Abstract
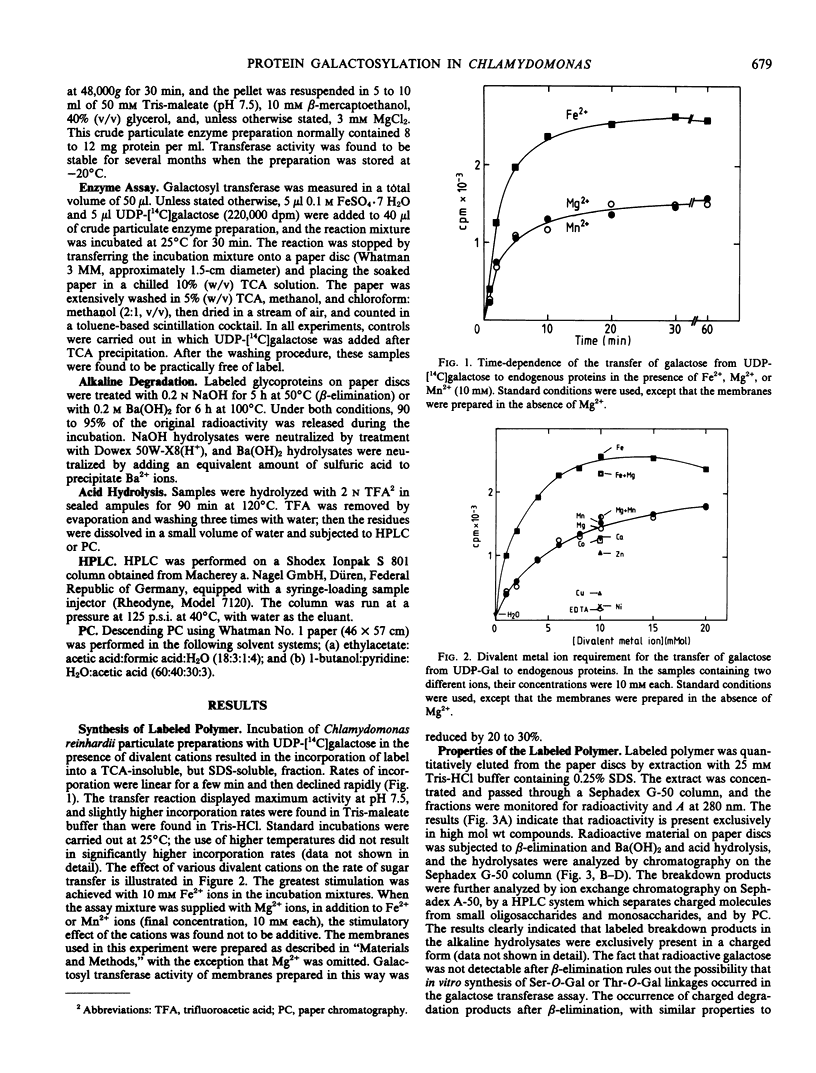
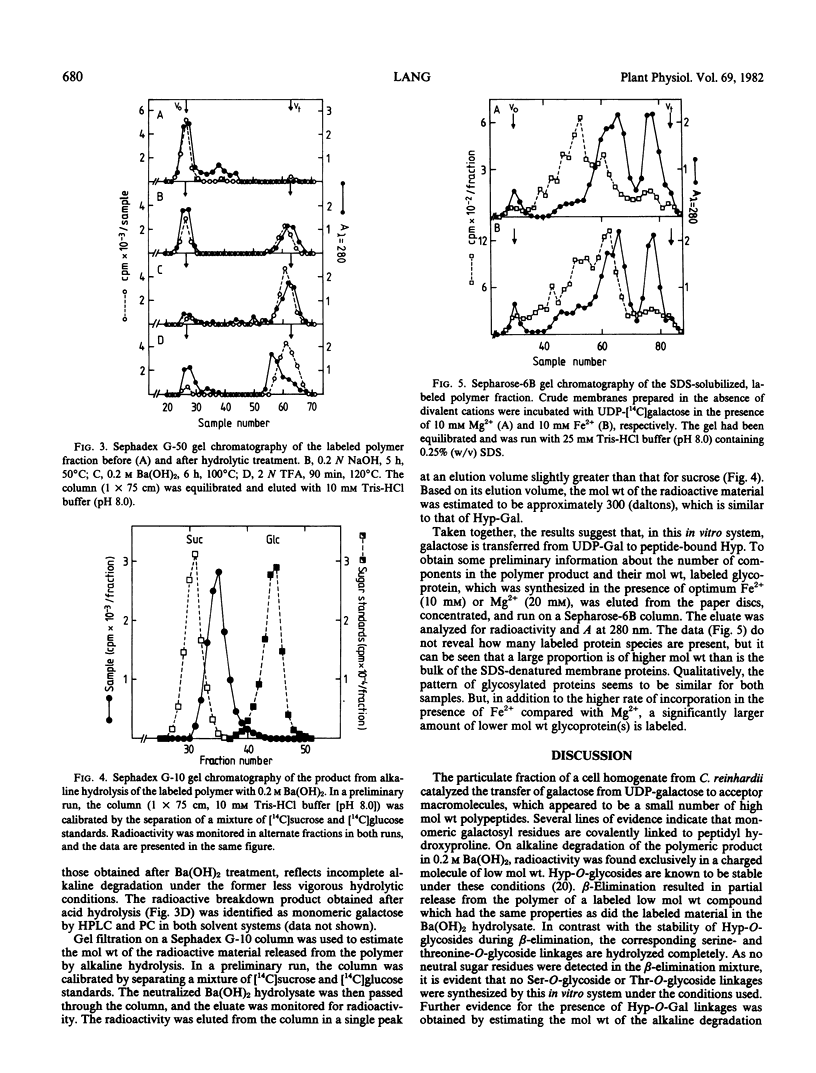
A crude membrane fraction from Chlamydomonas reinhardii was found to catalyze d-galactose transfer from UDP-galactose to endogenous proteins. Highest incorporation rates were achieved by incubation at 25°C and pH 7.5 in the presence of 10 millimolar Fe2+. Hydrolytic studies on the labeled polymer revealed that radioactivity was attached to protein via an alkali-stable and acid-labile linkage. Identification of galactose as the only labeled sugar in the acid hydrolysate and results of a tentative estimation of the molecular weight of the charged alkaline degradation product indicate that monomeric galactose units are transferred to form an O-glycosidic bond with peptidyl hydroxyproline. No indications were found for a similar linkage to serine which, in contrast to the hydroxyproline-O-glycoside linkage, is acid-stable but is cleaved by β-elimination. Chromatography of the sodium dodecyl sulfate-solubilized polymer on Sepharose-6B demonstrated that galactosyl residues are mainly associated with proteins which are of considerably higher molecular weight than are the majority of sodium dodecyl sulfate-denatured membrane proteins in this fraction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brysk M. M., Chrispeels M. J. Isolation and partial characterization of a hydroxyproline-rich cell wall glycoprotein and its cytoplasmic precursor. Biochim Biophys Acta. 1972 Feb 29;257(2):421–432. doi: 10.1016/0005-2795(72)90295-4. [DOI] [PubMed] [Google Scholar]
- Dashek W. V. Synthesis and Transport of Hydroxyproline-rich Components in Suspension Cultures of Sycamore-Maple Cells. Plant Physiol. 1970 Dec;46(6):831–838. doi: 10.1104/pp.46.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills G. J., Phillips J. M., Gay M. R., Roberts K. Self-assembly of a plant cell wall in vitro. J Mol Biol. 1975 Aug 15;96(3):431–441. doi: 10.1016/0022-2836(75)90170-9. [DOI] [PubMed] [Google Scholar]
- Karr A. L. Isolation of an enzyme system which will catalyze the glycosylation of extensin. Plant Physiol. 1972 Aug;50(2):275–282. doi: 10.1104/pp.50.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- LAMPORT D. T. Oxygen fixation into hydroxyproline of plant cell wall protein. J Biol Chem. 1963 Apr;238:1438–1440. [PubMed] [Google Scholar]
- Miller D. H., Lamport D. T., Miller M. Hydroxyproline heterooligosaccharides in Chlamydomonas. Science. 1972 May 26;176(4037):918–920. doi: 10.1126/science.176.4037.918. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Mellman I. S., Lamport D. T., Miller M. The chemical composition of the cell wall of Chlamydomonas gymnogama and the concept of a plant cell wall protein. J Cell Biol. 1974 Nov;63(2 Pt 1):420–429. doi: 10.1083/jcb.63.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D. G. Relationships between Hydroxyproline-containing Proteins Secreted into the Cell Wall and Medium by Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1977 May;59(5):894–900. doi: 10.1104/pp.59.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Comparative biochemistry of plant glycoproteins. Biochem Soc Trans. 1979 Aug;7(4):783–799. doi: 10.1042/bst0070783. [DOI] [PubMed] [Google Scholar]
- Soliday C. L., Kolattukudy P. E. Introduction of O-glycosidically linked mannose into proteins via mannosyl phosphoryl dolichol by microsomes from Fusarium soani f. pisi. Arch Biochem Biophys. 1979 Oct 15;197(2):367–378. doi: 10.1016/0003-9861(79)90258-3. [DOI] [PubMed] [Google Scholar]


