moderate activity.
The primary finding of this analysis is that dyspnea at rest on admission for acute HF was independently associated with worse short-term outcomes and higher costs. Dyspnea severity should be recognized for its role as a major prognostic marker in acute HF. The prognostic utility may be additive to current risk prediction models, given that the association with worse outcomes was present after robust adjustment for variables included in prior risk models.14,15 Although dyspnea severity is rigorously documented in acute HF clinical trials,9 quantification of baseline dyspnea is not routinely performed in current practice. Routine clinical assessment and grading of the severity of baseline dyspnea at hospital presentation may allow clinicians to target interventions to high-risk patients in order to improve outcomes and reduce costs.8
Our findings support and extend previous studies demonstrating associations between dyspnea and outcomes in acute HF. For example, dyspnea relief within 6 hours of study drug initiation in the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) was associated with fewer events for the composite of mortality or HF hospitalization at 30 days, compared with no early dyspnea relief.5 Early dyspnea relief was not associated with lower mortality. The PROTECT pilot study, the PROTECT trial, and the Relaxin for the Treatment of Patients With Acute Heart Failure (Pre-RELAX-AHF) study also had mixed results with regard to associations between dyspnea relief during an acute HF hospitalization and 30-day to 60-day outcomes, including mortality and composite outcomes including readmission.16–18 Thus, dyspnea severity on presentation may provide prognostic information distinct from dyspnea relief.
Future studies are needed to clarify whether the categories of dyspnea severity in our analysis or the more granular scales used in clinical trials (eg, Likert scale, visual analog scale) have more robust associations with outcomes.7 Dyspnea as a patient-reported outcome or health status in acute HF should receive greater attention, including robust methods of assessment as part of routine clinical practice. Previous studies explored associations between objective measures of dyspnea evaluation (eg, peak expiratory flow rate) and patient-reported measures of dyspnea by Likert scale.19 To our knowledge, no study has demonstrated that objective measures of dyspnea at baseline adequately quantify patient-reported dyspnea severity on admission. These data in combination with our analysis support the utility of assessing patient-reported dyspnea severity on admission for acute HF.
It is notable that a specific research instrument or standardized questionnaire was not utilized for dyspnea assessment in ADHERE. However, any decrease in precision or standardization with this approach to evaluate dyspnea severity would be expected to limit, not enhance, the ability to detect any differences in clinical outcomes. While there may be greater variability with this approach, the findings may be more applicable and generalizable to clinical practice as they represent those based on dyspnea severity as measured in routine clinical practice.
The prognostic utility of dyspnea status on admission should be distinguished from the established role of New York Heart Association (NYHA) classification in determining prognosis in outpatient settings. NYHA classification is related to symptoms of exercise intolerance in patients with chronic HF.20 Determination of NYHA class is based not only on symptoms of dyspnea, but also fatigue, palpitations, and angina. Patients admitted with acute HF typically have NYHA class III to IV symptoms with varying contributions related to these different symptoms. Previous studies demonstrated a lack of reproducibility and validity of NYHA classification.21
Contemporary acute HF trials targeting dyspnea relief have entry criteria related to baseline dyspnea severity. For example, the RELAX-AHF study enrolled patients with dyspnea at rest or with minimum exertion.8 Our analysis demonstrates that patients with dyspnea at rest represent approximately half of all patients hospitalized with acute HF with dyspnea. Thus, dyspnea at rest is fairly common in patients with acute HF, and the use of dyspnea at rest as an entry criterion is not a major factor limiting broad generalizability. Despite dyspnea at rest being relatively common on admission for HF, our analysis provides further support for the use of this criterion in clinical trials in order to enrich the study population with high-risk patients. Patients with dyspnea at rest on admission tend to be symptomatic at discharge, a profile known to be associated with worse outcomes.22
The study was a retrospective analysis of data from an acute HF US registry. Despite covariate adjustment, other measured and unmeasured factors may have influenced the findings. The study population was limited to patients 65 years or older enrolled in fee-for-service Medicare and admitted between 2001 and 2006, and these findings may not apply to patients with different baseline characteristics or to more recent time periods.
Supplementary Material
Highlights.
Of 48,616 patients with acute heart failure (HF) and dyspnea, 4022 (8.3%) had dyspnea with moderate activity, 19,619 (40.3%) with minimal activity, and 24,975 (51.4%) at rest.
Patients with dyspnea with minimal activity or at rest had greater comorbidity, including renal insufficiency.
Dyspnea severity, as assessed in clinical practice, is an independent predictor of outcomes and costs.
Acknowledgments
Funding/support: This project was supported by a research agreement between Duke University and Novartis Pharmaceuticals Corporation. RJM was supported by grant T32GM086330 from the National Institute of General Medical Sciences.
PPS and KWJ are employees of Novartis. GCF reported serving as a consultant for Bayer, Gambro, Medtronic, and Novartis. AFH reported receiving research funding from the AHA, Amgen, and Novartis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial disclosures: No other authors reported conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Braunschweig F, Cowie MR, Auricchio A. What are the costs of heart failure? Europace. 2011;13(Suppl 2):ii13–ii17. doi: 10.1093/europace/eur081. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Zannad F, Sopko G, Klein L, Piña IL, Konstam MA, Massie BM, Roland E, Targum S, Collins SP, Filippatos G, Tavazzi L. Acute heart failure syndromes: current state and framework for future research. Circulation. 2005;112:3958–3968. doi: 10.1161/CIRCULATIONAHA.105.590091. [DOI] [PubMed] [Google Scholar]
- 5.Mentz RJ, Hernandez AF, Stebbins A, Ezekowitz JA, Felker GM, Heizer GM, Atar D, Teerlink JR, Califf RM, Massie BM, Hasselblad V, Starling RC, O'Connor CM, Ponikowski P. Predictors of early dyspnoea relief in acute heart failure and the association with 30-day outcomes: findings from ASCEND-HF. Eur J Heart Fail. 2013;15:456–464. doi: 10.1093/eurjhf/hfs188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teerlink JR. Dyspnea as an end point in clinical trials of therapies for acute decompensated heart failure. Am Heart J. 2003;145:S26–S33. doi: 10.1067/mhj.2003.151. [DOI] [PubMed] [Google Scholar]
- 7.West RL, Hernandez AF, O’Connor CM, Starling RC, Califf RM. A review of dyspnea in acute heart failure syndromes. Am Heart J. 2010;160:209–214. doi: 10.1016/j.ahj.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Teerlink JR1, Cotter G, Davison BA, Felker GM, Filippatos G, Greenberg BH, Ponikowski P, Unemori E, Voors AA, Adams KF, Jr, Dorobantu MI, Grinfeld LR, Jondeau G, Marmor A, Masip J, Pang PS, Werdan K, Teichman SL, Trapani A, Bush CA, Saini R, Schumacher C, Severin TM, Metra M. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet. 2013;381:29–39. doi: 10.1016/S0140-6736(12)61855-8. [DOI] [PubMed] [Google Scholar]
- 9.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalán R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Méndez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 10.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM, Parent EM, Tu N, Vaccarino V, Wang Y, Radford MJ, Hennen J. Readmission after hospitalization for congestive heart failure among Medicare beneficiaries. Arch Intern Med. 1997;157:99–104. [PubMed] [Google Scholar]
- 12.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kociol RD, Greiner MA, Hammill BG, Eapen ZJ, Fonarow GC, Klaskala W, Mills RM, Curtis LH, Hernandez AF. B-type natriuretic peptide level and postdischarge thrombotic events in older patients hospitalized with heart failure: insights from the Acute Decompensated Heart Failure National Registry. Am Heart J. 2012;163:994–1001. doi: 10.1016/j.ahj.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 14.O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, Greenberg BH, Yancy CW, Young JB, Fonarow GC. Predictors of mortality after discharge in patients hospitalized with heart failure: an analysis from the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF) Am Heart J. 2008;156:662–673. doi: 10.1016/j.ahj.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 15.Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, Jr, Gheorghiade M, O'Connor CM. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. 2004;10:460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Metra M, Cleland JG, Weatherley BD, Dittrich HC, Givertz MM, Massie BM, O'Connor CM, Ponikowski P, Teerlink JR, Voors AA, Cotter G. Dyspnoea in patients with acute heart failure: an analysis of its clinical course, determinants, and relationship to 60-day outcomes in the PROTECT pilot study. Eur J Heart Fail. 2010;12:499–507. doi: 10.1093/eurjhf/hfq021. [DOI] [PubMed] [Google Scholar]
- 17.Metra M, O'Connor CM, Davison BA, Cleland JG, Ponikowski P, Teerlink JR, Voors AA, Givertz MM, Mansoor GA, Bloomfield DM, Jia G, DeLucca P, Massie B, Dittrich H, Cotter G. Early dyspnoea relief in acute heart failure: prevalence, association with mortality, and effect of rolofylline in the PROTECT Study. Eur Heart J. 2011;32:1519–1534. doi: 10.1093/eurheartj/ehr042. [DOI] [PubMed] [Google Scholar]
- 18.Metra M, Teerlink JR, Felker GM, Greenberg BH, Filippatos G, Ponikowski P, Teichman SL, Unemori E, Voors AA, Weatherley BD, Cotter G. Dyspnoea and worsening heart failure in patients with acute heart failure: results from the Pre-RELAX-AHF study. Eur J Heart Fail. 2010;12:1130–1139. doi: 10.1093/eurjhf/hfq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ezekowitz JA, Hernandez AF, O'Connor CM, Starling RC, Proulx G, Weiss MH, Bakal JA, Califf RM, McMurray JJ, Armstrong PW. Assessment of dyspnea in acute decompensated heart failure: insights from ASCEND-HF on the contributions of peak expiratory flow. J Am Coll Cardiol. 2012;59:1441–1448. doi: 10.1016/j.jacc.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 20.The Criteria Committee of the New York Heart Association. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed. Boston, ML: Brown & Co.; 1994. pp. 253–256. [Google Scholar]
- 21.Goldman L, Hashimoto B, Cook EF, Loscalzo A. Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 1981;64:1227–1234. doi: 10.1161/01.cir.64.6.1227. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC, Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–843. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.