Abstract
During virus infection and autoimmune disease, inflammatory dendritic cells (iDCs) differentiate from blood monocytes and infiltrate infected tissue. Following acute infection with hepatotropic viruses, iDCs are essential for re-stimulating virus-specific CD8+ T cells and therefore contribute to virus control. Here we used the lymphocytic choriomeningitis virus (LCMV) model system to identify novel signals, which influence the recruitment and activation of iDCs in the liver. We observed that intrinsic expression of Toso (Faim3, FcμR) influenced the differentiation and activation of iDCs in vivo and DCs in vitro. Lack of iDCs in Toso-deficient (Toso–/–) mice reduced CD8+ T-cell function in the liver and resulted in virus persistence. Furthermore, Toso–/– DCs failed to induce autoimmune diabetes in the rat insulin promoter-glycoprotein (RIP-GP) autoimmune diabetes model. In conclusion, we found that Toso has an essential role in the differentiation and maturation of iDCs, a process that is required for the control of persistence-prone virus infection.
More than 500 million people worldwide suffer from chronic infections with hepatitis B or hepatitis C viruses.1 Although both viruses are poorly cytopathic, persistence of either virus can lead to chronic liver inflammation and potentially cause liversteatosis, liver cirrhosis, end-stage liver failure or hepatocellular carcinoma. Virus-specific CD8+ T cells are a major determinant governing the outcome of viral hepatitis due to their antiviral activity against virus-infected hepatocytes.2, 3, 4, 5 However, during prolonged infection, virus-specific CD8+ T cells are exhausted, resulting in their loss of function and consequently virus persistence.1, 6 Regulators influencing CD8+ T-cell function during chronic virus infection still remain ill defined.
Inflammatory dendritic cells (iDCs) can develop from a subset of monocytes recruited to the site of inflammation.7, 8 This monocyte subset is characterized by the expression of CD115+/Ly6Chi/CCR2+.7 iDCs express CD11c, CD11b, and Ly6C.9, 10, 11 IDCs that exhibit tumor necrosis factor (TNF)-α production and inducible nitric oxide synthase (iNOS) were named TNF-α and iNOS producing DCs (Tip-DCs). iDCs contribute to the elimination of pathogens following bacterial infection.12, 13, 14 During infection with influenza virus, iDCs enhance CD8+ T-cell immunopathology, but have limited impact on viral replication.11, 15 According to recent observations, chronic activation of toll-like receptor 9 leads to intrahepatic myeloid-cell aggregates (iMATE).16 These aggregates, which contain iDCs, are essential for T-cell activation and therefore participate in virus control.16 Co-stimulatory signals from either direct cell contact or from cytokines in combination with continued antigen contact in iMATEs lead to proliferation and activation of virus-specific T cells.16 These observations suggest that infiltration of professional antigen-presenting cells into target organs is important for the maintenance of strong antiviral cytotoxic CD8+ T-cell activity. Factors regulating iDC infiltration into the liver remain poorly understood.
Toso is a membrane protein whose extracellular domain has homology to the immunoglobulin variable (IgV) domains. The cytoplasmic region has partial homology to the FAST kinase (Fas-activated serine/threonine kinase).17 Toso is expressed on B cells and activated T cells17 and is overexpressed in B-cell lymphomas.18, 19 Expression of Toso can influence survival of macrophages.20 Originally, Toso was described as an inhibitor of FAS signaling.17, 21 More recently, a role of Toso in IgM binding and TNFR signaling was also demonstrated22, 23, 24 and consistently, Toso-deficient animals are protected from lipopolysaccharide (LPS)-induced septic shock.24, 25 Recently, we identified a role of Toso in the activation of granulocytes, monocytes, and DCs.26, 27, 28 During infection with Listeria, the expression of Toso regulated granulocyte function.26, 27 The role of Toso in the function of monocytes and other myeloid cells still remains to be further elucidated.
In this study, we investigated the role of Toso during chronic viral infection by using the murine lymphocytic choriomeningitis virus (LCMV). We report that Toso promotes the differentiation and maturation of iDCs at virus-infected sites, which were essential for effector CD8+ T-cell function and in accelerating the control of the virus. We further tested the role of Toso in the rat insulin promoter-glycoprotein (RIP-GP) autoimmune diabetes model and found that Toso was required to trigger diabetes in RIP-GP mice. Taken together, we have identified an essential role of Toso in the differentiation and maturation of iDCs, which is essential for the control of persistence-prone virus infection and triggering of autoimmune disease.
Results
LCMV infection induces iDCs in the liver
To investigate signals important for iDCs generation in the liver, we first infected wild-type (WT) mice with 2x106 plaque-forming units (PFU) of LCMV strain WE. In confirmation of previous reports, we found that MHC-II+ CD45+ cells infiltrate the liver between days 4 and 6 (Figure 1a). MHC-II+ cells expressed CD11c (Figure 1a). Some of the CD11c+ cells expressed iNOS (Figure 1b). This suggests that iDCs infiltrate the liver during LCMV infection. Using flow cytometric analysis, we found that in addition to CD8+ T cells, CD11b+ myeloid cells also infiltrated the liver following LCMV infection. Most of those cells expressed Ly6C, and a subpopulation expressed CD11c (Figure 1c). Quantification of the fluorescence-activated cell sorting (FACS) data showed that on day 6, a time point when CD8+ T cells infiltrate the liver, iDCs also infiltrated the liver (Figure 1d). Furthermore, inflammatory signals derived by CD8+ T cells were responsible for infiltration of iDCs because lack of CD8+ T cells limited recruitment of iDCs (Figure 1d). Taken together, these data suggest that inflammatory signals in the liver recruit and activate CD11c+ cells during LCMV infection.
Figure 1.
LCMV infection induces iDCs in the liver. C57BL/6 mice were infected with 2 × 106 PFU of LCMV-WE on day 0. (a) After 0, 4 and 6 days, the livers were removed and analyzed using histology for expression of LCMV-NP, the pan leukocyte marker CD45, MHC-II and CD11c (one representative of n=5 is shown). (b) On day 0, 4 and 6, livers were analyzed using histology for expression of CD11c and iNOS (one representative of n=5 is shown). (c) Livers from uninfected (naive) mice and day 6 infected mice were digested and cell suspensions were stained for CD8, CD11b, Ly6C and CD11c. Top panel: Dot plots show expression of CD11b and CD8. Lower panel: expression of Ly6C and CD11c for cells gated on CD11b. Gate indicates iDCs. One of three representative dot plots is shown. (d) Livers from uninfected (naive) WT mice and day 6 infected WT and CD8a–/– mice were digested and cell suspension was stained for CD8, CD11b, Ly6C and CD11c. The Graph shows the number of CD8+ T cells and iDCs (CD11b+ Ly6C+ CD11c+, n=6). Scale bars, 25 μm (main image and inset)
Toso is necessary for the development of iDCs in the liver
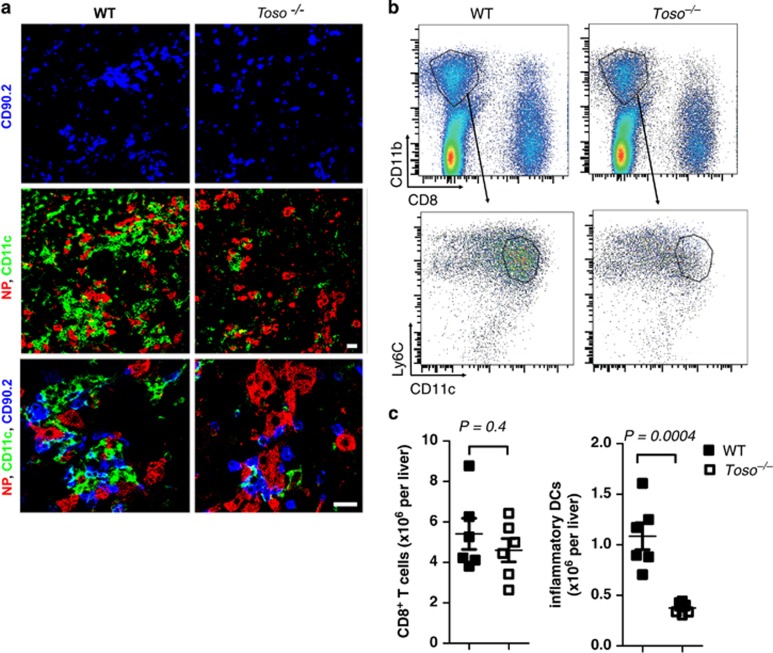
Next, we explored whether Toso influences the recruitment and differentiation of CD11c+ cells. We infected WT mice and Toso–/– mice with 2 × 106 PFU of LCMV-WE and analyzed the livers 6 days later. The infected hepatocytes in WT animals were surrounded by T cells (CD90.2+) and iDCs (Figure 2a). Toso–/– mice showed similar numbers of infected hepatocytes and CD8+ T cells (Figure 2a). In contrast to WT mice however, numbers of CD11c+ iDCs were limited in Toso–/– mice (Figure 2a). Using flow cytometric analysis we observed that iDCs were reduced in liver tissue of Toso–/– mice when compared with WT animals (Figures 2b and c), whereas CD8+ T cells on day 6 were comparable between WT and Toso–/– mice (Figure 2c). Toso was previously shown to influence signaling of IgM and CD95.17, 23, 24 To explore whether either of the signal was responsible for the lack of iDCs observed in the liver of Toso–/– mice, we analyzed mice that were deficient in soluble IgM (sIgM–/– mice) and Fas (Faslpr/lpr mice). We did not observe reduced numbers of iDCs in sIgM–/– or Faslpr/lpr mice (Supplementary Figure 1), suggesting that Toso influenced iDC generation independently of both signals. Taken together, these data indicate that iDCs infiltrate LCMV-infected liver tissue and this is dependent on the expression of the membrane protein Toso.
Figure 2.
Toso is necessary for development of iDCs in the liver. Toso–/– mice and corresponding WT control mice were infected with 2 × 106 PFU of LCMV-WE. (a) On day 6, livers were analyzed for T cells (CD90.2), dendritic cells (CD11c+) and virus-infected cells (NP+) by immunofluorescence (one representative slide of n=6 is shown). Scale bars, 20 μm. (b and c) On day 6, livers were examined for CD8+ T cells and iDCs (CD11b+ Ly6C+ CD11c+). Dot plots show expression of CD11b and CD8 (top panel), and expression of Ly6C and CD11c for cells gated on CD11b (lower panel). Statistical analysis of total CD8+ T cells and inflammatory DCs (CD11b+ Ly6C+ CD11c+) is shown (c), n=6, pooled from two independent experiments)
Intrinsic Toso expression regulates the differentiation of iDCs
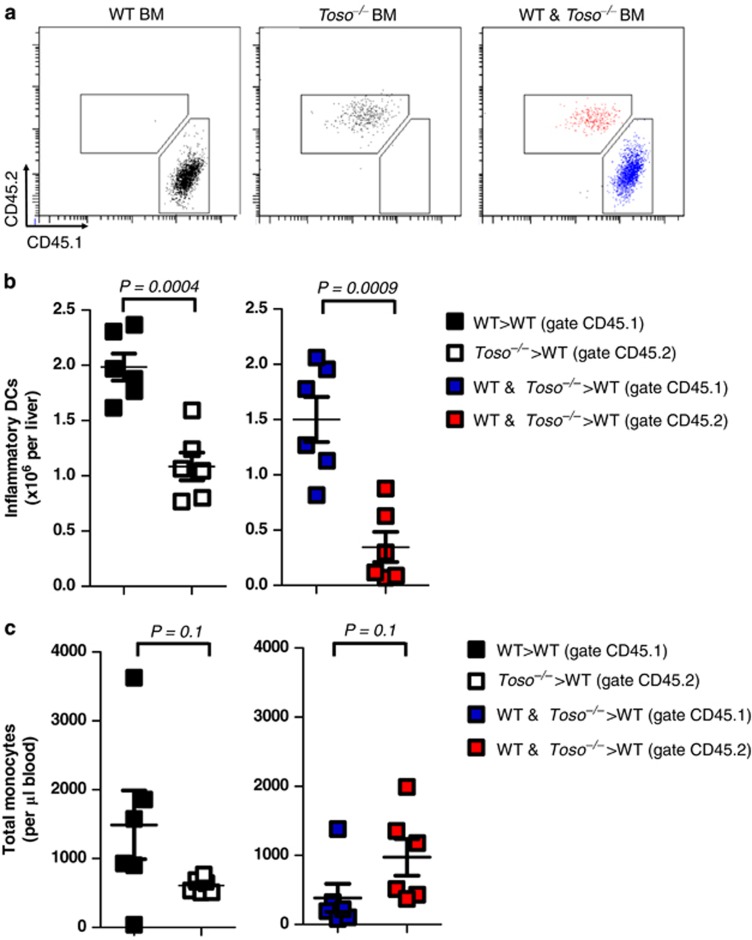
Next, we wondered whether cell intrinsic expression of Toso was critical for iDC infiltration into liver tissue. We generated mixed bone marrow chimeras by lethally irradiating recipient WT mice followed by reconstitution with either WT, Toso–/–, or a 1 : 1 mixture of WT and Toso–/– bone marrow. Cells derived from WT bone marrow were identified using the CD45.1 congenic marker. Cells derived from Toso–/– bone marrow were identified with CD45.2. Fifty days after bone marrow transplantation, mice were infected with 2 × 105 PFU of LCMV. On day 6 following infection, the number of iDCs of WT or Toso–/– origin in liver tissue was determined. As expected, iDCs in mice that received WT bone marrow were all of CD45.1 origin, whereas iDCs in mice which received Toso–/– bone marrow were all of CD45.2 origin (Figure 3a). Numbers of iDCs were reduced in mice reconstituted with Toso–/– bone marrow when compared with mice reconstituted with WT bone marrow (Figure 3b). Mixed bone marrow chimeras, which received a 1 : 1 ratio of WT and Toso–/– bone marrow, showed a reduced proportion of iDCs of Toso–/– (CD45.2) origin (Figures 3a and b). This was not due to a difference in hematopoietic cell development as no difference was observed in numbers of blood monocytes of WT (CD45.1) or Toso–/– (CD45.2) origin (Figure 3c). These data indicate that cell intrinsic Toso expression is critical for infiltration and differentiation of iDCs into the target tissue during LCMV infection.
Figure 3.
Intrinsic Toso expression regulates iDCs differentiation. CD45.2+ WT mice were irradiated and reconstituted with bone marrow from either CD45.1+ WT mice, CD45.2+ Toso–/– mice or with a 1 : 1 mixture of WT and Toso–/– bone marrow. Fifty days after bone marrow transplantation, mice were infected with 2x105 PFU of LCMV (a) representative FACS plots showing expression of CD45.2 and CD45.1 on iDCs (Ly6C+ CD11c+ cells) in the liver (n=6). (b) The total number of inflammatory DCs from the liver derived from CD45.1+ WT bone marrow and CD45.2+ Toso–/– bone marrow is shown from WT>WT chimeras (black), Toso–/–>WT chimeras (white), and WT & Toso–/–>WT chimeras (gate CD45.1=blue; gate CD45.2=red) (n=6, pooled from two independent experiments). (c) The total number of monocytes (Ly6Chi CD11b+) present per μl of blood derived from CD45.1+ WT bone marrow and CD45.2+ Toso–/– bone marrow is shown from WT>WT chimeras (black), Toso–/–>WT chimeras (white) and WT and Toso–/–>WT chimeras (gate CD45.1=blue; gate CD45.2=red) (n=6, pooled from two independent experiments)
Toso is critical for maturation of functional DCs in vitro
Next, we determined whether our in vivo phenotype was reproducible in vitro. We treated bone marrow cells with granulocyte macrophage colony-stimulating factor (GM-CSF) and measured expression of Ly6C and CD11c 6 days later. We found that cultures from WT cells showed a significantly higher percentage of CD11c+ cells when compared with bone marrow cultures from Toso–/– mice (Figures 4a and b). We did not detect a significant difference in total numbers of WT and Toso–/– CD11c+ cells/well on our day 6 DC cultures, although there is a trend of higher numbers of CD11c+ WT cells (Figure 4b). Co-staining revealed that the Ly6C-positive cells were Gr1+ CD11b+ CD80− cells (data not shown). To test the effect of Toso on the activation of DCs, we generated bone marrow DCs in vitro and activated them 9 days later with LPS. In WT mice we found that DCs expressed CD80 and CD86 and produced interleukin-6 (IL-6) after LPS challenge, whereas Toso–/– DCs displayed limited expression of CD80 and CD86 and limited production of IL-6 (Figures 4c and d). To validate the capacity of Toso–/– DCs to activate CD8+ T cells and induce co-stimulatory signals in vivo, we injected in vitro-generated bone marrow-derived dendritic cells of WT and Toso–/– mice into RIP-GP animals after pulsing with the LCMV peptides (GP33, GP276, and GP61).29 Induction of diabetes in this model depends on the induction of auto-reactive CD8+ T cells, which infiltrate the pancreas.30, 31, 32 WT DCs induced auto-reactive CD8+ T cells and mice developed diabetes (Figure 4e). In contrast, Toso–/– DCs could not induce diabetes in RIP-GP mice (Figure 4e). Next, we wanted to gain insights into the mechanism of reduced activation of Toso–/– DCs. Previously, we identified that Toso can influence nuclear factor ‘kappa-light-chain-enhancer' of activated B cells (NF-κB) signaling.26 Indeed, we found that after activation with LPS, Toso–/– DCs showed reduced phosphorylation of NF-κB p65 (Figure 4f), whereas phosphorylation of p38 and ERK was not affected by the absence of Toso (Figure 4f). In conclusion, we found that the absence of Toso on DCs resulted in reduced activation of DCs, an effect that was correlated with reduced phosphorylation of NF-κB.
Figure 4.
Toso is critical for maturation of functional dendritic cells. (a and b) Bone marrow cells from Toso–/–and corresponding WT control mice were cultured in the presence of 40 ng/ml GM-CSF. Numbers of CD11c+ cells were analyzed on day 6. Representative FACS plot (a) and quantification of CD11c+ cells in percentage of living cells (n=8) and absolute numbers of CD11c+ cells per well (b, n=4–6) are shown. (c and d) DCs were generated from WT and Toso–/–bone marrows. On day 9, DCs were stimulated with LPS. The MFI of CD80 and CD86 was analyzed on cells gated for CD11c at the indicated time points (c, n=6). Interleukin-6 was analyzed in the supernatant following 20 h stimulation with the indicated concentrations of LPS (d, a representative of two independent experiments is shown with three technical replicates). (e) WT or Toso–/–dendritic cells were generated from bone marrow cells cultured with 400 ng/ml GM-CSF. Ten days later, dendritic cells were incubated with GP33, GP276 and GP61 with or without addition of CpG (5 μM) and injected into RIP-GP mice. Induction of diabetes was analyzed (n=6). (f) Western blot of p-p65, p-p38, p-ERK and actin in bone marrow-derived dendritic cells isolated from WT and Toso–/– mice without or with stimulation with 50 ng/ml LPS at the indicated time points (one of two experiments is shown)
Toso influences function of CD8+ T cells in the liver
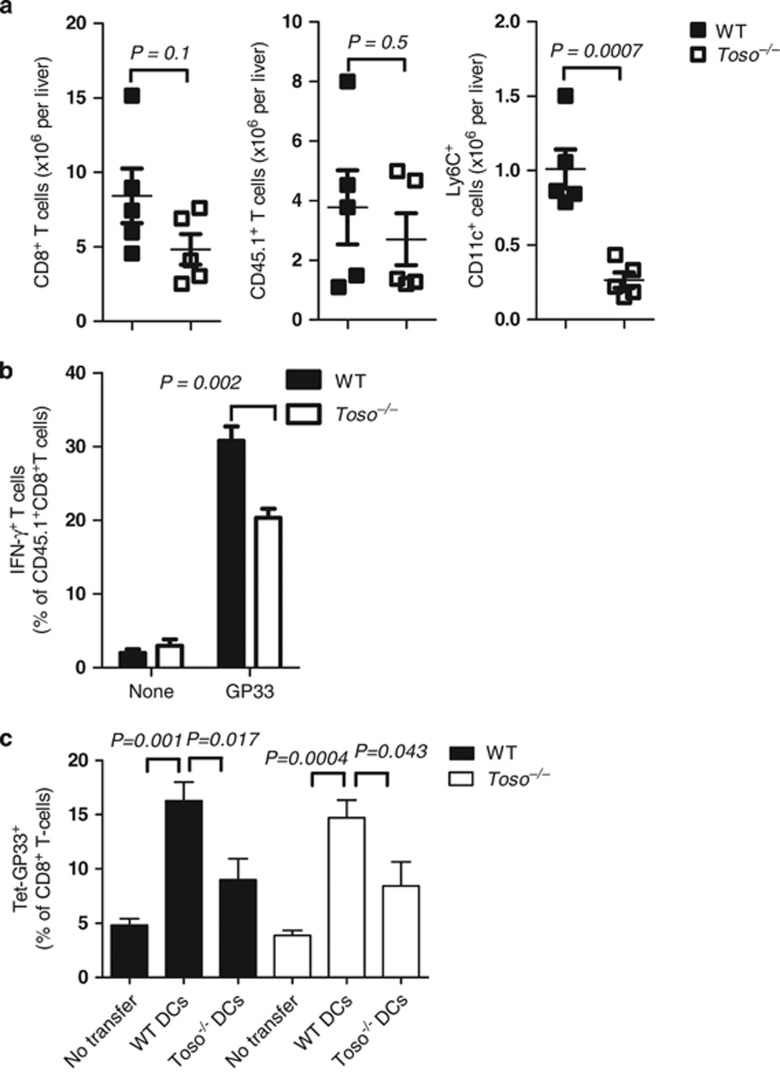
To further validate whether the lack of iDCs in Toso–/– mice results in attenuated CD8+ T-cell responses, we utilized the P14 mouse that expresses a transgenic T-cell receptor recognizing the LCMV epitope GP33.33 P14 TCR transgenic splenocytes expressing the congenic marker (CD45.1) were injected intravenously into naive CD45.2+ WT and Toso–/– recipients. Recipient mice were infected with LCMV, and the donor and host CD8+ T-cell response was monitored. Till day 6, the expansion of donor (CD45.1 positive) T cells was similar between WT and Toso–/– mice (Figure 5a). As expected, the numbers of iDCs in the liver were reduced in Toso–/– mice (Figure 5a). This strengthens our data that Toso has intrinsic functions in iDCs. Adoptively transferred virus-specific CD8+ T cells exhibited significantly reduced interferon-gamma (IFN-γ) production when transferred into Toso–/– recipients (Figure 5b). This indicates that reduced numbers and function of iDCs in Toso–/– recipient mice have an impact on CD8+ T-cell function in the liver. To analyze the direct impact of DCs on CD8+ T cells in the liver, we performed adoptive transfer experiments of WT and Toso–/– DCs into WT and Toso–/– mice. On day 6, we analyzed frequencies of virus-specific CD8+ T cells in the liver. Transfer of WT DCs enhanced the frequency of LCMV glycoprotein 33–41 (LCMV-GP33)-specific CD8+ T cells in WT mice (Figure 5c). In contrast,WT mice transferred with Toso–/– DCs showed reduced frequencies of LCMV-GP33-specific CD8+ T cells (Figure 5c). Similarly, Toso–/– mice showed increased LCMV-GP33-specific CD8+ T cells when transferred with WT DCs (Figure 5c). Transfer of Toso–/– DCs resulted in limited increase of LCMV-GP33-specific CD8+ T cells (Figure 5c). Collectively, these data demonstrate that lack of Toso in DCs rather than in T cells influences CD8+ T-cell function in the liver.
Figure 5.
Toso influences function of CD8+ T cells in the liver. 104 splenocytes from an LCMV-specific T-cell receptor transgenic mouse (P14 mouse expressing CD45.1) were injected intravenously into CD45.2+ WT and Toso–/– recipient mice on day −1. On day 0, recipient mice were infected with 2 x 106 PFU of LCMV-WE. (a) On day 6, following infection livers were analyzed for host CD8+ T cells, donor T cells (CD45.1+) and iDCs (Ly6C+CD11c+) (n=5, pooled from two independent experiments). (b) Spleen lymphocytes were re-stimulated with LCMV-derived GP33 peptide and IFN-γ produced by donor TCR transgenic CD8+ CD45.1+ cells was analyzed by intracellular staining (c), n=5, pooled from two independent experiments). (c) WT and Toso–/– mice were infected with 2 x 106 PFU of LCMV-WE. On day 3, WT and Toso–/– DCs (derived from the bone marrow) which were infected with LCMV 24 h earlier were transferred into infected mice. On day 6, frequencies of virus-specific CD8+ T cells were analyzed in the liver by GP33 tetramer staining (n=5–9)
Toso deficiency alters CD8+ T-cell-dependent virus control and immunopathology
To investigate the impact of Toso on the course of virus-induced hepatitis, virus control, and the systemic antiviral T-cell response, we infected WT and Toso–/– mice with 2 × 106 PFU LCMV-WE. On day 6, we phenotyped CD8+ T cells in the spleen and liver. WT CD8+ T cells of LCMV-infected mice showed expression of programmed cell death-1 (PD-1), CXCR3 and reduced expression of interleukin-7 receptor alpha chain (IL7Rα) when compared with CD8+ T cells of naive mice (Figure 6a). This suggests strong T-cell activation in WT animals.34 In contrast, CD8+ T cells in the spleen of Toso–/– mice showed reduced expression of PD-1, CXCR3 and higher expression of IL7Rα (Figure 6a), suggesting reduced activation of CD8+ T cells in Toso–/– mice. Accordingly, Toso–/– mice showed reduced numbers of virus-specific CD8+ T cells after day 6 when compared with WT animals (Figure 6b). This indicates that iDCs contribute to continuous activation and expansion of antiviral T cells. Consequently, Toso–/– mice failed to clear virus from blood, whereas WT mice eliminated LCMV from circulating blood 3 weeks after infection (Figure 6c). Next, we assessed liver cell damage by measuring the alanine-amino transferase (ALT) activity in the serum of LCMV-infected WT and Toso–/– mice. Consistent with the reduced T-cell function, virus-induced liver cell damage was reduced in Toso-deficient mice (Figure 6d). We speculated that the reduction in liver cell damage was due to limited T-cell function. To gain further insights we used the LCMV strain Docile, which also persists in WT mice. During infection, antiviral CD8+ T cells were again reduced in Toso–/– mice when compared with WT animals (Figure 6e). Nevertheless, LCMV established a persistent infection in WT and Toso–/– mice (Figure 6f). Consistently, the reduced CD8+ T-cell response corresponded to lower ALT levels in Toso-deficient mice when compared with WT controls (Figure 6g). Taken together, these data indicate that Toso promotes long-term in vivo CD8+ T-cell function and virus persistence.
Figure 6.
Toso deficiency alters CD8+ T-cell-dependent virus control and immunopathology. (a–c) Toso–/– mice or WT mice were infected with 2 × 106 PFU of LCMV-WE. The CD8+ T cells in the spleen and liver were analyzed by flow cytometry using the markers PD-1, CXCR3 and IL7R-α. Black line shows expression in CD8+ T cells of virus-infected WT and Toso–/– mice. Gray histograms show expression in CD8+ T cells of naive WT mice (a, n=3–6). Virus-specific CD8+ T cells were analyzed in the blood by GP33 tetramer staining (b, n=3–4). Titers of infectious virus were analyzed in the serum of LCMV-WE-infected mice (c, n=4–8, * P<0.05). At the indicated time points, serum ALT activity was analyzed (d, n=3–8, *P<0.05). (e–g) WT or Toso–/– mice were infected with 2x106 PFU of LCMV-Docile. Virus-specific CD8+ T cells were analyzed in the blood by GP33 tetramer staining (e, n=4). Titers of infectious virus were analyzed over time in the serum of WT or Toso–/– mice (f, n=3–4). At the indicated time points, serum ALT activity was analyzed (g, n=3–4, *P<0.05)
CD8+ T cells can be activated during bacterial infection in Toso –/– mice
DCs are needed for the generation of almost any CD8+ T-cell response. In contrast, iDCs in the liver impact the CD8+ T-cell response only under very specific conditions. Next, we analyzed the impact of Toso during subcutaneous bacterial infection. We infected WT and Toso–/– mice subcutaneously with Listeria-GP33. Under these conditions, Toso–/– mice generated a functional CD8+ T-cell response (Supplementary Figure 2) suggesting a specific role for Toso on the immune response during LCMV infection.
Discussion
In this study, we report a novel role of Toso in promoting effective immune responses during chronic virus infection using the well-defined LCMV model system. Infection of Toso–/–mice with LCMV led to reduced effector CD8+ T-cell function, resulting in delayed virus clearance. This did not result from an inherent defect in CD8+ T cells in the absence of Toso, but rather correlated with the impaired functionality of Toso–/– iDCs within infected liver and spleen.
iDCs in WT animals expressed high levels of CD11c, MHC-II and CD80. Some of these cells additionally expressed iNOS and TNF-α (also called Tip-DCs). CCR2 is essential for the exit of monocytes from the bone marrow into circulating blood15, 35 and thus is likely to impact iDC development. However, while CCR2 is important for release of mature monocytes from bone marrow, Toso appears to play a role in the differentiation of these cells after their entry into infected tissues. The differentiation of iDCs in lungs of influenza-infected mice has also been described.11 iDCs during influenza infection may also contribute to the development of fully functional effector CD8+ T cells.15 Although the absence of Toso may be expected to be disadvantageous for the host following infection with a cytopathic virus-like influenza, impaired function of Toso during infection with non-cytopathic viruses such as LCMV and hepatitis viruses may be beneficial for the host by preventing potentially damaging immunopathology.1, 36 Interestingly, the presence or absence of virally induced pathology following infection with persistent low cytopathic viruses such as HIV, Hepatitis B and Hepatitis C virus,1 and with cytopathic viruses such as severe acute respiratory syndrome 37, 38 and H5N1 influenza39 can differ between individuals, and the underlying cause for such differential outcomes remains unclear. We speculate that differences in expression or function of Toso may contribute to the inter-individual variability of disease outcome following viral infections.
Toso–/–mice infected with LCMV exhibited reductions in CD8+ T-cell activation as determined by decreased IFN-γ production. It remains unclear which signals contribute to the different functions of CD8+ T cells in Toso–/–mice. PD-1 is known to promote T-cell exhaustion, whereas IL7R provides essential survival signals to T cells.40 Thus, changes in the expression of these molecules on T cells or the ligands on antigen-presenting cells in Toso–/–mice may alter the ability of these cells to survive during chronic infection.41, 42, 43, 44 However, in the absence of Toso, the generation of memory T cells was impaired, suggesting that Toso, in promoting DC–T-cell interactions, impacts the generation and maintenance of effector CD8+ T cells. In humans, chronic virus infection enhances PD-1 expression and correlates with virus persistence.44, 45 Based on those findings and our own observations, we hypothesize that Toso, in addition to PD-1, may modulate T-cell effector function.
The molecular mechanisms affected by Toso signaling in DCs still remain elusive. While we identified NF-κB as an important downstream transcription factor affected by Toso expression, the upstream signaling remains unknown. Recently, Toso was discovered to be a receptor for IgM23 and while there is good evidence that IgM can activate Toso,46, 47 we also observed IgM-independent effects of Toso. Indeed, Toso has been described as influencing very diverse signaling events.17, 20, 21, 24, 48 In view of the diverse signaling pathways affected by Toso, we speculate that Toso might interfere with several pathways eventually converging on the downstream transcription factor NF-kB.
In conclusion, we have shown that Toso expression promotes functional maturation and activation of iDCs within virus-infected tissue. Impaired DC maturation resulted in impaired expansion and effector function of CD8+ T cells, increased viral replication and reduced immunopathology.
Materials and Methods
Virus, bacteria and mice
Toso–/–mice were used as previously described.26 sIgM–/– mice, lacking soluble IgM and Faslpr/lpr mice, lacking FAS signaling, were kept on C57BL/6 background. P14 mice that express LCMV-GP33-specific T-cell receptor as a transgene were used. For adoptive transfer experiments, mice congenic for CD45 (CD45.1) were used to distinguish between transferred cells and endogenous (CD45.2) cells. All experiments were performed in single ventilated cages. Animal experiments were carried out with authorization of the Veterinäramt of Nordrhein Westfalen, Germany and in accordance with the German law for animal protection and in accordance to the institutional guidelines at the Ontario Cancer Institute. LCMV strain WE was originally obtained from F. Lehmann-Grube (Heinrich Pette Institute, Hamburg, Germany) and was propagated in L929 cells. Virus titers were measured using focus-forming assays as previously described.49 Mice were infected with 2 × 106 PFUs LCMV-WE. For bacterial infections, recombinant Listeria expressing the LCMV-GP33 epitope was used. This recombinant strain of Listeria is highly attenuated compared with WT Listeria strains. For experiments with mixed chimeras, C57BL/6 mice were irradiated with 10.5 Gy. The next day, mice were reconstituted with either WT (CD45.1) bone marrow, Toso–/–(CD45.2) bone marrow or with a 1 : 1 mixture of WT (CD45.1) and Toso–/–(CD45.2) bone marrow. After 50 days, mice were infected with LCMV and analyzed 6 days post-infection.
DC cultures
To generate conventional DCs, we isolated bone marrow taken from femurs and tibias of mice. Following erythrocyte elimination, we cultured bone marrow cells in very low endotoxin Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum and 0.1% β-mercaptoethanol (β-ME) in the presence of GM-CSF. On day 3 of differentiation, an equal volume of growth medium was added. Growth medium was exchanged on day 6 of differentiation, and differentiation status was checked by FACS. On day 9 of differentiation, cells were harvested for use in stimulation experiments with LPS and for immunization of RIP-GP mice.
Histology
Histological analysis was performed on snap-frozen or formalin-fixed tissue as previously described31, 32 using anti-LCMV-NP antibody generated in-house (clone VL4, clone KL53). Antibodies against CD11b, CD11c, CD90.2 and F4/80 were purchased from eBiosciences (San Diego, CA, USA). Anti-iNOS antibody was purchased from AnaSpec (Fremont, CA, USA).
ALT
ALT levels were measured using a serum multiple biochemical analyzer (Ektachem DTSCII, Johnson & Johnson Inc., Rochester, NY, USA).
Western blot and ELISA
Proteins were isolated with trizol and solubilized with 10 M urea/50 mM DTT. Protein lysates were normalized for total protein (Bio-Rad). Proteins were analyzed by electrophoresis under denaturating conditions using 4–20% SDS Clear-PAGE and blotted onto nitrocellulose membranes (Whatman, Buckinghamshire, UK). The membranes were stained with antibodies against p-p65, p-p38, p-ERK and actin (Cell Signaling, Danvers, MA, USA). IL-6 in the serum of cultured DCs was detected using the mouse IL-6 Elisa kit (eBiosciences).
Flow cytometric analysis
Tetramers were kindly proved by the NIH. Surface and intracellular FACS staining was performed as previously described.31 For liver FACS the upper right liver lobe (lobus, representing 20% of the total liver) was digested with Liberase, DNAse (Roche, Basel, Switzerland) for 30 min at 37 °C and then smashed. Spleens were smashed without digestion. Anti-CD8a (BD Biosciences, San Jose, CA, USA), anti-PD1, anti-CXCR3, anti-IL-7 Rα, anti-CD11c, anti-CD11b, anti-Ly6C, anti-CD80, anti-CD86, anti-MHC-II (eBioscience) were used. For quantification of total cell numbers in the liver and spleen, calibrating beads were added to the cell suspensions and total numbers calculated back accordingly.
Statistical analysis
Data are expressed as mean±S.E.M. Statistically significant differences between two different groups were analyzed using Student's t-test. Analyses with several groups were tested using a one-way ANOVA with post-testing according to Bonferroni or Dunnett. Statistically significant differences between treatment groups in experiments involving more than one analysis time point were calculated using two-way ANOVA (repeated measurements). P-values <0.05 were considered statistically significant.
Acknowledgments
This study was supported by the Alexander von Humboldt Foundation (SKA2008 to KSL and SKA2010 to PAL). Furthermore, this study was supported by the German Research Council (TRR60, CRC974, LA2558-3-5/1, LA1419/5-1). This work was also supported by CIHR. DB was supported by a postdoctoral fellowship from the German Research Foundation (DFG).
Glossary
- ALT
Alanine-amino transferase
- β-ME
Beta-mercaptoethanol
- BMDC
Bone marrow-derived dendritic cell
- FACS
Fluorescence-activated cell sorting
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- GP
Glycoprotein
- IL-6
Interleukin-6
- iDC
inflammatory dendritic cell
- iNOS
inducible nitric oxide synthase
- IFN-γ
Interferon-gamma
- IL7Rα
Interleukin-7 receptor alpha chain
- iMATE
Intrahepatic myeloid cell aggregate
- LPS
Lipopolysaccharide
- LCMV
Lymphocytic choriomeningitis virus
- LCMV-GP
LCMV glycoprotein
- LCMV-GP33
LCMV glycoprotein 33–41
- MFI
Mean fluorescence intensity
- MHC
Major histocompatibility complex
- NF-κB
Nuclear factor ‘kappa-light-chain-enhancer' of activated B cells
- PD-1
Programmed cell death-1
- RIP
Rat insulin promoter
- PFU
Plaque-forming unit
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
- Tip-DCs
TNF-α and iNOS producing DCs
- WT
Wild type
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by RA Knight
Supplementary Material
References
- Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–229. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- Sobao Y, Tomiyama H, Sugi K, Tokunaga M, Ueno T, Saito S, et al. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J Hepatol. 2002;36:105–115. doi: 10.1016/s0168-8278(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B, Fowler P, Sidney J, Person J, Redeker A, Brown M, et al. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J Exp Med. 1995;181:1047–1058. doi: 10.1084/jem.181.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari C, Penna A, Bertoletti A, Valli A, Antoni AD, Giuberti T, et al. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J Immunol. 1990;145:3442–3449. [PubMed] [Google Scholar]
- Recher M, Lang KS, Navarini A, Hunziker L, Lang PA, Fink K, et al. Extralymphatic virus sanctuaries as a consequence of potent T-cell activation. Nat Med. 2007;13:1316–1323. doi: 10.1038/nm1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annu Rev Immunol. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Nakano H, Lin KL, Yanagita M, Charbonneau C, Cook DN, Kakiuchi T, et al. Blood-derived inflammatory dendritic cells in lymph nodes stimulate acute T helper type 1 immune responses. Nat Immunol. 2009;10:394–402. doi: 10.1038/ni.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, et al. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS Pathog. 6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JR, Jr., Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci USA. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters W, Scott HM, Chambers HF, Flynn JL, Charo IF, Ernst JD, et al. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer EG. Tipping the balance in favor of protective immunity during influenza virus infection. Proc Natl Acad Sci USA. 2009;106:4961–4962. doi: 10.1073/pnas.0901574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LR, Wohlleber D, Reisinger F, Jenne CN, Cheng RL, Abdullah Z, et al. Intrahepatic myeloid-cell aggregates enable local proliferation of CD8(+) T cells and successful immunotherapy against chronic viral liver infection. Nat Immunol. 2013;14:574–583. doi: 10.1038/ni.2573. [DOI] [PubMed] [Google Scholar]
- Hitoshi Y, Lorens J, Kitada SI, Fisher J, LaBarge M, Ring HZ, et al. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity. 1998;8:461–471. doi: 10.1016/s1074-7613(00)80551-8. [DOI] [PubMed] [Google Scholar]
- Pallasch CP, Schulz A, Kutsch N, Schwamb J, Hagist S, Kashkar H, et al. Overexpression of TOSO in CLL is triggered by B-cell receptor signaling and associated with progressive disease. Blood. 2008;112:4213–4219. doi: 10.1182/blood-2008-05-157255. [DOI] [PubMed] [Google Scholar]
- Proto-Siqueira R, Panepucci RA, Careta FP, Lee A, Clear A, Morris K, et al. SAGE analysis demonstrates increased expression of TOSO contributing to Fas-mediated resistance in CLL. Blood. 2008;112:394–397. doi: 10.1182/blood-2007-11-124065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigruener A, Buechler C, Bared SM, Grandl M, Aslanidis C, Ugocsai P, et al. E-LDL upregulates TOSO expression and enhances the survival of human macrophages. Biochem Biophys Res Commun. 2007;359:723–728. doi: 10.1016/j.bbrc.2007.05.169. [DOI] [PubMed] [Google Scholar]
- Song Y, Jacob CO. The mouse cell surface protein TOSO regulates Fas/Fas ligand-induced apoptosis through its binding to Fas-associated death domain. J Biol Chem. 2005;280:9618–9626. doi: 10.1074/jbc.M413609200. [DOI] [PubMed] [Google Scholar]
- Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- Kubagawa H, Oka S, Kubagawa Y, Torii I, Takayama E, Kang DW, et al. Identity of the elusive IgM Fc receptor (FcmuR) in humans. J Exp Med. 2009;206:2779–2793. doi: 10.1084/jem.20091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen XH, Lang PA, Lang KS, Adam D, Fattakhova G, Föger N, et al. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood. 2011;118:598–608. doi: 10.1182/blood-2010-10-313643. [DOI] [PubMed] [Google Scholar]
- Nguyen X, Lang PA, Lang KS, Adam D, Fattakhova G, Föger N, et al. Toso regulates death receptor-induced apoptosis by facilitating RIP1 ubiquitination. Blood. 2010;118 (3:598–608. doi: 10.1182/blood-2010-10-313643. [DOI] [PubMed] [Google Scholar]
- Lang KS, Lang PA, Meryk A, Pandyra AA, Boucher LM, Pozdeev VI, et al. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc Natl Acad Sci USA. 2013;110:2593–2598. doi: 10.1073/pnas.1222264110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang KS, Lang PA, Meryk A, Pandyra AA, Merches K, Lee KH, et al. Reply to Honjo et al.: Functional relevant expression of Toso on granulocytes. Proc Natl Acad Sci USA. 2013;110:E2542–E2543. doi: 10.1073/pnas.1306422110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D, Brüstle A, Lin GH, Lang PA, Duncan GS, Knobbe-Thomsen CB, et al. Toso controls encephalitogenic immune responses by dendritic cells and regulatory T cells. Proc Natl Acad Sci USA. 2014;111:1060–1065. doi: 10.1073/pnas.1323166111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi PS, Oehen S, Buerki K, Pircher H, Ohashi CT, Odermatt B, et al. Ablation of ‘‘tolerance'' and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- Lang KS, Recher M, Junt T, Navarini AA, Harris NL, Freigang S, et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat Med. 2005;11:138–145. doi: 10.1038/nm1176. [DOI] [PubMed] [Google Scholar]
- Honke N, et al. Usp18 driven enforced viral replication in dendritic cells contributes to break of immunological tolerance in autoimmune diabetes. PLoS pathog. 2013;9:e1003650. doi: 10.1371/journal.ppat.1003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honke N, Shaabani N, Cadeddu G, Sorg UR, Zhang DE, Trilling M, et al. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat Immunol. 2012;13:51–57. doi: 10.1038/ni.2169. [DOI] [PubMed] [Google Scholar]
- Ohashi PS, Pircher H, Mak TW, Bürki K, Zinkernagel RM, Hengartner H, et al. Ontogeny and selection of the T cell repertoire in transgenic mice. Semin Immunol. 1989;1:95–104. [PubMed] [Google Scholar]
- Cui W, Kaech SM. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol Rev. 2010;236:151–166. doi: 10.1111/j.1600-065X.2010.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, et al. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltman AM, Boonstra A, Janssen HL. Dendritic cells in chronic viral hepatitis B and C: victims or guardian angels. Gut. 59:115–125. doi: 10.1136/gut.2009.181040. [DOI] [PubMed] [Google Scholar]
- Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133:101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris JS, Cheung CY, Leung CY, Nicholls JM. Innate immune responses to influenza A H5N1: friend or foe. Trends Immunol. 2009;30:574–584. doi: 10.1016/j.it.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PA, Recher M, Haussinger D, Lang KS. Genes determining the course of virus persistence in the liver: lessons from murine infection with lymphocytic choriomeningitis virus. Cell Physiol Biochem. 2010;26:263–272. doi: 10.1159/000320549. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Harris NL, Löhning M, Junt T, et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur J Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- Radziewicz H, Hanson HL, Ahmed R, Grakoui A. Unraveling the role of PD-1/PD-L interactions in persistent hepatotropic infections: potential for therapeutic application. Gastroenterology. 2008;134:2168–2171. doi: 10.1053/j.gastro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Shima H, Takatsu H, Fukuda S, Ohmae M, Hase K, Kubagawa H, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. doi: 10.1093/intimm/dxp121. [DOI] [PubMed] [Google Scholar]
- Vire B, David A, Wiestner A. TOSO, the Fcmicro receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J Immunol. 2011;187:4040–4050. doi: 10.4049/jimmunol.1100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen XH, Fattakhova G, Lang PA, Lang KS, Adam D, Föger N, et al. Antiapoptotic function of Toso (Faim3) in death receptor signaling. Blood. 2012;119:1790–1791. doi: 10.1182/blood-2011-11-386839. [DOI] [PubMed] [Google Scholar]
- Lang KS, Recher M, Navarini AA, Freigang S, Harris NL, van den Broek M, et al. Requirement for neutralizing antibodies to control bone marrow transplantation-associated persistent viral infection and to reduce immunopathology. J Immunol. 2005;175:5524–5531. doi: 10.4049/jimmunol.175.8.5524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.