Abstract
Background
HCV kinetic analysis and modeling during antiviral therapy have not been performed in decompensated cirrhotic patients awaiting liver transplantation. Here, viral and host parameters were compared in patients treated with daily intravenous silibinin (SIL) monotherapy for 7 days according to the severity of their liver disease.
Methods
Data were obtained from 25 patients, 12 non-cirrhotic, 8 with compensated cirrhosis and 5 with decompensated cirrhosis. The standard-biphasic model with time-varying SIL effectiveness (from 0 to εmax) was fit to viral kinetic data.
Results
Baseline viral load and age were significantly associated with the severity of liver disease (p<0.0001). A biphasic viral decline was observed in most patients with a higher first phase decline patients with less severe liver disease. The maximal effectiveness, εmax, was significantly (p≤0.032) associated with increasing severity of liver disease (εmax[s.e.]=0.86[0.05], εmax=0.69[0.06] and εmax=0.59[0.1]).. The 2nd phase decline slope was not significantly different among groups (mean 1.88±0.15 log10IU/ml/wk, p=0.75) as was the rate of change of SIL effectiveness (k=2.12/day[standard error, s.e.=0.18/day]). HCV-infected cell loss rate (δ[s.e.]=0.62/day[0.05/day]) was high and similar among groups.
Conclusions
The high loss rate of HCV-infected cells suggests that sufficient dose and duration of SIL might achieve viral suppression in advanced liver disease.
Introduction
Hepatitis C virus (HCV) infection is a serious public health concern affecting approximately 150 million persons worldwide and causing approximately 350,000 deaths annually from cirrhosis and hepatocellular carcinoma [1]. HCV cirrhosis and related complications are the leading cause for liver transplantation (LT) in the Western World [2]. Unfortunately, HCV infects the graft universally, immediately after LT [3], and the disease course is accelerated in around one third of HCV LT recipients. The recent approval of direct-acting antiviral agents (DAAs), the HCV NS3/4A protease inhibitors boceprevir, telaprevir, and simeprevir and the HCV nucleotide polymerase inhibitor sofosbuvir, for use in combination with pegylated-interferon (pegIFN) and ribavirin (RBV), represents an important milestone in achieving higher sustained virological response (SVR) rates in HCV genotype-1 infected patients with compensated liver disease [4]. However, there are important safety concerns in treating compensated cirrhotic patients [5] and decompensated cirrhotic patients with a pegIFN/RBV treatment backbone [6, 7]. As such detailed HCV kinetic analysis has not been performed in patients with decompensated cirrhosis [8].
Legalon SIL (SIL) is a chemically hydrophilized version of silibinin that has exhibited high antiviral effectiveness against HCV in patients with compensated liver disease during the first 7 days of SIL monotherapy [9, 10]. A recent study indicated that daily intravenous SIL has promising antiviral properties and is well tolerated in the peri-LT period [11]. The aim of the current study was to estimate and compare viral kinetic parameters and SIL effectiveness using a standard biphasic mathematical model in non-cirrhotic patients, patients with compensated cirrhosis, and, for the first time, in patients with decompensated cirrhosis.
Material and Methods
Patients
The data analyzed in this study were obtained from two published studies where 20 mg/kg/d of SIL was administered intravenously to a total of 12 non-cirrhotic patients, 8 with compensated cirrhosis (including 3 with hepatocellular carcinoma awaiting LT), and 5 with decompensated cirrhosis awaiting LT [10, 11]. All patients were infected with HCV genotype 1 except two who were infected with HCV genotype 4 (Table 1). In both studies, viral load was measured once a day during the first 7 days of SIL monotherapy [10, 11]. Although in [11] patients were treated up to 21 days, for comparison purposes we only use the treatment data to day 7.
Table 1.
Baseline characteristics.
| Severity of liver disease [n] |
Age [yr] |
HCV genotype [gen1:gen4] |
Baseline HCV [log10 IU/ml] |
|
|---|---|---|---|---|
| Mean (sd) | Chronic hepatitis [12] | 49 (14.74) | 10:2 | 6.39 (0.54) |
| Mean (sd) | Compensated cirrhosis [8] | 57.0 (7.0) | 8:0 | 6.3 (0.44) |
| Mean (sd) | Decompensated cirrhosis [5] | 64.0 (6.0) | 5:0 | 4.72 (0.64) |
| p-value* | 0.53 | 0.006 |
, p-value to reject the null hypothesis that the distribution between the three groups is the same (Kruskal-Wallis test). Individual characteristics and kinetics are shown in Table*p-value for the difference among the three patient groups (Kruskal-Wallis test).
Mathematical modeling
HCV viral kinetics under SIL therapy was assumed to follow the standard biphasic model [12]:
| (Eq. 1) |
where T represents target cells, I, infected cells and V, free virus. Since the analysis focused on 7 days of therapy, we assume the target cell level was constant and represented by the steady state pre-treatment level of T0=cδ/βp. Virus, V, infects target cells with rate constant β, generating infected cells, I, which produce new virions at rate p per infected cell. Infected cells are lost at a rate δ per infected cell and virions are assumed to be cleared at rate c per virion. SIL effectiveness in blocking infection is modeled by a factor (1−η), where η is defined as the drug effectiveness in blocking infection. Similarly, the effect of SIL on viral production and/or secretion from infected cells is modeled by a factor (1−ε(t)), where ε(t) is defined as the effectiveness at time t of drug in preventing viral production/secretion.
We used either a constant effectiveness (CE), i.e., ε(t)=constant, or a time-varying effectiveness (VE), ε(t). When ε(t) is taken to be a constant, this model has been called a constant effectiveness (CE) model [13]. We also considered a VE model where, ε(t) = εmax (1 − e−kt) and the rate of change in treatment effectiveness from 0 to the final effectiveness (εmax) is described by a rate constant k.
Parameters estimation
The effectiveness of SIL in blocking HCV entry, η, and the HCV clearance rate, c, were fixed to 0.6 and 6 d−1 as described previously [10]. The method we used to estimate the transition time from the 1st to the 2nd phase of viral decline is described in the Supplementary Material.
Nonlinear mixed-effect models were used in order to take into account the between-subject variability and to compute individual parameters (see Supplementary Material). Parameters were estimated by likelihood maximization using the SAEM algorithm, implemented in MONOLIX 4.2 (http://www.lixoft.eu/). The Akaike Information Criteria (AIC) is a measure of the relative quality of a model fit to a given dataset. It is possible to increase the likelihood of a model by adding parameters. The AIC penalizes models depending on the number of parameters to satisfy the principle of parsimony. Models were selected after parameter estimation based on AIC, using the rule “the lower the better” [14].
Statistical Methods
We used the nonparametric test for trend (termed here the Trend test) across ordered groups developed by Cuzick [15] to assess the tendency in the data to vary with or inversely with the severity of liver disease. The Wald test was used to determine if the effect of a covariate on a given model parameter was significant. Non-parametric Mann-Whitney U and Kruskal-Wallis tests were used to determine if a difference exists between two and multiple groups, respectively. For all analyses, a P-value of 0.05 was considered as statistically significant.
Results
Baseline characteristics and viral kinetics
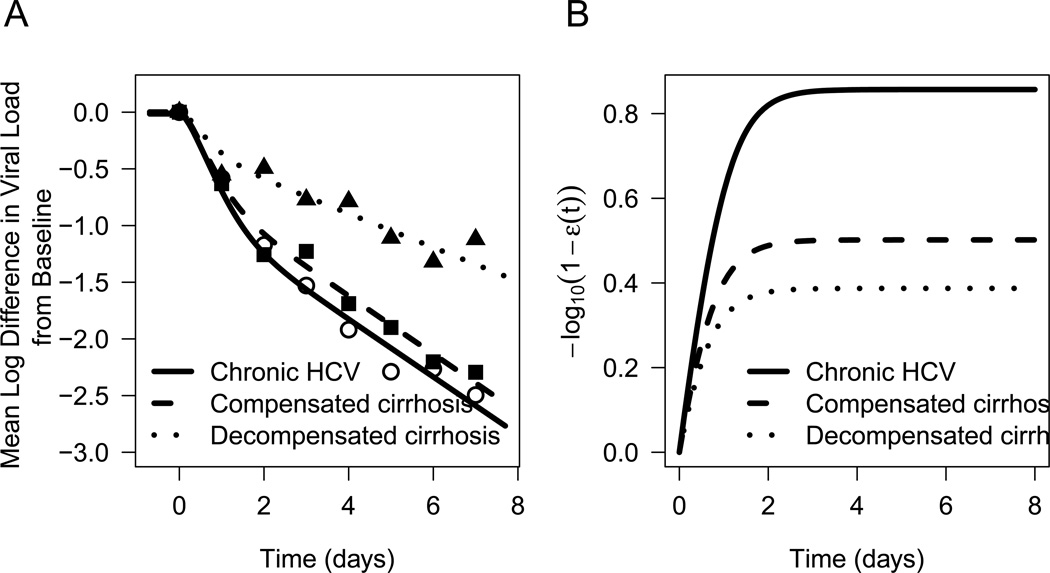
The liver disease significantly varied with age (p<0.0001), with an average age of 64.0 ± 6.0 years in patients with decompensated cirrhosis, 57.0 ± 7.0 years in patients with compensated cirrhosis and 49.0 ± 14.7 years in non-cirrhotic patients (Tables 1 and S1). The baseline viral load varied inversely with liver disease severity (p<0.0001), with V0=4.72 ± 0.64 log10 IU/mL in decompensated cirrhotic patients, V0=6.33 ± 0.44 log10 IU/mL in patients with compensated cirrhosis and V0=6.39 ± 0.54 log10 IU/mL in non-cirrhotic patients (Tables 1 and S1). After therapy was initiated, a biphasic HCV RNA decline was observed in most of the patients (Fig. 1A and Fig. 2).
Figure 1. Viral kinetics and time to reach maximum effectiveness.
A) Predicted viral load decline from baseline for non-cirrhotic patients (solid line), patients with compensated cirrhosis (dashed line) and decompensated cirrhosis (dotted line) as well as measured viral loads in non-cirrhotic patients (open circles), patients with compensated cirrhosis (filled squares) and decompensated cirrhosis (filled triangles). B) Predicted SIL effectiveness for non-cirrhotic patients (solid line), patients with compensated cirrhosis (dashed line) and decompensated cirrhosis (dotted line).
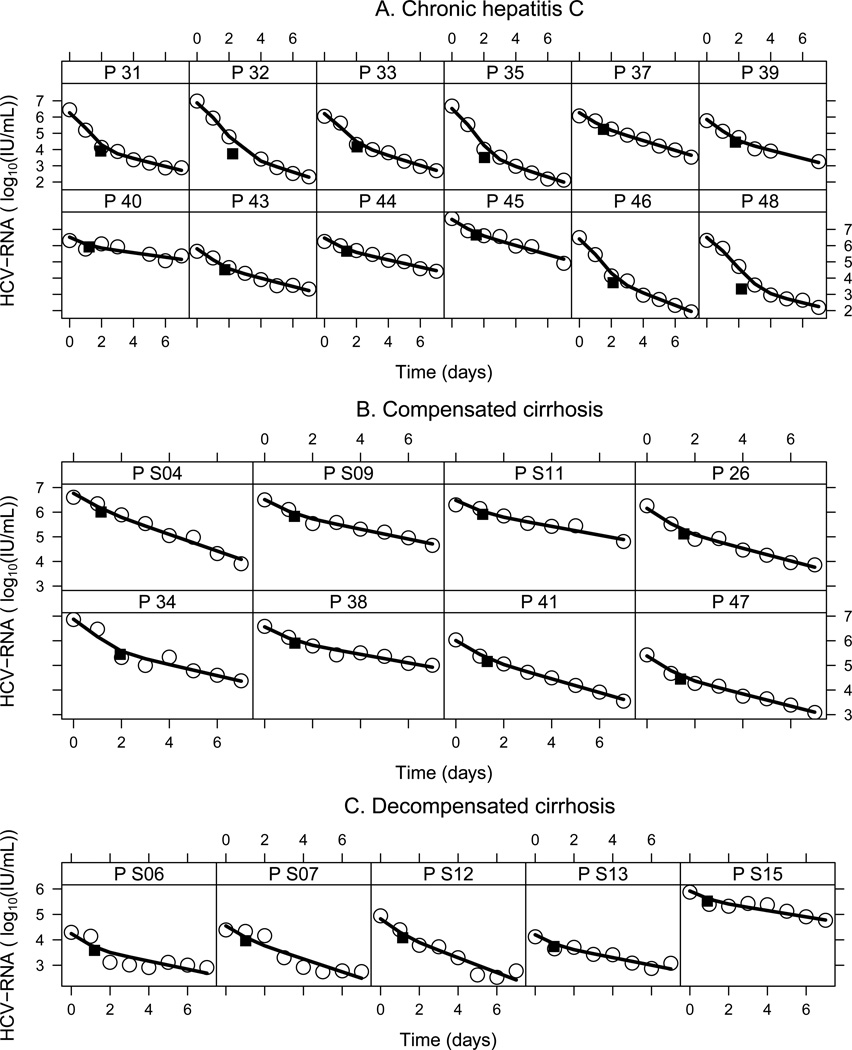
Figure 2.
Viral kinetics of individual patients based on predictions of the mathematical model (Eq. 1) under SIL monotherapy. Best fit curves (solid lines) of the model to the observed HCV RNA kinetic data (open circles) during daily 20 mg/kg/d dosing of SIL. The black square represents the predicted transition time between the first and second phases of viral decline (Table S2). A) Non-cirrhotic group. B) Compensated cirrhosis group. C) Decompensated cirrhosis group.
Mathematical modeling
The varying effectiveness (VE) model of SIL provided a better fit (AIC=117) than the constant-effectiveness model (AIC=132; Fig S1). Therefore, the VE model (Eq. 1), which assumes a gradual increase in SIL effectiveness with treatment time, was used for the remainder of the calculations (Fig. 1A and B and Fig. 2).
The estimated initial viral loads, V0, (Table 2) were consistent with baseline measurements (Table 1). The maximum SIL effectiveness was inversely associated with the severity of liver disease, being lowest in patients with decompensated cirrhosis (εmax=0.59±0.1, p=0.03), intermediate in patients with compensated cirrhosis (εmax=0.69±0.06, p=0.032), and the highest in non-cirrhotic patients (εmax=0.86, s.e.=0.05). The same rate of change of effectiveness (k=2.12 d−1, s.e.=0.18 d−1) was found among the 3 groups (Tables 2 and S2). The transition time between the first and second phase of viral decline was predicted longer in non-cirrhotic patients, occurring at 1.99±0.90 days, than in compensated cirrhotic patients, 1.12±0.25 days, and in decompensated cirrhotic patients, 1.00±0.16 days (Table S2). While the transition time was found significantly different among the 3 groups (p=0.015; a detailed analysis showed that this significance caused only by the difference between the non-cirrhotic group and each other cirrhotic groups), the Trend test indicated that transition time was not associated with the severity of liver disease (p=0.46). Interestingly, we did not find a difference in the infected cell loss rate (δ=0.62 d−1, s.e.=0.05 d−1) among the three groups (Tables 2 and S2).
Table 2.
Population model parameter estimates using the VE model
| Parameter |
V0 [Log10 IU/mL] |
εmax |
k [d−1] |
δ [d−1] |
||||
|---|---|---|---|---|---|---|---|---|
| Severity of liver disease |
Non- cirrhotic |
Compensated cirrhosis |
Decompensated cirrhosis # |
Non- cirrhotic |
Compensated cirrhosis |
Decompensated cirrhosis |
||
| Estimate (Standard error S.E) | 6.4 (0.3) | 6.3 (0.6) | 4.7 (0.8) | 0.861 (0.047) | 0.685 (0.064) | 0.59 (0.098) | 2.12 (0.18) | 0.62 (0.05) |
| - | p=0.68 | p<0.0001 | - | p=0.032 | p=0.03 | |||
| Between-subject variability [%] (SE) | 133 (20) | 16.6 (3.7) | 13.9 (9.7) | 31.8 (6.4) | ||||
V0, HCV baseline viral load; εmax, maximal SIL efficacy; k, rate constant describing the time to reach maximal drug effectiveness, and δ the infected cell loss rate. Individual parameter estimates are shown in Table S2. p, p-value for the difference from the non-cirrhotic group (Wald test).
Based on the predicted transition time the magnitude of the first phase decline and the 2nd phase slope were calculated (Tables 3 and S2). The magnitude of the first phase decline from baseline varied inversely with the disease severity: 1.51 ± 1.07 log10 IU/mL in non-cirrhotic patients, 0.62 ± 0.43 log10 IU/mL in patients with compensated cirrhosis and 0.34 ± 0.22 log10 IU/mL in patients with decompensated cirrhosis (Table 3). However, there was no significant difference in the 2nd phase slopes among the three groups (Table 3), and no significant association (p=0.75, Trend test). Since the 2nd slope is approximately δεmax, the slower mean 2nd phase slopes observed in compensated and decompensated cirrhotic patients (1.81±0.53 and 1.31±0.59 log10 IU/ml/wk, respectively) compared to non-cirrhotic patients (1.91±0.62 IU/ml/wk) reflect the association of εmax and the severity of liver disease.
Table 3.
Predicted viral kinetic characteristics
| Severity of liver disease |
Transition time [d] |
1st phase decline from baseline [log10 IU/mL] |
2nd phase slope [log10/wk] |
|
|---|---|---|---|---|
| Mean (sd) | Non-cirrhotic [n=12] | 1.99 (0.90) | 1.51 (1.07) | 1.91 (0.62) |
| Mean (sd) | Compensated cirrhosis [n=8] | 1.12 (0.16) | 0.62 (0.43) | 1.81 (0.53) |
| Mean (sd) | Decompensated cirrhosis [n=5] | 1.00 (0.16) | 0.34 (0.22) | 1.31 (0.59) |
| p-value* | 0.015 | 0.042 | 0.24 |
We performed a sensitivity analysis by estimating the parameters only in patients with genotype 1, i.e., excluding 2 subjects infected with HCV genotype 4. In general, similar results were obtained with or without the 2 patients with genotype-4. However, we did not find that εmax was significantly higher in patients with compensated cirrhosis than in non-cirrhotic patients (Table S3), probably due to the lack of power associated with a smaller number of subjects.
Discussion
This study provides a unique viral kinetic comparison of patients with different severity of liver disease. The findings are made even more interesting by the use of a daily IV infusion of 20 mg/ml/kg of Legalon SIL to treat hepatitis C in patients with decompensated cirrhosis who were awaiting LT. Modeling results show that SIL had a significantly higher effectiveness in non-cirrhotic patients than in those with compensated and decompensated cirrhosis and predict that the HCV-infected cells loss rate is high and similar among patients with different severity of liver disease.
A significantly (p<0.0001) lower baseline viral load in patients with decompensated cirrhosis (4.72±0.64 log10 IU/ml) was found compared to the compensated cirrhosis patients (6.33±0.44 log10 IU/ml) and non-cirrhotic patients (6.39±0.54 log10 IU/ml). In order to verify that this finding is not due to the small sample size (i.e., n=5) of the group with decompensated cirrhosis, we included the baseline HCV levels of 14 patients with decompensated cirrhosis and 12 patients with compensated cirrhosis from our previous study in patients who underwent LT [16]. The analysis shows that patients with compensated cirrhosis had significantly (p=0.0001) higher baseline viral load compared to decompensated patients (6.0±0.5 vs. 5.2±0.7 log10 IU/ml). The reason for the lower baseline viral load in patients with decompensated cirrhosis is not known, but might in part reflect reduced viral production due to excessive loss of hepatocytes in advanced cirrhosis [17] with a higher infection rate [18].
Modeling results suggest that SIL’s maximum effectiveness in blocking HCV production and/or release and the time to reach SIL maximal effectiveness varied inversely with the severity of liver disease (Fig. 1B). This might be partially explained by the presence of a functional intrahepatic shunt due to sinusoidal capillarization in patients with advanced cirrhosis, which could lead to a lower drug delivery to hepatocytes [17].
While the first phase of viral decline was significantly associated with the severity of liver disease, the loss rate of HCV-infected cells was high and similar between groups (δ=0.62/day which translates into an average viral load decline of ~1.88±0.15 log10 IU/ml/wk) indicating that SIL has favorable antiviral activity in HCV infected patients with compensated and decompensated cirrhosis. Interestingly, an approximately similar rapid 2nd slope was observed with DAA-based therapies with high effectiveness in blocking HCV production/release (i.e., ~4-fold higher than with pegIFN/RBV alone) [8, 19, 20]. The origin of the high loss rate of HCV-infected cells, δ, seen with SIL is not known. Possibilities include an immunomodulatory effect of SIL [21], and/or an effect of SIL in increasing the loss rate of intracellular viral RNA [22] and/or in blocking hepatocyte proliferation [23]. Further studies are needed to elucidate the high apparent HCV-infected cell loss rate, δ, regardless of the severity of liver disease.
A third phase corresponding to a viral plateau (or extremely slow decline) was previously observed in most of the patients with decompensated cirrhosis studied here who were treated with SIL for 14 to 21 days [11]. The beginning of the third phase may be observed between days 4 and 7 in three of the decompensated cirrhotic patients, i.e., S06, S07 and S12 [Fig. 2C] [11]. The final plateau phase suggests that viral load reached a new set point, in spite of a high loss rate, δ, of HCV-infected cells [18, 23]. If one fit the standard CE or VE models to viral kinetic data with a new set point i.e., viral plateau (or extremely slow decline) it may unrealistically suggest that the infected cell loss rate is close to 0. To model viral kinetic patterns with a plateau, as seen under SIL beyond day 7, more complex models will be needed, such as those that include hepatocyte proliferation [24], as we previously showed that the final plateau phase does not necessarily indicate a low HCV-infected loss rate, δ [23]. Similar to the findings of the current study, a model that included hepatocyte proliferation predicted a high δ in patients awaiting LT, in whom a final plateau (or extremely slow decline) phase was seen beyond day 7 [25] (manuscript in preparation).
The management of recurrent HCV infection after LT is a major challenge. Antiviral treatment with pegIFN and ribavirin can be effective in up to 25% of carefully selected patients awaiting LT, but such treatment is limited due to safety and tolerability concerns [26]. Interestingly, treatment with sofosbuvir (an HCV polymerase inhibitor) and ribavirin in patients awaiting-LT patients with hepatocellular carcinoma (Child A score) was safe and prevented post-transplant HCV recurrence in 69% of patients who achieved HCV RNA <25 IU/mL at the end of treatment and prior to transplant [27].
SIL treatment during the peri-transplant setting and/or immediately after graft reperfusion in 2 patients with baseline viral load below 4.5 log10 UI/ml was reported to be safe and prevented HCV infection of the graft [28, 29]. Administration of SIL during the anhepatic phase and immediately after reperfusion might further prevent viral infection of the graft by blocking HCV entry [30]. Very recently, IFN-free treatment with SIL+ribavirin with on treatment mathematical modeling of viral kinetics for individualized duration of therapy was recently performed in a non-cirrhotic previous non-responder to pegylated-IFN and ribavirin. Based on viral kinetic data obtained during the first 4 weeks of therapy the model predicted 34 weeks of therapy to achieve cure. Provided with this information, the patient accepted to be treated for 34 weeks and achieved SVR (Dahari et al. in preparation). This proof-of-concept case suggests that sufficient duration of SIL-based therapy can achieve clearance of HCV. In decompensated cirrhotic patients, in whom a viral plateau (or extremely slower decline) was observed under SIL monotherapy [11], a higher SIL dose (i.e., >20 mg/kg/day) might give rise to a continuous decline in viral load and with sufficient duration to cure.
In conclusion, the effectiveness of 20 mg/kg/day of SIL in blocking HCV production and/or release was inversely associated with increasing severity of liver disease. Nevertheless, the 2nd phase slope (or the loss rate of infected cells) with SIL was similar in non-cirrhotic, compensated, and decompensated cirrhosis (~1.9 log10 IU/ml/wk). This high infected cell loss rate indicates that SIL therapy has favorable antiviral activity in patients with hepatitis C including those with decompensated cirrhosis and that sufficient dose and duration of SIL might suppress viral replication and reduce the risk of graft infection post-LT.
Supplementary Material
Acknowledgement statement
We thank Steve Polyak (University of Washington) for discussions and insights. Portions of this work were performed under the auspices of the US Department of Energy under contract DE-AC52-06NA25396, and supported by NIH grants R01-AI07881, P20-GM103452, R01-AI028433 and R01-OD011095.
MD is a Madaus–Rottapharm employee (the firm producing Legalon SIL). PF is a member of the global advisory board of Madaus–Rottapharm. LC and HD have received partial travel support from Madaus–Rottapharm to attend scientific meetings.
Footnotes
Disclosure statement
Other authors have nothing to disclose.
References
- 1.Kwong JC, Ratnasingham S, Campitelli MA, et al. The impact of infection on population health: results of the Ontario burden of infectious diseases study. PloS one. 2012;7:e44103. doi: 10.1371/journal.pone.0044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatzakis A, Wait S, Bruix J, et al. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference*. J Viral Hepat. 2011;18(Suppl 1):1–16. doi: 10.1111/j.1365-2893.2011.01499.x. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Retortillo M, Forns X, Feliu A, et al. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680–687. doi: 10.1053/jhep.2002.31773. [DOI] [PubMed] [Google Scholar]
- 4.Dugum M, O'Shea R. Hepatitis C virus: Here comes all-oral treatment. Cleve Clin J Med. 2014;81:159–172. doi: 10.3949/ccjm.81a.13155. [DOI] [PubMed] [Google Scholar]
- 5.Hézode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC)– NCT01514890. J Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 6.Carrion JA, Martinez-Bauer E, Crespo G, et al. Antiviral therapy increases the risk of bacterial infections in HCV-infected cirrhotic patients awaiting liver transplantation: A retrospective study. J Hepatol. 2009;50:719–728. doi: 10.1016/j.jhep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 7.Iacobellis A, Siciliano M, Perri F, et al. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206–212. doi: 10.1016/j.jhep.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Guedj H, Guedj J, Negro F, et al. The impact of fibrosis and steatosis on early viral kinetics in HCV genotype 1–infected patients treated with Peg-IFN-alfa-2a and ribavirin. J Viral Hepat. 2012;19:488–496. doi: 10.1111/j.1365-2893.2011.01569.x. [DOI] [PubMed] [Google Scholar]
- 9.Ferenci P, Scherzer TM, Kerschner H, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 10.Guedj J, Dahari H, Pohl RT, Ferenci P, Perelson AS. Understanding silibinin's modes of action against HCV using viral kinetic modeling. J Hepatol. 2012;56:1019–1024. doi: 10.1016/j.jhep.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marino Z, Crespo G, D'Amato M, et al. Intravenous silibinin monotherapy shows significant antiviral activity in HCV-infected patients in the peri-transplantation period. J Hepatol. 2013;58:415–420. doi: 10.1016/j.jhep.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 12.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 13.Shudo E, Ribeiro RM, Perelson AS. Modeling HCV kinetics under therapy using PK and PD information. Expert Opin Drug Metab Toxicol. 2009;5:321–332. doi: 10.1517/17425250902787616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akaike H. A new look at the statistical model identification. IEEE T Automat Contr. 1974;19:716–723. [Google Scholar]
- 15.Cuzick J. A Wilcoxon type test for trend. Stat Med. 1985;4:543–547. doi: 10.1002/sim.4780040416. [DOI] [PubMed] [Google Scholar]
- 16.Dahari H, Feliu A, Garcia-Retortillo M, Forns X, Neumann AU. Second hepatitis C replication compartment indicated by viral dynamics during liver transplantation. J Hepatol. 2005;42:491–498. doi: 10.1016/j.jhep.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Wolf D. Clinical methods: The history, physical, and laboratory examination. City: Butterworths; 1990. Evaluation of the size, shape, and consistency of the liver; pp. 478–481. [Google Scholar]
- 18.Dahari H, Layden–Almer JE, Kallwitz E, et al. A mathematical model of hepatitis C virus dynamics in patients with high baseline viral loads or advanced liver disease. Gastroenterology. 2009;136:1402–1409. doi: 10.1053/j.gastro.2008.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adiwijaya BS, Kieffer TL, Henshaw J, et al. A viral dynamic model for treatment regimens with direct-acting antivirals for chronic hepatitis C infection. PLoS Comput Biol. 2012;8:e1002339. doi: 10.1371/journal.pcbi.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rong L, Guedj J, Dahari H, et al. Analysis of hepatitis C virus decline during treatment with the protease inhibitor danoprevir using a multiscale model. PLoS Comput Biol. 2013;9:e1002959. doi: 10.1371/journal.pcbi.1002959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer K, Myers R, Lee S. Silymarin treatment of viral hepatitis: a systematic review. J Viral Hepat. 2005;12:559–567. doi: 10.1111/j.1365-2893.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 22.Guedj J, Neumann A. Understanding hepatitis C viral dynamics with direct-acting antiviral agents due to the interplay between intracellular replication and cellular infection dynamics. J Theor Biol. 2010;267:330–340. doi: 10.1016/j.jtbi.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Dahari H, Shudo E, Cotler SJ, Layden TJ, Perelson AS. Modelling hepatitis C virus kinetics: the relationship between the infected cell loss rate and the final slope of viral decay. Antivir Ther. 2009;14:459–464. [PMC free article] [PubMed] [Google Scholar]
- 24.Dahari H, Lo A, Ribeiro RM, Perelson AS. Modeling hepatitis C virus dynamics: Liver regeneration and critical drug efficacy. J Theor Biol. 2007;247:371–381. doi: 10.1016/j.jtbi.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DebRoy S, Mariño Z, Crespo G, et al. Modeling HCV kinetics during intravenous silibinin monotherapy in the peri-transplant period. J Hepatol. 2013;58:S330–S331. doi: 10.1016/j.jhep.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 26.Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57:1752–1762. doi: 10.1002/hep.25976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curry MP, Forns X, Chung RT, et al. Pretransplant Sofosbuvir and Ribavirin to Prevent Recurrence of HCV Infection after Liver Transplantation. Hepatology. 2013;58(Suppl S1):86A. doi: 10.1053/j.gastro.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 28.Neumann UP, Biermer M, Eurich D, Neuhaus P, Berg T. Successful prevention of hepatitis C virus (HCV) liver graft reinfection by silibinin mono-therapy. J Hepatol. 2010;52:951–952. doi: 10.1016/j.jhep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Beinhardt S, Rasoul-Rockenschaub S, Scherzer TM, Ferenci P. Silibinin monotherapy prevents graft infection after orthotopic liver transplantation in a patient with chronic hepatitis C. J Hepatol. 2011;54:591–592. doi: 10.1016/j.jhep.2010.09.009. author reply 592–593. [DOI] [PubMed] [Google Scholar]
- 30.Blaising J, Lévy PL, Gondeau C, et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol. 2013 doi: 10.1111/cmi.12155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.