Abstract
Background:
Varenicline (VAR) has demonstrated superior efficacy over other smoking cessation pharmacotherapies, though 50-60% of those treated do maintain abstinence. Some preclinical findings suggest that new nicotine dependence pharmacotherapies should target the glutamatergic system, given its demonstrated role in addiction. Attention has been given to N-acetylcysteine (NAC), which appears to restore normal glutamate signaling in animal models. It is possible that NAC and VAR may work in concert to promote abstinence at higher rates than with either medication alone.
Objective:
The aim of the current study was to demonstrate the feasibility and safety of co-administering NAC and VAR in nicotine-dependent participants.
Methods:
Participants (N=19) were daily cigarette smokers, and did not need to be seeking treatment. They received 4 weeks of open-label treatment with NAC (1200 mg twice daily) and VAR (1 mg twice daily, following titration) and were assessed weekly for adverse events (AEs), smoking, craving and withdrawal.
Results:
Sixteen participants reported a total of 40 AEs, and most were mild (88%). The most commonly reported AE was nausea (15%). Medication adherence, assessed via self-reports and pill counts, was excellent (98%). Exploratory analyses showed reductions in cigarettes per day, though point prevalence abstinence at the end of the study was low.
Conclusions:
These preliminary data provide the first demonstration of safety and feasibility of the co-administration of NAC and VAR in cigarette smokers. AEs were consistent with those typically reported for VAR and NAC. These data support future efficacy research on NAC and VAR for smoking cessation.
Keywords: N-Acetylcysteine, varenicline, pharmacotherapy, smoking cessation, safety, tolerability
Introduction
Long-term abstinence remains difficult to attain for the vast majority of cigarette smokers, with successful cessation prevalence ranging from 4-7% among unassisted quitters (1-3). Varenicline (Chantix®), an α4β2 nicotinic receptor partial agonist, is arguably the most efficacious smoking cessation pharmacotherapy available (4-6). VAR appears to promote abstinence through decreasing withdrawal symptoms and blunting the reward derived from smoking (7). Smokers treated with VAR compared to placebo reported cigarette smoking to be less satisfying and rewarding (8-10). The most common adverse events reported with VAR include nausea, insomnia, abnormal dreams and headache (11, 12). Concerns regarding serious adverse events associated with VAR have emerged, including neuropsychiatric and cardiovascular events, though a recent meta-analysis showed no difference in serious adverse events in placebo-controlled VAR trials (12). Despite the demonstrated efficacy and tolerability of VAR as a first-line pharmacotherapy for smoking, 50-60% of those treated do not maintain abstinence following 12 weeks of treatment (4-6), suggesting the need for improvements in cessation pharmacotherapies.
Within the preclinical literature, glutamate has emerged as a potential pharmacotherapeutic target in the treatment of addiction (13-15). Specific attention has been given to N-acetylcysteine (NAC) as a pharmacotherapeutic agent. NAC appears to restore normal glutamate signaling and decrease reinstatement of heroin, cocaine, and nicotine seeking in animal models (16-22). Clinical data also support the efficacy of NAC as a pharmacotherapy to reduce compulsive behavior (23) and reverse addiction pathology (24, 25). Preliminary data with cigarette smokers has demonstrated that NAC reduces smoking and smoking-related reward (17, 26). Oral NAC is well-tolerated, with the majority of side effects involving gastrointestinal events that typically do not require the termination of medication (27). It has also been suggested that NAC may work best under conditions of abstinence (28), thus functioning to promote relapse prevention. NAC may have particular benefit when co-administered with other cessation medication with known efficacy; e.g., VAR. NAC and VAR may be a potentially synergistic combination pharmacotherapeutic regimen to promote long-term abstinence at higher rates than with either medication alone. No prior studies have demonstrated the feasibility and safety of co-administering these medications in cigarette smokers. Prior to examining efficacy, safety must first be established. The purpose of this study was to conduct a short-term, single-arm, open-label feasibility and safety trial of NAC and VAR in adult, daily cigarette smokers.
Methods
Participants
Participants (N=19) were daily smokers (≥10 cigarettes per day for ≥6 months) between the ages of 18-65 years. They did not need to be seeking smoking cessation treatment to be eligible for study procedures. Participants were excluded if they had any unstable psychiatric or medical disorder, were pregnant or breastfeeding, or taking other smoking cessation medications. Study recruitment and procedures took place from July 2013 through February 2014. The Medical University of South Carolina Institutional Review Board approved all procedures. In total, 33 participants consented to participate, 10 were excluded during screening procedures (unstable psychiatric or medical conditions [n=9], <10 cigarettes/day [n=1]). Of the 23 participants enrolled in the study, 19 completed all study procedures (83%) and are included in the current analysis. Four participants were lost to follow-up.
Procedures
Eligibility was assessed through a brief telephone screening, followed by a thorough in-person screening assessment. Following informed consent procedures, participants completed a medical history, physical exam, self-report questionnaires, and semi-structured interviews to determine eligibility. Eligible participants were enrolled in the study following screening procedures and provided with active study medication at the baseline visit (Assessment/Day 0). Participants completed a 1-week standard titration period for VAR (0.5 mg once daily for 3 days, followed by 0.5 mg dosing twice daily for 4 days). Participants were then maintained at 1 mg twice daily for 3 weeks. Participants concurrently took NAC 1200 mg twice daily, in approximately 12-hour intervals (no titration required). This dose was chosen due to its demonstrated tolerability in prior studies (25, 29). Participants returned for weekly clinic visits over the next four weeks. Participants were given a one week supply of study medication at each weekly visit and an additional one week supply of medication (rescue pack) in the case that they could not attend a weekly visit. Participants were only given one rescue pack throughout the course of the study. A follow-up visit was conducted at Week 5. No target quit date was set for participants in this trial, but research staff did provide brief smoking cessation counseling (3-5 minutes) at each visit to assess and enhance motivation to quit or reduce smoking. Participants received $40 cash for each weekly study visit and a $40 bonus for completing all study procedures ($280 maximum for study participation).
The screening visit included the following assessments: demographic information, smoking history, medical history and physical, the Fagerström Test for Nicotine Dependence (FTND) (30), a Timeline Follow-Back (TLFB) (31) assessment capturing cigarettes per day during the past 30 days, and Readiness and Confidence to Quit Scales. Safety assessments were conducted at screening and at all clinic visits during Weeks 1-5 and included: the Beck Depression Inventory (BDI) (32), the Columbia-Suicide Severity Rating Scale (33), urine pregnancy tests and vitals (only collected through Week 4), and adverse events (AEs) assessed by the medical clinician. Medication adherence was assessed through daily medication logs and pills counts (from returned medication blister packs). Medication adherence was not financially incentivized, but was reviewed and encouraged at each visit by research staff and medical clinicians. Smoking assessments completed at screening and at clinic visits during Weeks 1-5 included: Daily smoking diaries, the Questionnaire on Smoking Urges—Brief (QSU-B) (34), the Minnesota Nicotine Withdrawal Scale (MNWS) (35), the Modified Cigarette Evaluation Questionnaire (mCEQ) (36), and biochemical measures of smoking through Carbon Monoxide (CO) Breathalyzer (Bedfont) breath samples. Smoking outcomes were assessed as secondary and exploratory. Study data were managed using REDCap electronic data capture tools hosted at the Medical University of South Carolina (37).
Statistical Analyses
Standard descriptive statistics were used to summarize demographic and smoking characteristics, as well as the prevalence of AEs. Smoking outcomes (cigarettes per day and CO), physiological outcomes (heart rate, weight, blood pressure), and mood outcomes (BDI) were analyzed across the study. Prior to model development, demographic, clinical, and smoking characteristics were tested for individual association with the smoking outcomes. Only cigarettes per day during the 30 days prior to study enrollment was significantly associated with smoking outcomes (p<0.001). Simple growth models were developed to explore linear and quadratic trends in cigarettes smoked per day and CO measures over the course of the study. Likelihood ratio tests were used to determine the best model structure (linear vs. quadratic). All analyses and descriptive statistics were calculated using the SAS System version 9.3.
Results
Demographic and Smoking Characteristics
Participants (N=19) averaged 32.2 ± SD 9.1 years of age, and 53% were African American, 47% were male, 90% had a high school degree/GED or higher, and 48% were employed full- or part-time. Participants smoked 15.9±9.7 cigarettes per day, with FTND scores of 5.1±2.3, and baseline CO of 15.8±9.2. Participants rated their readiness and confidence to quit smoking as 8.1±2.2 and 6.9±2.9 respectively on a 10-point Likert scale (1=not at all ready/confident, 10=extremely ready/confident).
Medication Adherence
Medication logs and pill counts showed the participants took 98.5% of all NAC doses (upper limit: 110) and 98.4% of all VAR doses (upper limit: 52) throughout the course of the 4-week active treatment phase. The majority of scheduled doses were confirmed by pill counts (88%).
Safety and Tolerability
Sixteen participants (84%) reported a total of 40 AEs during the course of active treatment or at the 1-week post-medication follow-up visit (Table 1). The majority of AEs were mild (88%) and the remainder were moderate (12%). The three most common AEs were nausea (15%), increased appetite (12.5%), and headache (12.5%). Only one participant reduced VAR dose (0.5 mg twice daily) due to AEs (insomnia and irritability), which resolved with dose reduction. No other participants required dose reduction or termination. Only 23 out of the reported 40 AEs (58%) were considered by the medical clinician to be either probably or possibly related to study medication.
Table 1.
All adverse events reported during 4 weeks of NAC and VAR combined treatment and during follow-up.
| Overall | Mild | Moderate | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Adverse Event | N | % | N | % | N | % |
| Nausea | 6 | 15 | 4 | 10 | 2 | 5 |
| Increased Appetite | 5 | 12.5 | 5 | 12.5 | 0 | 0 |
| Headache | 5 | 12.5 | 5 | 12.5 | 0 | 0 |
| Insomnia | 3 | 7.5 | 2 | 5 | 1 | 2.5 |
| Taste Perversion | 3 | 7.5 | 3 | 7.5 | 0 | 0 |
| Vivid Dreams | 2 | 5 | 2 | 5 | 0 | 0 |
| Tired/Fatigue | 2 | 5 | 2 | 5 | 0 | 0 |
| Vomiting | 1 | 2.5 | 0 | 0 | 1 | 2.5 |
| Diarrhea | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Heartburn | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Acid Reflux | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Bloating | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Stomach Pain | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Depressed Mood | 1 | 2.5 | 0 | 0 | 1 | 2.5 |
| Irritability | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Drowsiness | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Restlessness | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Back Ache | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Dry Mouth | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Influenza | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Upper Respiratory | 1 | 2.5 | 1 | 2.5 | 0 | 0 |
| Infection | 0 | 0 | 0 | 0 | 0 | 0 |
|
| ||||||
| Total | 40 | 100 | 35 | 87.5 | 5 | 12.5 |
To assess the effect of combination VAR and NAC on physiologic measures, changes in blood pressure, heart rate, and weight were assessed throughout the study. No changes in systolic blood pressure or weight were found during the treatment phase of the study. There was a moderate increase in both diastolic blood pressure (74.4±2.3 vs. 77.8±2.5) and heart rate (76.8±1.9 vs. 82.3±2.8) during the first 2 weeks of treatment. Any BDI total score of 16 or higher (ranges from 0-63) was reviewed by the medical clinician during the study visit. Throughout the study, only two participants had scores higher than 16, which were situational (i.e., death of a family member and anxieties surrounding unemployment). The majority of participants did not show an increase in BDI scores or report any changes in mood throughout the study. Mean BDI scores were generally low, but variable throughout the study (3.8±6.4 at Screening and 2.8±9.2 at Week 4). Additionally, no participants endorsed any suicidal ideation on the BDI (item #9) at any point during study procedures.
Smoking Outcomes
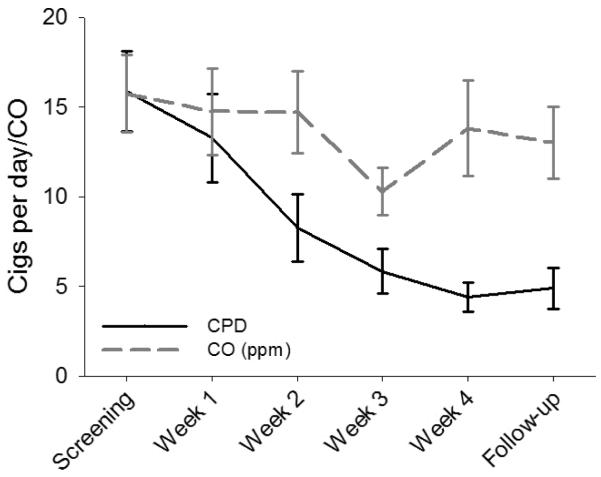
During the course of the study, participants showed a significant reduction in the mean number of self-reported cigarettes smoked per day (±SEM) from 16±2 at the screening visit to 5±1 at the follow-up visit (p<0.001). Weekly CO measures declined by greater than 30% during the Week 3 study visit but increased to near screening levels during the Week 4 visit (end of active treatment) as well as at the follow-up visit (15.8±2.1 to 13.8±2.7; p=0.504). Changes in self-reported cigarettes per day and in expired CO are shown in Figure 1. Additionally, there was a decrease in ratings on the mCEQ and QSU-B during the study. Reported mCEQ scores dropped from 1.9±0.2 at screening to 0.1±0.2 at study completion. QSU-B scores also decreased significantly from screening (4.0±0.3) to study completion (1.6±0.2). There was a moderate decline in MNWS scores during the treatment phase of the study (4.1±0.8 to 2.4±1.2). Despite reductions in cigarettes per day during the treatment phase, very few participants achieved abstinence, with 7-day point prevalence abstinence rates at the end of treatment only reaching 11% (2/19).
Figure 1.
Changes in cigarettes per day (CPD) and breath carbon monoxide (CO) during 4 weeks of active treatment and follow-up. Data are shown as model-based means and associated standard errors. PPM = Parts per million.
Discussion
The purpose of the current study was to assess the safety and feasibility of co-administering NAC and VAR in adult cigarette smokers. In this single-arm, open-label pilot study, results showed that combination NAC and VAR was well tolerated over the 4-week study period. AEs were generally mild, and no participants terminated medication. Some AEs reported here (i.e., nausea, headache, insomnia, and vivid dreams) are consistent with AEs reported in placebo-controlled trials of VAR (11), while gastrointestinal events, such as, vomiting, diarrhea, heartburn, etc., are consistent with the safety literature on NAC (27). Of the remaining reported AEs that emerged in this study, most seemed to be related to nicotine withdrawal rather than medication side effects, such as increased appetite, restlessness, and irritability (38). None of the reported AEs in the current study were unexpected. It does not appear that the addition of NAC to VAR exacerbated AEs, though proper placebo-controlled comparisons are required to support this statement. It also appears that there were no harmful interactions of VAR and NAC in the current study, suggesting that future trials could safely co-administer these medications.
Study results revealed high medication adherence (98% of scheduled doses were taken) when confirmed via medication logs and pill counts. VAR and NAC pills were taken twice daily and doses consisted of three capsules and pills (1 VAR tablet twice daily and 2 NAC capsules twice daily). Thus, our data suggest that adult cigarette smokers are willing and able to comply with the dual medication regimen, indicating that this combined pharmacotherapy is reasonable for use in future protocols. Research staff and medical clinicians carefully reviewed and encouraged medication adherence throughout the study. This provided an opportunity to discuss side effects, troubleshoot any issues with taking medication, and encourage continued compliance, which we feel contributed to excellent adherence rates. While four capsules of NAC per day was feasible for participants to take in the current study, formulations of NAC that are more bioavailable, thus requiring less drug for optimal therapeutic effects, would be ideal for future studies.
Efficacy data from the current study found a reduction in cigarettes per day, smoking reward, and smoking urges during active treatment, but continuous abstinence and point prevalence abstinence at the end of treatment was low. These results are interpreted cautiously since efficacy was not the purpose of this study, but are not surprising given previous studies that have shown similar results with VAR and NAC as monotherapies (4-6, 8-10, 17, 26). Given that participants had varying readiness to quit motivation and were provided with only minimal encouragement to quit smoking during the study, it is not unexpected that we failed to identify continuous abstinence. Also, the short half-life of CO (39) creates difficulties in accurately capturing reductions in smoking and is influenced greatly by the time since last cigarette. As such, it is also not unexpected that we failed to see reductions in CO, even with reductions in cigarettes per day. Future studies should circumvent these issues by using urinary cotinine measures, as well employing more intense counseling procedures and motivational enhancement to promote quit attempts, which were not used in the current study. Additionally, NAC may work most effectively as a relapse prevention aid (28). In this case, VAR may be most useful when administered initially to promote abstinence, followed by NAC on the target quit date under conditions of abstinence. These are questions that future studies should work to address.
This study has several important limitations that should be noted. First, this study had a small sample size (N=19), though this seems to be a reasonable number of participants to support the feasibility and safety of the co-administration of NAC and VAR. Second, this study was an open-label, single-arm pilot and did not utilize any control group to compare AEs and smoking-related measures. This open-label design cannot adequately address efficacy, and the presented efficacy results are largely exploratory. Only safety and feasibility can be adequately captured through this study design. Therefore, reductions in smoking across the course of the study should be interpreted cautiously. Third, the therapeutic utility of these results are limited given the lack of cessation support and target quit date. These aspects of study design typically used in cessation studies were intentionally avoided in the current study to simplify the protocol and expand recruitment to include smokers with varying levels of quit interest. While this design limits conclusions regarding efficacy, the primary aim of assessing safety and feasibility was still accomplished with the study design. Finally, the trial was only four weeks in duration. It is possible that with longer co-administration of NAC and VAR, more AEs may have emerged. Future efficacy studies with NAC and VAR should carefully assess outcomes for 12 weeks or longer.
Despite these limitations, the preliminary data from the current study provide the first demonstration of safety and feasibility of the co-administration of NAC and VAR in adult cigarette smokers. This preliminary study supports the need for placebo-controlled studies on NAC and VAR as a combined pharmacotherapy for smoking cessation. Next steps should include human laboratory studies assessing mechanisms of action of this combined pharmacotherapy through relapse analogs and imaging techniques. These results may then inform a smaller, randomized clinical trial to assess efficacy with the inclusion of formalized cessation counseling, behavioral support, a target quit date, and the inclusion of smokers interested in quitting.
Acknowledgements
The authors wish to acknowledge the funding sources for this study. Funding was provided by NIDA grants P50DA015369 (PI, Peter W. Kalivas) and U01DA031779 (PI, Kevin M. Gray). Additional support was also provided by the South Carolina Clinical and Translational Institute at the Medical University of South Carolina (UL1TR000062). The funding source had no role other than financial support. The authors would like to thank the medical and research staff of the Clinical Neuroscience Division at the Medical University of South Carolina. Specifically, we would like to thank Jessica Hinton, Danielle Paquette, Priscilla Muldrow, Casy Johnson, Christine Horne, Lori Ann Ueberroth, and Elisabeth Kryway for their assistance with data collection, management, study coordination, and participant safety.
Footnotes
Declaration of Interest. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper. All authors contributed to the design and execution of the study, analyses of data, and manuscript preparation. All authors have read and approved the manuscript.
References
- 1.Centers for Disease Control and Prevention Quitting smoking among adults--United States, 2001-2010. MMWR.Morbidity and mortality weekly report. 2011;60(44):1513–1519. [PubMed] [Google Scholar]
- 2.Cohen S, Lichtenstein E, Prochaska JO, Rossi JS, Gritz ER, Carr CR, Orleans CT, Schoenbach VJ, Biener L, Abrams D. Debunking myths about self-quitting. Evidence from 10 prospective studies of persons who attempt to quit smoking by themselves. The American Psychologist. 1989;44(11):1355–1365. doi: 10.1037//0003-066x.44.11.1355. [DOI] [PubMed] [Google Scholar]
- 3.Hughes JR. Motivating and helping smokers to stop smoking. Journal of general internal medicine. 2003;18(12):1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study G Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 5.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR, Varenicline Phase 3 Study G Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 6.Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, Anziano R, Reeves K. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine. 2006;166(15):1571–1577. doi: 10.1001/archinte.166.15.1571. [DOI] [PubMed] [Google Scholar]
- 7.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007;28(7):316–325. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 8.McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2013;15(1):139–148. doi: 10.1093/ntr/nts101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C. Varenicline improves mood and cognition during smoking abstinence. Biological psychiatry. 2009;65(2):144–149. doi: 10.1016/j.biopsych.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West R, Baker CL, Cappelleri JC, Bushmakin AG. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology. 2008;197(3):371–377. doi: 10.1007/s00213-007-1041-3. [DOI] [PubMed] [Google Scholar]
- 11.Cahill K, Stead L, Lancaster T. A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug safety : an international journal of medical toxicology and drug experience. 2009;32(2):119–135. doi: 10.2165/00002018-200932020-00005. [DOI] [PubMed] [Google Scholar]
- 12.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Molecular psychiatry. 2011;16(10):974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(22):9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(12):3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biological psychiatry. 2009;65(10):841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12(2):182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez-Nino AM, D'Souza MS, Markou A. N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology. 2013;225(2):473–482. doi: 10.1007/s00213-012-2837-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE. Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. The Journal of pharmacology and experimental therapeutics. 2011;337(2):487–493. doi: 10.1124/jpet.111.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63(3):338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW. Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A. 2011;108(1):385–390. doi: 10.1073/pnas.1011265108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berk M, Malhi GS, Gray LJ, Dean OM. The promise of N-acetylcysteine in neuropsychiatry. Trends in pharmacological sciences. 2013;34(3):167–177. doi: 10.1016/j.tips.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.McClure EA, Gipson CD, Malcolm RJ, Kalivas PW, Gray KM. Potential Role of N-Acetylcysteine in the Management of Substance Use Disorders. CNS drugs. 2014 doi: 10.1007/s40263-014-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. The American Journal of Psychiatry. 2012;169(8):805–812. doi: 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W. Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. European addiction research. 2011;17(4):211–216. doi: 10.1159/000327682. [DOI] [PubMed] [Google Scholar]
- 27.Grandjean EM, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clinical therapeutics. 2000;22(2):209–221. doi: 10.1016/S0149-2918(00)88479-9. [DOI] [PubMed] [Google Scholar]
- 28.LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ. A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict. 2013;22(5):443–452. doi: 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaRowe SD, Mardikian P, Malcolm R, Myrick H, Kalivas P, McFarland K, Saladin M, McRae A, Brady K. Safety and tolerability of N-acetylcysteine in cocaine-dependent individuals. The American Journal on Addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2006;15(1):105–110. doi: 10.1080/10550490500419169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 31.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. British journal of addiction. 1988;83(4):393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT, SR A, BG K. Manual for Beck Depression Inventory II (BDI-II) Psychology Corporation; San Antonio, TX: 1996. [Google Scholar]
- 33.Posner KB, Lucas C, Gould M, Stanley B, Brown G, Fisher P, Zelazny J, Burke A, Oquendo M, Mann J. Columbia-Suicide Severity Rating Scale (C-SSRS) 2007. D.
- 34.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 35.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 36.Brauer LH, Behm FM, Lane JD, Westman EC, Perkins C, Rose JE. Individual differences in smoking reward from de-nicotinized cigarettes. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2001;3(2):101–109. doi: 10.1080/14622200123249. [DOI] [PubMed] [Google Scholar]
- 37.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th American Psychiatric Publishing; Arlington, VA: 2013. [Google Scholar]
- 39.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. In Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2002;4 doi: 10.1080/14622200210123581. England. [DOI] [PubMed] [Google Scholar]