Abstract
The finding that smoking is inversely correlated with Parkinson's disease and that nicotine attenuates nigrostriatal damage in parkinsonian animals supports the idea that nicotine may be neuroprotective. Nicotine is thought to exert this effect by acting at nicotinic receptors (nAChRs), including the α7 subtype. The objective of this study was twofold: first, to test the protective potential of ABT-107, an agonist with high selectivity for α7 nAChRs; and second, to investigate its cellular mechanism of action. Rats were implanted with minipumps containing ABT-107 (0.25 mg/kg/d). In addition, we tested the effect of nicotine (1 mg/kg/d) as a positive control, and also DMXB (2 mg/kg/d) which acts primarily with α7 but also α4β2* nAChRs. Two wk after minipump placement, the rats were lesioned by unilateral administration of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle. Lesioning alone decreased contralateral forelimb use and adjusted stepping, two measures of parkinsonism. ABT-107 and nicotine treatment significantly improved these behaviors at all wk tested, with variable improvement with DMXB. We next investigated the cellular mechanism involved. The striatal dopamine transporter (DAT), a marker of dopaminergic integrity, was reduced ~70% with lesioning. ABT-107 or nicotine treatment significantly increased DAT levels in lesioned striatum; these drugs did not alter DAT levels in intact striatum. ABT-107 and nicotine also significantly improved basal dopamine release from lesioned striatum, as well as nicotine-stimulated dopamine release mediated via α4β2* and α6β2* nAChRs. These data suggest that α7 nAChR agonists may improve motor behaviors associated with nigrostriatal damage by enhancing striatal dopaminergic function.
Keywords: ABT-107, DMXB, neuroprotection, nicotine, nicotinic receptors, Parkinson's disease
Introduction
Parkinson’s disease is a devastating neurological disorder, characterized by rigidity, tremor and bradykinesia, as well as autonomic deficits, sleep disturbances, and dementia in the latter stages (Hirsch et al., 2013; Kieburtz and Wunderle, 2013; Olanow and Schapira, 2013; Sprenger and Poewe, 2013; Sulzer and Surmeier, 2013). Pathologically there is a primary loss of nigrostriatal dopaminergic neurons, as well as other deficits throughout the brain. Currently marketed therapeutics for Parkinson’s disease are symptomatic only, with an inevitable disease progression. There is thus a critical need for novel therapeutics, preferably drugs that slow or halt Parkinson’s disease progression.
A large pre-clinical literature suggests that protection against nigrostriatal damage may be achieved via numerous approaches, including stimulation of nAChRs (Hickey and Stacy, 2013; Kerr, 2010; Marques-Aleixo et al., 2012; Pascual et al., 2011; Quik et al., 2012; Smeyne and Smeyne, 2013; Sutachan et al., 2012; Wirdefeldt et al., 2011). Initial evidence for a role for the nicotinic cholinergic system stemmed from epidemiologic studies, which showed a decreased incidence of Parkinson’s disease in smokers (Fahn, 2010; Nicoletti et al., 2010; Tanner, 2010). Work dating back to the 1960’s demonstrated an overall 50% decline in the risk for Parkinson’s disease in tobacco users. This inverse relationship is reproducible, dose related and not due to selective mortality (Chen et al., 2010; Gorell et al., 1999; Morens et al., 1995; Ritz et al., 2007; Thacker et al., 2007; Wirdefeldt et al., 2011). Studies in cultured cells and in parkinsonian animal models showed that nicotine is neuroprotective against a wide variety of toxic insults including dopamine neuron degeneration, suggesting that the nicotine in tobacco may underlie the reduced incidence of Parkinson’s disease in smokers (Picciotto and Zoli, 2008; Quik et al., 2012). Indeed, a Michael J Fox Foundation funded trial is in progress to evaluate the protective potential of nicotine in early Parkinson's disease.
Neuronal nAChRs in mammalian systems are pentameric ion channels composed of varying combinations of different α (α2 to α7) and β (β2 to β4) subunits, with the α subunit expressing the acetylcholine recognition site (Millar and Gotti, 2009; Quik and Wonnacott, 2011). The predominant nAChRs in the CNS are the β2* and α7 nAChR populations (Millar and Gotti, 2009; Quik and Wonnacott, 2011). The asterisk indicates the possible presence of other subunits in the receptor complex. β2* nAChRs are selectively present in the brain. α7 nAChRs are present in both the peripheral and central nervous system; however, α7 nAChR drugs have no appreciable functional effects in the peripheral nervous system (Freedman et al., 2008; Othman et al., 2011). Thus β2* and α7 nAChRs represent potential targets for the development of neuroprotective therapies for Parkinson’s disease.
We focused on a role for α7 nAChRs based on accumulating evidence showing that these receptors play a role in protection against a wide variety of toxic insults in culture and in vivo. For instance, nicotine protects against glutamate-, ethanol-, NMDA-, oxygen-deprivation- and β-amyloid-induced cytotoxicity in different neuronal culture systems via α7 nAChRs (Dajas-Bailador et al., 2000; de Fiebre and de Fiebre, 2003; Kaneko et al., 1997; Li et al., 2000; Rosa et al., 2006; Toulorge et al., 2011; Wang et al., 2000). In addition, work in parkinsonian animal models show that drugs such as the α7 nAChR allosteric modulator galantamine or the relatively selective α7 agonist DMXB protect against 6-OHDA-induced nigrostriatal damage in rats (Suzuki et al., 2013; Yanagida et al., 2008), while the α7 agonist PNU-282987 attenuated MPTP-induced nigrostriatal damage in mice (Stuckenholz et al., 2013).
For the present experiments, we investigated the α7 nAChR agonist ABT-107 because it interacts selectively with α7 nAChRs and has shown to be neuroprotective against glutamate neurotoxicity in culture (Malysz et al., 2010). Additionally, α7 nAChR drugs have been used in clinical trials and appear safe and well tolerated (Freedman et al., 2008; Othman et al., 2011). The present results show that ABT-107 improved striatal dopaminergic function and that this is associated with a reduction in motor deficits in 6-OHDA-lesioned rat. These combined findings suggest that α7 nAChR agonists may be a useful therapy in Parkinson's disease.
Materials and methods
Animals
Male Sprague-Dawley rats (120–140 g) were purchased from Charles River (Livermore, CA). They were housed 3–4 per cage under a 12/12 hr light-dark cycle in a temperature-controlled room, with free access to food and water.
nAChR agonist treatment
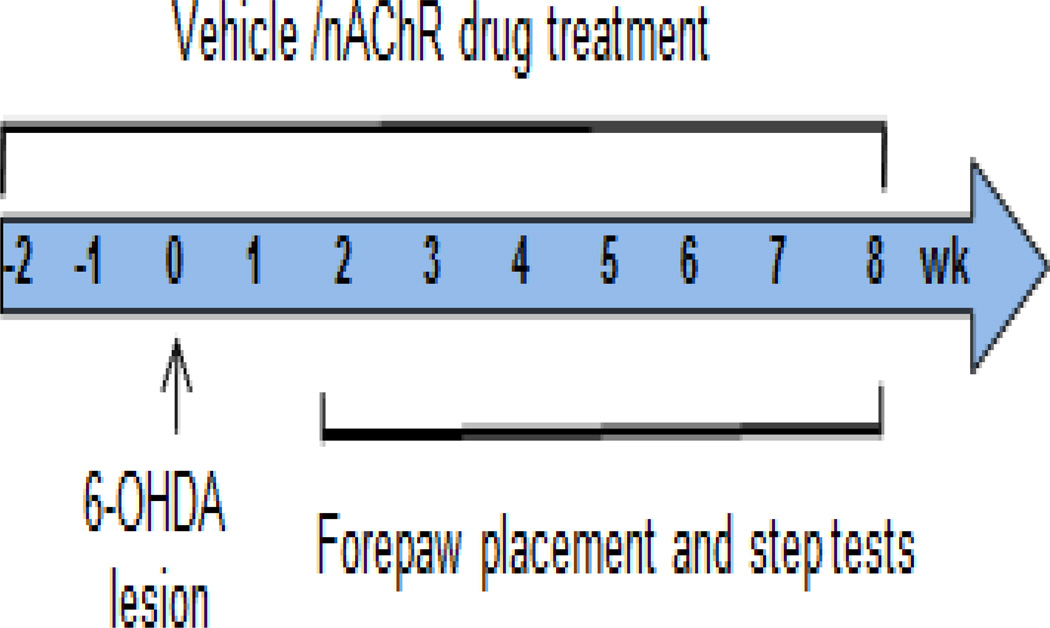
In study 1, rats were surgically implanted with osmotic minipumps (Fig. 1) (Alzet model 2006, delivery rate: 0.15 µl/hr; Durect Corporation, Cupertino, CA) containing vehicle or nicotine (1 mg/kg/d free base, Sigma-Aldrich Co., St. Louis, MO). The rats were euthanized 8 wk after lesioning with depleted minipumps replaced as necessary.
Fig. 1.
NAChR agonist treatment timeline of 6-OHDA-lesioned rats. Rats were pretreated with vehicle or nAChR agonists via an osmotic minipump and lesioned 2 wk later as detailed in Materials and Methods. The forepaw placement and adjusted stepping test were done 2 to 8 wk after lesioning to determine the extent of motor deficits.
In study 2, rats were implanted with minipumps containing vehicle, ABT-107 (0.25 mg/kg/d, AbbVie Inc. North Chicago, IL) or DMXB (2 mg/kg/day, from R.L. Papke). The ABT-107-treated and DMXB-treated rats were euthanized 8 and 4 wk after lesioning, respectively, with depleted minipumps replaced as necessary.
6-OHDA lesioning
Two wk after minipump implantation, rats were unilaterally lesioned with 6-OHDA (Sigma-Aldrich, St. Louis, MO) under isofluorane anesthesia, as previously described (Bordia et al., 2008; Bordia et al., 2010; Cenci and Lundblad, 2007). 6-OHDA was dissolved in 0.02% ascorbic acid/saline at a concentration of 1.5 µg free base/µl. A 2 µl aliquot was stereotaxically injected into the medial forebrain bundle at the following coordinates relative to bregma and the dural surface: 1) anteroposterior bregma, −4.4; lateral midline, 1.2; dorsoventral dura, 7.8; tooth bar at −2.4; and 2) anteroposterior bregma, −4.0; lateral midline, 0.75; dorsoventral dura, 8.0; tooth bar at +3.4. The cannula was lowered into the injection site over a 2 min period. 6-OHDA was then infused over a 2 min period with the cannula maintained at the site of injection for an additional 2 min prior to removal, which was also done over 2 min. After surgery, the rats were given buprenorphine (0.02 mg/kg sc) for postoperative pain. All procedures conformed to the Guide for the Care and Use of Laboratory Animals and were approved by SRI Institutional Animal Care and Use Committee.
Assessment of motor impairment
The severity of 6-OHDA-induced motor impairment was assessed 2 to 8 wk after 6-OHDA lesioning, using two behavioral tests: the forepaw placement or cylinder test and the adjusted stepping test (Schallert et al., 2000; Bordia et al., 2008; Bordia et al., 2010). The forepaw placement test was performed by individually placing animals in a transparent, plexiglass cylinder. The number of upward contacts during exploratory rearing was then counted for 5 min for both forelimbs by a blinded rater. The use of the paw contralateral to the lesion was assessed as a percent of total contacts made, as previously described (Bordia et al. 2008, Bordia et al. 2010). The step test, another measure of contralateral forelimb use, was performed on the same day as the forepaw placement test, as described (Schallert et al., 1982, Chang et al., 1999, Fleming et al., 2012). The rat was held with the torso slightly raised and the hind limbs and one forelimb gently tucked. The rat was lifted above the table such that the weight of the body was supported by one forelimb alone. The rat was then moved slowly sideways with the forelimb bearing weight in the backhand and then forehand direction across a ~90 cm distance in ~5 sec. The number of steps made in each direction was recorded for both forelimbs. Three trials were performed and then averaged. The use of the paw contralateral to the lesion in the forehand direction was expressed as the percent of total forehand steps.
Tissue preparation
The rats were killed by decapitation using a guillotine. Brains were promptly removed and placed on ice. They were then bisected coronally at a mid-striatal level, with the anterior striatum used for 3H-dopamine release and the striatum from the caudal portion frozen in isopentane on dry ice and stored at −80°C. The caudal portion was later cut into 8 µm sections at −15 to −20°C in a cryostat (Leica Microsystems Inc., Deerfield, IL). The sections were thaw mounted onto poly-L-lysine coated slides, dried and stored at −80°C until use.
DAT autoradiography
Striatal DAT binding was performed using 125I-RTI-121 (specific activity, 2200 Ci/mmol; PerkinElmer Life and Analytical Sciences, Waltham, MA) as described (Quik et al. 2003). The sections were preincubated at 22°C for two 15 min periods in buffer containing 50 mM Tris-HCl, pH 7.4, 120 mM NaCl, and 5 mM KCl. Next, the sections were incubated for 2 h in the same buffer also containing 0.025% bovine serum albumin (BSA), 1 µM fluoxetine, and 50 pM 125I-RTI-121. Nonspecific binding was determined in the presence of the uptake inhibitor nomifensine (100 µM). Afterwards, slides were washed four times for 15 min in ice-cold buffer, once for 10 s in ice-cold water and air dried. After drying, the slides were exposed to Kodak MR Film (Easterman Kodak Co., Rochester, NY) for several days along with 125I-microscale standards (American Radiolabeled chemicals, Inc., Saint Louis, MO).
Nicotine-evoked 3H-dopamine release
Nicotine-evoked 3H-dopamine release was performed as described (Bordia et al., 2013b; Quik et al., 2003). Approximately 5 mg (wet weight) of the intact and lesioned striatum was homogenized in 2 ml of ice-cold 0.32 M sucrose buffered with 5.0 M HEPES (pH 7.5) and centrifuged at 12,000 g at 4°C for 15 min. The pellets obtained were re-suspended in 0.8 ml of uptake buffer (128 mM NaCl, 2.4 mM KCl, 3.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES, pH 7.5, 10 mM glucose, 1 mM ascorbic acid, 0.01 mM pargyline) and incubated for 10 min at 37°C. 3H-Dopamine (100 nM, specific activity, 30–60 Ci/mmol; PerkinElmer Life and Analytical Sciences, Waltham, MA) was then added to the synaptosomes, followed by a 5 min incubation period. Next, 80 µl of re-suspended synaptosomes were pipetted onto glass-fiber filters mounted on polypropylene platforms and perfused for 10 min with perfusion buffer (uptake buffer with 0.1% BSA and 10 µM nomifensine). 3H-Dopamine release was evoked by an 18 s exposure to 1 µM (sub-maximal) and 10 µM (maximal) nicotine concentrations. A separate set of filters were perfused with 50 nM α-CtxMII for 3 min prior to nicotine stimulation to determine the α4β2* and α6β2* nAChR-mediated components of total release. A total of twelve 18 s fractions were collected, which included basal release before and after stimulation. Radioactivity was counted using a liquid scintillation counter. The filters were dissolved in organic solvent and radioactivity also counted as a measure of 3H-dopamine uptake into synaptosomes.
Data analyses
Autoradiographic images were quantitated to determine the optical density values using the ImageQuant system (GE Healthcare (Chalfront, St. Giles, Buckinghamshire, UK). Background tissue values were subtracted from total tissue binding to evaluate specific binding of the radioligands. Specific binding values were then converted to fmol/mg tissue using standard curves generated from the 125I-microscale standards. The sample OD readings were within the linear range of the standards. Data from the 3H-dopamine release were analyzed using the curve-fitting algorithm of SigmaPlot (Systat Software Inc, San Jose, CA), as previously described (Bordia et al., 2013b; McCallum et al., 2005). Statistical comparisons were done with GraphPad Prism (GraphPad Software Inc, San Diego, CA) using student’s t-test or ANOVA followed by a Dunnett or Bonferroni post hoc test. A value of p = 0.05 was considered statistically significant. Values are expressed as the mean ± S.E.M. of the indicated number of rats.
Results
α7 nAChR agonist treatment improve 6-OHDA-induced motor deficits
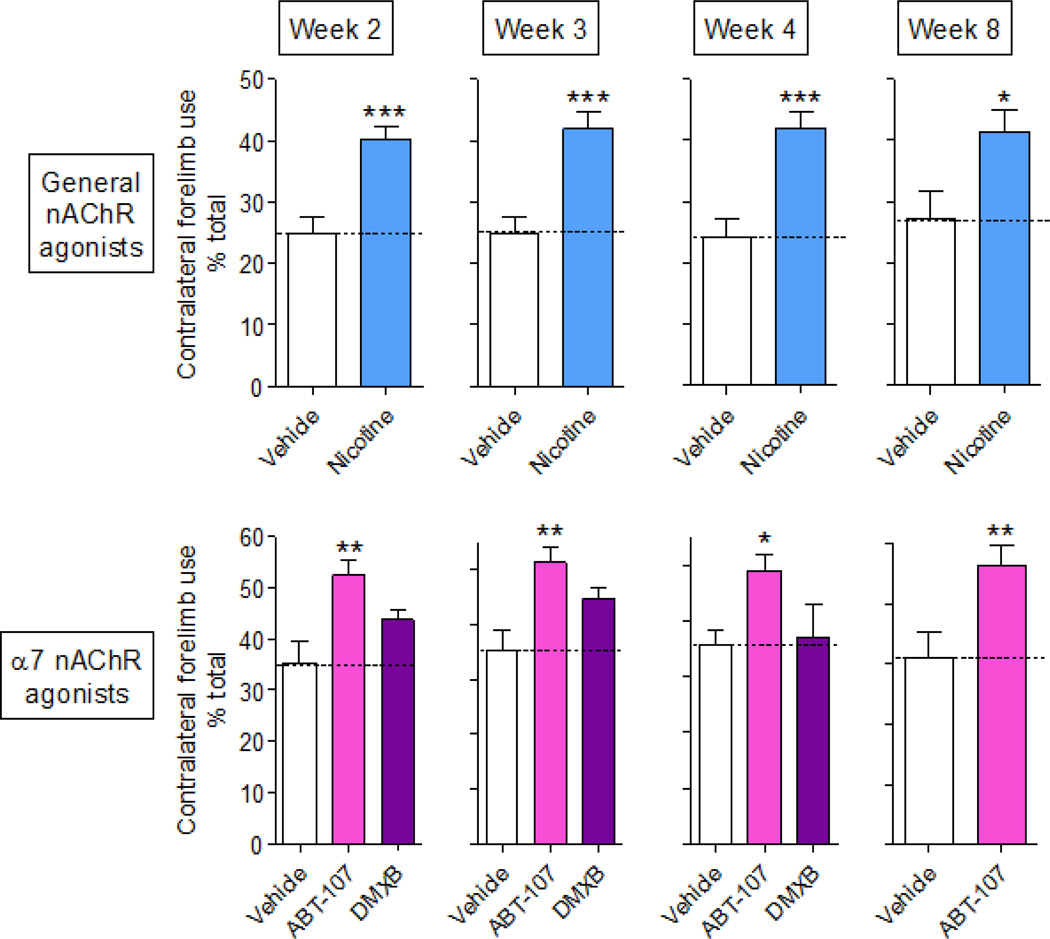
The forepaw placement or cylinder test was used to measure 6-OHDA-induced motor impairment during the rat’s natural exploratory activity, according to the timeline depicted in Fig. 1. Contralateral forepaw use was reduced in 6-OHDA lesioned vehicle-treated rats 2 to 8 wk after lesioning (Fig. 2). In study 1 (Fig. 2, top panel), nicotine (1 mg/kg/d) treatment significantly improved forelimb use from 25% to 40% of total limb use at 2 wk after lesioning, with similar results at wk 3, 4 and 8. In study 2 (Fig. 2 bottom panel), rats receiving the α7 nAChR agonist ABT-107 (0.25 mg/kg/d) also exhibited improved motor function compared to the vehicle-treated lesioned group, with contralateral forelimb improved from 35% to 50% of total limb use at 2 through 8 wk after lesioning. Motor function in rats treated with the α7 nAChR agonist DMXB did not differ significantly from vehicle over the 4 wk period tested.
Fig. 2.
α7 nAChR agonist treatment improves forepaw placement in lesioned rats. Rats pretreated with vehicle or nAChR agonists via an osmotic minipump were rendered parkinsonian by unilateral 6-OHDA treatment. Two to 8 wk post-lesion, rats were tested for deficits in the use of their contralateral limb using the forepaw placement or cylinder test. Top panel, the effect of nicotine (1 mg/kg/d) (Study 1). Bottom panel, effect of the α7 nAChR agonists ABT-107 (0.25 mg/kg/d) and DMXB (2 mg/kg/d) (Study 2). Values are the mean ± SEM of 12–25 rats per group. Significance of difference from the vehicle-treated group, *p < 0.5; **p < 0.01; ***p < 0.001.
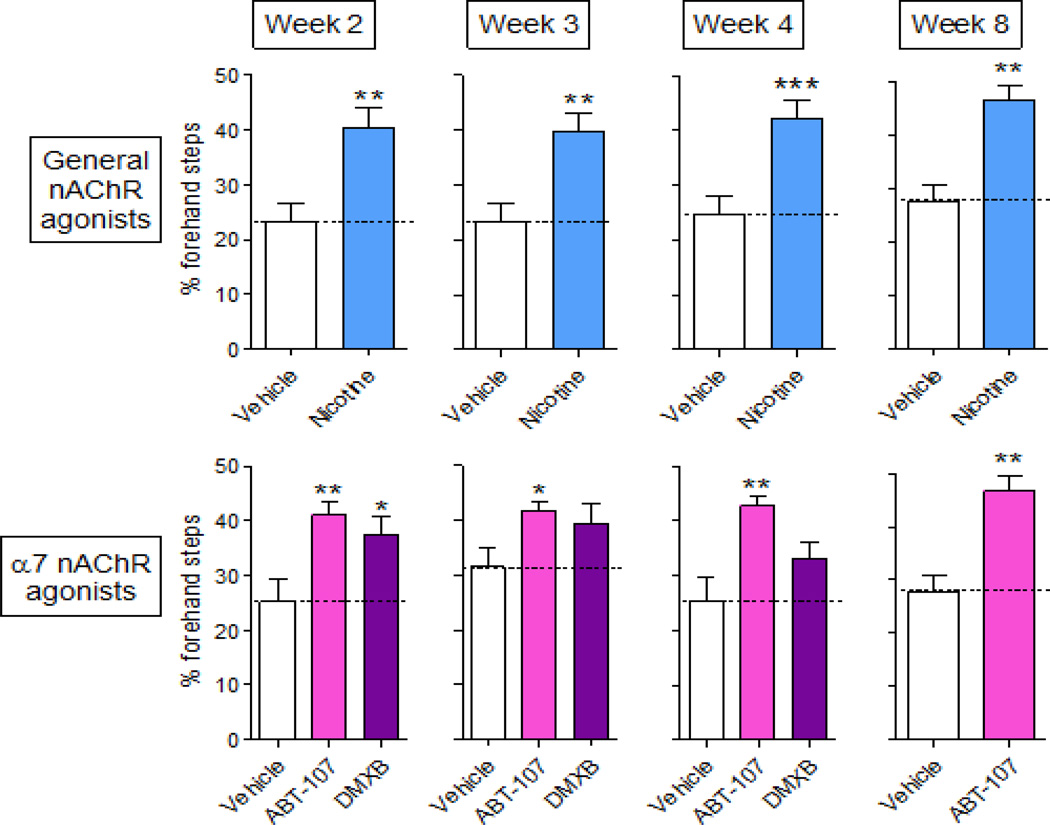
In addition to the forepaw placement test, we also performed the adjusted stepping test. At 2 through 8 wk, lesioning reduced the number of adjusted contralateral forehand steps in vehicle-treated rats (Fig. 3). In study 1 (Fig. 3, top panel), rats treated with nicotine exhibited a significantly greater number of contralateral steps (40 to 47% of total) at wk 2 to 8. In study 2 (Fig. 3, bottom panel), treatment with the α7 nAChR agonist ABT-107 also significantly improved deficits in forehand steps, with contralateral steps averaging ~44% of total. The DMXB-mediated improvement in the stepping test was significantly higher at wk 2 with no increase at wk 3 and 4.
Fig. 3.
α7 nAChR agonist treatment improves contralateral forepaw use in the adjusted stepping test in lesioned rats. Rats treated with vehicle or a nAChR agonist via minipump were rendered parkinsonian by unilateral injection of 6-OHDA. Two to 8 wk post-lesion, they were tested for postural instability using the adjusted stepping test. The number of forehand adjustment steps made by each forelimb as the animal was moved laterally across the table top was counted. Top panel, the effect of nicotine (1 mg/kg/d) (Study 1). Bottom panel, effect of α7 nAChR agonists ABT-107 (0.25 mg/kg/d) and DMXB (2 mg/kg/d) (Study 2). Values are the mean ± SEM of 12–25 rats per group. Significance of difference from the vehicle-treated group, *p < 0.5; **p < 0.01; ***p < 0.001.
Since DMXB did not significantly improve contralateral forepaw and improved adjusted stepping at wk 2 but not wk 3 and 4, further studies were only conducted with nicotine and ABT-107.
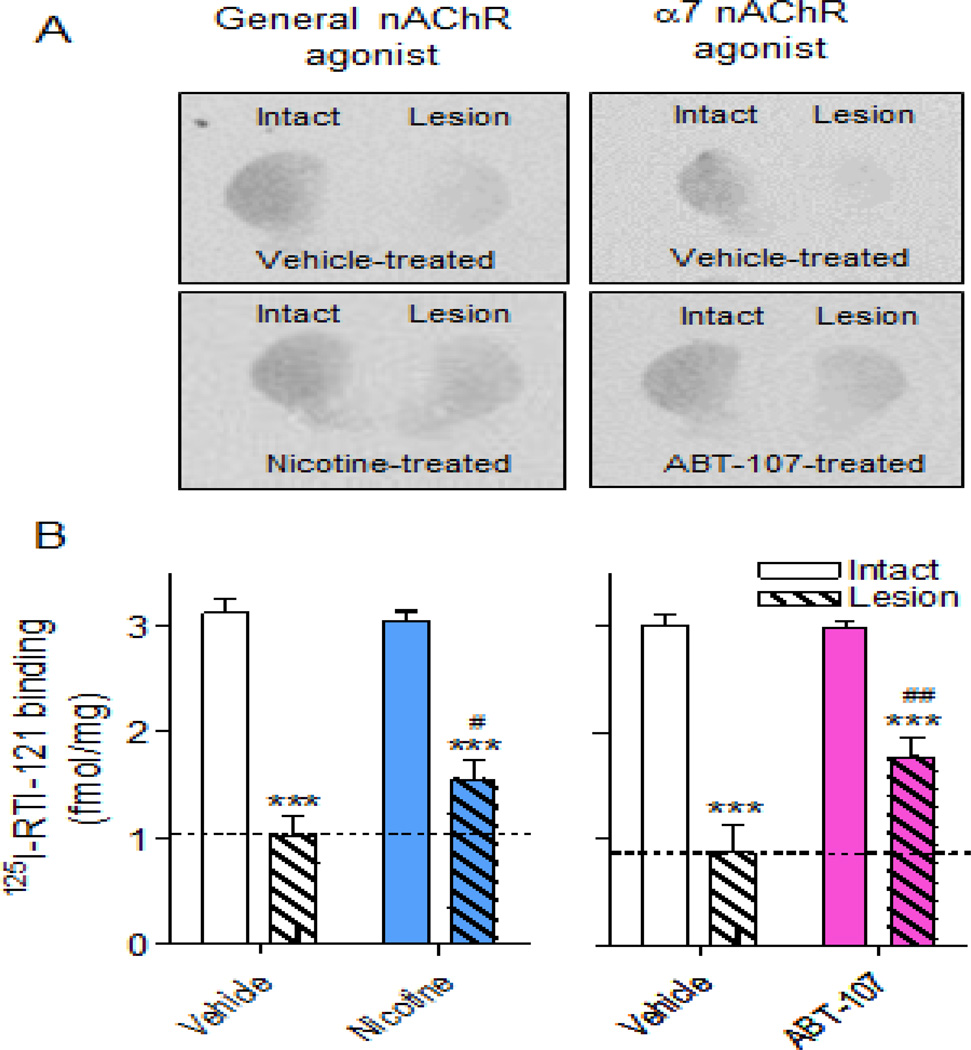
α7 nAChR agonist treatment attenuates lesion-induced declines in the striatal DAT
To understand the molecular changes linked to the improvement in behavior, we measured the DAT in striatum, a region critical to motor function. Fig. 4A shows autoradiographic images of the effect of unilateral 6-OHDA lesioning on striatal DAT. Quantitation revealed a ~70% reduction in DAT levels in vehicle-treated lesioned rats in both study 1 (Fig. 4B left panel) and 2 (Fig. 4B right panel). DAT levels were significantly higher in the lesioned striatum of rats treated with the general nAChR agonist nicotine (Fig. 4B left panel). In study 2 (Fig. 4B right panel), there were also significantly increased DAT levels in the lesioned striatum of rats treated with the α7 nAChR agonists ABT-107 compared to the vehicle-treated group. There were no changes in DAT levels in the intact striatum.
Fig. 4.
α7 nAChR agonist treatment improves the 6-OHDA-induced loss in DAT levels. Rats were administered vehicle or nAChR agonist via minipump and lesioned using 6-OHDA, as described. (A) DAT was measured in the intact and lesioned striatum using 125I-RTI-121 autoradiography. Quantitative analysis (B) shows that 6-OHDA lesioning led to ~70% loss in DAT levels in the lesioned striatum of vehicle-treated rats. However, DAT levels in the agonisttreated group were significantly higher in the lesioned but not intact striatum. Values are the mean ± SEM of 12–25 rats per group. Significance of difference from the vehicle-treated intact side, ***p < 0.001; from vehicle-treated lesioned side, #p < 0.05, ##p < 0.01 using two-way ANOVA followed by a Bonferroni post hoc test.
α7 nAChR agonist treatment prevents the 6-OHDA-induced loss in dopamine release
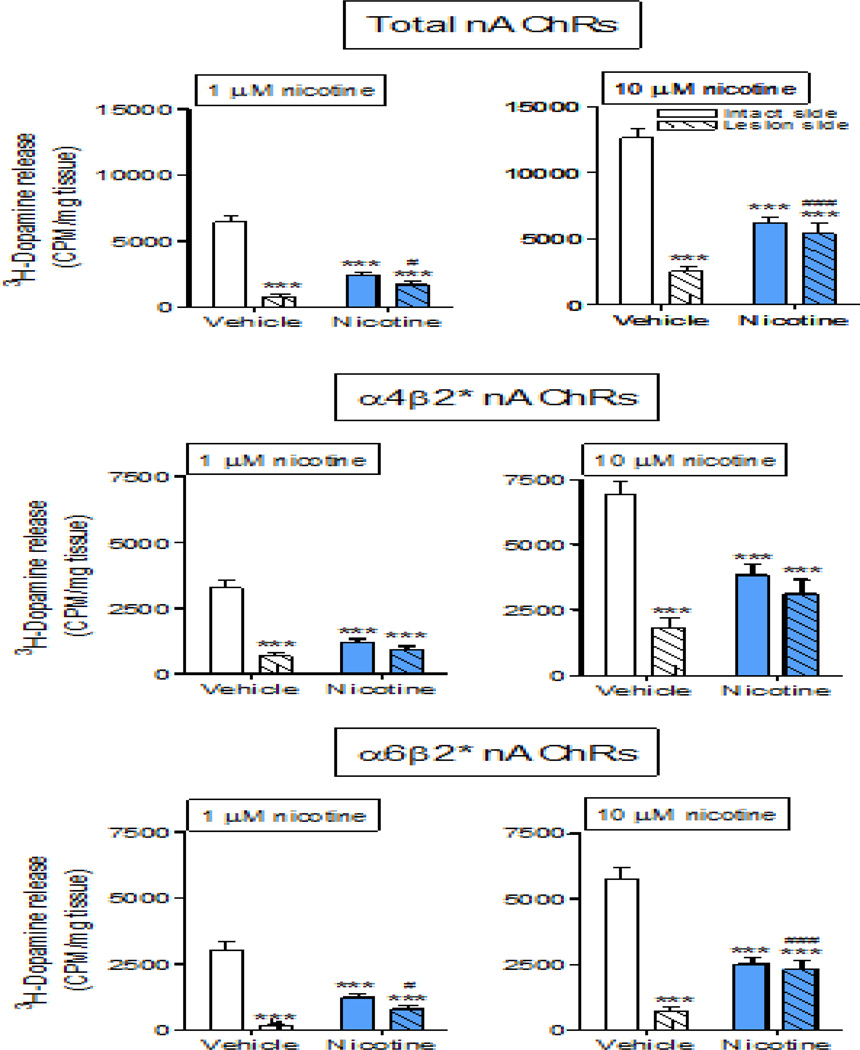
Nicotine-evoked 3H-dopamine release was measured from synaptosomes prepared from intact and lesioned striatum of rats treated with vehicle or nAChR agonists (Fig. 5, Table 1), as described (Bordia et al., 2013b; Quik et al., 2003). We used 1.0 and 10 µM nicotine to evaluate submaximal and maximal nAChR-evoked striatal 3H-dopamine release, respectively. In vehicle-treated rats, total dopamine release was decreased from 6062 ± 322 (n =25) cpm/mg tissue on the intact side to 838 ± 258 (n = 25) cpm/mg tissue on the lesioned side at 1.0 µM nicotine, that is, to 14% of own intact side (Fig. 5, Table 1). At 10 µM nicotine, release decreased from 12,366 ± 440 (n = 25) to 2862 ± 377 cpm/mg tissue with lesioning or was 23% of own intact side.
Fig. 5.
Nicotine prevents the 6-OHDA-induced loss in striatal nAChR-evoked dopamine release. Rats were administered vehicle or nicotine via minipump and lesioned using 6-OHDA. Synaptosomal nicotine-evoked 3H-dopamine release was determined in response to 1 and 10 µM nicotine in intact and lesioned striatum. Release was done in the absence (total nAChR-mediated release) and presence of 50 nM α-CtxMII (α4β2* nAChR-mediated release) with the difference in release defined as α6β2* nAChR-mediated release. Values are the mean ± SEM of 22–25 rats per group. Significance of difference from the vehicle-treated intact side, ***p < 0.001; from vehicle-treated lesioned side, #p < 0.05, ###p < 0.001 using two-way ANOVA followed by a Bonferroni post hoc test.
Table 1.
nAChR agonist treatment improves synaptosomal nAChR-mediated 3H-dopamine release from lesioned striatum
| Nicotine -evoked release |
nAChR- mediated release |
Chronic treatment |
Nicotine evoked-3H-dopamine release (CPM/mg tissue) |
|||
|---|---|---|---|---|---|---|
| Intact side | % own vehicle | Lesioned side | % own vehicle | |||
| 1 µM | Total | Vehicle | 6062 ± 332 | 100 ± 5.5 | 838 ± 130### | 100 ±15 |
| Nicotine | 2413 ± 196*** | 40 ± 3.2*** | 1696 ± 258* | 202 ± 30* | ||
| ABT-107 | 2946 ± 358*** | 41 ± 5.9*** | 1818 ± 495* | 217 ± 59* | ||
| α4β2* | Vehicle | 3159 ± 228 | 100 ± 7.2 | 758 ± 118### | 100 ± 16 | |
| Nicotine | 1191 ± 147*** | 38 ± 4.7*** | 895 ± 183 | 118 ± 24 | ||
| ABT-107 | 1954 ± 218*** | 62 ± 6.9*** | 1327 ± 386 | 175 ± 51 | ||
| α6β2* | Vehicle | 2914 ± 281 | 100 ± 9.6 | 106 ± 41### | 100 ± 39 | |
| Nicotine | 1237 ± 154*** | 43 ± 5.3*** | 801 ± 129*** | 755 ± 122*** | ||
| ABT-107 | 1121 ± 235*** | 38 ± 8.1*** | 540 ± 162* | 509 ± 152* | ||
| 10 µM | Total | Vehicle | 12366 ± 440 | 100 ± 0.4 | 2862 ± 377### | 100 ± 13 |
| Nicotine | 6229 ± 447*** | 50 ± 0.4*** | 5394 ± 805 | 188 ± 30* | ||
| ABT-107 | 7066 ± 348*** | 57 ± 0.3*** | 5317 ± 1020 | 186 ± 36* | ||
| α4β2* | Vehicle | 7003 ± 385 | 100 ± 5.5 | 2291 ± 349### | 100 ± 15 | |
| Nicotine | 3853 ± 396*** | 55 ± 5.6*** | 3120 ± 553 | 136 ± 24 | ||
| ABT-107 | 4147 ± 309*** | 59 ± 4.4*** | 3748 ± 754 | 164 ± 33 | ||
| α6β2* | Vehicle | 5410 ± 306 | 100 ± 5.7 | 692 ± 108### | 100 ± 16 | |
| Nicotine | 2509 ± 255*** | 46 ± 4.7*** | 2324 ± 335*** | 336 ± 48*** | ||
| ABT-107 | 2919 ± 328*** | 54 ± 6.1*** | 1609 ± 465 | 233 ± 67 | ||
3H-Dopamine release was measured from synaptosomes from intact and lesioned striatum as described. Release was stimulated by either 1.0 or 10 µM nicotine to obtain a measure of submaximal and maximal 3H-dopamine release, respectively. Nicotine-stimulated release was measured in the absence (total) and presence of the α6β2* nAChR blocker α-CtxMII. Release in the presence of α-CtxMII was defined as α4β2* nAChR-mediated, while α6β2* nAChR-mediated release was determined by subtracting α4β2* nAChR-mediated release from total release.
Values represent the mean ± SEM of 12–25 rats. Significance of difference from own vehicle-treated side,
p < 0.05,
p < 0.001; from intact vehicle-treated side,
p < 0.001, using one-way ANOVA followed by a Newman-Keuls post hoc test.
Long term nicotine treatment alone decreased total dopamine release on the intact side compared to vehicle-treated rats (6062 ± 322 to 2413 ± 196 cpm/mg tissue, n = 22–25), as previously shown (Bordia et al., 2013a). However, nicotine treatment increased total dopamine release in striatum of lesioned rats from 838 ± 130 to 1696 ± 258 cpm/mg tissue, n = 22–25) at 1.0 µM nicotine stimulation (Fig. 5, Table 1). Thus, dopamine release expressed as % own vehicle was decreased on the intact side with nicotine treatment but significantly increased on the lesioned side (Table 1). Similar results were obtained for α6β2* nAChR-mediated release with lesioning and long term nicotine treatment (Table 1). For α4β2* nAChR-mediated dopamine release, long term nicotine treatment alone also decreased α4β2* dopamine release on the intact side compared to vehicle-treated rats, as previously shown (Bordia et al., 2013a). In this case, there was a trend for an increase in total striatal dopamine release on the lesioned side although it was not significant (Fig. 5, Table 1). Thus, for all modes of release, dopamine release expressed as % own vehicle was decreased on the intact side with nicotine treatment but increased or not changed on the lesioned side (Table 1), suggesting an improvement in function. The α7 nAChR agonist ABT-107 alone decreased total release from 6062 ± 332 (vehicle-treated intact side) to 2946 ± 358 (n = 12) cpm/mg tissue on the intact side at 1.0 µM nicotine stimulation. However, ABT-107 treatment increased total dopamine release in striatum of lesioned rats from 838 ± 130 to 1818 ± 495 cpm/mg/tissue, n = 22–25) at 1.0 µM nicotine stimulation. The data at 1 µM nicotine for α4β2* and α6β2* nAChR-mediated release was similar with ABT-107 treatment as compared to that with chronic nicotine treatment (Fig. 5, Table 1).
At 10 µM nicotine, there was a trend or a significant increase in striatal dopamine release on the lesioned side for all components of release with chronic nicotine or ABT-107 treatment (Fig. 5, Table 1). Again this suggests an improvement in dopaminergic activity with nicotine treatment.
α7 nAChR agonist treatment reduces basal dopaminergic tone
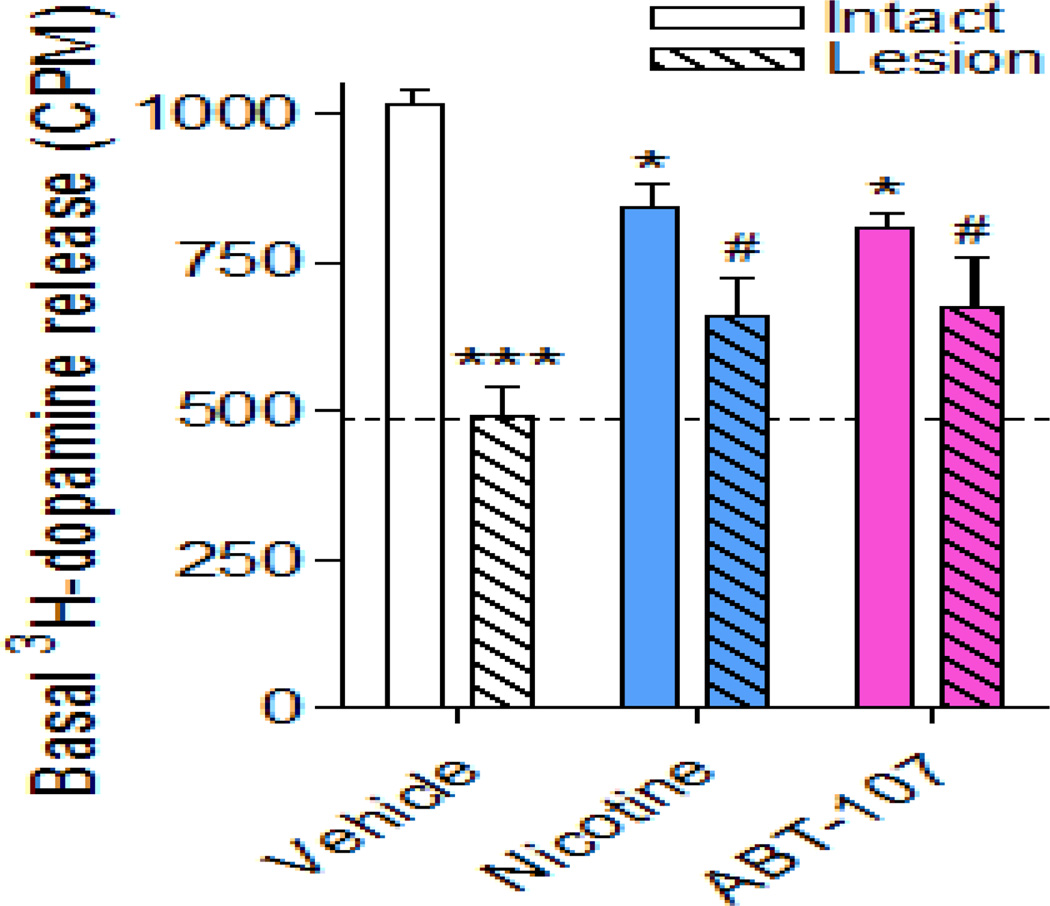
Basal 3H-dopamine before and after the nicotine stimulus was also measured. The results in Fig. 6 show that lesioning alone decreased basal 3H-dopamine release by 50% in the vehicle-treated rats, in agreement with previous studies (Quik et al., 2003, Bordia et al., 2013). In rats treated with nicotine there was a significant overall decrease in basal release on the intact side alone with similar results with ABT-107 treatment. Notably, the basal release on the lesioned side of these agonist-treated rats was significantly higher compared to the lesioned side of the vehicle-treated rats.
Fig. 6.
α7 nAChR agonists reduce the 6-OHDA-induced loss in basal dopamine release in striatum. Rats were administered vehicle or nAChR agonist via minipump and lesioned using 6-OHDA. Basal 3H-dopamine release was measured from synaptosomes prepared from intact and lesioned striatum. Values are the mean ± SEM of 12–25 rats per group. Significance of difference from the vehicle-treated intact side, *p < 0.05, ***p < 0.001; from the vehicle-treated lesioned side, #p < 0.05 using two-way ANOVA followed by a Bonferroni post hoc test.
Discussion
The present studies are the first to show that ABT-107, an agonist exhibiting high affinity and selectivity for α7 nAChRs (Bitner et al., 2010; Malysz et al., 2010), may improve motor behaviors associated with nigrostriatal damage by enhancing dopaminergic function in the striatum. Evidence for this possibility stems from our experiments showing that ABT-107 treatment significantly improved two measures of parkinsonism in unilaterally lesioned 6-OHDA rats. Studies to understand the molecular changes that contribute to the improvement in behavior showed that there were significantly higher DAT levels in lesioned striatum from ABT-107 compared to vehicle-treated rats. Chronic ABT-107 treatment also significantly improved both basal and nicotine-stimulated 3H-dopamine release from striatal synaptosomes. These data suggest that α7 nAChR drugs such as ABT-107 may improve motor behaviors associated with nigrostriatal damage by enhancing dopaminergic function in the striatum.
To assess the effect of the α7 agonist ABT-107 on alterations in 6-OHDA-induced motor impairments, we used two behavioral assays. These include the forepaw placement and the adjusted stepping test, which model varying deficits related to Parkinson’s disease including limb use for weight bearing movements and postural stability, respectively (Chang et al., 1999; Fleming et al., 2012; Schallert et al., 1982). These two tests offer the advantage that together they provide a drug-independent measure of voluntary and challenged motor function (Betts et al., 2012; Fleming et al., 2012). Treatment with ABT-107 improved motor deficits in both the forepaw placement and adjusted stepping test. By contrast, the effects of the α7 agonist DMXB were more variable. The reason for this inconsistency is not clear. The plasma half-lives are somewhat similar being about 2–3 h for DMXB and 3–4 h for ABT-107 (Freedman et al., 2008; Othman et al., 2011). However, DMXB is less potent at α7 nAChRs as compared to ABT-107, as evident from the higher doses of DMXB used (Freedman et al., 2008; Othman et al., 2011). This may lead to differential effects on α7 nAChR activation, desensitization and chaperoning, although such studies remain to be done. A point of note is that ABT-107 is more selective for α7 nAChRs whereas DMXB is also known to act at α4β2* nAChRs (Briggs et al., 1997). An interaction at multiple nAChRs may lead to opposing effects on dopaminergic activity and complicate outcomes in studies using DMXB.
Notably, the α7 nAChR agonist ABT-107 was as effective in improving behavioral measures linked to parkinsonism as the general agonist nicotine, which interacts with multiple nAChRs (Quik and Wonnacott, 2011). Since both ABT-107 and nicotine acted similarly, these data may suggest that the improvement afforded by the general agonist nicotine is mediated via α7 receptors. It is also possible that nicotine induces its effect through an interaction at multiple nAChR subtypes, but that they converge on a final common mechanism. α4β2* nAChRs may be involved based on studies showing no protection against methamphetamine-induced toxicity in α4 nAChR subunit null mutant mice as compared to control mice (Ryan et al., 2001). Moreover, the α4β2* nAChR agonist ABT-089 protected against glutamate-induced cytotoxicity in rat cortical cultures and differentiated human IMR32 cells (Sullivan et al., 1997), although the α4β2* nAChR RJR2403 did not protect against 6-OHDA induced nigrostriatal damage in rats (Visanji et al., 2006). Further work is thus necessary to understand the role of α4β2* nAChRs in neuroprotection.
To investigate the molecular changes that underlie the ABT-107-mediated improvement in motor function, we measured striatal DAT a marker that correlates with the level of dopaminergic denervation (Miller et al., 1999; Perez et al., 2010; Quik et al., 2003). ABT-107 treatment led to significantly higher DAT levels in the striatum of 6-OHDA lesioned compared to vehicle-treated lesioned rats. The magnitude of the effect was similar to that observed with nicotine. Notably, no changes in DAT levels were observed on the intact side of ABT-107 or nicotine-treated rats. These latter results suggest that the elevated DAT levels in lesioned striatum are not due to DAT up-regulation, consistent with previous work (Bordia et al., 2013b; Huang et al., 2009).
We next examined evoked 3H-dopamine release from striatal synaptosomes to evaluate changes in cellular dopaminergic function associated with agonist treatments and lesioning. This technique provides a direct measure of pre-synaptic modulation of terminal function and has proved very useful in providing information on the functional characteristics of presynaptic nAChRs and the mechanisms by which they modulate neurotransmitter release (Grady et al., 2002; Quik et al., 2003; Wonnacott et al., 2000). Long-term nicotine treatment led to a reduction in nAChR-evoked 3H-dopamine release on the intact side, in agreement with previous studies (Bordia et al., 2013b; Marks et al., 1993). ABT-107 treatment resulted in a similar decline in nAChR-evoked dopamine release on the intact side, suggesting the effect of nicotine may be mediated by α7 nAChRs, at least in part.
Consistent with previous findings, 6-OHDA treatment led to ~80% decrease in nAChR-evoked 3H-dopamine release in vehicle-treated rats, due to a loss of dopaminergic nerve terminal (Quik et al., 2003). Notably, in ABT-107 treated rats, nAChR-mediated 3H-dopamine release on the lesioned side was reduced by only 38% compared to its own intact side. There was a similar improvement in nAChR-mediated 3H-dopamine release with long-term nicotine treatment, with release decreased by only 30% compared to its own intact side. Studies to elucidate the nAChR subtypes that contribute to the enhanced 3H-dopamine release show that both α4β2* and α6β2* nAChR-mediated release was improved with chronic ABT-107 treatment.
Since lesioning not only affects nAChR-evoked 3H-dopamine release but also reduces baseline dopaminergic activity, basal 3H-dopamine release was measured in striatal synaptosomes. Notably, basal release in the lesioned striatum of ABT-107-treated rats was significantly higher compared to that on the lesioned side of the vehicle-treated rats. Thus alterations in non-nAChR mediated dopaminergic tone may also contribute to the enhanced dopaminergic function.
A question that arises is how an α7 agonist such as ABT-107 enhances striatal dopamine function since these receptors do not appear to be present on striatal dopamine nerve terminals. One possible explanation may relate to the presence of a small population of α7 nAChRs on striatal glutamatergic afferents that project from the cortex to the striatum. Stimulation of these α7 nAChRs increases glutamate release, which in turn acts at glutamate receptors on dopamine terminals to modulate dopamine release/turnover and to promote neuronal integrity (Kaiser and Wonnacott, 2000; Zigmond et al., 1990). Nicotine is also well known to enhance neuronal growth, maintenance and survival via multiple molecular mechanisms (Picciotto and Zoli, 2008; Quik et al., 2007; Shimohama, 2009). ABT-107 may exert its effects in a similar manner specifically via an interaction at α7 nAChRs. Another possible mechanism may relate to ABT-107’s ability to protect against glutamate-induced toxicity that may arise with 6-OHDA-induced nigrostriatal degeneration (Malysz et al., 2010).
In summary, the present results show that ABT-107, a high affinity α7 selective nAChR agonist attenuates 6-OHDA-induced loss in motor deficits and that this is associated with enhanced striatal dopaminergic function. Since α7 nAChR drugs are associated with few side effects compared to nicotine, these results suggest that α7 nAChR agonists, such as ABT-107, may represent a promising class of drugs for Parkinson's disease therapy.
Highlights.
The α7 agonist ABT-107 improves parkinsonism using two behavioral tests
ABT-107 ameliorates basal and nicotinic receptor-mediated dopamine release
ABT-107 partially prevents lesioned-induced losses in the dopamine transporter
Drugs targeting α7 nAChRs, such as ABT-107, may improve motor behaviors associated with nigrostriatal damage by enhancing striatal dopaminergic function
Acknowledgements
These studies were supported by NIH grants NS59910 and 65851 to MQ; GM57481 to RLP; and GM103801 and GM48677 to JMM.
Abbreviations
- ANOVA
Analysis of variance
- α-CtxMII
α-conotoxinMII
- DAT
dopamine transporter
- nAChRs
nicotinic receptors
- 6-OHDA
6-hydroxydopamine
- 125I-RTI-121
125I-3β-(4-iodophenyl)tropane-2β-carboxylic acid isopropyl ester
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relevant conflict of interest.
ABT-107 is an AbbVie compound; MWD is employed by and owns shares of AbbVie. There are no conflicts of interest for the other authors.
References
- Betts MJ, et al. Allosteric modulation of the group III mGlu(4) receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson's disease. Br J Pharmacol. 2012;166:2317–2330. doi: 10.1111/j.1476-5381.2012.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitner RS, et al. In vivo pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107: preclinical considerations in Alzheimer's disease. J Pharmacol Exp Ther. 2010;334:875–886. doi: 10.1124/jpet.110.167213. [DOI] [PubMed] [Google Scholar]
- Bordia T, et al. Continuous and intermittent nicotine treatment reduces L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther. 2008;327:239–247. doi: 10.1124/jpet.108.140897. [DOI] [PubMed] [Google Scholar]
- Bordia T, et al. Nicotinic receptor-mediated reduction in L-dopa-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther. 2010;333:929–938. doi: 10.1124/jpet.109.162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, et al. The nicotine-mediated decline in l-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release. J Neurochem. 2013a doi: 10.1111/jnc.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, et al. The nicotine-mediated decline in L-dopa-induced dyskinesias is associated with a decrease in striatal dopamine release. J Neurochem. 2013b doi: 10.1111/jnc.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs CA, et al. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997;57:231–241. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M. Ratings of L-DOPA-induced dyskinesia in the unilateral 6-OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci. Chapter. 2007;9:1–23. doi: 10.1002/0471142301.ns0925s41. [DOI] [PubMed] [Google Scholar]
- Chang JW, et al. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- Chen H, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dajas-Bailador FA, et al. The alpha7 nicotinic acetylcholine receptor subtype mediates nicotine protection against NMDA excitotoxicity in primary hippocampal cultures through a Ca(2+) dependent mechanism. Neuropharmacology. 2000;39:2799–2807. doi: 10.1016/s0028-3908(00)00127-1. [DOI] [PubMed] [Google Scholar]
- de Fiebre NC, de Fiebre CM. Alpha 7 nicotinic acetylcholine receptor-mediated protection against ethanol-induced neurotoxicity. Alcohol. 2003;31:149–153. doi: 10.1016/j.alcohol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Fahn S. Parkinson's disease: 10 years of progress, 1997–2007. Mov Disord. 2010;25(Suppl 1):S2–S14. doi: 10.1002/mds.22796. [DOI] [PubMed] [Google Scholar]
- Fleming SM, et al. Cranial and related sensorimotor impairments in rodent models of Parkinson's disease. Behav Brain Res. 2012;231:317–322. doi: 10.1016/j.bbr.2012.02.034. [DOI] [PubMed] [Google Scholar]
- Freedman R, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165:1040–1047. doi: 10.1176/appi.ajp.2008.07071135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorell JM, et al. Smoking and Parkinson's disease: a dose-response relationship. Neurology. 1999;52:115–119. doi: 10.1212/wnl.52.1.115. [DOI] [PubMed] [Google Scholar]
- Grady SR, et al. Characterization of nicotinic agonist-induced [(3)H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Hickey P, Stacy M. AAV2-neurturin (CERE-120) for Parkinson's disease. Expert Opin Biol Ther. 2013;13:137–145. doi: 10.1517/14712598.2013.754420. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, et al. Pathogenesis of Parkinson's disease. Mov Disord. 2013;28:24–30. doi: 10.1002/mds.25032. [DOI] [PubMed] [Google Scholar]
- Huang LZ, et al. Nicotine is neuroprotective when administered before but not after nigrostriatal damage in rats and monkeys. J Neurochem. 2009;109:826–837. doi: 10.1111/j.1471-4159.2009.06011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S, Wonnacott S. alpha-bungarotoxin-sensitive nicotinic receptors indirectly modulate [(3)H]dopamine release in rat striatal slices via glutamate release. Mol Pharmacol. 2000;58:312–318. doi: 10.1124/mol.58.2.312. [DOI] [PubMed] [Google Scholar]
- Kaneko S, et al. Nicotine protects cultured cortical neurons against glutamate-induced cytotoxicity via alpha7-neuronal receptors and neuronal CNS receptors. Brain Res. 1997;765:135–140. doi: 10.1016/s0006-8993(97)00556-8. [DOI] [PubMed] [Google Scholar]
- Kerr DS. Treatment of mitochondrial electron transport chain disorders: a review of clinical trials over the past decade. Mol Genet Metab. 2010;99:246–255. doi: 10.1016/j.ymgme.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Wunderle KB. Parkinson's disease: evidence for environmental risk factors. Mov Disord. 2013;28:8–13. doi: 10.1002/mds.25150. [DOI] [PubMed] [Google Scholar]
- Li Y, et al. alpha7 nicotinic receptor-mediated protection against ethanol-induced oxidative stress and cytotoxicity in PC12 cells. Brain Res. 2000;861:165–167. doi: 10.1016/s0006-8993(99)02457-9. [DOI] [PubMed] [Google Scholar]
- Malysz J, et al. In vitro pharmacological characterization of a novel selective alpha7 neuronal nicotinic acetylcholine receptor agonist ABT-107. J Pharmacol Exp Ther. 2010;334:863–874. doi: 10.1124/jpet.110.167072. [DOI] [PubMed] [Google Scholar]
- Marks MJ, et al. Downregulation of nicotinic receptor function after chronic nicotine infusion. J Pharmacol Exp Ther. 1993;266:1268–1276. [PubMed] [Google Scholar]
- Marques-Aleixo I, et al. Physical exercise as a possible strategy for brain protection: evidence from mitochondrial-mediated mechanisms. Prog Neurobiol. 2012;99:149–162. doi: 10.1016/j.pneurobio.2012.08.002. [DOI] [PubMed] [Google Scholar]
- McCallum SE, et al. Decrease in alpha3*/alpha6* nicotinic receptors but not nicotine-evoked dopamine release in monkey brain after nigrostriatal damage. Mol Pharmacol. 2005;68:737–746. doi: 10.1124/mol.105.012773. [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Miller GW, et al. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Morens DM, et al. Cigarette smoking and protection from Parkinson's disease: false association or etiologic clue? Neurology. 1995;45:1041–1051. doi: 10.1212/wnl.45.6.1041. [DOI] [PubMed] [Google Scholar]
- Nicoletti A, et al. Voluptuary habits and clinical subtypes of Parkinson's disease: the FRAGAMP case-control study. Mov Disord. 2010;25:2387–2394. doi: 10.1002/mds.23297. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Schapira AH. Therapeutic prospects for Parkinson disease. Ann Neurol. 2013;74:337–347. doi: 10.1002/ana.24011. [DOI] [PubMed] [Google Scholar]
- Othman AA, et al. Single- and multiple-dose pharmacokinetics, safety, and tolerability of the selective alpha7 neuronal nicotinic receptor agonist, ABT-107, in healthy human volunteers. J Clin Pharmacol. 2011;51:512–526. doi: 10.1177/0091270010370460. [DOI] [PubMed] [Google Scholar]
- Pascual A, et al. GDNF and protection of adult central catecholaminergic neurons. J Mol Endocrinol. 2011;46:R83–R92. doi: 10.1530/JME-10-0125. [DOI] [PubMed] [Google Scholar]
- Perez XA, et al. {Alpha}6{beta}2* and {alpha}4{beta}2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson's disease. Mol Pharmacol. 2010;78:971–980. doi: 10.1124/mol.110.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Quik M, et al. Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci. 2007;28:229–235. doi: 10.1016/j.tips.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Quik M, et al. Nicotine as a potential neuroprotective agent for Parkinson's disease. Mov Disord. 2012;27:947–957. doi: 10.1002/mds.25028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, et al. Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol. 2003;63:1169–1179. doi: 10.1124/mol.63.5.1169. [DOI] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. {alpha}6{beta}2* and {alpha}4{beta}2* Nicotinic Acetylcholine Receptors As Drug Targets for Parkinson's Disease. Pharmacol Rev. 2011;63:938–966. doi: 10.1124/pr.110.003269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz B, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol. 2007;64:990–997. doi: 10.1001/archneur.64.7.990. [DOI] [PubMed] [Google Scholar]
- Rosa AO, et al. Neuroprotection by nicotine in hippocampal slices subjected to oxygen-glucose deprivation: involvement of the alpha7 nAChR subtype. J Mol Neurosci. 2006;30:61–62. doi: 10.1385/JMN:30:1:61. [DOI] [PubMed] [Google Scholar]
- Ryan RE, et al. Dose-related neuroprotective effects of chronic nicotine in 6-hydroxydopamine treated rats, and loss of neuroprotection in alpha4 nicotinic receptor subunit knockout mice. Br J Pharmacol. 2001;132:1650–1656. doi: 10.1038/sj.bjp.0703989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, et al. Tactile extinction: distinguishing between sensorimotor and motor asymmetries in rats with unilateral nigrostriatal damage. Pharmacol Biochem Behav. 1982;16:455–462. doi: 10.1016/0091-3057(82)90452-x. [DOI] [PubMed] [Google Scholar]
- Shimohama S. Nicotinic receptor-mediated neuroprotection in neurodegenerative disease models. Biol Pharm Bull. 2009;32:332–336. doi: 10.1248/bpb.32.332. [DOI] [PubMed] [Google Scholar]
- Smeyne M, Smeyne RJ. Glutathione metabolism and Parkinson's disease. Free Radic Biol Med. 2013;62:13–25. doi: 10.1016/j.freeradbiomed.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger F, Poewe W. Management of motor and non-motor symptoms in Parkinson's disease. CNS Drugs. 2013;27:259–272. doi: 10.1007/s40263-013-0053-2. [DOI] [PubMed] [Google Scholar]
- Stuckenholz V, et al. The alpha7 nAChR Agonist PNU-282987 Reduces Inflammation and MPTP-Induced Nigral Dopaminergic Cell Loss in Mice. J Parkinsons Dis. 2013;3:161–172. doi: 10.3233/JPD-120157. [DOI] [PubMed] [Google Scholar]
- Sullivan JP, et al. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties. J Pharmacol Exp Ther. 1997;283:235–246. [PubMed] [Google Scholar]
- Sulzer D, Surmeier DJ. Neuronal vulnerability, pathogenesis, and Parkinson's disease. Mov Disord. 2013;28:715–724. doi: 10.1002/mds.25187. [DOI] [PubMed] [Google Scholar]
- Sutachan JJ, et al. Cellular and molecular mechanisms of antioxidants in Parkinson's disease. Nutr Neurosci. 2012;15:120–126. doi: 10.1179/1476830511Y.0000000033. [DOI] [PubMed] [Google Scholar]
- Suzuki S, et al. 3-[(2,4-Dimethoxy)benzylidene]-anabaseine dihydrochloride protects against 6-hydroxydopamine-induced parkinsonian neurodegeneration through alpha7 nicotinic acetylcholine receptor stimulation in rats. J Neurosci Res. 2013;91:462–471. doi: 10.1002/jnr.23160. [DOI] [PubMed] [Google Scholar]
- Tanner CM. Advances in environmental epidemiology. Mov Disord. 2010;25(Suppl 1):S58–S62. doi: 10.1002/mds.22721. [DOI] [PubMed] [Google Scholar]
- Thacker EL, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulorge D, et al. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+ FASEB J. 2011;25:2563–2573. doi: 10.1096/fj.11-182824. [DOI] [PubMed] [Google Scholar]
- Visanji NP, et al. Nicotine, but neither the alpha4beta2 ligand RJR2403 nor an alpha7 nAChR subtype selective agonist, protects against a partial 6-hydroxydopamine lesion of the rat median forebrain bundle. Neuropharmacology. 2006;51:506–516. doi: 10.1016/j.neuropharm.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Wang HY, et al. beta-Amyloid(1–42) Binds to alpha7 Nicotinic Acetylcholine Receptor with High Affinity. Implications for alzheimer's disease pathology. J Biol Chem. 2000;275:5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- Wirdefeldt K, et al. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–S58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, et al. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur J Pharmacol. 2000;393:51–58. doi: 10.1016/s0014-2999(00)00005-4. [DOI] [PubMed] [Google Scholar]
- Yanagida T, et al. Synergistic effect of galantamine on nicotine-induced neuroprotection in hemiparkinsonian rat model. Neurosci Res. 2008;62:254–261. doi: 10.1016/j.neures.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, et al. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]